An Animal Study to Compare Hepatoprotective Effects Between Fermented Rice Bran and Fermented Rice Germ and Soybean in a Sprague-Dawley Rat Model of Alcohol-Induced Hepatic Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setting and Design
- (1)
- The 15% FRB group (n = 6): The SD rats which were given 30% ethanol (v/v) and 1.5% FRB (w/w).
- (2)
- The 30% FRB group (n = 6): The SD rats which were given 30% ethanol (v/v) and 3.0% FRB (w/w).
- (3)
- The 15% FRS group (n = 6): The SD rats which were given 30% ethanol (v/v) and 1.5% FRS (w/w).
- (4)
- The 30% FRS group (n = 6): The SD rats which were given 30% ethanol (v/v) and 3.0% FRS (w/w).
2.2. Experimental Procedures
2.2.1. Validation of an SD Rat Model of AIHI
2.2.2. Quantification of Serum Hepatic Enzymes
2.2.3. Preparation of Liver Homogenate Fractions
2.2.4. Quantification of Lipid Peroxidation
2.2.5. Quantification of Hepatic Enzymes Involved in The Alcohol Metabolism
2.2.6. Western Blot Analysis
2.2.7. Quantification of GSH
2.3. Statistical Analysis
3. Results
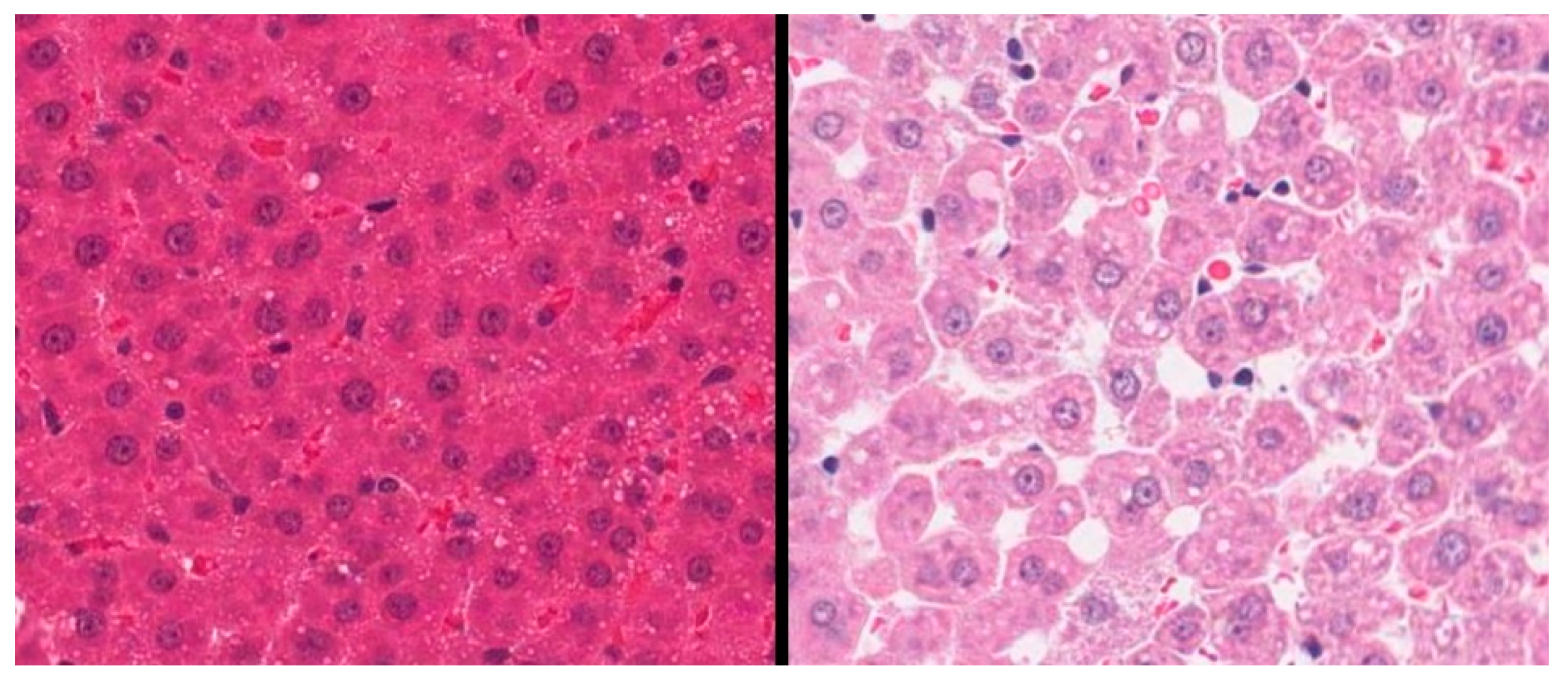
3.1. Validation of an SD Model of AIHI
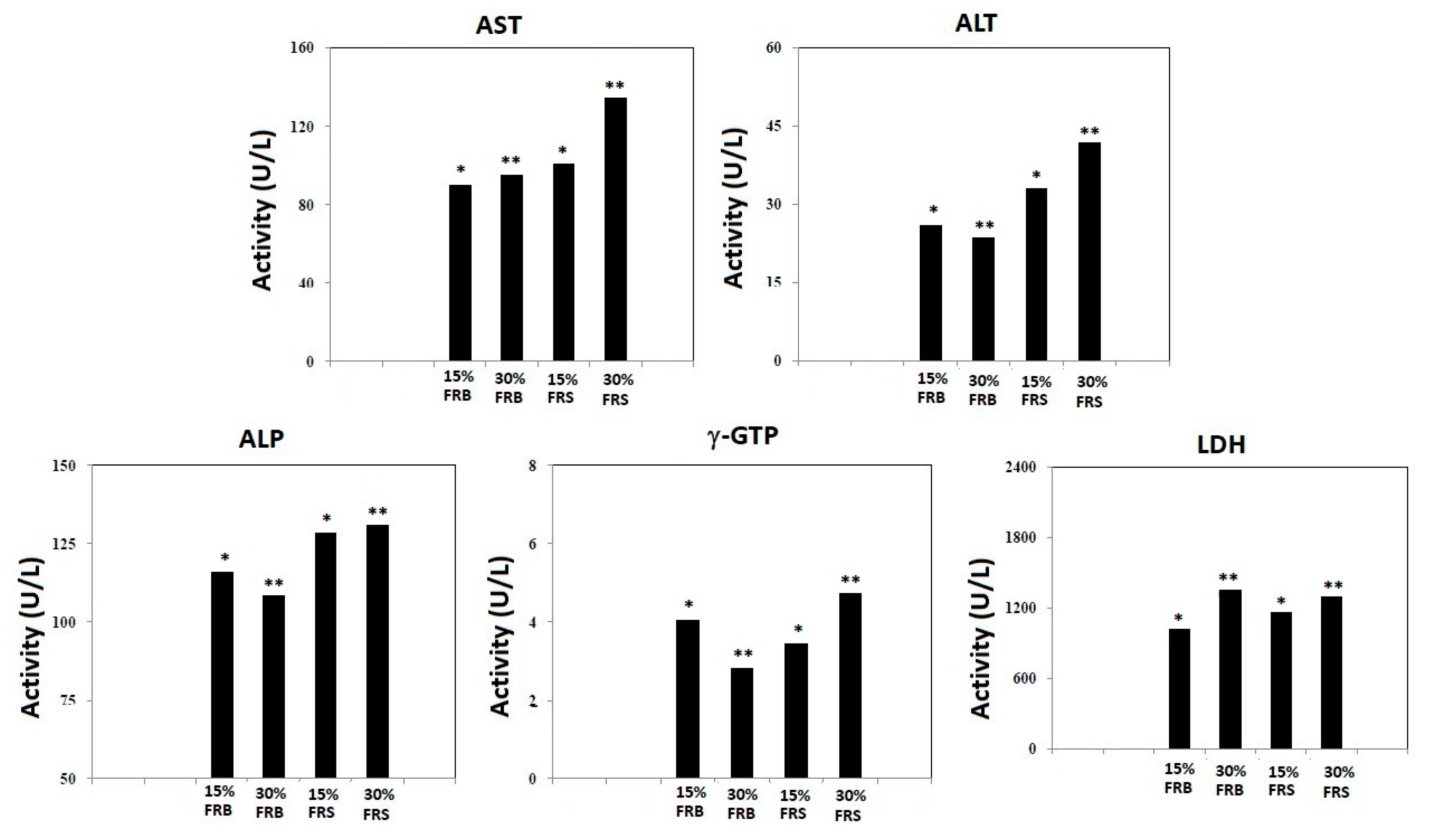
3.2. Effects of FRB and FRS on Serum Levels of ALT, AST, ALP, γ-GTP and LDH
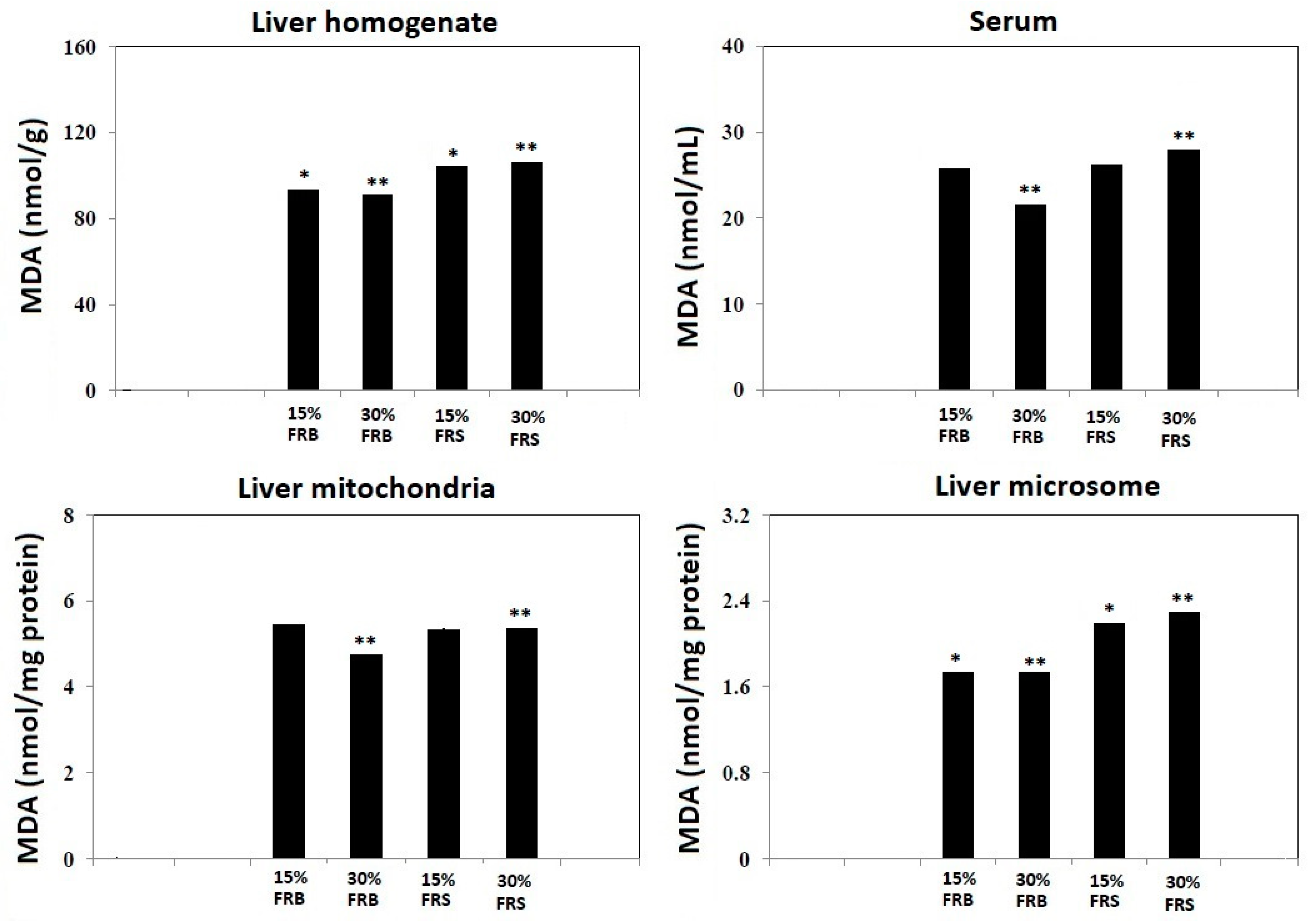
3.3. Effects of FRB and FRS on TBARS
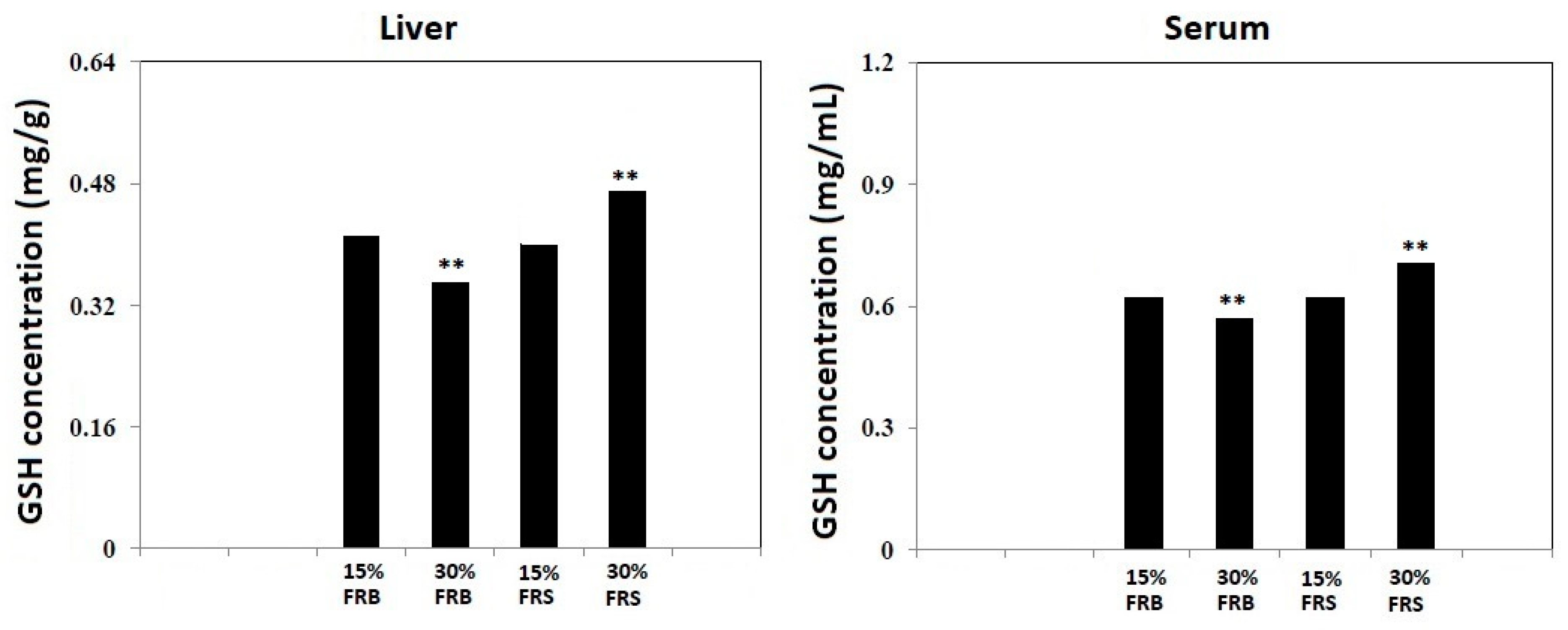
3.4. Effects of FRB and FRS on GSH
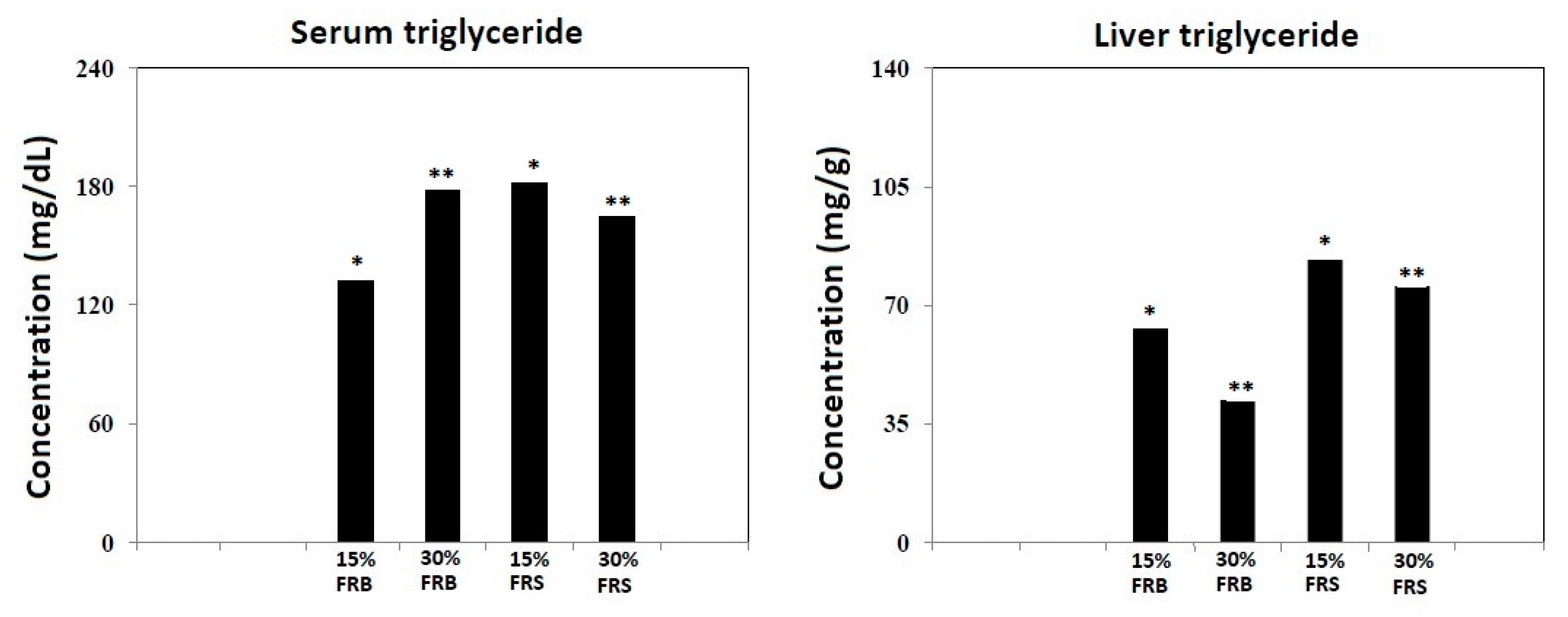
3.5. Effects of FRB and FRS on Levels of Triglyceride
3.6. Effects of FRB and FRS on Levels of ADH and ALDH
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K. Quality Ingredients and Safety Concerns for Traditional Fermented Foods and Beverages from Asia: A Review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. From Microbial Ecology to Innovative Applications in Food Quality Improvements: The Case of Sourdough as a Model Matrix. J Multidiscip. Sci. J. 2020, 3, 9–19. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented Food and Non-Communicable Chronic Diseases: A Review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef]
- Russo, P.; Fares, C.; Longo, A.; Spano, G.; Capozzi, V. Lactobacillus plantarum with Broad Antifungal Activity as a Protective Starter Culture for Bread Production. Foods 2017, 6, 110. [Google Scholar] [CrossRef]
- Berbegal, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of Autochthonous Yeasts and Bacteria in Order to Control Brettanomyces bruxellensis in Wine. Fermentation 2017, 3, 65. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.-A.; Park, G.-G.; Jang, J.-K.; Park, Y.-S. Semi-Continuous Fermentation of Onion Vinegar and Its Functional Properties. Molecules 2017, 22, 1313. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Martini, S.; Solieri, L. Bioprospecting for Bioactive Peptide Production by Lactic Acid Bacteria Isolated from Fermented Dairy Food. Fermentation 2019, 5, 96. [Google Scholar] [CrossRef]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented foods: Patented approaches and formulations for nutritional supplementation and health promotion. Recent Pat. Food Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef] [PubMed]
- de Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Verardo, V.; Gómez-Caravaca, A.M.; Tabanelli, G. Bioactive Components in Fermented Foods and Food By-Products. Foods 2020, 9, 153. [Google Scholar] [CrossRef]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Blanchard, C.L.; Schwarz, L.J.; Santhakumar, A.B. Rice Bran Phenolic Compounds Regulate Genes Associated with Antioxidant and Anti-Inflammatory Activity in Human Umbilical Vein Endothelial Cells with Induced Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 4715. [Google Scholar] [CrossRef]
- Islam, M.S.; Nagasaka, R.; Ohara, K.; Hosoya, T.; Ozaki, H.; Ushio, H.; Hori, M. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr. Top. Med. Chem. 2011, 11, 1847–1853. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; De La Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- Azizah, A.H.; Luan, Y.S. Functional properties of dietary fibre prepared from defatted rice bran. Food Chem. 2000, 68, 15–19. [Google Scholar]
- Kahlon, T.S.; Chow, F.I.; Sayre, R.N.; Betschart, A.A. Cholesterol-lowering in hamsters fed rice bran at various levels, defatted rice bran and rice bran oil. J. Nutr. 1992, 122, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.K.; Betschart, A.A.; Newman, C.W.; Hofer, P.J. Effect of full-fat or defatted rice bran on serum cholesterol. Plant Foods Hum. Nutr. 1992, 42, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, R.J.; Ausman, L.M.; Hegsted, D.M. Rice bran oil lowers serum total and low density lipoprotein cholesterol and apo B levels in nonhuman primates. Atherosclerosis 1991, 88, 133–142. [Google Scholar] [CrossRef]
- Shirakawa, H.; Shimeno, T.; Koseki, T.; Shiono, Y.; Murayama, T.; Hatakeyama, E.; Komai, M. Adenosine, an identified active component from the Driselase-treated fraction of rice bran, is effective at improving metabolic syndrome in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2009, 57, 2558–2564. [Google Scholar]
- Palou, M.; Sánchez, J.; García-Carrizo, F.; Palou, A.; Picó, C. Pectin supplementation in rats mitigates age-related impairment in insulin and leptin sensitivity independently of reducing food intake. Mol. Nutr. Food Res. 2015, 59, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and γ-oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef]
- Candiracci, M.; Justo, M.L.; Castaño, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472. [Google Scholar] [CrossRef]
- Justo, M.L.; Candiracci, M.; Dantas, A.P.; de Sotomayor, S.M.A.; Parrado, J.; Vila, E.; Herrera, M.D.; Rodriguez-Rodriguez, R. Rice bran enzymatic extract restores endothelial function and vascular contractility in obese rats by reducing vascular inflammation and oxidative stress. J. Nutr. Biochem. 2013, 24, 1453–1461. [Google Scholar] [CrossRef]
- Boonloh, K.; Kukongviriyapan, V.; Kongyingyoes, B.; Kukongviriyapan, U.; Thawornchinsombut, S.; Pannangpetch, P. Rice Bran Protein Hydrolysates Improve Insulin Resistance and Decrease Pro-inflammatory Cytokine Gene Expression in Rats Fed a High Carbohydrate-High Fat Diet. Nutrients 2015, 7, 6313–6329. [Google Scholar] [CrossRef]
- Ryan, E.P.; Heuberger, A.L.; Weir, T.L.; Barnett, B.; Broeckling, C.D.; Prenni, J.E. Rice bran fermented with saccharomyces boulardii generates novel metabolite profiles with bioactivity. J. Agric. Food Chem. 2011, 59, 1862–1870. [Google Scholar] [CrossRef]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement Altern. Med. 2016, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Yu, K.W.; Kang, D.H.; Suh, H.J. Anti-stress and anti-fatigue effect of fermented rice bran. Phytother. Res. 2002, 16, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Ogasa, S.; Kuwahara, T.; Bando, Y.; Hagiwara, M.; Arimochi, H.; Nakanishi, S.; Iwasaki, T.; Ohnishi, Y. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig. Dis. Sci. 2008, 53, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Nam, Y.; Yi, S.; Seo, M.; Lim, S. Hepatoprotective Effect of Fermented Rice Bran against Carbon Tetrachloride-Induced Toxicity in Mice. Food Sci. Biotechnol. 2014, 23, 165–171. [Google Scholar] [CrossRef]
- Lee, H.S.; Song, J.; Kim, T.M.; Joo, S.S.; Park, D.; Jeon, J.H.; Shin, S.; Park, H.K.; Lee, W.K.; Ly, S.Y.; et al. Effects of a preparation of combined glutathione-enriched yeast and rice embryo/soybean extracts on ethanol hangover. J. Med. Food 2009, 12, 1359–1367. [Google Scholar] [CrossRef]
- Ahn, H.; Choe, D.; Kim, B.; Lee, J.; Cho, Y. Bioactive Materials and Antioxidant Properties of Fermented Rice-bran Extract. J. Life Sci. 2015, 25, 1014–1020. [Google Scholar] [CrossRef][Green Version]
- Féher, J.; Lengyel, G. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr. Pharm. Biotechnol. 2012, 13, 210–217. [Google Scholar] [CrossRef]
- Comar, J.F.; de Sá-Nakanishi, A.B.; de Oliveira, A.L.; Marques, N.W.M.; Bersani, A.C.A.; Ishii, I.E.L.; Peralta, R.M.; Bracht, A. Oxidative state of the liver of rats with adjuvant-induced arthritis. Free Radic. Biol. Med. 2013, 58, 144–153. [Google Scholar] [CrossRef]
- Trejo-Solís, C.; Chagoya, D.S.V.; Aranda-Fraustro, A.; Sánchez-Sevilla, L.; Gómez-Ruíz, C.; Hernández-Muñoz, R. Inhibitory effect of vitamin e administration on the progression of liver regeneration induced by partial hepatectomy in rats. Lab. Investig. 2003, 83, 1669–1679. [Google Scholar] [CrossRef]
- Hernández-Muñoz, R.; Olguín-Martínez, M.; Aguilar-Delfín, I.; Sánchez-Sevilla, L.; García-García, N.; Díaz-Muñoz, M. Oxidant status and lipid composition of erythrocyte membranes in patients with type 2 diabetes, chronic liver damage, and a combination of both pathologies. Oxid. Med. Cell Longev. 2013, 2013, 657387. [Google Scholar] [CrossRef]
- Josan, S.; Xu, T.; Yen, Y.F.; Hurd, R.; Ferreira, J.; Chen, C.H.; Mochly-Rosen, D.; Pfefferbaum, A.; Mayer, D.; Spielman, D. In vivo measurement of aldehyde dehydrogenase-2 activity in rat liver ethanol model using dynamic MRSI of hyperpolarized [1-(13) C]pyruvate. NMR Biomed. 2013, 26, 607–612. [Google Scholar] [PubMed]
- Gong, J.; Gan, J.; Masson, E.; Syed, S.; Xia, Y.Q.; Williams, D.; Pursley, J.; Jemal, M.; Humphreys, W.G.; Iyer, R.A. Metabolic chiral inversion of brivanib and its relevance to safety and pharmacology. Drug Metab. Dispos. 2012, 40, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K.; Omori, K.; Yanaka, N. Binding and phosphorylation of a novel male germ cell-specific cGMP-dependent protein kinase-anchoring protein by cGMP-dependent protein kinase Ialpha. J. Biol. Chem. 2000, 275, 4897–4905. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.L.; Fuller, S.; Patel, M.; Kersaitis, C.; Ooi, L.; Münch, G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox. Biol. 2013, 1, 441–445. [Google Scholar] [CrossRef]
- Rusyn, I.; Bataller, R. Alcohol and toxicity. J. Hepatol. 2013, 59, 387–388. [Google Scholar] [CrossRef]
- Molina, P.E.; Gardner, J.D.; Souza-Smith, F.M.; Whitaker, A.M. Alcohol abuse: Critical pathophysiological processes and contribution to disease burden. Physiology 2014, 29, 203–215. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef]
- Capozzi, V.; Makhoul, S.; Aprea, E.; Romano, A.; Cappellin, L.; Sanchez Jimena, A.; Spano, G.; Gasperi, F.; Scampicchio, M.; Biasioli, F. PTR-MS Characterization of VOCs Associated with Commercial Aromatic Bakery Yeasts of Wine and Beer Origin. Molecules 2016, 21, 483. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Mota de Carvalho, N.; Costa, E.M.; Silva, S.; Pimentel, L.; Fernandes, T.H.; Pintado, M.E. Fermented Foods and Beverages in Human Diet and Their Influence on Gut Microbiota and Health. Fermentation 2018, 4, 90. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Suo, H.; Wang, W.; Wang, H.; Zhang, Y.; Hu, Q.; Zhao, X.; Li, J. Preventive Effects of Different Fermentation Times of Shuidouchi on Diphenoxylate-Induced Constipation in Mice. Foods 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Wu, B.-H.; Chu, Y.-L.; Chang, W.-C.; Wu, M.-C. Effects of Tempeh Fermentation with Lactobacillus plantarum and Rhizopus oligosporus on Streptozotocin-Induced Type II Diabetes Mellitus in Rats. Nutrients 2018, 10, 1143. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How Fermentation Affects the Antioxidant Properties of Cereals and Legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Ali, M.W.; Kim, I.-D.; Bilal, S.; Shahzad, R.; Saeed, M.T.; Adhikari, B.; Nabi, R.B.S.; Kyo, J.R.; Shin, D.-H. Effects of Bacterial Fermentation on the Biochemical Constituents and Antioxidant Potential of Fermented and Unfermented Soybeans Using Probiotic Bacillus subtilis (KCTC 13241). Molecules 2017, 22, 2200. [Google Scholar] [CrossRef]
- Michitaka, N.; Xiaohong, W.U.; Jer-Min, L.; Akihiko, K.; Michiteru, K.; Atsushi, T.; Toshitaka, O.; Toshihiko, O. Anti-Atherogenic Effects of Fermented Fresh Coffee Bean, Soybean and Rice Bran Extracts. Food Sci. Technol. Res. 2003, 9, 170–175. [Google Scholar]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, X.; Shang, Y.; Chen, K. Radical Scavenging Activity of Puerarin: A Theoretical Study. Antioxidants 2019, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is Chickpea a Potential Substitute for Soybean? Phenolic Bioactives and Potential Health Benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Seong, K.; Song, S.; Hwang, J.; Lee, B. Effect of rice bran and soybean fermented by Bacillus spp. on lipid profiles of liver, serum, and feces in rats fed high fat diet. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 237–242. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, S.B.; Jeon, J.J.; Kim, H.S.; Kim, S.H.; Hong, E.C.; Kang, H.K. Effects of Dietary Supplementation of Fermented Rice Bran (FRB) or Fermented Broken Rice (FBR) on Laying Performance, Egg Quality, Blood Parameter, and Cholesterol in Egg Yolk of Hy-Line Brown Laying Hens. Korean J. Poult. Sci. 2017, 44, 235–243. [Google Scholar] [CrossRef]
- Islam, J.; Koseki, T.; Watanabe, K.; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; Shirakawa, H. Dietary Supplementation of Fermented Rice Bran Effectively Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. Rice Bran Derived Bioactive Compounds Modulate Risk Factors of Cardiovascular Disease and Type 2 Diabetes Mellitus: An Updated Review. Nutrients 2019, 11, 2736. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Gunaratne, A.; Sui, Z.Q.; Corke, H. Effects of Fermented Edible Seeds and Their Products on Human Health: Bioactive Components and Bioactivities. Compr. Rev. Food Sci. Food Saf. 2017, 16, 489–531. [Google Scholar] [CrossRef]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef]
- Kuno, T.; Takahashi, S.; Tomita, H.; Hisamatsu, K.; Hara, A.; Hirata, A.; Kobayashi, H.; Mori, H. Preventive effects of fermented brown rice and rice bran against N-nitrosobis (2-oxopropyl) amine-induced pancreatic tumorigenesis in male hamsters. Oncol. Lett. 2015, 10, 3377–3384. [Google Scholar] [CrossRef]
- Yeap, S.K.; Beh, B.K.; Ali, N.M.; Mohd, Y.H.; Ho, W.Y.; Koh, S.P.; Alitheen, N.B.; Long, K. In vivo antistress and antioxidant effects of fermented and germinated mung bean. Biomed. Res. Int. 2014, 2014, 694842. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, G.W.; Lee, S.H.; Han, G.D. Ligularia fischeri extract attenuates liver damage induced by chronic alcohol intake. Pharm. Biol. 2016, 54, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Phutthaphadoong, S.; Yamada, Y.; Hirata, A.; Tomita, H.; Hara, A.; Limtrakul, P.; Iwasaki, T.; Kobayashi, H.; Mori, H. Chemopreventive effect of fermented brown rice and rice bran (FBRA) on the inflammation-related colorectal carcinogenesis in ApcMin/+ mice. Oncol. Rep. 2010, 23, 53–59. [Google Scholar] [PubMed]
- Kim, D.; Han, G.D. Ameliorating effects of fermented rice bran extract on oxidative stress induced by high glucose and hydrogen peroxide in 3T3-L1 adipocytes. Plant Foods Hum. Nutr. 2011, 66, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Casas, G.A.; Overholt, M.F.; Dilger, A.C.; Boler, D.D.; Stein, H.H. Effects of full fat rice bran and defatted rice bran on growth performance and carcass characteristics of growing-finishing pigs. J. Anim. Sci. 2018, 96, 2293–2309. [Google Scholar] [CrossRef]
- Zare-Sheibani, A.A.; Arab, M.; Zamiri, M.J.; Rezvani, M.R.; Dadpasand, M.; Ahmadi, F. Effects of extrusion of rice bran on performance and phosphorous bioavailability in broiler chickens. J. Anim. Sci. Technol. 2015, 57, 26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Law, B.M.H.; Waye, M.M.Y.; So, W.K.W.; Chair, S.Y. Hypotheses on the Potential of Rice Bran Intake to Prevent Gastrointestinal Cancer through the Modulation of Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 1352. [Google Scholar]
- Rahman, M.; Yang, D.K.; Kim, G.B.; Lee, S.J.; Kim, S.J. Nigella sativa seed extract attenuates the fatigue induced by exhaustive swimming in rats. Biomed. Rep. 2017, 6, 468–474. [Google Scholar] [CrossRef][Green Version]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A Comprehensive Review on Functional Properties of Fermented Rice Bran. Pharmacogn. Rev. 2018, 12, 218. [Google Scholar] [CrossRef]
- Seo, Y.K.; Jung, S.H.; Song, K.Y.; Park, J.K.; Park, C.S. Anti-photoaging effect of fermented rice bran extract on UV-induced normal skin fibroblasts. Eur. Food Res. Technol. 2010, 231, 163–169. [Google Scholar] [CrossRef]
- Cheng, J.; Choi, B.; Yang, S.H.; Suh, J. Effect of Fermentation on the Antioxidant Activity of Rice Bran by Monascus pilosus KCCM60084. J. Appl. Biol. Chem. 2016, 59, 57–62. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A Mini Review on Antidiabetic Properties of Fermented Foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.S.; Wung, B.S.; Lin, Y.C.; Hsieh, C.W. Isolating a cytoprotective compound from Ganoderma tsugae: Effects on induction of Nrf-2-related genes in endothelial cells. Biosci. Biotechnol. Biochem. 2009, 73, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; An, J.E.; Jeong, H.W. Study of Fermentation Extract Made from Rice Bran and Dendropanax on the Whitening Effects in B16F10 Cell Line. J. Physiol. Pathol. Korean Med. 2016, 30, 301. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Rosique-Oramas, D.; Medina-Avila, Z.; Álvarez-Torres, T.; Falcón, D.; Higuera-de la, T.F.; Béjar, Y.L.; Cordero-Pérez, P.; Muñoz-Espinosa, L.; Pérez-Hernández, J.L.; et al. Behavior of Oxidative Stress Markers in Alcoholic Liver Cirrhosis Patients. Oxid. Med. Cell Longev. 2016, 2016, 9370565. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Chen, Y.; Singh, S.; Matsumoto, A.; Manna, S.K.; Abdelmegeed, M.A.; Golla, S.; Murphy, R.C.; Dong, H.; Song, B.J.; Gonzalez, F.J.; et al. Chronic Glutathione Depletion Confers Protection against Alcohol-induced Steatosis: Implication for Redox Activation of AMP-activated Protein Kinase Pathway. Sci. Rep. 2016, 6, 29743. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Public. Health. 2010, 7, 4281–4304. [Google Scholar] [CrossRef]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Martínez, O.; Valente-Godínez, H.; Quintanar-Stephano, A.; Gasca-Martínez, D.; López-Cervantes, M.L.; Palma-Tirado, L.; de Jesús Guerrero-Carrillo, M.; Pérez-Solís, M.; Díaz-Muñoz, M. Reduced Liver Lipid Peroxidation in Subcellular Fractions Is Associated with a Hypometabolic State in Rats with Portacaval Anastomosis. Oxid. Med. Cell Longev. 2019, 2019, 4565238. [Google Scholar] [CrossRef] [PubMed]
- Srilaxmi, P.; Sareddy, G.R.; Kavi, K.P.B.; Setty, O.H.; Babu, P.P. Protective efficacy of natansnin, a dibenzoyl glycoside from Salvinia natans against CCl4 induced oxidative stress and cellular degeneration in rat liver. BMC Pharmacol. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A.; Howarth, F.C. Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell Physiol. Biochem. 2015, 35, 1241–1251. [Google Scholar] [CrossRef]
- Lamid, M.; Al-Arif, M.A.; Amin, M.; Warsito, S.H. Decreasing triglyceride, LDL―c and increasing HDL―c contents in broiler meat by partial replacement of commercial feed with fermented rice bran and turmeric flour. Biocatal. Agric. Biotechnol. 2020, 23, 101450. [Google Scholar] [CrossRef]
- Park, N.Y.; Rico, C.W.; Lee, S.C.; Kang, M.Y. Comparative effects of doenjang prepared from soybean and brown rice on the body weight and lipid metabolism in high fat-fed mice. J. Clin. Biochem. Nutr. 2012, 51, 235–240. [Google Scholar] [CrossRef][Green Version]
- Govindarajan, S.; Vellingiri, K. Effect of Red Yeast Rice and Coconut, Rice Bran or Sunflower Oil Combination in Rats on Hypercholesterolemic Diet. J. Clin. Diagn. Res. 2016, 10, BF05–BF07. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.-Y.; Cho, Y.-S. An Animal Study to Compare Hepatoprotective Effects Between Fermented Rice Bran and Fermented Rice Germ and Soybean in a Sprague-Dawley Rat Model of Alcohol-Induced Hepatic Injury. J 2020, 3, 54-66. https://doi.org/10.3390/j3010006
Ahn H-Y, Cho Y-S. An Animal Study to Compare Hepatoprotective Effects Between Fermented Rice Bran and Fermented Rice Germ and Soybean in a Sprague-Dawley Rat Model of Alcohol-Induced Hepatic Injury. J. 2020; 3(1):54-66. https://doi.org/10.3390/j3010006
Chicago/Turabian StyleAhn, Hee-Young, and Young-Su Cho. 2020. "An Animal Study to Compare Hepatoprotective Effects Between Fermented Rice Bran and Fermented Rice Germ and Soybean in a Sprague-Dawley Rat Model of Alcohol-Induced Hepatic Injury" J 3, no. 1: 54-66. https://doi.org/10.3390/j3010006
APA StyleAhn, H.-Y., & Cho, Y.-S. (2020). An Animal Study to Compare Hepatoprotective Effects Between Fermented Rice Bran and Fermented Rice Germ and Soybean in a Sprague-Dawley Rat Model of Alcohol-Induced Hepatic Injury. J, 3(1), 54-66. https://doi.org/10.3390/j3010006




