Cancer Risk in Children and Young Adults (Offspring) Born after Medically Assisted Reproduction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Source
2.2. Statistical Analysis
3. Results
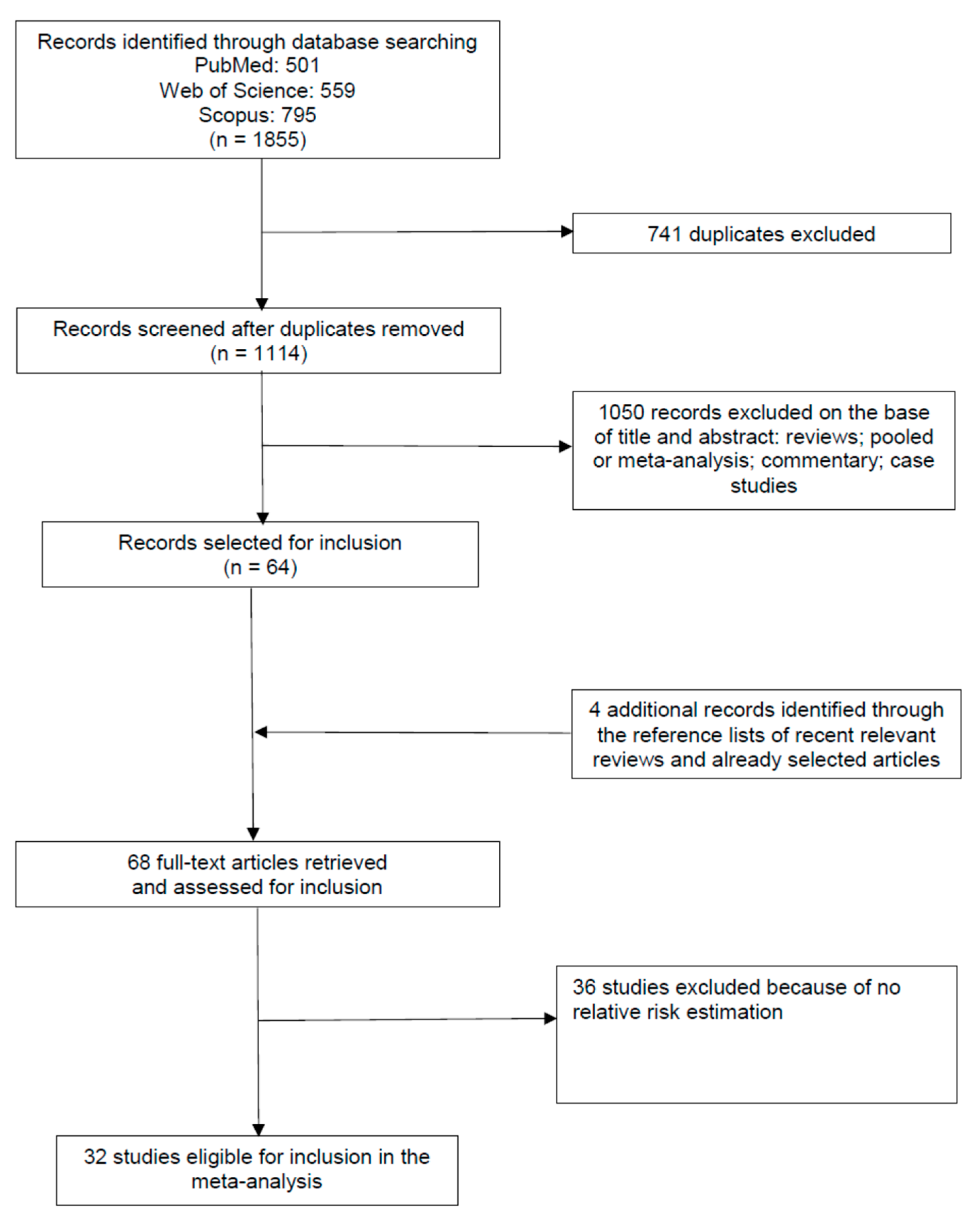
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Meta-Analysis
3.5. Overall Analysis
3.5.1. Hematological Tumors
3.5.2. Neural Tumors
3.5.3. Neuroblastoma
3.5.4. Retinoblastoma
3.5.5. Renal Tumors
3.5.6. Hepatic Tumors
3.5.7. Bone Tumors
3.5.8. Soft Tissue and Other Extraosseous Sarcomas
3.5.9. Germ Cell Tumors
3.6. Publication Bias and Sensitivity Analyses
4. Discussion
Study Strenghts and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferraretti, A.P.; Nygren, K.; Andersen, A.N.; de Mouzon, J.; Kupka, M.; Calhaz-Jorge, C.; Wyns, C.; Gianaroli, L.; Goossens, V. Trends over 15 Years in ART in Europe: An analysis of 6 million cycles. Hum. Reprod. Open 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V.; et al. ART in Europe, 2014: Results generated from European registries by ESHRE. Hum. Reprod. 2018, 33, 1586–1601. [Google Scholar] [CrossRef] [PubMed]
- Kissin, D.M.; Jamieson, D.J.; Barfield, W.D. Monitoring health outcomes of assisted reproductive technology. N. Engl. J. Med. 2014, 371, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The international glossary on infertility and fertility care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Kurinczuk, J.J.; Milne, E.; de Klerk, N.; Bower, C. Assisted reproductive technology and birth defects: A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 330–353. [Google Scholar] [CrossRef]
- Farhi, A.; Reichman, B.; Boyko, V.; Hourvitz, A.; Ron-El, R.; Lerner-Geva, L. Maternal and neonatal health outcomes following assisted reproduction. Reprod. Biomed. Online 2013, 26, 454–461. [Google Scholar] [CrossRef][Green Version]
- Liang, Y.; Chen, L.; Yu, H.; Wang, H.; Li, Q.; Yu, R.; Qin, J. Which type of congenital malformations is significantly increased in singleton pregnancies following after in vitrofertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Oncotarget 2018, 9, 4267–4278. [Google Scholar] [CrossRef]
- Pinborg, A.; Wennerholm, U.B.; Romundstad, L.B.; Loft, A.; Aittomaki, K.; Soderstrom-Anttila, V.; Nygren, K.G.; Hazekamp, J.; Bergh, C. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 87–104. [Google Scholar] [CrossRef]
- Davies, M.J.; Moore, V.M.; Willson, K.J.; Van Essen, P.; Priest, K.; Scott, H.; Haan, E.A.; Chan, A. Reproductive technologies and the risk of birth defects. N. Engl. J. Med. 2012, 366, 1803–1813. [Google Scholar] [CrossRef]
- Kettner, L.O.; Henriksen, T.B.; Bay, B.; Ramlau-Hansen, C.H.; Kesmodel, U.S. Assisted reproductive technology and somatic morbidity in childhood: A systematic review. Fertil. Steril. 2015, 103, 707–719. [Google Scholar] [CrossRef]
- Kaatsch, P. Epidemiology of childhood cancer. Cancer Treat. Rev. 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, T.J.; Roman, E. Causes of childhood leukaemia and lymphoma. Toxicol. Appl. Pharmacol. 2004, 199, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Hargreave, M.; Jensen, A.; Toender, A.; Andersen, K.K.; Kjaer, S.K. Fertility treatment and childhood cancer risk: A systematic meta-analysis. Fertil. Steril. 2013, 100, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.; Berlin, J.; Morton, S.; Olkin, I.; Williamson, G.; Rennie, D.; Moher, D.; Becker, B.; Sipe, T.; Thacker, S. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.; Ioannidis, J.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]
- Sundh, K.J.; Henningsen, A.K.A.; Källen, K.; Bergh, C.; Romundstad, L.B.; Gissler, M.; Pinborg, A.; Skjaerven, R.; Tiitinen, A.; Vassard, D.; et al. Cancer in children and young adults born after assisted reproductive technology: A nordic cohort study from the committee of nordic ART and safety (CoNARTaS). Hum. Reprod. 2014, 29, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Hargreave, M.; Jensen, A.; Nielsen, T.S.S.; Colov, E.P.; Andersen, K.K.; Pinborg, A.; Kjaer, S.K. Maternal use of fertility drugs and risk of cancer in children—A nationwide population-based cohort study in Denmark. Int. J. Cancer 2015, 136, 1931–1939. [Google Scholar] [CrossRef]
- Petridou, E.T.; Sergentanis, T.N.; Panagopoulou, P.; Moschovi, M.; Polychronopoulou, S.; Baka, M.; Pourtsidis, A.; Athanassiadou, F.; Kalmanti, M.; Sidi, V.; et al. In vitro fertilization and risk of childhood leukemia in Greece and Sweden. Pediatr. Blood Cancer 2012, 58, 930–936. [Google Scholar] [CrossRef]
- Reigstad, M.M.; Larsen, I.K.; Myklebust, T.Å.; Robsahm, T.E.; Oldereid, N.B.; Brinton, L.A.; Storeng, R. Risk of cancer in children conceived by assisted reproductive technology. Pediatrics 2016, 137, e20152061. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International classification of childhood cancer. Cancer 2005, 103, 1457–1467. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. 2015. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 14 May 2018).
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Measuring inconsistency in meta-analyses. BMJ Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thompson, S. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Roman, E.; Ansell, P.; Bull, D. Leukaemia and non-Hodgkin’s lymphoma in children and young adults: Are prenatal and neonatal factors important determinants of disease? Br. J. Cancer 1997, 76, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Doyle, P.; Bunch, K.J.; Beral, V.; Draper, G.J. Cancer incidence in children conceived with assisted reproduction technology. Lancet 1998, 352, 452–453. [Google Scholar] [CrossRef]
- Schuz, J.; Kaatsch, P.; Kaletsch, U.; Meinert, R.; Michaelis, J. Association of childhood cancer with factors related to pregnancy and birth. Int. J. Epidemiol. 1999, 2, 631–639. [Google Scholar] [CrossRef]
- Olshan, A.F.; Smith, J.; Cook, M.N.; Grufferman, S.; Pollock, B.H.; Stram, D.O.; Seeger, R.C.; Look, A.T.; Cohn, S.L.; Castleberry, R.P.; et al. Hormone and fertility drug use and the risk of neuroblastoma: A report from the children’s cancer group and the pediatric oncology group. Am. J. Epidemiol. 1999, 150, 930–938. [Google Scholar] [CrossRef]
- Bruinsma, F.; Venn, A.; Lancaster, P.; Speirs, A.; Healy, D. Incidence of cancer in children born after in-vitro fertilization. Hum. Reprod. 2000, 15, 604–607. [Google Scholar] [CrossRef][Green Version]
- Klip, H.; Burger, C.W.; de Kraker, J.; van Leeuwen, F.E. Risk of cancer in the offspring of women who underwent ovarian stimulation for IVF. Hum. Reprod. 2001, 16, 2451–2458. [Google Scholar] [CrossRef][Green Version]
- McLaughlin, C.C.; Baptiste, M.S.; Schymura, M.J.; Nasca, P.C.; Zdeb, M.S. Maternal and infant birth characteristics and hepatoblastoma. Am. J. Epidemiol. 2006, 163, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Puumala, S.E.; Ross, J.A.; Olshan, A.F.; Robison, L.L.; Smith, F.O.; Spector, L.G. Reproductive history, infertility treatment, and the risk of acute leukemia in children with down syndrome: A report from the children’s oncology group. Cancer 2007, 110, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Mallol-Mesnard, N.; Menegaux, F.; Lacour, B.; Hartmann, O.; Frappaz, D.; Doz, F.; Bertozzi, A.-I.; Chastagner, P.; Hémon, D.; Clavel, J. Birth characteristics and childhood malignant central nervous sytem tumors: The ESCALE study (French society for childhood cancer). Cancer Detect. Prev. 2008, 32, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Munzer, C.; Menegaux, F.; Lacour, B.; Valteau-Couanet, D.; Michon, J.; Coze, C.; Bergeron, C.; Auvrignon, A.; Bernard, F.; Thomas, C.; et al. Birth-related characteristics, congenital malformation, maternal reproductive history and neuroblastoma: The ESCALE study (SFCE). Int. J. Cancer 2007, 122, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Marees, T.; Dommering, C.J.; Imhof, S.M.; Kors, W.A.; Ringens, P.J.; van Leeuwen, F.E.; Moll, A.C. Incidence of retinoblastoma in Dutch children conceived by IVF: An expanded study. Hum. Reprod. 2009, 24, 3220–3224. [Google Scholar] [CrossRef]
- Källén, B.; Finnström, O.; Lindam, A.; Nilsson, E.; Nygren, K.-G.; Olausson, P.O. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics 2010, 126, 270–276. [Google Scholar] [CrossRef]
- Puumala, S.E.; Spector, L.G.; Wall, M.M.; Robison, L.L.; Heerema, N.A.; Roesler, M.A.; Ross, J.A. Infant leukemia and parental infertility or its treatment: A children’s oncology group report. Hum. Reprod. 2010, 25, 1561–1568. [Google Scholar] [CrossRef][Green Version]
- Puumala, S.E.; Ross, J.A.; Wall, M.M.; Spector, L.G. Pediatric Germ Cell Tumors and Parental Infertility and Infertility Treatment: A Children’s Oncology Group Report. Cancer Epidemiol. 2011, 35, 25–31. [Google Scholar] [CrossRef]
- Foix-L’Helias, L.; Aerts, I.; Marchand, L.; Lumbroso-Le Rouic, L.; Gauthier-Villars, M.; Labrune, P.; Bouyer, J.; Doz, F.; Kaminski, M. Are children born after infertility treatment at increased risk of retinoblastoma? Hum. Reprod. 2012, 27, 2186–2192. [Google Scholar] [CrossRef]
- Puumala, S.E.; Ross, J.A.; Feusner, J.H.; Tomlinson, G.E.; Malogolowkin, M.H.; Krailo, M.D.; Spector, L.G. Parental infertility, infertility treatment and hepatoblastoma: A report from the children’s oncology group. Hum. Reprod. 2012, 27, 1649–1656. [Google Scholar] [CrossRef][Green Version]
- Rudant, J.; Amigou, A.; Orsi, L.; Althaus, T.; Leverger, G.; Baruchel, A.; Bertrand, Y.; Nelken, B.; Plat, G.; Michel, G.; et al. Fertility treatments, congenital malformations, fetal loss, and childhood acute leukemia: The ESCALE study (SFCE). Pediatr. Blood Cancer 2013, 60, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Bunch, K.J.; Stiller, C.A.; Murphy, M.F.G.; Botting, B.J.; Wallace, W.H.; Davies, M.; Sutcliffe, A.G. Cancer risk among children born after assisted conception. N. Engl. J. Med. 2013, 369, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Hargreave, M.; Jensen, A.; Deltour, I.; Brinton, L.A.; Andersen, K.K.; Kjaer, S.K. Increased risk for cancer among offspring of women with fertility problems. Int. J. Cancer 2013, 133, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Ajrouche, R.; Rudant, J.; Orsi, L.; Petit, A.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bergeron, C.; Ducassou, S.; et al. Maternal reproductive history, fertility treatments and folic acid supplementation in the risk of childhood acute leukemia: The ESTELLE study. Cancer Causes Control 2014, 25, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Luke, B.; Brown, M.B.; Wantman, E.; Richardson, M.; Baker, V.L.; Letterie, G.S.; Schymura, M.J. ART and the risk of childhood cancer—Preliminary results. Fertil. Steril. 2014, 102, e51. [Google Scholar] [CrossRef]
- Spaan, M.; van den Belt-Dusebout, A.W.; van den Heuvel-Eibrink, M.M.; Hauptmann, M.; Lambalk, C.B.; Burger, C.W.; van Leeuwen, F.E.; OMEGA-Steering Group. Risk of cancer in children and young adults conceived by assisted reproductive technology. Hum. Reprod. 2019, 34, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Lerner-Geva, L.; Boyko, V.; Ehrlich, S.; Mashiach, S.; Hourvitz, A.; Haas, J.; Margalioth, E.; Levran, D.; Calderon, I.; Orvieto, R.; et al. Possible risk for cancer among children born following assisted reproductive technology in Israel. Pediatr. Blood Cancer 2017, 64, e26292. [Google Scholar] [CrossRef]
- Wainstock, T.; Walfisch, A.; Shoham-Vardi, I.; Segal, I.; Harlev, A.; Sergienko, R.; Landau, D.; Sheiner, E. Fertility treatments and pediatric neoplasms of the offspring: Results of a population-based cohort with a median follow-up of 10 years. Am. J. Obstet. Gynecol. 2017, 216, 314. [Google Scholar] [CrossRef]
- Williams, C.L.; Bunch, K.J.; Murphy, M.F.G.; Stiller, C.A.; Botting, B.J.; Wallace, W.H.; Davies, M.C.; Sutcliffe, A.G. Cancer risk in children born after donor ART. Hum. Reprod. 2018, 33, 140–146. [Google Scholar] [CrossRef]
- Brinton, L.A.; KrügerKjær, S.; Thomsen, B.L.; Sharif, H.F.; Graubard, B.I.; Olsen, J.H.; Bock, J.E. Childhood tumor risk after treatment with ovulation-stimulating drugs. Fertil. Steril. 2004, 81, 1083–1091. [Google Scholar] [CrossRef]
- Michalek, A.M.; Buck, G.M.; Nasca, P.C.; Freedman, A.N.; Baptiste, M.S.; Mahoney, M.C. Gravid health status, medication use, and risk of neuroblastoma. Am. J. Epidemiol. 1996, 143, 996–1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wennerholm, U.B.; Jerhamre-Sundh, K.; Henningsen, A.K.; Romundstad, L.B.; Gissler, M.; Pinborg, A.; Tiitinen, A.; Skjaerven, R.; Bergh, C. Risk of cancer in children and young adults born after ART: A nordic cohort study from the CoNARTaSgroup. Am. J. Obstet. Gynecol. 2014, 210, S54. [Google Scholar] [CrossRef]
- Qin, J.B.; Sheng, X.Q.; Wu, D.; Gao, S.Y.; You, Y.P.; Yang, T.B.; Wang, H. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Amoako, A.A.; Nafee, T.M.; Ola, B. Epigenetic influences during the periconception period and assisted reproduction. Adv. Exp. Med. Biol. 2017, 1014, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Fauque, P.; Jouannet, P.; Lesaffre, C.; Ripoche, M.-A.; Dandolo, L.; Vaiman, D.; Jammes, H. Assisted reproductive technology affects developmental kinetics, H19 imprinting control region methylation and H19 gene expression in individual mouse embryos. BMC Dev. Biol. 2007, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Doornbos, M.E.; Maas, S.M.; McDonnell, J.; Vermeiden, J.P.W.; Hennekam, R.C.M. Infertility, assisted reproduction technologies and imprinting disturbances: A Dutch study. Hum. Reprod. 2007, 22, 2476–2480. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Bergmann, M.; Bohle, R.M.; Weidner, W.; Steger, K. Genetic imprinting during impaired spermatogenesis. Mol. Hum. Reprod. 2006, 12, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B.; Ludwig, M. Assisted reproduction: The epigenetic perspective. Hum. Reprod. Update 2005, 11, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, M.; Ferguson-Smith, A.C. DNA methylation in genomic imprinting, development, and disease. J. Pathol. 2001, 195, 97–110. [Google Scholar] [CrossRef]
- Lazaraviciute, G.; Kauser, M.; Bhattacharya, S.; Haggarty, P.; Bhattacharya, S. A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Hum. Reprod. Update 2014, 20, 840–852. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Yang, T.; Wang, L.; Zhao, L.; Zhang, S.; Ye, Z.; Chen, L.; Zheng, Z.; Qin, J. Cancer risk among children conceived by fertility treatment. Int. J. Cancer 2019, 144, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, B.D.; Jick, H. In vitro fertilization and childhood retinoblastoma. Br. J. Clin. Pharmacol. 2004, 58, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Källén, B.; Finnström, O.; Nygren, K.-G.; Olausson, P.O. In vitro fertilization in Sweden: Child morbidity including cancer risk. Fertil. Steril. 2005, 84, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Lerner-Geva, L.; Toren, A.; Chetrit, A.; Modan, B.; Mandel, M.; Rechavi, G.; Dor, J. The risk for cancer among children of women who underwent in vitro fertilization. Cancer 2000, 88, 2845–2847. [Google Scholar] [CrossRef]
- Lidegaard, O.; Pinborg, A.; Andersen, A.N. Imprinting diseases and IVF: Danish national IVF cohort study. Hum. Reprod. 2005, 20, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Loft, A.; Aaris Henningsen, A.K.; Rasmussen, S.; Andersen, A.N. Infant outcome of 957 singletons born after frozen embryo replacement: The Danish national cohort study 1995–2006. Fertil. Steril. 2010, 94, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Pankratz, N.; Marcotte, E.L. Genetic and nongenetic risk factors for childhood cancer. Pediatr. Clin. N. Am. 2015, 62, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, F.M.; Perquin, D.A.M.; Donker, D.; Keirse, M.J.N.C. Perinatal outcome of singletons and twins after assisted conception: A systematic review of controlled studies. BMJ 2004, 328, 261. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Han, Z.; Mulla, S.; Ohlsson, A.; Beyene, J.; Murphy, K.E.; Knowledge Synthesis Group. Preterm birth and low birth weight among in vitro fertilization twins: A systematic review and meta-analyses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 148, 105–113. [Google Scholar] [CrossRef]
- Spector, L.G.; Puumala, S.E.; Carozza, S.E.; Chow, E.J.; Fox, E.E.; Horel, S.; Johnson, K.J.; McLaughlin, C.C.; Reynolds, P.; Behren, J.V.; et al. Cancer risk among children with very low birth weights. Pediatrics 2009, 124, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Johnson, K.J.; Soler, J.T.; Puumala, S.E. Perinatal risk factors for hepatoblastoma. Br. J. Cancer 2008, 98, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
| Models | Studies | Effect Size | Combined Risk Estimate | Test of Heterogeneity | Publication Bias | ||||
|---|---|---|---|---|---|---|---|---|---|
| MAR | N° | N° | Value (95% CI) | p | Q | I2% | p | p (Egger Test) | p (Begg Test) |
| All cancer sites | 33 | 218 | 1.21 (1.14–1.28) | <0.0001 | 342.30 | 36.61 | <0.0001 | 0.101 | 0.897 |
| Study design | |||||||||
| Case-control | 15 | 73 | 1.16 (1.04–1.30) | 0.010 | 100.92 | 28.66 | 0.014 | 0.070 | 0.329 |
| Cohort | 18 | 145 | 1.22 (1.14–1.31) | <0.0001 | 240.80 | 40.20 | <0.0001 | 0.019 | 0.603 |
| Cohort excluding William et al., 2013 | 17 | 128 | 1.20 (1.12–1.28) | <0.0001 | 192.02 | 33.86 | <0.0001 | 0.098 | 0.993 |
| Age | |||||||||
| <6 years | 5 | 11 | 1.06 (0.74–1.52) | 0.735 | 20.08 | 50.20 | 0.029 | 0.844 | 0.436 |
| <15 years | 22 | 128 | 1.20 (1.09–1.32) | 0.0001 | 235.80 | 46.16 | <0.0001 | 0.336 | 0.699 |
| All others | 11 | 90 | 1.20 (1.13–1.28) | <0.0001 | 106.50 | 16.43 | 0.100 | 0.108 | 0.840 |
| All cancers (not specified) | 16 | 43 | 1.09 (1.03–1.16) | 0.005 | 47.27 | 11.15 | 0.266 | 0.771 | 0.746 |
| Study design | |||||||||
| Case-control | 0 | 0 | - | - | - | - | - | - | - |
| Cohort | 16 | 43 | 1.09 (1.03–1.16) | 0.005 | 47.27 | 11.15 | 0.266 | 0.771 | 0.746 |
| Age | |||||||||
| <6 years | 1 | 3 | - | - | - | - | - | - | - |
| <15 years | 9 | 14 | 1.00 (0.90–1.11) | 0.989 | 10.55 | 0.00 | 0.649 | 0.182 | 0.324 |
| All others | 8 | 29 | 1.13 (1.05–1.23) | 0.001 | 33.32 | 15.97 | 0.224 | 0.505 | 0.896 |
| ART | |||||||||
| All cancer sites | 20 | 95 | 1.34 (1.22–1.47) | <0.0001 | 160.36 | 41.38 | <0.0001 | 0.003 | 0.293 |
| Study design | |||||||||
| Case-control | 6 | 13 | 1.29 (1.02–1.63) | 0.036 | 9.46 | 0.00 | 0.663 | 0.490 | 0.180 |
| Cohort | 14 | 82 | 1.35 (1.22–1.50) | <0.0001 | 150.71 | 46.25 | <0.0001 | 0.003 | 0.203 |
| Age | |||||||||
| <6 years | 2 | 2 | 1.40 (0.78–2.50) | 0.258 | 0.01 | 0.00 | 0.934 | - | - |
| <15 years | 15 | 72 | 1.30 (1.16–1.47) | <0.0001 | 132.67 | 46.48 | <0.0001 | 0.009 | 0.201 |
| All others | 5 | 23 | 1.38 (1.21–1.56) | <0.0001 | 24.46 | 10.05 | 0.324 | 0.040 | 0.895 |
| All others excluding Wennerholm et al., 2014 | 4 | 21 | 1.44 (1.25–1.66) | <0.0001 | 19.92 | 0.00 | 0.463 | 0.240 | 0.904 |
| All cancers (not specified) | 13 | 15 | 1.11 (1.02–1.20) | 0.015 | 11.76 | 0.00 | 0.625 | 0.524 | 0.520 |
| Study design | |||||||||
| Case-control | 0 | 0 | - | - | - | - | - | - | - |
| Cohort | 13 | 15 | 1.11 (1.02–1.20) | 0.015 | 11.76 | 0.00 | 0.625 | 0.524 | 0.520 |
| Age | |||||||||
| <6 years | 0 | 0 | - | - | - | - | - | - | - |
| <15 years | 8 | 10 | 1.03 (0.92–1.14) | 0.623 | 4.97 | 0.00 | 0.837 | 0.927 | 0.929 |
| All others | 5 | 5 | 1.22 (1.00–1.38) | 0.002 | 2.45 | 0.00 | 0.654 | 0.058 | 0.142 |
| IVF | |||||||||
| All cancer sites | 10 | 18 | 1.41 (1.11–1.78) | 0.005 | 22.57 | 24.67 | 0.164 | 0.963 | 0.570 |
| Study design | |||||||||
| Case-control | 5 | 12 | 1.28 (1.00–1.63) | 0.051 | 9.39 | 0.00 | 0.586 | 0.493 | 0.170 |
| Cohort | 5 | 6 | 1.80 (1.05–3.09) | 0.031 | 11.37 | 56.01 | 0.045 | 0.530 | 0.573 |
| Age | |||||||||
| <6 years | 1 | 1 | - | - | - | - | - | - | - |
| <15 years | 9 | 17 | 1.37 (1.07–1.76) | 0.012 | 21.93 | 27.05 | 0.145 | 0.994 | 0.742 |
| All others | 1 | 1 | - | - | - | - | - | - | - |
| All cancers (not specified) | 3 | 3 | 1.16 (0.68–1.98) | 0.598 | 2.61 | 23.47 | 0.271 | 0.923 | 0.602 |
| Study design | |||||||||
| Case-control | - | - | - | - | - | - | - | - | - |
| Cohort | 3 | 3 | 1.16 (0.68–1.98) | 0.598 | 2.61 | 23.47 | 0.271 | 0.923 | 0.602 |
| Age | |||||||||
| <6 years | - | - | - | - | - | - | - | - | - |
| <15 years | 2 | 2 | 0.87 (0.49–1.54) | 0.632 | 0.002 | 0.00 | 0.881 | - | - |
| All others | 1 | 1 | - | - | - | - | - | - | - |
| Models | Studies | Effect Size | Combined Risk Estimate | Test of Heterogeneity | Publication Bias | ||||
|---|---|---|---|---|---|---|---|---|---|
| MAR | N° | N° | Value (95% CI) | p | Q | I2% | p | p (Egger Test) | p (Begg Test) |
| Hematological Tumors | 16 | 70 | 1.21 (1.07–1.36) | 0.002 | 120.57 | 42.77 | 0.0001 | 0.541 | 0.883 |
| -Hematopoietic | 1 | 3 | - | - | - | - | - | - | - |
| -Lymphatic and hematopoietic tissue | 1 | 1 | - | - | - | - | - | - | - |
| Leukemias (ICCC-3 I) | 14 | 59 | 1.17 (1.03–1.34) | 0.018 | 111.22 | 47.85 | <0.0001 | 0.263 | 0.534 |
| -Leukemia | 14 | 26 | 1.13 (0.96–1.34) | 0.135 | 47.16 | 46.99 | 0.005 | 0.208 | 0.567 |
| -Other leukemia | 1 | 1 | - | - | - | - | - | - | - |
| -non-ALL | 2 | 2 | 1.34 (0.38–4.69) | 0.651 | 0.65 | 0.00 | 0.420 | - | - |
| -ALL | 7 | 18 | 1.07 (0.82–1.40) | 0.632 | 39.93 | 57.43 | 0.001 | 0.096 | 0.088 |
| -AML | 5 | 12 | 1.41 (1.02–1.95) | 0.039 | 10.22 | 0.00 | 0.511 | 0.693 | 0.583 |
| Lymphomas (ICCC-3 II) | 5 | 7 | 1.22 (0.88–1.67) | 0.232 | 7.04 | 14.83 | 0.317 | 0.203 | 0.024 |
| -Lymphoma | 4 | 4 | 1.13 (0.84–1.53) | 0.412 | 2.96 | 0.00 | 0.397 | 0.249 | 0.042 |
| -Lymphoma NH | 2 | 2 | 0.91 (0.44–1.88) | 0.802 | 0.01 | 0.00 | 0.930 | - | - |
| -Lymphoma Hodgkins | 1 | 1 | - | - | - | - | - | - | - |
| Hematological | 16 | 70 | 1.21 (1.07–1.36) | 0.002 | 120.57 | 42.77 | 0.0001 | 0.541 | 0.883 |
| Study design | |||||||||
| Case-control | 7 | 49 | 1.12 (0.95–1.32) | 0.164 | 88.93 | 46.03 | 0.0003 | 0.072 | 0.648 |
| Cohort | 9 | 21 | 1.32 (1.11–1.56) | 0.001 | 31.39 | 36.28 | 0.050 | 0.128 | 0.415 |
| Age | |||||||||
| <6 years | 1 | 3 | - | - | - | - | - | - | - |
| <15 years | 11 | 51 | 1.09 (0.94–1.26) | 0.255 | 91.57 | 45.39 | 0.0003 | 0.097 | 0.559 |
| All others | 5 | 19 | 1.50 (1.25–1.81) | <0.0001 | 22.90 | 21.41 | 0.194 | 0.038 | 0.074 |
| All others excluding Hargreave et al., 2013 | 4 | 16 | 1.77 (1.40–2.25) | <0.0001 | 15.95 | 5.96 | 0.385 | 0.466 | 0.242 |
| Leukemias (ICCC-3 I) | 16 | 59 | 1.17 (1.03–1.34) | 0.018 | 111.22 | 47.85 | <0.0001 | 0.263 | 0.534 |
| Study design | |||||||||
| Case-control | 8 | 48 | 1.13 (0.96–1.33) | 0.156 | 88.39 | 46.83 | 0.0002 | 0.078 | 0.625 |
| Cohort | 8 | 11 | 1.29 (1.01–1.64) | 0.038 | 22.78 | 56.11 | 0.012 | 0.574 | 0.876 |
| Age | |||||||||
| <6 years | 1 | 3 | - | - | - | - | - | - | - |
| <15 years | 12 | 48 | 1.08 (0.93–1.26) | 0.300 | 89.25 | 47.34 | 0.0002 | 0.087 | 0.516 |
| All others | 4 | 11 | 1.61 (1.22–2.14) | 0.001 | 13.31 | 38.71 | 0.091 | 0.167 | 0.186 |
| ALL | 7 | 18 | 1.07 (0.82–1.40) | 0.632 | 39.93 | 57.43 | 0.001 | 0.096 | 0.088 |
| Study design | |||||||||
| Case-control | 5 | 16 | 1.04 (0.76–1.42) | 0.829 | 39.92 | 62.42 | 0.0004 | 0.118 | 0.150 |
| Cohort | 2 | 2 | 1.18 (0.79–1.78) | 0.415 | 0.01 | 0.00 | 0.937 | ||
| Age | |||||||||
| <6 years | 1 | 1 | - | - | - | - | - | - | - |
| <15 years | 5 | 16 | 1.06 (0.79–1.44) | 0.693 | 39.57 | 62.09 | 0.001 | 0.129 | 0.150 |
| All others | 2 | 2 | 1.06 (0.62–1.81) | 0.836 | 0.21 | 0.00 | 0.646 | - | - |
| AML | 5 | 12 | 1.41 (1.02–1.95) | 0.039 | 10.22 | 0.00 | 0.511 | 0.693 | 0.583 |
| Study design | |||||||||
| Case-control | 4 | 11 | 1.29 (0.91–1.82) | 0.149 | 9.23 | 0.00 | 0.606 | 0.848 | 0.938 |
| Cohort | 1 | 1 | - | - | - | - | - | - | - |
| Age | |||||||||
| <6 years | 1 | 1 | - | - | - | - | - | - | - |
| <15 years | 3 | 9 | 1.18 (0.80–1.74) | 0.393 | 6.29 | 0.00 | 0.617 | 0.634 | 0.677 |
| All others | 2 | 3 | 2.15 (1.18–3.93) | 0.012 | 1.24 | 0.00 | 0.538 | 0.462 | 0.602 |
| Lymphomas (ICCC-3 II) | 5 | 7 | 1.22 (0.88–1.67) | 0.232 | 7.04 | 14.83 | 0.317 | 0.203 | 0.024 |
| Study design | |||||||||
| Case-control | 1 | 1 | - | - | - | - | - | - | - |
| Cohort | 4 | 6 | 1.32 (0.90–1.94) | 0.157 | 6.56 | 23.73 | 0.256 | 0.187 | 0.091 |
| Age | |||||||||
| <6 years | 0 | 0 | - | - | - | - | - | - | - |
| <15 years | 3 | 3 | 1.09 (0.67–1.80) | 0.724 | 2.26 | 11.34 | 0.324 | 0.197 | 0.117 |
| All others | 2 | 4 | 1.46 (0.82–2.58) | 0.199 | 4.63 | 35.23 | 0.201 | 0.347 | 0.497 |
| ART | |||||||||
| Hematological Tumors | 11 | 25 | 1.30 (1.08–1.58) | 0.006 | 37.68 | 36.31 | 0.037 | 0.234 | 0.726 |
| Leukemias (ICCC-3 I) | 11 | 20 | 1.27 (1.03–1.56) | 0.025 | 31.85 | 40.34 | 0.033 | 0.453 | 0.820 |
| -Leukemia | 9 | 10 | 1.10 (0.90–1.34) | 0.365 | 10.66 | 15.60 | 0.299 | 0.849 | 0.531 |
| -Other leukemia | 1 | 1 | - | - | - | - | - | - | - |
| -non-ALL | 1 | 2 | - | - | - | - | - | - | - |
| -ALL | 5 | 6 | 1.25 (0.94–1.64) | 0.123 | 4.11 | 0.00 | 0.534 | 0.407 | 0.188 |
| -AML | 1 | 1 | - | - | - | - | - | - | - |
| Lymphomas (ICCC-3 II) | 3 | 5 | 1.58 (0.94–2.66) | 0.083 | 4.97 | 19.55 | 0.290 | 0.457 | 0.327 |
| -Lymphoma | 3 | 3 | 1.36 (0.78–2.37) | 0.278 | 2.39 | 16.19 | 0.303 | 0.071 | 0.117 |
| -Lymphoma NH | 1 | 1 | - | - | - | - | - | - | - |
| -Lymphoma Hodgkins | 1 | 1 | - | - | - | - | - | - | - |
| Hematological | 10 | 25 | 1.30 (1.08–1.58) | 0.006 | 37.68 | 36.31 | 0.037 | 0.234 | 0.726 |
| Study design | |||||||||
| Case-control | 3 | 10 | 1.26 (0.96–1.65) | 0.095 | 9.36 | 3.81 | 0.405 | 0.513 | 0.245 |
| Cohort | 7 | 15 | 1.37 (1.05–1.77) | 0.019 | 28.28 | 50.50 | 0.013 | 0.151 | 0.553 |
| Age | |||||||||
| <6 years | 0 | 0 | - | - | - | - | - | - | - |
| <15 years | 9 | 18 | 1.09 (0.94–1.27) | 0.240 | 15.54 | 0.00 | 0.556 | 0.658 | 0.850 |
| All others | 1 | 7 | - | - | - | - | - | - | - |
| Leukemias (ICCC-3 I) | 11 | 20 | 1.27 (1.03–1.56) | 0.025 | 31.85 | 40.34 | 0.033 | 0.453 | 0.820 |
| Study design | |||||||||
| Case-control | 4 | 10 | 1.26 (0.96–1.65) | 0.095 | 9.36 | 3.81 | 0.405 | 0.513 | 0.245 |
| Cohort | 7 | 10 | 1.31 (0.96–1.78) | 0.085 | 22.34 | 59.71 | 0.008 | 0.351 | 0.721 |
| Age | |||||||||
| <6 years | 0 | 0 | - | - | - | - | - | - | - |
| <15 years | 10 | 16 | 1.09 (0.93–1.27) | 0.293 | 13.52 | 0.00 | 0.562 | 0.848 | 0.653 |
| All others | 1 | 4 | - | - | - | - | - | - | - |
| Lymphomas (ICCC-3 II) | 3 | 5 | 1.58 (0.94-2.66) | 0.083 | 4.97 | 19.55 | 0.290 | 0.457 | 0.327 |
| Study design | |||||||||
| Case-control | 0 | 0 | - | - | - | - | - | - | - |
| Cohort | 3 | 5 | 1.58 (0.94–2.66) | 0.083 | 4.97 | 19.55 | 0.290 | 0.457 | 0.327 |
| Age | |||||||||
| <6 years | 0 | 0 | - | - | - | - | - | - | - |
| <15 years | 2 | 2 | 1.30 (0.56–3.03) | 0.547 | 1.92 | 47.99 | 0.166 | - | - |
| All others | 1 | 3 | - | - | - | - | - | - | - |
| IVF | |||||||||
| Hematological Tumors | 3 | 10 | 1.26 (0.96–1.65) | 0.095 | 9.36 | 3.81 | 0.405 | 0.513 | 0.245 |
| Leukemias (ICCC-3 I) | 3 | 10 | 1.26 (0.96–1.65) | 0.095 | 9.36 | 3.81 | 0.405 | 0.513 | 0.245 |
| -ALL | 3 | 4 | 1.29 (0.80–2.07) | 0.291 | 3.96 | 24.15 | 0.266 | 0.007 | 0.042 |
| -Leukemia | 3 | 4 | 1.18 (0.75–1.86) | 0.483 | 4.63 | 35.26 | 0.201 | 0.245 | 0.174 |
| -non-ALL | 1 | 2 | - | - | - | - | - | - | - |
| Models | Studies | Effect Size | Combined Risk Estimate | Test of Heterogeneity | Publication Bias | ||||
|---|---|---|---|---|---|---|---|---|---|
| MAR | N° | N° | Value (95% CI) | p | Q | I2% | p | p (Egger Test) | p (Begg Test) |
| Neural Tumors (ICCC-3 III) | 11 | 22 | 1.05 (0.88-1.26) | 0.562 | 29.50 | 28.81 | 0.103 | 0.026 | 0.108 |
| -Astrocytomas | 3 | 3 | 1.13 (0.64–2.00) | 0.664 | 0.66 | 0.00 | 0.718 | 0.707 | 0.602 |
| -CNS tumors | 10 | 13 | 1.00 (0.80–1.24) | 0.968 | 24.03 | 50.07 | 0.020 | 0.004 | 0.028 |
| -Embryonal CNS tumors | 2 | 2 | 1.06 (0.38–2.92) | 0.916 | 1.73 | 42.26 | 0.188 | - | - |
| -Ependymomas | 1 | 1 | - | - | - | - | - | - | - |
| -Other CNS tumors | 1 | 1 | - | - | - | - | - | - | - |
| -Other glioma | 2 | 2 | 0.99 (0.30–3.28) | 0.992 | 0.05 | 0.00 | 0.826 | - | - |
| Neuroblastoma (ICCC-3 IV) | 9 | 14 | 1.21 (0.98–1.50) | 0.078 | 8.73 | 0.00 | 0.793 | 0.280 | 0.702 |
| Retinoblastoma (ICCC-3 V) | 8 | 9 | 1.49 (0.92–2.44) | 0.106 | 20.84 | 61.60 | 0.008 | 0.843 | 1.000 |
| Renal Tumors (ICCC-3 VI) | 8 | 8 | 1.22 (0.79–1.88) | 0.367 | 13.09 | 46.51 | 0.070 | 0.686 | 0.322 |
| -Nephroblastoma | 1 | 1 | - | - | - | - | - | - | - |
| -Renal tumors | 6 | 6 | 1.18 (0.64–2.17) | 0.601 | 12.66 | 60.51 | 0.027 | 0.753 | 0.348 |
| -Wilms tumors | 1 | 1 | - | - | - | - | - | - | - |
| Hepatic Tumors (ICCC-3 VII) | 7 | 11 | 2.77 (1.72–4.49) | <0.0001 | 19.85 | 49.61 | 0.031 | 0.044 | 0.102 |
| Hepatic Tumors (ICCC-3 VII) excluding Puumala et al., 2012 | 6 | 9 | 3.59 (2.31–5.57) | <0.0001 | 9.11 | 12.19 | 0.333 | 0.434 | 0.404 |
| -Hepatoblastoma | 4 | 6 | 3.03 (1.31–6.99) | 0.009 | 16.05 | 68.85 | 0.007 | 0.171 | 0.348 |
| -Hepatic tumors | 5 | 5 | 2.63 (1.60–4.31) | 0.0001 | 3.36 | 0.00 | 0.500 | 0.415 | 0.327 |
| Bone Tumors (ICCC-3 VIII) | 5 | 8 | 1.50 (0.92–2.46) | 0.105 | 15.20 | 53.94 | 0.034 | 0.508 | 0.216 |
| -Bone tumors | 5 | 6 | 1.28 (0.70–2.33) | 0.422 | 13.75 | 63.64 | 0.017 | 0.297 | 0.188 |
| -Ewing’s sarcoma | 1 | 1 | - | - | - | - | - | - | - |
| -Osteosarcoma | 1 | 1 | - | - | - | - | - | - | - |
| Sarcomas (ICCC-3 IX) | 6 | 10 | 1.54 (1.18–2.02) | 0.002 | 9.46 | 4.90 | 0.396 | 0.523 | 0.325 |
| -Mesothelium and connective tissue | 1 | 1 | - | - | - | - | - | - | - |
| -Other sarcomas | 1 | 1 | - | - | - | - | - | - | - |
| -Rhabdomyosarcoma | 1 | 3 | - | - | - | - | - | - | - |
| -Soft tissue sarcomas | 5 | 5 | 1.14 (0.81–1.60) | 0.441 | 0.21 | 0.00 | 0.995 | 0.766 | 0.624 |
| Germ Cell Tumors (ICCC-3 X) | 4 | 5 | 1.13 (0.76–1.67) | 0.541 | 1.22 | 0.00 | 0.875 | 0.329 | 0.327 |
| -GCT | 3 | 3 | 0.92 (0.47–1.82) | 0.819 | 0.61 | 0.00 | 0.736 | 0.262 | 0.117 |
| -Gonadal tumors | 2 | 2 | 1.24 (0.77–2.00) | 0.366 | 0.11 | 0.00 | 0.742 | - | - |
| ART | |||||||||
| Neural Tumors (ICCC-3 III) | 7 | 11 | 1.21 (1.01–1.46) | 0.040 | 8.88 | 0.00 | 0.543 | 0.188 | 0.312 |
| -Astrocytomas | 2 | 2 | 1.17 (0.65–2.10) | 0.609 | 0.54 | 0.00 | 0.461 | - | - |
| -CNS tumors | 6 | 6 | 1.17 (0.90–1.52) | 0.245 | 7.11 | 29.71 | 0.212 | 0.192 | 0.188 |
| -Embryonal CNS tumors | 1 | 1 | - | - | - | - | - | - | - |
| -Other CNS tumors | 1 | 1 | - | - | - | - | - | - | - |
| -Other glioma | 1 | 1 | - | - | - | - | - | - | - |
| Neuroblastoma (ICCC-3 IV) | 6 | 6 | 1.13 (0.81–1.58) | 0.477 | 2.57 | 0.00 | 0.766 | 0.193 | 0.573 |
| Retinoblastoma(ICCC-3 V) | 7 | 7 | 1.65 (0.83–3.27) | 0.154 | 19.14 | 68.65 | 0.004 | 0.903 | 0.881 |
| Renal Tumors (ICCC-3 VI) | 6 | 6 | 1.30 (0.67–2.50) | 0.440 | 12.46 | 59.89 | 0.029 | 0.871 | 0.573 |
| -Renal tumors | 5 | 5 | 1.26 (0.55–2.89) | 0.588 | 12.30 | 67.47 | 0.015 | 0.882 | 0.624 |
| -Wilms tumors | 1 | 1 | - | - | - | - | - | - | - |
| Hepatic Tumors (ICCC-3 VII) | 5 | 8 | 3.14 (1.95–5.06) | <0.0001 | 8.25 | 15.15 | 0.311 | 0.540 | 0.458 |
| -Hepatoblastoma | 3 | 4 | 3.31 (1.28–8.57) | 0.014 | 6.01 | 50.07 | 0.111 | 0.744 | 0.497 |
| -Hepatic tumors | 4 | 4 | 3.18 (1.73–5.83) | 0.0002 | 2.22 | 0.00 | 0.528 | 0.751 | 1.000 |
| Bone Tumors (ICCC-3 VIII) | 3 | 5 | 1.86 (0.93–3.69) | 0.078 | 9.99 | 59.97 | 0.041 | 0.650 | 0.142 |
| -Bone tumors | 3 | 3 | 1.46 (0.48–4.51) | 0.507 | 9.54 | 79.03 | 0.008 | 0.619 | 0.117 |
| -Ewing’s sarcoma | 1 | 1 | - | - | - | - | - | - | - |
| -Osteosarcoma | 1 | 1 | - | - | - | - | - | - | - |
| Sarcomas (ICCC-3 IX) | 4 | 7 | 1.92 (1.34–2.74) | 0.0003 | 5.28 | 0.00 | 0.508 | 0.693 | 0.881 |
| -Other sarcomas | 1 | 1 | - | - | - | - | - | - | - |
| -Rhabdomyosarcoma | 1 | 3 | - | - | - | - | - | - | - |
| -Soft tissue sarcomas | 3 | 3 | 1.25 (0.71–2.21) | 0.436 | 0.05 | 0.00 | 0.976 | 0.569 | 0.602 |
| Germ Cell Tumors (ICCC-3 X) | 2 | 2 | 0.98 (0.41–2.31) | 0.595 | 0.57 | 0.00 | 0.451 | - | - |
| IVF | |||||||||
| Neuroblastoma (ICCC-3 IV) | 1 | 1 | - | - | - | - | - | - | - |
| Retinoblastoma (ICCC-3 V) | 2 | 2 | 1.83 (1.00–3.35) | 0.049 | 1.17 | 14.50 | 0.279 | - | - |
| Hepatic Tumors (ICCC-3 VII) | 1 | 1 | - | - | - | - | - | - | - |
| -Hepatoblastoma | 1 | 1 | - | - | - | - | - | - | - |
| Sarcomas (ICCC-3 IX) | 1 | 1 | - | - | - | - | - | - | - |
| -Rhabdomyosarcoma | 1 | 1 | - | - | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavarini, M.; Ostorero, A.; Naldini, G.; Fabiani, R. Cancer Risk in Children and Young Adults (Offspring) Born after Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. J 2019, 2, 430-448. https://doi.org/10.3390/j2040028
Chiavarini M, Ostorero A, Naldini G, Fabiani R. Cancer Risk in Children and Young Adults (Offspring) Born after Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. J. 2019; 2(4):430-448. https://doi.org/10.3390/j2040028
Chicago/Turabian StyleChiavarini, Manuela, Andrea Ostorero, Giulia Naldini, and Roberto Fabiani. 2019. "Cancer Risk in Children and Young Adults (Offspring) Born after Medically Assisted Reproduction: A Systematic Review and Meta-Analysis" J 2, no. 4: 430-448. https://doi.org/10.3390/j2040028
APA StyleChiavarini, M., Ostorero, A., Naldini, G., & Fabiani, R. (2019). Cancer Risk in Children and Young Adults (Offspring) Born after Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. J, 2(4), 430-448. https://doi.org/10.3390/j2040028







