An Innovative Industrial Complex for Sustainable Hydrocarbon Production with Near-Zero Emissions
Abstract
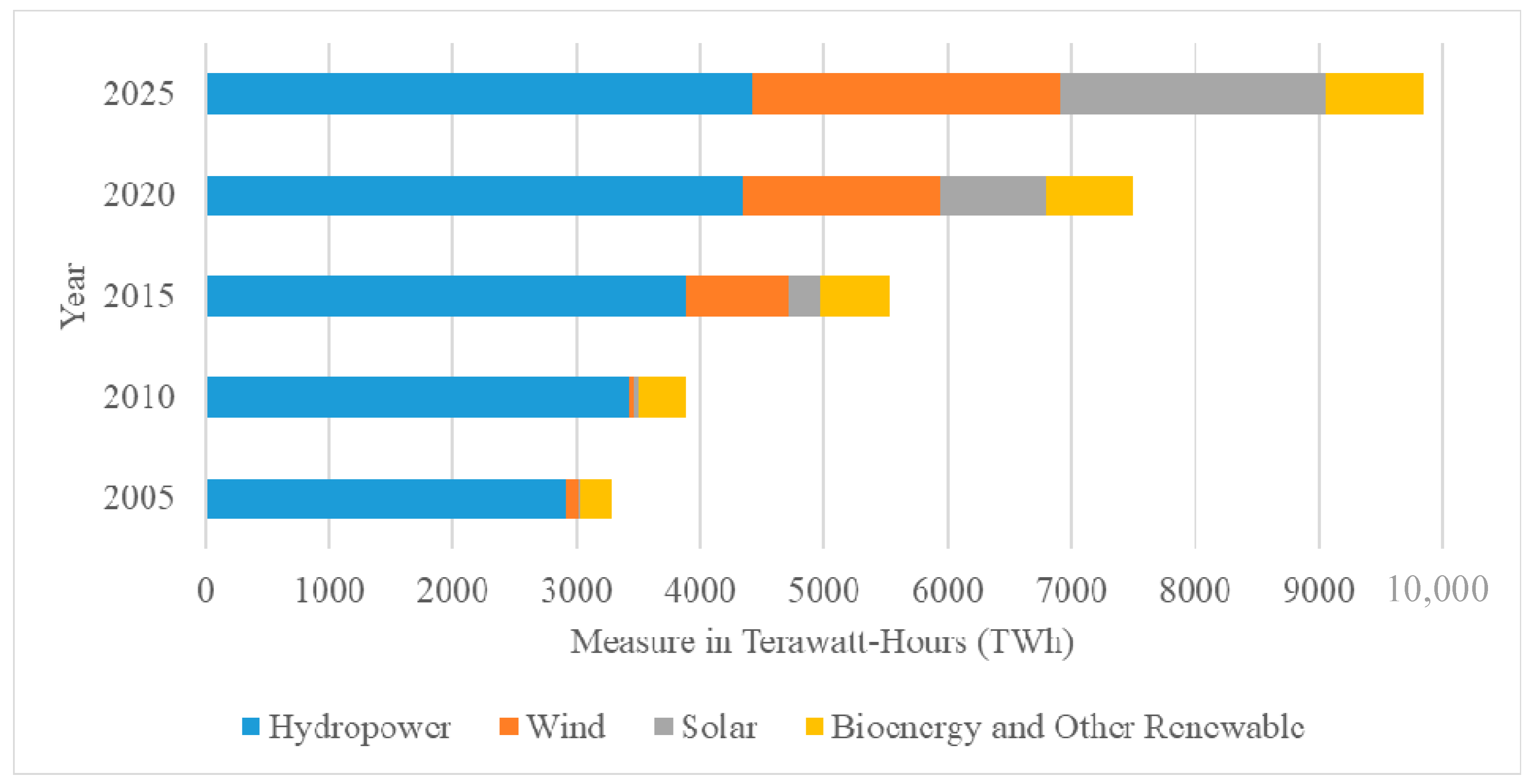
1. Introduction
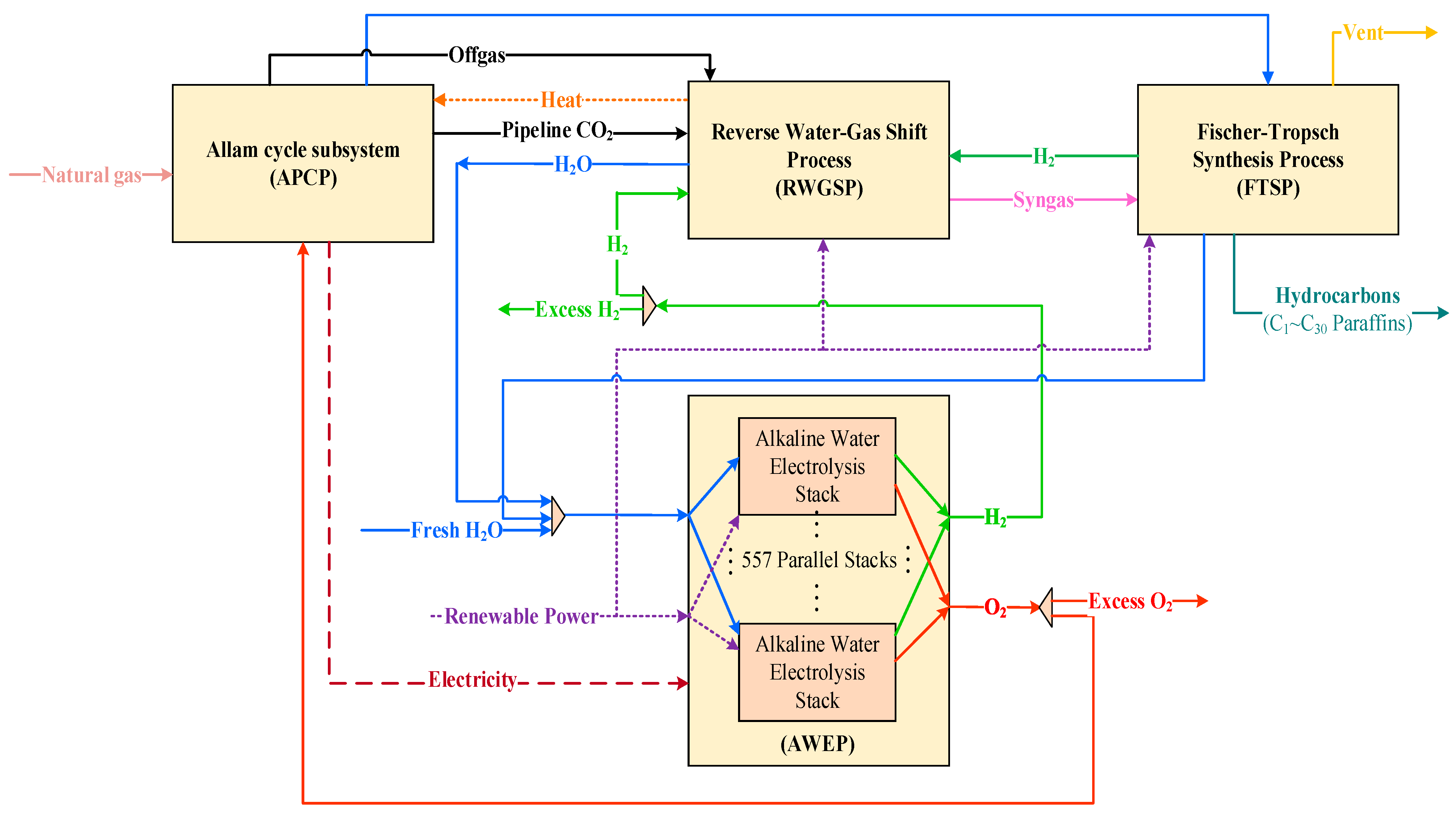
2. Overview of the Developed FAAR Industrial Complex
3. Modeling of the FAAR Complex
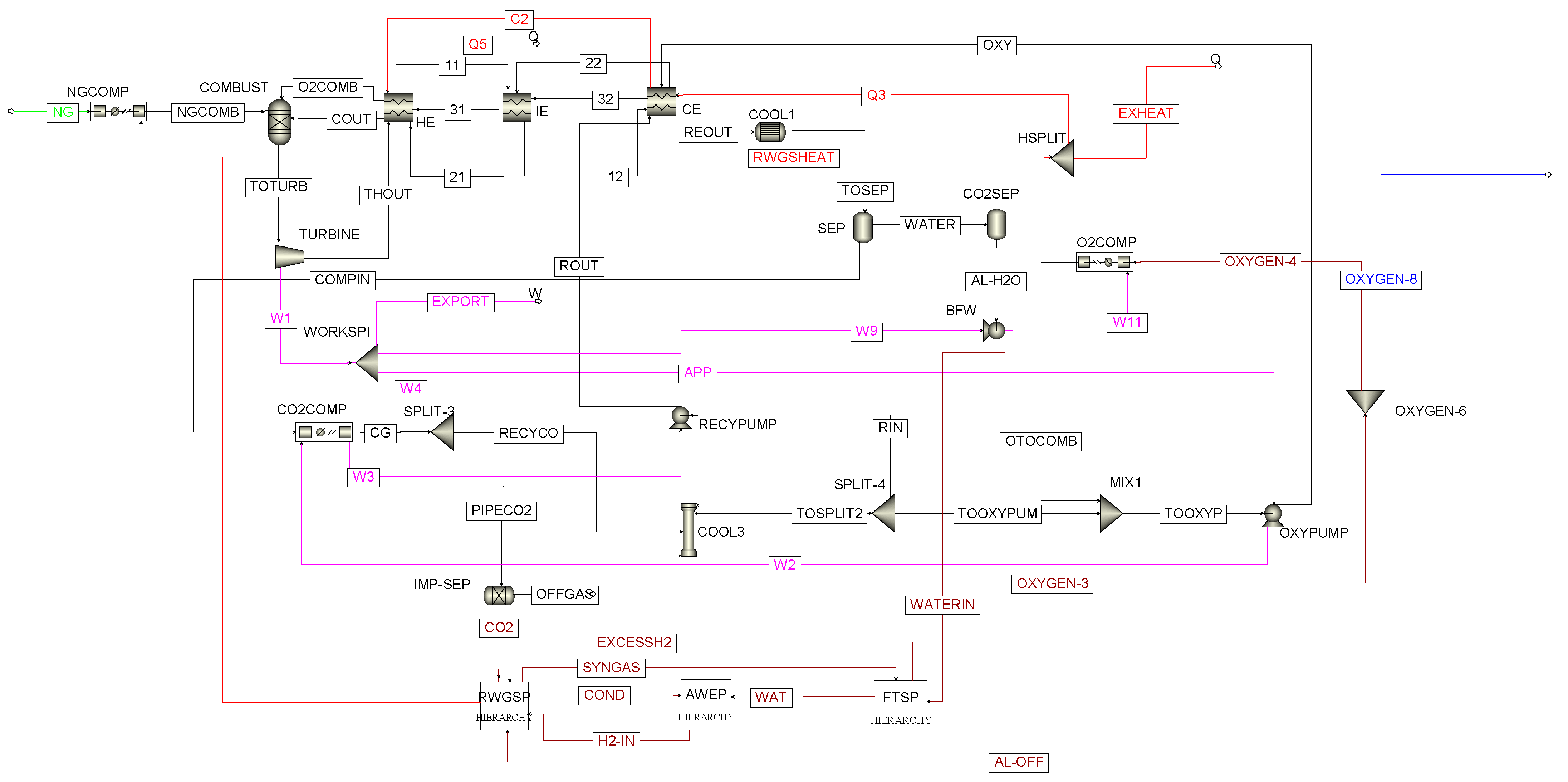
3.1. Modeling of the APCP Subsystem
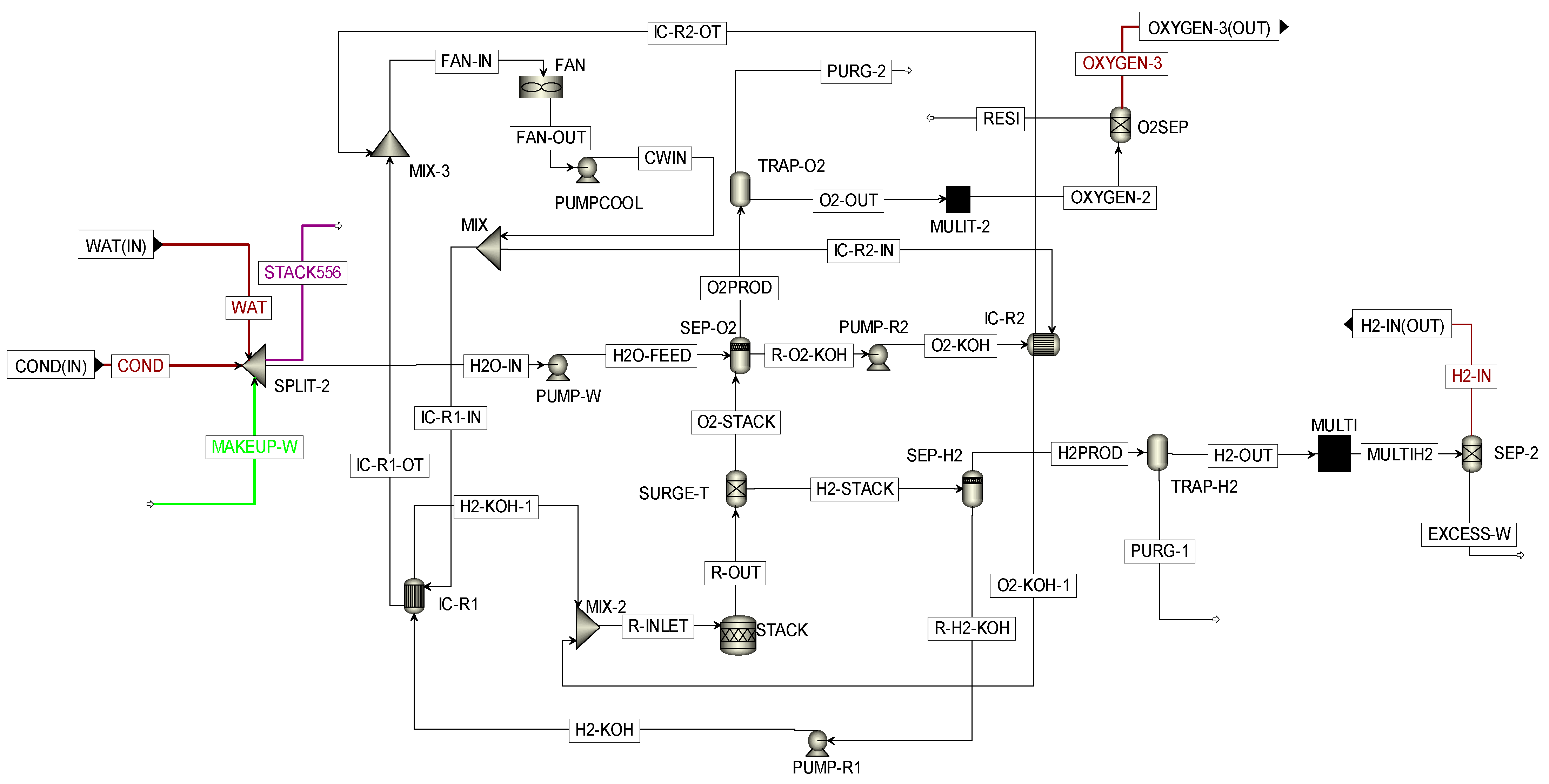
3.2. Modeling of the AWEP Subsystem
3.3. Modeling of the RWGSP Subsystem
3.4. Modeling of the FTSP Subsystem
4. Economic Analysis
5. Sensitivity Analysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M.; Rosado, P. Renewable Energy. Our World in Data. Available online: https://ourworldindata.org/renewable-energy (accessed on 15 October 2025).
- Allam, R.J.; Fetvedt, J.E.; Forrest, B.A.; Freed, D.A. The Oxy-Fuel, Supercritical CO2 Allam Cycle: New Cycle Developments to Produce Even Lower-Cost Electricity from Fossil Fuels without Atmospheric Emissions. In Proceedings of the ASME Turbo Expo 2014: Turbine Technical Conference and Exposition. Volume 3B: Oil and Gas Applications; Organic Rankine Cycle Power Systems; Supercritical CO2 Power Cycles; Wind Energy, Düsseldorf, Germany, 16–20 June 2014. [Google Scholar] [CrossRef]
- Allam, R.; Martin, S.; Forrest, B.; Fetvedt, J.; Lu, X.; Freed, D.; Brown, G.W., Jr.; Sasaki, T.; Itoh, M.; Manning, J. Demonstration of the Allam cycle: An update on the development status of a high efficiency supercritical carbon dioxide power process employing full carbon capture. Energy Procedia 2017, 114, 5948–5966. [Google Scholar] [CrossRef]
- Fernandes, D.; Wang, S.; Xu, Q.; Buss, R.; Chen, D. Process, and carbon footprint analyses of the Allam cycle power plant integrated with an air separation unit. Clean Technol. 2019, 1, 325–340. [Google Scholar] [CrossRef]
- Fernandes, D.; Wang, S.; Xu, Q.; Chen, D. Dynamic simulations of the Allam cycle power plant integrated with an air separation unit. Int. J. Chem. Eng. 2019, 6035856. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Z.; Liu, Y.; Wu, Y.; Wen, Z.; Xu, H.; Zhu, P.; Zhou, J.; Xiao, G. Energy Transfer and Conversion in the Allam Cycle with a Multi-Stream Heat Exchanger. Appl. Therm. Eng. 2024, 255, 124022. [Google Scholar] [CrossRef]
- Bocandé, D.; Rohardt, J.; Schatz, M. The Hydrogen-Fueled Allam Cycle: Thermodynamic Evaluation and Optimization after a Fuel Switch to Hydrogen. In Proceedings of the ASME Turbo Expo 2025: Turbomachinery Technical Conference and Exposition. Volume 4: Controls, Diagnostics & Instrumentation; Cycle Innovations; Education; Electric Power, Memphis, TN, USA, 16–20 June 2025. [Google Scholar] [CrossRef]
- Wang, S.; Fernandes, D.; Xu, Q.; Chen, D. New Conceptual Design of an Integrated Allam-Cycle Power Complex Coupling Air Separation Unit and Ammonia Plant. Ind. Eng. Chem. Res. 2021, 60, 18007–18017. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Xu, Q.; Ho, T.C. Eco-Friendly Natural Gas Monetization Complex for Simultaneous Power Generation and Nitrogen-Based Fertilizer Production. Ind. Eng. Chem. Res. 2023, 62, 489–499. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Xu, Q.; Huang, Y. Comprehensive Utilization of Natural Gas for Simultaneous Production of Electricity, Urea, and Liquefied Natural Gas with Inherent Carbon Capture. Clean Technol. Environ. Policy 2024, 27, 2535–2546. [Google Scholar] [CrossRef]
- Kato, T.; Kubota, M.; Kobayashi, N.; Suzuoki, Y. Effective Utilization of By-Product Oxygen from Electrolysis Hydrogen Production. Energy 2005, 30, 2580–2595. [Google Scholar] [CrossRef]
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen Production with CO2 Capture. Int. J. Hydrogen Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Rothschild, A.; Grader, G.; Shter, G.; Landman, A.; Dotan, H. Methods and System for Hydrogen Production by Water Electrolysis. U.S. Patent 10,487,408 B2, 26 November 2019. Available online: https://patents.google.com/patent/US10487408B2/en (accessed on 20 May 2024).
- Sánchez, M.; Amores, E.; Abad, D.; Rodríguez, L.; Clemente-Jul, C. Aspen plus Model of an Alkaline Electrolysis System for Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 3916–3929. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of Water Electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13814. [Google Scholar] [CrossRef]
- Campbell-Stanway, C.; Becerra, V.; Prabhu, S.; Bull, J. Investigating the Role of Byproduct Oxygen in UK-Based Future Scenario Models for Green Hydrogen Electrolysis. Energies 2024, 17, 281. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, L. Recent Advances in Hybrid Seawater Electrolysis for Hydrogen Production. Adv. Mater. 2023, 36, 2308647. [Google Scholar] [CrossRef]
- Ernst, K.-H.; Campbell, C.T.; Moretti, G. Kinetics of the reverse water-gas shift reaction over Cu(110). J. Catal. 1992, 134, 66–74. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Kinetics of Reverse Water-Gas Shift Reaction over Pt/Al2O3Catalyst. Can. J. Chem. Eng. 2015, 94, 101–106. [Google Scholar] [CrossRef]
- Daza, Y.A.; Kuhn, J.N. CO2 Conversion by Reverse Water Gas Shift Catalysis: Comparison of Catalysts, Mechanisms and Their Consequences for CO2 Conversion to Liquid Fuels. RSC Adv. 2016, 6, 49675–49691. [Google Scholar] [CrossRef]
- Kirsch, H.; Sommer, U.; Pfeifer, P.; Dittmeyer, R. Power-To-Fuel Conversion Based on Reverse Water-Gas-Shift, Fischer-Tropsch Synthesis and Hydrocracking: Mathematical Modeling and Simulation in Matlab/Simulink. Chem. Eng. Sci. 2020, 227, 115930. [Google Scholar] [CrossRef]
- González-Castaño, M.; Dorneanu, B.; Arellano-Garcia, H. The Reverse Water Gas Shift Reaction: A Process Systems Engineering Perspective. React. Chem. Eng. 2021, 6, 954–976. [Google Scholar] [CrossRef]
- Zhang, Q.; Bown, M.; Pastor-Pérez, L.; Duyar, M.S.; Reina, T.R. CO2 Conversion via Reverse Water Gas Shift Reaction Using Fully Selective Mo–P Multicomponent Catalysts. Ind. Eng. Chem. Res. 2022, 61, 12857–12865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Ma, W.-P.; Lu, Y.-J.; Yang, J.; Xu, Y.-Y.; Xiang, H.-W.; Li, Y.-W.; Zhao, Y.-L.; Zhang, B.-J. Kinetics Modelling of Fischer–Tropsch Synthesis over an Industrial Fe–Cu–K Catalyst. Fuel 2003, 82, 195–213. [Google Scholar] [CrossRef]
- Marion, M.-C.; Bertoncini, F.; Hugues, F.; Forestiere, A. Comprehensive Characterisation of Products from Cobalt Catalysed Fischer-Tropsch Reaction. In Proceedings of the DGMK/SCI-Conference, Synthesis Gas Chemistry, Dresden, Germany, 4–6 October 2006; Available online: https://www.osti.gov/etdeweb/servlets/purl/20840761 (accessed on 18 May 2024).
- Visconti, C.G.; Tronconi, E.; Lietti, L.; Zennaro, R.; Forzatti, P. Development of a Complete Kinetic Model for the Fischer–Tropsch Synthesis over Co/Al2O3 Catalysts. Chem. Eng. Sci. 2007, 62, 5338–5343. [Google Scholar] [CrossRef]
- Swanson, R.M.; Platon, A.; Satrio, J.A.; Brown, R.C.; Hsu, D.D. Techno-Economic Analysis of Biofuels Production Based on Gasification. OSTI OAI; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2010. [CrossRef]
- Todic, B.; Nowicki, L.; Nikacevic, N.; Bukur, D.B. Fischer–Tropsch Synthesis Product Selectivity over an Industrial Iron-Based Catalyst: Effect of Process Conditions. Catal. Today 2016, 261, 28–39. [Google Scholar] [CrossRef]
- Rafiee, A.; Panahi, M.; Khalilpour, K.R. CO2 Utilization through Integration of Post-Combustion Carbon Capture Process with Fischer-Tropsch Gas-To-Liquid (GTL) Processes. J. CO2 Util. 2017, 18, 98–106. [Google Scholar] [CrossRef]
- Theampetch, A.; Prapainainar, C.; Tungkamani, S.; Narataruksa, P.; Sornchamni, T.; Árnadóttir, L.; Jovanovic, G.N. Detailed Microkinetic Modelling of Syngas to Hydrocarbons via Fischer Tropsch Synthesis over Cobalt Catalyst. Int. J. Hydrogen Energy 2021, 46, 24721–24741. [Google Scholar] [CrossRef]
- Davies, I.; Möller, K.P. Development of a Kinetic Model for Low Temperature Fischer-Tropsch Synthesis. Chem. Eng. Sci. 2021, 241, 116666. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Green Liquid Fuel and Synthetic Natural Gas Production via CO2 Hydrogenation Combined with Reverse Water-Gas-Shift and Co-Based Fischer-Tropsch Synthesis. J. CO2 Util. 2021, 51, 101619. [Google Scholar] [CrossRef]
- Ahmed, H.S. Power-to-X: Modelling of Fischer-Tropsch Synthesis in Aspen Plus. Master’s Thesis, Master’s Programme in Advanced Energy Solutions. Aalto University, Espoo, Finland, 2021. Available online: https://aaltodoc.aalto.fi/bitstream/handle/123456789/112650/master_Sayed_Ahmed_Hassan_2022.pdf?sequence=1 (accessed on 10 May 2024).
- Repasky, J.M.; Zeller, R.L., III. Process for the Conversion of Carbon Dioxide; World Intellectual Property Organization: Geneva, Switzerland, 2021; Available online: https://patentimages.storage.googleapis.com/5a/d5/48/81c7e5318737af/WO2021062384A1.pdf (accessed on 10 November 2023).
- Zang, G.; Sun, P.; Elgowainy, A.A.; Bafana, A.; Wang, M. Performance and Cost Analysis of Liquid Fuel Production from H2 and CO2 Based on the Fischer-Tropsch Process. J. CO2 Util. 2021, 46, 101459. [Google Scholar] [CrossRef]
- Zang, G.; Sun, P.; Delgado, H.E.; Cappello, V.; Ng, C.; Elgowainy, A.; Systems Assessment Center, Energy Systems Division, Argonne National Laboratory. The Modeling of the Synfuel Production Process; Argonne National Laboratory: Argonne, IL, USA, 2022.
- Azhari, N.J.; Nurdini, N.; Mardiana, S.; Ilmi, T.; Fajar, A.T.N.; Makertihartha, I.G.B.N.; Subagjo; Kadja, G.T.M. Zeolite-Based Catalyst for Direct Conversion of CO2 to C2+ Hydrocarbon: A Review. J. CO2 Util. 2022, 59, 101969. [Google Scholar] [CrossRef]
- Singh, H.; Li, C.; Cheng, P.; Wang, X.; Liu, Q. A Critical Review of Technologies, Costs, and Projects for Production of Carbon-Neutral Liquid E-Fuels from Hydrogen and Captured CO2. Energy Adv. 2022, 1, 580–605. [Google Scholar] [CrossRef]
- Modi, V.A.; Xu, Q. Modeling, and Integration of Green-Hydrogen-Assisted Carbon Dioxide Utilization for Hydrocarbon Manufacturing. Ind. Eng. Chem. Res. 2024, 63, 18043–18052. [Google Scholar] [CrossRef]
- EPRI Home. Epri.com. Available online: https://www.epri.com/research/products/3002008149 (accessed on 5 October 2025).
- Richardson, J.F. Coulson and Richardson’s Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Capex Rates|Electrolysis Techno-Economic Analysis. Epri.com. Available online: https://apps.epri.com/lcri-electrolysis-tea/en/capex-rates.html (accessed on 10 October 2025).
- U.S. DOE. 2030 Solar Cost Targets. 2021. Available online: https://www.energy.gov/eere/solar/articles/2030-solar-cost-targets (accessed on 18 March 2024).
- Green, D.W.; Southard, M.Z. Perry’s Chemical Engineers’ Handbook. Choice Rev. Online 1998, 35, 35–307935–3079. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Clean Hydrogen Production Tax Credit (45V) Resources. Energy.gov. Available online: https://www.energy.gov/articles/clean-hydrogen-production-tax-credit-45v-resources (accessed on 10 October 2025).

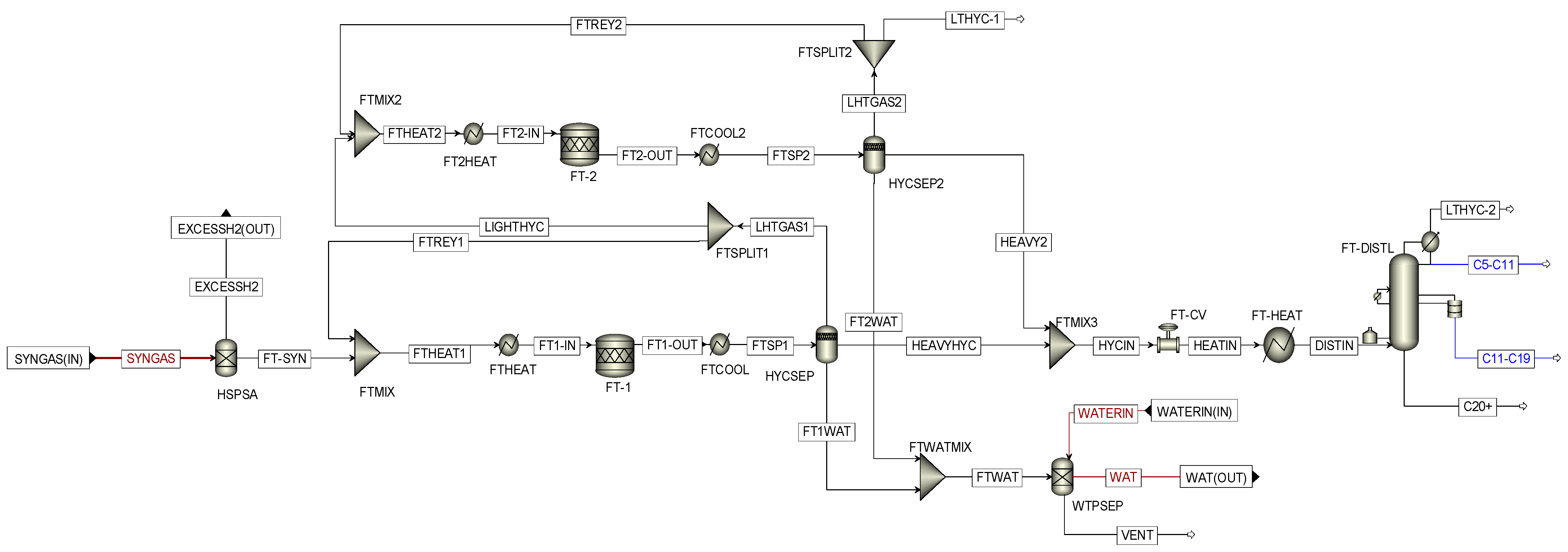
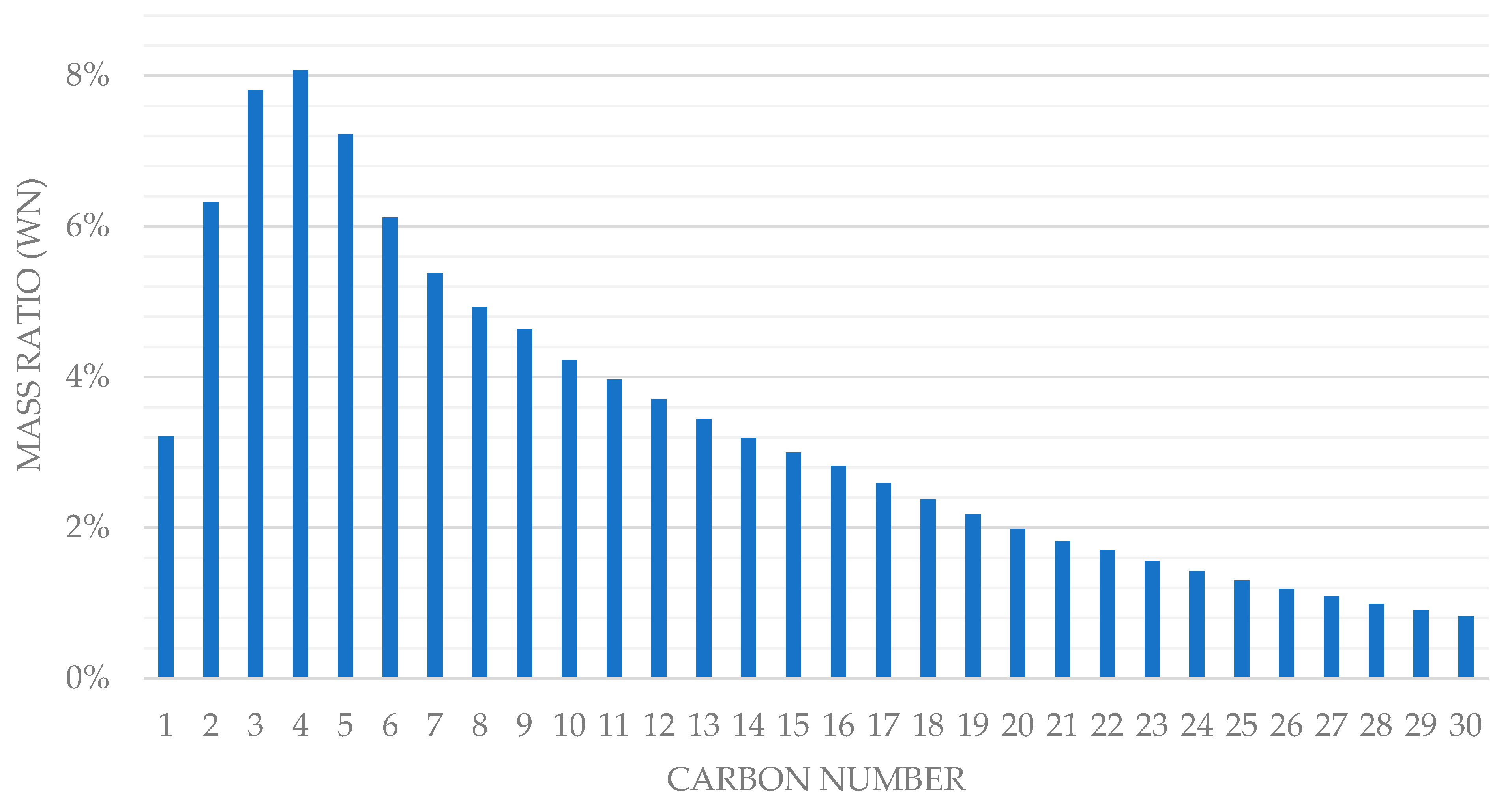

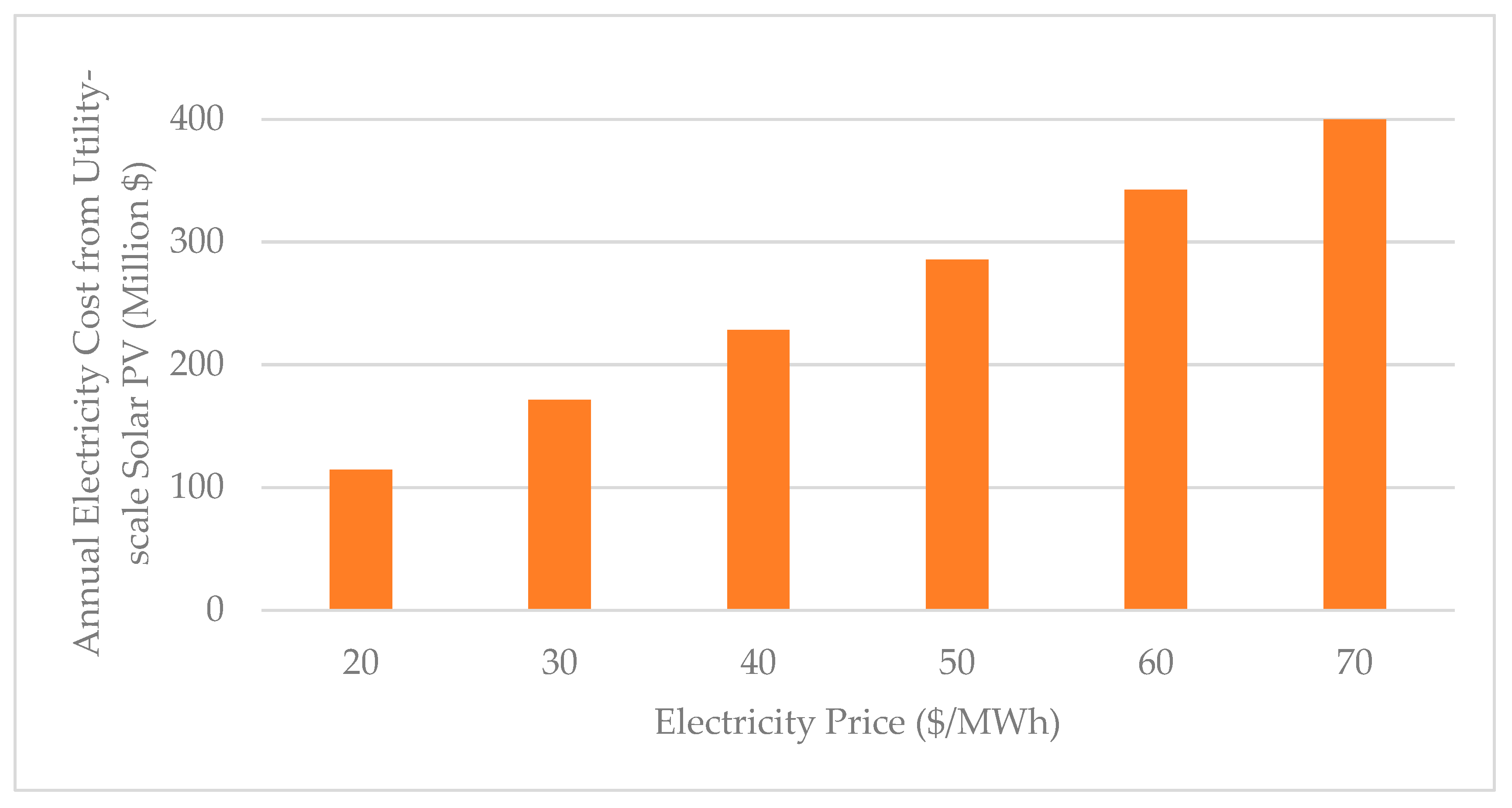

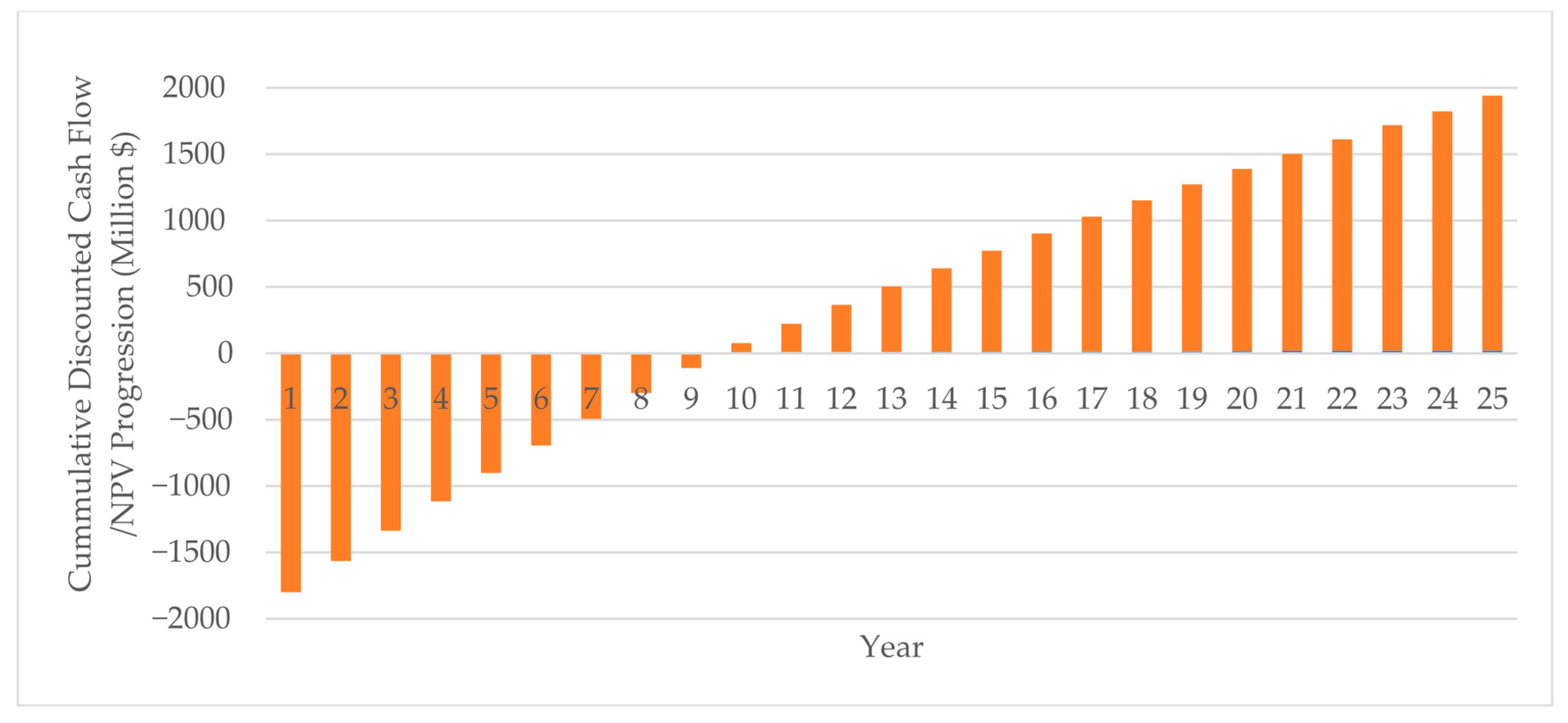
| Subsystem | Power Generation (MWh) | Power Consumption (MWh) | Power Source | |
|---|---|---|---|---|
| APCP | 490.89 | NGCOMP | 3.45 | 122.43 MW internal consumption and remaining 368.46 MW exported to stacks in AWEP subsystem |
| BFW | 0.03 | |||
| O2COMP | 20.86 | |||
| OXYPUMP | 10.55 | |||
| CO2COMP | 61.57 | |||
| RECYPUMP | 25.97 | |||
| RWGSP | 0 | H2COMP | 48.71 | Renewable energy |
| AWEP | 0 | PUMP-W | 0.04 | Renewable energy |
| PUMP-R2 | 0.01 | |||
| PUMP-R1 | 0.01 | |||
| PUMPCOOL | 0.01 | |||
| Stacks * | 971.04 | Renewable energy plus power from the APCP subsystem | ||
| FTSP | 0 | -- | 0 | -- |
| Stream Index | T (°C) | P (bar) | Flow (tonne/h) | Mass Fraction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | H2O | N2 | O2 | H2 | C1–C4 | CO | ||||
| AL-OFF | 5.00 | 0.03 | 0.09 | 0.89 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CG | 23.00 | 100.00 | 3266.15 | 0.97 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| COUT | 717.00 | 312.00 | 2480.57 | 0.97 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| NGCOMB | 270.00 | 330.00 | 36 | 0.03 | 0.00 | 0.03 | 0.00 | 0.00 | 0.95 | 0.00 |
| O2COMB | 717.00 | 310.00 | 824.48 | 0.81 | 0.00 | 0.01 | 0.18 | 0.00 | 0.00 | 0.00 |
| OFFGAS | 23.43 | 100.00 | 2.66 | 0.00 | 0.02 | 0.35 | 0.63 | 0.00 | 0.00 | 0.00 |
| OXYGEN-4 | 25.00 | 1.00 | 136.88 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 |
| OXYGEN-8 | 25.00 | 1.00 | 0.07 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 |
| PIPECO2 | 23.00 | 100.00 | 97.98 | 0.97 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| RIN | 16.00 | 99.30 | 2480.57 | 0.97 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| ROUT | 44.99 | 312.00 | 2480.57 | 0.97 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| TOOXYPUM | 16.00 | 99.30 | 687.60 | 0.97 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| TOSEP | 17.00 | 29.50 | 3341.04 | 0.95 | 0.02 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| TOSPLIT2 | 16.00 | 99.30 | 3168.17 | 0.97 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| TOTURB | 1250.00 | 300.00 | 3341.04 | 0.95 | 0.02 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 |
| WATERIN | 19.07 | 38.00 | 74.81 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Stream Index | T (°C) | P (bar) | Flow (tonne/h) | Mass Fraction | ||
|---|---|---|---|---|---|---|
| H2O | O2 | H2 | ||||
| COND | 25.00 | 1.00 | 38.96 | 1.00 | 0.00 | 0.00 |
| CWIN | 35.02 | 2.60 | 0.35 | 1.00 | 0.00 | 0.00 |
| FAN-IN | 38.98 | 2.60 | 0.35 | 1.00 | 0.00 | 0.00 |
| FAN-OUT | 35.00 | 2.30 | 0.35 | 1.00 | 0.00 | 0.00 |
| H2-IN | 19.00 | 1.00 | 17.25 | 0.00 | 0.00 | 1.00 |
| H2-OUT | 25.00 | 1.00 | 0.04 | 0.19 | 0.00 | 0.81 |
| H2PROD | 75.00 | 6.70 | 0.05 | 0.33 | 0.00 | 0.67 |
| IC-R1-IN | 35.02 | 2.60 | 0.18 | 1.00 | 0.00 | 0.00 |
| IC-R1-OT | 36.61 | 2.60 | 0.18 | 1.00 | 0.00 | 0.00 |
| IC-R2-IN | 35.02 | 2.60 | 0.18 | 1.00 | 0.00 | 0.00 |
| IC-R2-OT | 41.35 | 2.60 | 0.18 | 1.00 | 0.00 | 0.00 |
| MAKEUP-W | 25.00 | 1.00 | 16.79 | 1.00 | 0.00 | 0.00 |
| MULTIH2 | 25.00 | 1.00 | 21.37 | 0.19 | 0.00 | 0.81 |
| O2-OUT | 25.00 | 1.00 | 0.25 | 0.01 | 0.99 | 0.00 |
| O2PROD | 75.00 | 6.70 | 0.25 | 0.03 | 0.97 | 0.00 |
| OXYGEN-3 | 25.00 | 1.00 | 136.95 | 0.00 | 1.00 | 0.00 |
| R-INLET | 72.80 | 7.30 | 0.63 | 1.00 | 0.00 | 0.00 |
| R-OUT | 779.05 | 7.00 | 0.63 | 0.56 | 0.39 | 0.05 |
| STACK556 | 20.30 | 1.00 | 166.86 | 1.00 | 0.00 | 0.00 |
| WAT | 17.93 | 1.00 | 111.41 | 1.00 | 0.00 | 0.00 |
| WATER-IN | 18.80 | 1.00 | 0.30 | 1.00 | 0.00 | 0.00 |
| Stream Index | T (°C) | P (bar) | Flow (tonne/h) | Mass Fractions | |||
|---|---|---|---|---|---|---|---|
| CO2 | H2O | H2 | CO | ||||
| CO2-IN | 64.52 | 33.00 | 95.32 | 1.00 | 0.00 | 0.00 | 0.00 |
| OFFGAS | 57.00 | 33.00 | 0.08 | 1.00 | 0.00 | 0.00 | 0.00 |
| PURGE | 5 | 0.03 | 0.009 | 0.00 | 1.00 | 0.00 | 0.00 |
| H2 | 25.10 | 1.00 | 20.52 | 0.00 | 0.00 | 1.00 | 0.00 |
| EXCESSH2 | 25.00 | 1.00 | 3.67 | 0.00 | 0.00 | 1.00 | 0.00 |
| CO2+H2 | 87.32 | 33.00 | 135.07 | 0.87 | 0.00 | 0.12 | 0.01 |
| COND | 25.00 | 30.00 | 38.96 | 0.00 | 1.00 | 0.00 | 0.00 |
| REC-CO2 | 25.00 | 30.00 | 22.81 | 0.97 | 0.00 | 0.00 | 0.03 |
| RWGS-IN | 950.00 | 33.00 | 135.07 | 0.87 | 0.00 | 0.12 | 0.01 |
| RWGS-PR | 950.00 | 33.00 | 135.07 | 0.17 | 0.29 | 0.09 | 0.45 |
| SYNGAS | 25.00 | 30.00 | 73.56 | 0.00 | 0.00 | 0.17 | 0.83 |
| Stream Index | T (°C) | P (bar) | Flow (tonne/h) | Mass Fraction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | H2O | H2 | CO | C1–C4 | C5–C10 | C11–C19 | C20+ | ||||
| SYNGAS | 25.00 | 30.00 | 73.29 | 0.00 | 0.00 | 0.17 | 0.83 | 0.00 | 0.00 | 0.00 | 0.00 |
| FT1-IN | 230.00 | 20.00 | 92.24 | 0.01 | 0.00 | 0.12 | 0.82 | 0.04 | 0.01 | 0.00 | 0.00 |
| FT1-OUT | 230.00 | 20.00 | 92.24 | 0.01 | 0.33 | 0.04 | 0.30 | 0.08 | 0.10 | 0.09 | 0.05 |
| FT2-IN | 240.00 | 20.00 | 25.04 | 0.01 | 0.01 | 0.09 | 0.62 | 0.21 | 0.06 | 0.00 | 0.00 |
| FT2-OUT | 230.00 | 20.00 | 25.04 | 0.01 | 0.25 | 0.03 | 0.23 | 0.24 | 0.13 | 0.07 | 0.04 |
| DISTIN | 240.00 | 1.00 | 25.40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.37 | 0.38 | 0.20 |
| C5–C11 | 25.00 | 1.00 | 9.84 | 0.00 | 0.01 | 0.00 | 0.00 | 0.06 | 0.88 | 0.05 | 0.00 |
| C11–C19 | 214.33 | 1.00 | 9.63 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.91 | 0.05 |
| C20+ | 369.38 | 1.00 | 5.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.92 |
| EXCESSH2 | 25.00 | 30.00 | 3.27 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| VENT | 17.96 | 20.00 | 0.0003 | 0.51 | 0.00 | 0.05 | 0.16 | 0.28 | 0.00 | 0.00 | 0.00 |
| LTHYC-1 | 43.00 | 20.00 | 8.01 | 0.03 | 0.00 | 0.05 | 0.43 | 0.41 | 0.07 | 0.00 | 0.00 |
| LTHYC-2 | 25.00 | 1.00 | 0.79 | 0.01 | 0.02 | 0.00 | 0.08 | 0.59 | 0.29 | 0.00 | 0.00 |
| WAT | 17.96 | 1.00 | 111.41 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Subsystem | Capex (Million USD) |
|---|---|
| 1. APCP | 726.52 |
| 2. RWGSP + FTSP | 466.26 |
| 3. AWEP | 753.53 |
| Total | 1946.31 |
| Items | Value (in USD M) |
|---|---|
| 1. Annual Variable Operating Cost (AVOC) | 543.98 |
| 1.1 Annual Raw Material Cost | 1.11 |
| 1.1.1 Natural Gas | 0.91 |
| 1.1.2 Water | 0.20 |
| 1.2 Annual Utility Cost | 320.45 |
| 1.2.1 Cooling water | 4.21 |
| 1.2.2 Steam | 2.05 |
| 1.2.3 Electricity (Renewable energy) | 257.12 |
| 1.2.4 Fired Heat | 57.07 |
| 1.3 Operating labor | 43.52 |
| 1.4 Maintenance (labor + material) | 116.72 |
| 1.5 Operating Supplies | 17.52 |
| 1.6 Plant Overhead | 26.11 |
| 1.7 General and Administrative expenses (G&A) | 3.26 |
| 1.8 Laboratory Charges | 6.53 |
| 1.9 Supervision | 8.70 |
| 2. Annual Total Product Revenue (ATPR) | 743.15 |
| 2.1 Hydrogen (EXCESS-H2) | 144.77 |
| 2.2 Oxygen (OXYGEN-8) | 0.06 |
| 2.3 FT Fuel (C5–C11), (C11–C19), (C20+) | 598.31 |
| Parameters | Value | Notes |
|---|---|---|
| Initial Fixed Investment (USD M) | 1946.31 | |
| Working Capital (USD M) | 97.31 | 5% of Initial Fixed Investment |
| Total Capital Investment (USD M) | 2043.62 | |
| Total Product Sales Revenue (USD M) | 743.15 | 5% annual increase every year |
| Total Operating Cost (USD M) | 543.98 | 2.5% annual increase every year |
| Salvage Value (USD M) | 194.63 | 10% of Initial Fixed Investment |
| Annual Depreciation (USD M) | 70.06 | Straight-line method over 25 years |
| Tax (%) | 30 | |
| IRS Section 45V Tax Credit (USD M) | 90.66 | Base rate considered USD 0.60 per kg of H2 produced with carbon intensity less <0.45 (kg CO2e/kg H2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modi, V.A.; Xu, Q.; Wang, S. An Innovative Industrial Complex for Sustainable Hydrocarbon Production with Near-Zero Emissions. Clean Technol. 2025, 7, 93. https://doi.org/10.3390/cleantechnol7040093
Modi VA, Xu Q, Wang S. An Innovative Industrial Complex for Sustainable Hydrocarbon Production with Near-Zero Emissions. Clean Technologies. 2025; 7(4):93. https://doi.org/10.3390/cleantechnol7040093
Chicago/Turabian StyleModi, Viral Ajay, Qiang Xu, and Sujing Wang. 2025. "An Innovative Industrial Complex for Sustainable Hydrocarbon Production with Near-Zero Emissions" Clean Technologies 7, no. 4: 93. https://doi.org/10.3390/cleantechnol7040093
APA StyleModi, V. A., Xu, Q., & Wang, S. (2025). An Innovative Industrial Complex for Sustainable Hydrocarbon Production with Near-Zero Emissions. Clean Technologies, 7(4), 93. https://doi.org/10.3390/cleantechnol7040093






