Abstract
In this work a multi-criteria analysis and an optimization tool were developed, which allows the substitution of fossil-based solvents with bio-based alternatives based on Hansen solubility parameters and various physical parameters, such as the boiling point, evaporation rate, viscosity or wetting behavior. The proof of concept was achieved by formulating two different paints used in coil coatings using the bio-based solvents, and they performed equally as well as their fossil-based counterparts. A potential decrease in CO2 emissions was determined by a life cycle assessment and cradle-to-grave analysis of bio- and fossil-based solvents, which showed a large sustainability bonus when using solvents based on biomass. The introduced methodology provides initial insights into substituting currently used solvents systematically. Overall, implementing bio-based solvents is a viable drop-in method to decrease the environmental impact of paints and coatings, while maintaining the same performance.
1. Introduction
Increasing worldwide concerns about climate change and ambitious neutral targets have driven interest in finding sustainable alternatives and decarbonization options in the coil coatings industry. Therefore, a steady decrease in fossil-based compound use during formulations is of high priority. The utilization of products stemming from regrowing resources, like polymers [1], pigments [2], solvents [3] and additives [4], has been investigated to substitute current paint and coating systems. Bio-based solvents could offer a significant chance to minimize the introduction of fossil carbon to the atmosphere while maintaining the proven performance of solvent-borne thermoset coating systems. As the bio-market steadily increases, technological and economic viability might be enhanced in the upcoming years. The development of novel drop-in methods could be an interesting economical and sustainable opportunity, as, ideally, no further investments have to be made in the production or formulation plant. Since the paint and coating industry represents more than 45% of the global solvent market [5], the impact of a substitution towards more sustainable solutions is particularly high.
Besides the positive impact on CO2 emissions, improving work safety might be viable, as legislation for certain solvents is getting stricter. In 2022, the European Union released a regulation tightening the classification of several solvents. Butyl glycol, a solvent commonly used in the formulation of organic coatings, has been labeled as an H331 (toxic by inhalation) since November 2023 [6]. New solutions, including solvents with less toxic and greener properties, are therefore of high interest. Many replacement guides for pharmaceutic processes, as well as recently for the paints and coatings industry, were developed to simplify the general replacement of currently applied solvents [7,8,9]. Most solvent guides provide a good overview of less problematic solvents but do not provide clear indications of which bio-based or green solvents have the best chance of replacing their fossil-based counterparts. This gets more complicated when multiple solvents or even mixtures need replacements. Furthermore, guidelines rarely discuss availability and pricing, although these economically driven factors have a huge impact on the usage of such products.
The composition of coating systems varies with specific applications, making the substitution especially complex. Solutions to overcome this problem have been published in recent years and show the potential of computer-aided formulations compared to experience-based or “trial and error” methods [10,11,12]. These methodologies contain large amounts of information on individual components and their properties and can predict potential characteristics of novel formulations, which is resource- and time-efficient.
Within Microsoft Excel, solvents were characterized according to their physicochemical and safety parameters relevant to the formulation and application of organic coatings. The substitution of petro-based solvents was performed by a multi-criteria decision analysis and Microsoft Excel’s optimization tool Solver (LTSC 2021 MSO). To verify this concept, fossil-based solvents of two commercially available top coatings were substituted with solvents suggested by the new replacement tool, named the solvent catalog. The paints were applied onto primer-coated metal, and all solvent-relevant characteristics, including economic factors, the sustainability influence and functional properties, were evaluated.
2. Materials and Methods
2.1. Solvent Catalog
The solvent catalog was created in Microsoft Excel and works as a tool to substitute individual fossil-based solvents (direct substitution) or mixtures (indirect substitution) with bio-based solvents in paints and coatings. The catalog comprises solvents that are bio-based and available on an industrial scale. More than 40 solvents and their physicochemical parameters (including relative evaporation rate (RER), boiling point (BP), Hansen solubility parameters (HSPs), dynamic and kinematic viscosity, molar volume, surface tension, Chem 21-based safety parameters [9]) and references from chemical retailers are included.
Based on the technique for order of preference by similarity to the ideal solution (TOPSIS) [13,14], the catalog enables the user to compare numerous physicochemical properties based on one value and provides suggestions on direct substitution. The indirect substitution is a more complex optimization problem since the number of variables rises drastically with each solvent and physicochemical parameter. As an optimization tool, the catalog uses the Microsoft Excel add-in Solver (LTSC 2021 MSO). The calculations for the indirect substitution differ partially from the direct approach since TOPSIS cannot be applied to a complex multi-component system. Every physicochemical parameter in mixtures was estimated according to a linear mixing model based on molar composition [12]. Here the add-in Solver is used to find the optimum or a preliminary set value, by changing the volume percentage of each solvent in the data set.
2.2. Chemicals, Coating Formulation and Curing
The Purasolv-series (EL: ethyl lactate, BL: butyl lactate and EHL: 2-ethylhexyl lactate), the Nxt Solv-series (100: ethyl levulinate, 200: butyl levulinate, 300: ethyl 3-(2,4-dimethyl-1,3-dioxolan-2-yl) propanoate) and the bio-based solvent mixture Elsol XR were kindly provided by Corbion (Amsterdam, The Netherlands), Nxtlevvel Biochem (Geleen, The Netherlands) and Vertec Biosolvents (West Chicagoo, IL, USA). All other solvents were purchased from common chemical retailers with purity grades ≥ 97%.
Two polyester–hexamethoxymethylmelamine coatings in white and gray with different solvent combinations representative for coil coatings were provided by Akzo Nobel Hilden GmbH (Hilden, Germany). To formulate a version of each paint with bio-based solvents, about 50% of fossil-based solvents was substituted by applying the solvent catalog, resulting in four samples, two fossil-based ones (Paint 1 and Paint 2) and two paints with bio-based solvents (Bio-Solvent Paint 1 and Bio-Solvent Paint 2). All paints were produced in kilogram scale. Further information on the paint formulation can be found in Tables S1–S4.
The viscosity of every formulation was tested with DIN 4 Cup at 20 °C to achieve a viscosity of 500 mm2 s−1. Paint 1 did not need any adjustment, while Paint 2 (2.5 wt.%) and Bio-Solvent Paints 1 (3.5 wt.%) and 2 (3.5 wt.%) were adjusted with Solvesso 150 (Brenntag, Guntramsdorf, Austria) or its bio-based counterpart.
All four paints were applied on primer-coated metal with a doctor blade (52 µm), cured at a peak metal temperature of 245 °C for 30 s and tested for optical, mechanical and thermal characteristics.
2.3. Optical, Mechanical and Thermal Characterization of Cured Organic Coatings
To characterize all solvent-relevant properties of the cured organic coatings, optical, mechanical and thermal tests were performed. The optical appearance was tested three times for each sample with a gloss meter (Micro-Gloss, Byk Gardner, Geretsried, Germany) at 60°. Mechanical tests were performed based on functionality and their respective standards. Functionality (test/standard/device name): solvent resistance (MEK-Hammer/DIN EN 13523-11) [15], adhesion and flexibility (T Bend/DIN 13523-7) [16], ductility (Erichsen Cupping/DIN EN 13523-6/Byk Gardner Mechanical Cupping Tester (Byk Gardner, Geretsried, Germany)) [17], hardness (Pendulum hardness according to König/DIN EN ISO 1522/TQC SPO500 (TQC, Capelle aan den IJssel, The Netherlands)) [18], deformation (Micro Hardness and Contact Indentation Toughness (CIT)/DIN EN ISO 14577-1/Fischerscope H100C (Fischerscope, Lymington, UK)) [19], impact and penetration resistance (>7 mm and >18 J/DIN EN 13523-5/Byk Mallinckrodt Impact Tester (Byk, Geretsried, Germany)) [20] and scratch resistance (DIN EN 13523-12) [21]. All potential structure failures were monitored with a microscope (Leica M205C (Leica Microsystems, Wetzlar, Germany)). A detailed description of each mechanical test and the replicate numbers is discussed in the Supplementary Information.
Thermal properties of the cured free films were evaluated for each sample once via thermomechanical analysis (TA Instruments, TMA Q400EM, (TA instruments, New Castle, DE, USA)) and differential scanning calorimetry (Netzsch, DSC214 Polyma, (Netzsch, Selb, Germany)). During TMA measurements, a first heating stage up to 80 °C at 10 °C min−1 was performed to erase the physical aging of the sample then cooled down to 0 °C at 10 °C min−1. The glass transition temperature is then measured with the second heating stage heated up to 120 °C at 5 K min−1 with a modulated force of 70 ± 10 mN at 0.1 Hz. Tg was determined from the maximum of the tan delta curve.
For the DSC measurements, also consisting of two heating stages, in the first one the sample was heated from 20 °C to 80 °C at 20 °C min−1, then decreased to −30 °C at 20 °C min−1 and held for 3 min. Lastly, the sample was heated up to 200 °C at 10 °C min−1, where Tg was measured.
Thermogravimetric analysis (TGA 4000, Perkin Elmer, Waltham, MA, USA)) of solvent mixtures was performed on each mixture by applying 20 mL min−1 N2-flow and a temperature program of 20 °C min−1 from 30 °C to 300 °C (Figure S1).
2.4. Life Cycle Assessment (LCA) of Solvents
To assess the potential impact of newly introduced bio-based solvents, a cradle-to-gate and end-of-life LCA was performed. The environmental impact was calculated for butyl glycol acetate, naphtha, ethyl lactate and ethyl levulinate. The software Life Cycle Assessment for Experts (LCA FE 10, DB 2023.02) was used. The impact assessment was performed according to the environmental footprint method 3.1 (EF 3.1) published by the European Commission. Data modeling based on literature research and publicly available databases and life cycle assessment were provided by Daxner & Merl GmbH. The LCA does not represent the actual production where solvents were obtained and produced but should represent a trend on decreased CO2 emissions by the utilization of bio-based solvents.
2.5. Assessment of Economic Aspects
The impact on coating prices was evaluated by comparing target prices of solvents, which were provided by chemical retailers.
Since the heat of combustion drastically changes with exchange in solvent mixtures, the heat of combustion was evaluated for all mixtures. Therefore, the model according to Garvin was used [22,23].
3. Results and Discussion
3.1. Solvent Catalog
The solvent catalog was created in Microsoft Excel and comprises more than 40 bio-based solvents, which were assessed as bio-based and available on an industrial scale based on the literature research. It categorizes these solvents according to the most critical physicochemical parameters attributed to certain functionalities used in coating formulations [12]: the spray-ability (dynamic viscosity, molar volume and surface tension), spread-ability (kinematic viscosity and molar volume), wetting (surface tension and molar volume), solubility (Hansen Solubility Parameters), drying time (relative evaporation rate and boiling point), safety and environmental impact (safety estimation according to CHEM21 [9]). All physicochemical parameters were sourced from the database within the software Hansen Solubility Parameters in Practice (HSPiP, 5th Edition, 2015), while those that were not available were estimated with the Yamamoto molecular break method [24].
The exchange of fossil-based solvents in organic coatings in this tool can be divided into two parts: direct and indirect substitution.
Direct substitution describes the replacement of one solvent with another one, in this work, based on the similarity of physicochemical properties. Here, complex mixtures can be disintegrated into individual components and replaced. If a match is found, identical quantities of the substitute are used. To utilize the solvent catalog, the first physicochemical parameters of the solvent to be replaced need to be inserted. These can be found in datasheets or on governmental chemical databases. After adding these values, the tool calculates the absolute difference between the parameters inserted and the parameters from every solvent described in the catalog, except for the HSPs. Since HSPs comprise three parameters (dispersion δD, polarity δP and hydrogen bonding δH), the spatial difference Ra is calculated according to Equation (1).
Ra and all resulting differences from the physicochemical parameters are then compared using a technique for ordering the preference by the similarity to the ideal solution (TOPSIS). First, all parameters are normalized according to Equation (2).
Depending on the application, individual parameters can have different priorities. Therefore, a weighting factor is introduced in Equation (3) that can be changed by the user. The introduction of a weighing factor might be viable, for example, if the dataset used is not reliable or if specific physicochemical parameters should have a higher impact on the result. In this work, all physicochemical parameters had the same weighing factor, leading to equal contributions to the result. As , the contribution to the C-value of each weighing factor can be interpreted relatively to all other factors.
Since is introduced in Equation (2), the best solution is always the minimum for every parameter. Therefore, an m-dimensional matrix is calculated for the ideal and the anti-ideal solution according to Equation (4).
A separation measure is determined by calculating the m dimensional euclidic distance between the ideal solution and the negative solution in Equation (5).
The relative distance to the ideal solution is calculated according to Equation (6). The value Ci characterizes the similarity between the target solvent and solvents within the catalog. The closer to one, the more similar the solvents are.
After the insertion of the target solvent, potentially appropriate substitutes are chosen from the five highest C-values. Figure 1 and Table 1 show the results after implementing all data of fossil-based solvents to be substituted. Here, solvents with high similarity and potentially low safety concerns were chosen. Bio-based solvents were evaluated based on C-Values and their environmental health safety (EHS) scores. If there was no EHS data available for a solvent, EHS data of proxy structures were assessed. This was only necessary for 2-ethylhexyl lactate and Ethyl 3-(2,4-dimethyl-1,3-dioxolan-2-yl) propanoate, where lactate esters and levulinate esters were chosen as proxy structures. Figure 1A depicts the substitution of butyl diglycol acetate (BDGA) with 2-ethylhexyl lactate (EHL). While EHL has the highest C-value, its EHS data does not provide enough information to generate a proper ranking. Since lactic esters have comparably low safety concerns, EHL was chosen as a replacement for BFGA. Figure 1B,C display the substitution of Butyl glycol acetate (BGA) and dimethyl succinate. According to the C-values, ethyl levulinate and butyl lactate could be substitutes for both chemicals. Ethyl levulinate is categorized as a problematic solvent, according to CHEM21. The difference in EHS scores can be explained by the boiling point of ethyl levulinate. CHEM21 declares solvents with boiling points >200 °C as an environmental concern, since recycling, work-up and downstream unit operations are more energy intensive. This is mostly no concern during coil coating as latent solvents can influence drying times positively. Since both solvents showed high C-values, to increase the overall solvent portfolio within the formulation both solvents were found to be adequate substitutes. In Table 1 entries 1–8 are single compounds or mixtures of known compositions, enabling direct substitution. For entries 9–11, the indirect substitution method was used.
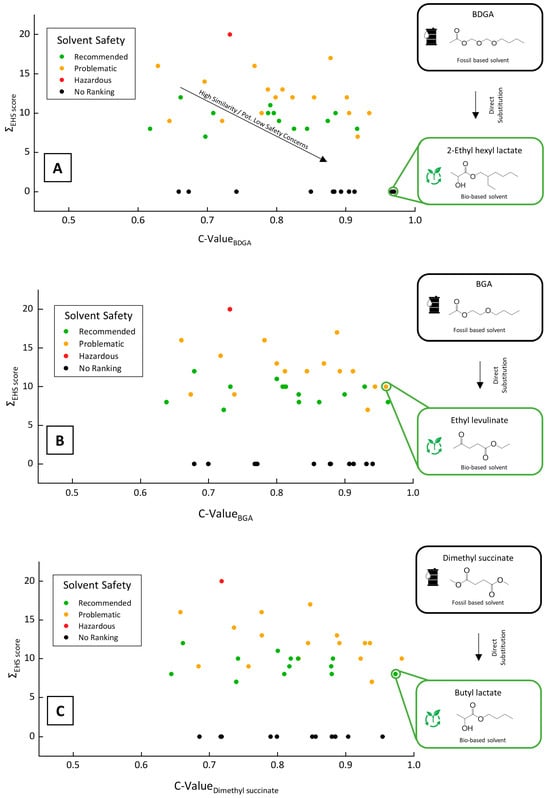
Figure 1.
Direct substitution of three solvents: (A) butyl diglycol acetate (BDGA), (B) butyl glycol acetate (BGA) and (C) dimethyl succinate. Each data point represents a bio-based solvent listed in the solvent catalog and is characterized by the sum of EHS (environment, health, safety) score according to CHEM21 solvent guide and the TOPSIS-C-value. All solvents are assigned to certain safety groups depending on EHS data according to CHEM21 (recommended: green, problematic: yellow, hazardous: red and no Ranking: black). The solvent to be substituted is shown on the top right, while the replacing bio-solvent is displayed on the bottom right.

Table 1.
Fossil-based solvents were substituted by direct substitution with bio-solvents. The proposed bio-based solvents are displayed with their weight contribution in Paints 1 and 2, and the C-valueDirect is determined using the TOPSIS algorithm. The C-valueindirect is determined by indirect substitution using Excel Solver. Abbreviations used: Butyl diglycol acetate (BDGA), Butyl glycol acetate (BGA), Butyl glycol (BG), Butyl diglycol (BDG), Propylene glycol methyl ether (PGME), Dibasic esters (DBEs), 2-Ethyl hexyl lactate (EHL), Ethyl levulinate (ELE), Ethyl lactate (EL) and Ethyl 3-(2,4-dimethyl-1,3-dioxloan-2-yl-propanoate) (EDDP).
Indirect substitution describes the replacement of fossil-based mixtures by a bio-based one with a calculated composition that tries to mimic the properties of the fossil-based one. Every physicochemical parameter in each bio-based mixture is estimated according to a linear mixing model based on the molar composition in Equation (7). Although the linear mixing model is adequate for most parameters, more complex models are commonly used for the RER [12]. Using linear models showed satisfying results in this work, leading to similar evaporation curves as fossil-based mixtures (Figure S1). As this may vary with specific mixture compositions or solvents, a practical assessment of the RER of mixtures is recommended.
and, therefore, the value of each physicochemical property can be varied by changing the volume percentage of solvents. Identical characteristics of the target mixture are found if Ci is equal to zero. By varying the volume fraction of each solvent, a global minimum can be calculated. Since each of more than 40 solvents has up to nine physicochemical parameters that can be changed, an optimization tool is needed. Excel provides three solving methods: Simplex Linear Programming (LP), Generalized Reduced Gradient (GRG) Nonlinear and Evolutionary. GRG Nonlinear and Evolutionary are both adequate for nonlinear optimizations. While Evolutionary is known to find global extrema, the method is rather slow. GRG Nonlinear is highly dependent on its starting values and often finds local extrema but is fast. Another option is GRG Nonlinear Multistart, which compromises both GRG Nonlinear and Evolutionary features since it uses multiple starting variables to avoid local extrema [25]. To decrease the number of variables and wrong solutions, boundaries were introduced. All boundaries set in the solvent catalog are found in Equation (8), where pp is the physicochemical parameter of a solvent.
The indirect substitution was used to replace Solvesso 100, 150 and 200. The exact composition of fossil-derived mixtures is hard to quantify based on one sample, since their composition and therefore their physicochemical properties can vary depending on the geographic source of the oil [26]. Therefore, adequate proxy structures like pseudocumenes were chosen as well as representative data for physical and chemical properties of Solvesso 100, 150 and 200 from chemical retailers.
In coating formulations, hydrocarbons are used to lower prices for viscosity reduction [27] and to increase the heating value, which also leads to higher emissions of CO2. Nevertheless, the solubility of most resins used for paints and coatings consists of various polar groups contributing to the overall polarity of the resin. Some resins are only soluble in non-polar solvents if a polar co-solvent is added. The solubility of the bio-based alternative can therefore differ compared to all the other parameters. Before the paint formulation, all solvents were tested to see if they were compatible with the pre-solubilized resin and additives in a 3:1 ratio. Most solvents except 2-octanol performed satisfactorily. Here phase separation and polymer swelling lead to a thick dispersion. The successive closest solvent according to the TOPSIS calculation was chosen to exchange butyl glycol acetate fully, nonetheless. The substitute for 2-octanol, ethyl lactate, was an adequate alternative since it could solubilize the tested components.
3.2. Optical, Mechanical and Thermal Characterization of Cured Coatings
The substitution of solvents can result in several unwanted effects, like blistering, air trapping, pinholes or cissing [28]. It is therefore necessary to perform optical, mechanical and thermal tests that are related to coating failure due to the solvent exchange. As shown in Table 2, no major differences in the adhesive or mechanical stability were found, and all paints showed satisfying results. The gloss was the only value that significantly decreased for Paint 1 and increased for Paint 2 compared to its bio-based counterparts. In practice this deviation could be easily compensated with appropriate matting agents. Overall, all tests are satisfying, and the paints could be formulated with this setup on a larger scale.

Table 2.
Optical and mechanical test results from two fossil and bio-solvent paints.
Residual solvents can lead to the plastification of the coating, decreasing its mechanical and thermal stability. A harsh decrease in drying time might lead to skinning, causing the upper layer to cure while the layers beneath remain undried [29,30]. The DSC and TMA were performed to assess changes in the glass transition temperature (Table 3 and Figures S2 and S3). Overall, no significant difference in thermal performance was found between fossil- and bio-based paints.

Table 3.
Thermal analysis with a thermomechanical analysis (TMA) and differential scanning calorimetry (DSC) of two fossil- and bio-based paints.
3.3. Economical and Sustainability Aspects
Prices of bio-based solvents are still high. For example, the solvent cyrene is currently still 2.8 times more expensive than the solvent 1-methyl-2-pyrrolidone (NMP), which it should replace [31,32]. Our research based on target prices from chemical retailers verifies this. When comparing the paint price per kilogram, Paint 1 has a 30% lower price, and Paint 2 has a 20% lower price compared to the bio-based mixtures (Table 4). We would like to mention that the price of individual solvents can vary largely and was not part of the current optimization process. Despite the price, bio-based substances show several advantages that could lower the overall investment in production: the replacement of toxic substances, lowering the content of hazardous volatile organic substances and increased work safety [33,34]. In the last few years, the price for allowances within the European Union emission trading system has increased drastically, potentially making an investment in sustainable products economically viable [35].

Table 4.
Price comparison of two pains based on fossil-based solvents and their bio-based counterparts. Here industrial prices were evaluated.
During coil coating, high temperatures are needed to cure the paint. This process leads to the spontaneous evaporation of all solvents, which are then incinerated to generate reusable heat. Many coatings contain carbon-rich solvents that contribute to higher heating values. The heating value of solvent mixtures is lowered by solvents with a larger content of non-carbon-like atoms, such as oxygen or hydrogen. Especially, bio-based solvents, like alcohols, ethers or esters, are known to have a high percentage of heteroatoms. This could lead to a lower energy supply, which then has to be compensated for by the production line with the addition of natural gas. Especially for novel solvent systems, the heating value can be estimated using group contribution methods [22,23]. Table 5 shows that both bio-based paints have a lower heating value than petro-based ones. The optimization of heating values was not a research target and could be improved by using new mixtures with carbon-rich bio-solvents.

Table 5.
Estimated heat of combustion for two paints compared to their bio-based alternatives, where 50% of solvents were substituted.
Most solvents, even comparably new ones, can be assigned to certain environmental, health and safety (EHS) hazards. Therefore, many replacement strategies, as well as the ones in this paper, are based on guidelines that categorize these hazards. Life cycle assessments are based on complex inventories, which need a strong data quality for each compound. Depending on the production or resource strategy of a process, the data can vary for the same chemical. Currently, there is still a large data gap that prevents the inclusion of life cycle assessments in solvent guides. LCA data is of great importance, since the long-optimized processes working with fossil-based educts could outperform bio-based ones, although the performance might be equal or even better. Based on already established inventories or synthesis modeling, CO2 emissions for two fossil-based solvents, butyl glycol acetate and naphtha, and two bio-based solvents, ethyl lactate and ethyl levulinate, were estimated in accordance with EF 3.1 by Daxner & Merl GmbH. Table 6 displays the cradle-to-grave analysis of these solvents. The data for the total climate change includes the fossil, biogenic, land use and land use change. When evaluating biogenic carbon storage within the product, climate change decreases drastically for bio-based solvents. While the carbon in ethyl lactate contributes more than 50% to the overall molar mass, ethyl levulinate is close to 60%, increasing the storage potential even more. In the production of coated coil, solvents are incinerated for energy regain. When incineration is included, carbon-rich structures, like aromatic hydrocarbons, increase CO2 emissions drastically, despite having low production emissions. In ethyl lactate or ethyl levulinate, all carbon is from biogenic sources, leaving only emissions coming from production. In our study, depending on the substitute, the cradle-to-grave analysis shows a decrease in CO2 emissions of 30–50% when a direct substitution is used. Since only four solvents were characterized by the LCA, it is important to mention that regarding “true” sustainability, the ongoing evaluation and characterization of new solvent mixtures is of great importance.

Table 6.
Life cycle assessment of butyl glycol acetate, naphtha, ethyl acetate and ethyl levulinate according to foot print method, 3.1 per kg paint.
4. Conclusions
Based on physicochemical and safety parameter modeling, a multi-criteria decision analysis was used to create a bio-based substitution mix for fossil-based solvents of two paints representative of coil coatings. The technical feasibility of the substitution was demonstrated in pre-formulated systems with an exchange of up to 50%. Considering the biogenic carbon stored in bio-based solvents from cradle-to-grave, we could show that the CO2 emissions can be reduced by 30–50%. From an economical perspective, these solvents cover a broad range of material prices, and specific price calculations must be made for each formulation. However, keeping in mind the anticipated growth in this field together with the economy of scale and increasing costs for CO2 emissions, the application of bio-based solvents is a topic that will continue to gain importance in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cleantechnol7040088/s1.
Author Contributions
Conceptualization, E.R.; methodology, E.R. and C.S.; formal analysis, E.R.; investigation, E.R.; resources, H.R.M.R., B.S. and C.S.; writing—original draft preparation, E.R.; writing—review and editing, H.R.M.R., B.S. and C.S.; visualization, E.R.; supervision, C.S.; project administration, B.S. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the FFG (Austrian Research Promotion Agency) under grant number 918850. The APC was funded by the Johannes Kepler University Open Access Publishing Fund and the federal state Upper Austria.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author. The Excel sheet for the solvent catalog and its manual can be downloaded at https://www.jku.at/en/institute-for-chemical-technology-of-organic-materials/publications/software/solvent-catalog/, accessed on 5 September 2025.
Acknowledgments
Supported by Johannes Kepler University Open Access Publishing Fund and the federal state Upper Austria. The authors would like to thank Akzo Nobel Hilden GmbH for providing the investigated coatings and for technical discussions and the FFG (Austrian Research Promotion Agency) for funding this work under grant 918850.
Conflicts of Interest
The authors Hector Rolando Mendez Rossal and Bernhard Strauß are affiliated with Voestalpine GmbH; however, the article received no funding from the company, and there is no financial or non-financial conflict of interest between Voestalpine GmbH and the publication.
References
- Caretto, A.; Passoni, V.; Brenna, N.; Sitta, M.; Ogliosi, L.; Catel, G.; Turri, S.; Griffini, G. Fully Biobased Polyesters Based on an Isosorbide Monomer for Coil Coating Applications. ACS Sustainable Chem. Eng. 2018, 6, 14125–14134. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. From wood waste to wood protection: New application of black bio renewable water-based dispersions as pigment for bio-based wood paint. Prog. Org. Coat. 2023, 180, 107577. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef] [PubMed]
- Marturano, V.; Marotta, A.; Salazar, S.A.; Ambrogi, V.; Cerruti, P. Recent advances in bio-based functional additives for polymers. Prog. Mater. Sci. 2023, 139, 101186. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Commission Delegated Regulation (EU) 2022/692 of 16 February amending, the puropses of its adapfor the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures. OJ L 129. 2022, pp. 1–17. Available online: https://eur-lex.europa.eu/eli/reg_del/2022/692/oj/eng (accessed on 26 August 2025).
- Pilon, L.; Day, D.; Maslen, H.; Stevens, O.P.J.; Carslaw, N.; Shaw, D.R.; Sneddon, H.F. Development of a solvent sustainability guide for the paints and coatings industry. Green Chem. 2024, 26, 9697–9711. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Tsakovski, S.; Simeonov, V.; Namieśnik, J.; Pena-Pereira, F. A solvent selection guide based on chemometrics and multicriteria decision analysis. Green Chem. 2015, 17, 4773–4785. [Google Scholar] [CrossRef]
- Conte, E.; Gani, R.; Ng, K.M. Design of formulated products: A systematic methodology. AIChE J. 2011, 57, 2431–2449. [Google Scholar] [CrossRef]
- Enekvist, M.; Liang, X.; Zhang, X.; Dam-Johansen, K.; Kontogeorgis, G.M. Computer-aided design and solvent selection for organic paint and coating formulations. Prog. Org. Coat. 2022, 162, 106568. [Google Scholar] [CrossRef]
- Yoon, K. A Reconciliation Among Discrete Compromise Solutions. J. Oper. Res. Soc. 1987, 38, 277–286. [Google Scholar] [CrossRef]
- Hwang, C.-L.; Lai, Y.-J.; Liu, T.-Y. A new approach for multiple objective decision making. Comput. Oper. Res. 1993, 20, 889–899. [Google Scholar] [CrossRef]
- DIN EN 13523-11: 2019-12; Coil coated metals—Test methods—Part 11: Resistance to solvents (rubbing test). Beuth Verlag: Berlin, Germany, 2019.
- DIN EN 13523-7: 2022-01; Coil coated metals—Test methods—Part 7: Resistance to cracking on bending (T-bend test). Beuth Verlag: Berlin, Germany, 2022.
- DIN EN 13523-6: 2020-08; Coil coated metals—Test methods—Part 6: Adhesion after indentation (cupping test). Beuth Verlag: Berlin, Germany, 2020.
- DIN EN ISO 1522: 2007-04; Paints and varnishes—Pendulum damping test. Beuth Verlag: Berlin, Germany, 2007.
- DIN EN ISO 14577-1: 2015-11; Metallic materials—Instrumented indentation test for hardness and materials parameters—Part 1: Test method. Beuth Verlag: Berlin, Germany, 2015.
- DIN EN 13523-5: 2014-08; Coil coated metals—Test methods—Part 5: Resistance to rapid deformation (impact test). Beuth Verlag: Berlin, Germany, 2014.
- DIN EN 13523-12: 2024-06; Coil coated metals—Test methods—Part 12: Resistance to scratching. Beuth Verlag: Berlin, Germany, 2024.
- Garvin, J. Calculate heats of combustion for organics. Chem. Eng. Prog. 1998, 94, 43–45. [Google Scholar]
- Marlair, G.; Vwiklinski, C.; Tewarson, A. An analysis of some practical methods for estimating heats of combustion in fire safety studies. Interflam 1999, 99. Available online: https://ineris.hal.science/ineris-00972167/ (accessed on 26 August 2025).
- Abbott, S.; Hansen, C.M.; Hiroshi, Y. Hansen Solubility Parameters in Practice, version 5; Hansen-Solubility: Boca Raton, FL, USA, 2015. [Google Scholar]
- Díaz de los Ríos, M.; Hernández Ramos, E. Determination of the Hansen solubility parameters and the Hansen sphere radius with the aid of the solver add-in of Microsoft Excel. SN Appl. Sci. 2020, 2, 676. [Google Scholar] [CrossRef]
- Takashi, S.; Sadaro, A.; Masato, M.; Ryuzo, T.; Hideki, Y. Comparison of Hansen solubility paramter of asphaltenes extracted from bitumen produced in different geographical regions. Energy Fuels 2014, 28, 891–897. [Google Scholar]
- Freitag, W.; Stoye, D. Paints, coatings and solvents; Wiley-VCH: Weinheim, Germany, 1993. [Google Scholar]
- Fitzsimons, B.; Parry, T. Coating Failures and Defects; ASM International: Novelty, OH, USA, 2015. [Google Scholar]
- Johansson, K.; Johansson, M. The effect of fatty acid methyl esters on the curing performance and final properties of thermally cured solvent-borne coil coatings. Prog. Org. Coat. 2007, 59, 146–151. [Google Scholar] [CrossRef]
- Patra, N.; Ramesh, P. Investigating the role of solvents in modulating glass transition and dynamic mechanical characteristics of polystyrene film. Colloids Surf. A Physiochem. Eng. Asp. 2024, 703, 135230. [Google Scholar] [CrossRef]
- 1-Methyl-2-pyrrolidinone ReagentPlus, 99 872-50-4. Available online: https://www.sigmaaldrich.com/AT/en/product/sigald/m79603 (accessed on 23 July 2025).
- Cyrene BioRenewable 53716-82-8. Available online: https://www.sigmaaldrich.com/AT/en/product/sial/807796?srsltid=AfmBOopq2-bLYxuNk6_HtsEpUdC_0wlBwpsbyxRjSDLmFaAF8fcK8NmD (accessed on 23 July 2025).
- Gao, F.; Bai, R.X.; Ferlin, F.; Vaccaro, L.; Li, M.H.; Gu, Y.L. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Papasavva, S.; Kia, S.; Claya, J.; Gunther, R. Characterization of automotive paints: An environmental impact analysis. Prog. Org. Coat. 2001, 43, 193–206. [Google Scholar] [CrossRef]
- EUR-Lex–52023DC0654–EN–EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52023DC0654 (accessed on 23 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).