1. Introduction
The transition to a hydrogen-based economy is widely recognized as a critical pathway toward sustainable, low-carbon energy systems. Hydrogen, as a versatile energy carrier, can be produced from renewable sources, stored, transported and converted into electricity, heat, or other energy forms with minimal environmental impact, thereby supporting global climate goals and net-zero commitments [
1,
2,
3,
4,
5,
6]. However, despite its promise, the large-scale deployment of hydrogen technologies faces critical challenges, including low operational efficiency, high production costs, limited durability, and complex infrastructure requirements. In other words, achieving this transition requires not only large-scale hydrogen production from renewable sources but also efficient, safe and cost-effective systems for storage, distribution, and utilization.
Additive manufacturing (AM), also known as 3D printing, offers transformative opportunities to address these challenges by providing layer-wise fabrication of complex energy infrastructure requirements. AM enables unprecedented design freedom, including complex internal structures, lightweight components, and multi-material integration. These capabilities can improve efficiency, durability, and performance while reducing material waste and assembly complexity [
7,
8,
9,
10]. AM facilitates near-net shape, topology-optimized components suitable for electrolyzer cells, fuel cells, steam reformers, storage systems, and carbon capture devices, while enabling functional integration of multiple parts within a single build [
11,
12]. AM has demonstrated notable performance gains in other sectors, such as aerospace, where its adoption in aircraft engines has improved propulsion efficiency, reduced fuel consumption, and lowered emissions [
12]. In hydrogen energy systems, AM reduces material waste, enables lightweight components, and supports localized production, all contributing to lower greenhouse gas emissions [
1,
13]. Optimization of electrolyzers and fuel cell designs through AM enhances energy conversion efficiency and the sustainability of hydrogen infrastructure [
3,
4].
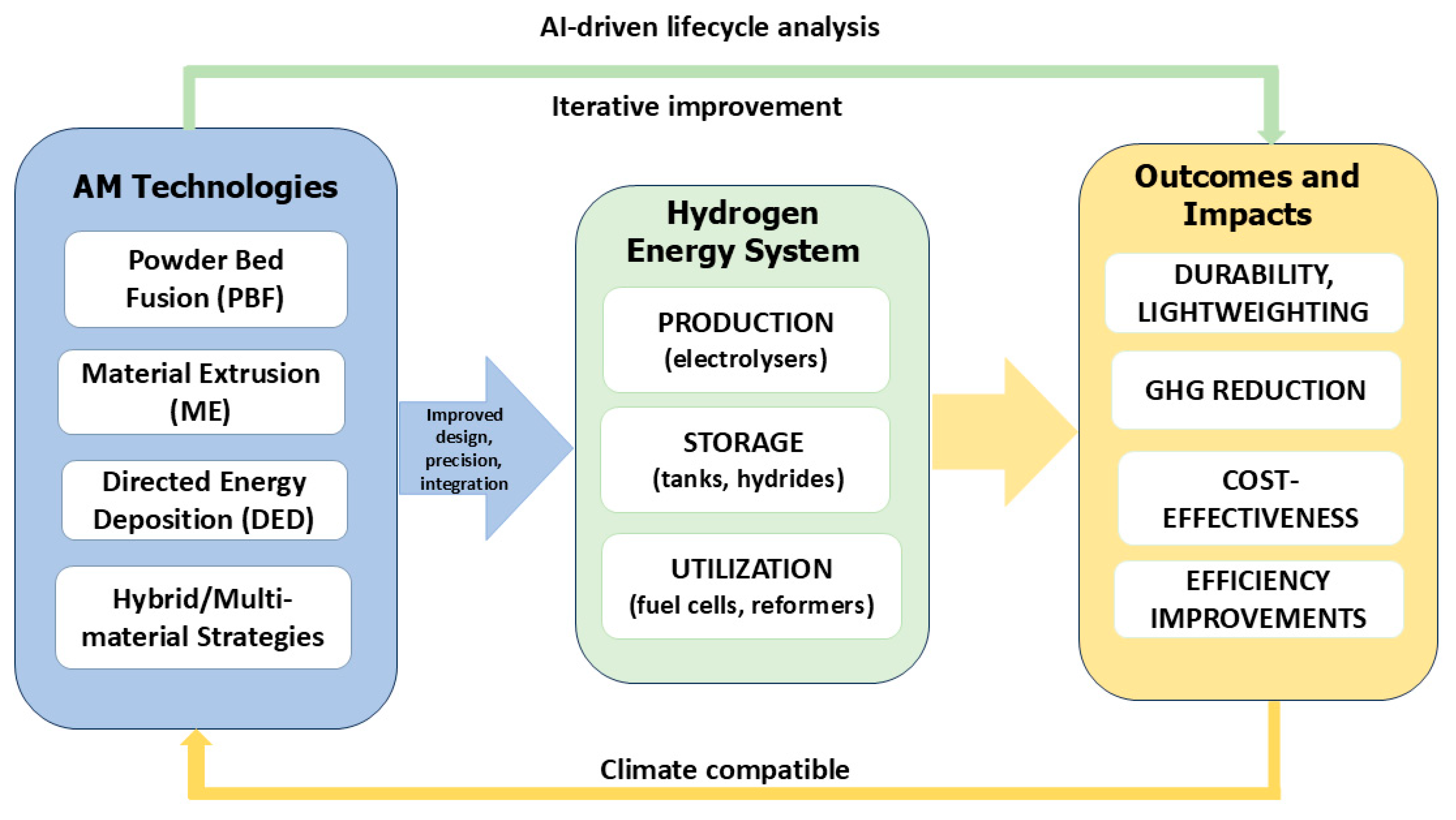
The present study focuses on the role of AM in enhancing the performance and functionality of hydrogen energy components across production, storage, and utilization systems. AM leads to significant improvements in operational efficiency by increasing the active surface area of components, introducing selective porosity to optimize reaction and mass transport, enhancing fluid and gas flow, extending the durability of electrochemical cells, and improving resistance to mechanical and environmental stresses (
Figure 1). Beyond performance optimization, AM facilitates material reduction, providing lightweight components that reduce structural loads and transportation energy requirements. The use of novel feedstocks and additive layer-by-layer fabrication further contributes to cost savings and resource efficiency. Additionally, AM streamlines production cycles by eliminating the need for traditional assembly steps, supporting functional integration of multiple components within a single build, and allowing for rapid prototyping and iterative design optimization. In particular, AM can enhance the performance of proton exchange membrane electrolyzers (PEMECs) through optimized flow fields and catalyst support structures, improve solid oxide electrolyzer cells (SOECs) via integrated interconnects and thermally resilient architectures, and advance alkaline electrolyzers (AELs) with lightweight, corrosion-resistant electrode designs. These improvements not only increase hydrogen yield and energy conversion efficiency but also reduce production and operational costs, addressing barriers to industrial-scale deployment. These capabilities collectively provide a transformative approach for advancing hydrogen production, storage, and utilization technologies while simultaneously reducing environmental impacts and enhancing economic viability [
8,
9]. Despite previous studies having explored AM or hydrogen technologies individually, few have systematically examined how AM can enhance hydrogen energy components across production, storage, and utilization. Key gaps remain for the integration of multi-material designs, optimization of electrochemical performance, durability under harsh conditions, and techno-economic feasibility for PEM, SOEC, and AEL systems. This study addresses these gaps by providing a comprehensive analysis of AM-enabled hydrogen energy components, highlighting technological innovations, assessing limitations, and proposing solutions to advance sustainable, scalable, and efficient hydrogen systems. In doing so, this work contributes novel insights into how AM can accelerate the hydrogen economy and support global net-zero objectives.
This review aims to (i) highlight the major contributions of AM in fabricating hydrogen energy components; (ii) identify key challenges limiting large-scale adoption; and (iii) propose solutions to advance AM-driven designs toward achieving global net-zero goals. By integrating current knowledge on design and material innovations, technological readiness, and cost-effectiveness, this work provides guidance for future research and industrial strategies, underscoring AM as a key enabler of the global hydrogen economy.
2. Hydrogen Energy Components
The term hydrogen economy was first introduced by Appleby in 1972 [
14], referring to a system built around hydrogen energy components, including devices for production, storage, and utilization [
15,
16,
17]. To better understand the strategic role of additive manufacturing (AM) in advancing the hydrogen economy, it is important to map how different AM technologies contribute across these three pillars of hydrogen systems (
Table 1). Each pillar faces unique technical challenges—from efficiency, durability in electrolysers, safety and density in storage systems, to cost and performance in fuel cells.
Hydrogen production technologies include electrolytic systems such as proton exchange membrane electrolyser cells (PEMEC;
Figure 2), microbial electrolyser cells (MEC), solid oxide electrolyser cells (SOEC) and alkaline electrolysers (AEL) cells [
18]. AM directly addresses challenges in these systems by providing layer-by-layer fabrication of complex geometries, lightweight structures, enhanced surface characteristics, and multi-material integration, improving overall efficiency, durability and cost-effectiveness.
Hydrogen storage faces challenges such as low volumetric density, high-pressure containment, diffusion losses, and cyclic degradation. AM provides opportunities to fabricate lightweight lattice tanks with optimized wall thickness, porous gyroid scaffolds for adsorption, and multicomponent hydrides with integrated thermal pathways and protective coatings to enhance resistance to embrittlement [
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30].
Hydrogen utilization components, including fuel cells, burners, and reformers, require resilience under high-temperature and corrosive environments, efficient thermal management, and prevention of hydrogen crossover. AM provides the integration of optimized flow channels, cermet structures for thermal stability, lattice supports for catalysts with large surface area, and embedded cooling channels, enhancing performance and reliability [
22,
23,
24,
25,
27,
30,
31,
32]. Across all hydrogen energy applications, AM supports rapid prototyping of tailored geometries, functional integration (e.g., cooling and monitoring), reduced part counts, and streamlined assembly. Topology optimization enabled by AM further ensures that hydrogen energy components meet demanding performance, cost, and sustainability targets.
Table 1 summarizes key hydrogen energy components, associated challenges, and the contribution of AM toward improving efficiency, durability, and cost-effectiveness. The integration of AM in this hydrogen system represents a critical pathway for supporting global net-zero goals, though large-scale adoption depends on continued advances in materials, standards, and cost reduction, alongside coordinated effort among academia, industry, and policymakers.
Figure 2.
Schematic representation of the main components in PEMEC fuel cell [
33].
Figure 2.
Schematic representation of the main components in PEMEC fuel cell [
33].
Table 1.
Hydrogen energy components, associated challenges, and additive manufacturing (AM) contributions.
Table 1.
Hydrogen energy components, associated challenges, and additive manufacturing (AM) contributions.
| Hydrogen Energy Area | Key Components | Challenges | Potential AM Contributions | Ref |
|---|
| Production (Electrolysers: PEMECs, SOECs, AELs, MECs) | Bipolar plates, membranes, gas diffusion layers, end plates | Corrosion, hydrogen embrittlement, thermal instability, high cost of precision parts | High-density, corrosion-resistant alloys via PBF; hierarchical porosity for improved gas/heat transfer; multi-material builds for protective coatings; reduced assembly through monolithic fabrication | [14,15,16,17,34,35,36,37,38,39,40,41] |
| Storage | Pressure tanks, porous adsorbents, chemical hydrides | Low volumetric density; high-pressure containment; diffusion losses; cyclic degradation | Lightweight lattice tanks with optimized wall thickness; porous gyroid scaffolds for adsorption; multi-material hydrides with integrated thermal pathways; coatings for embrittlement resistance | [19,20,21,22,23,24,25,26,27,28,29] |
| Utilization (Fuel cells, burners, reformers) | Bipolar plates, flow channels, catalytic supports, housings | High-temperature/corrosive environments; thermal management; hydrogen crossover | Integrated flow channels for optimized mass transport; cermet structures for thermal stability; lattice supports for catalysts with large surface area; embedded cooling channels | [22,23,24,25,27,30,31,32] |
| Cross-cutting Applications | Catalysts, sensors, housings, connectors | High cost, long production times, design inflexibility | Rapid prototyping of tailored geometries; functional integration (cooling, monitoring); reduced part count and assembly time; design optimization via AM-enabled topology control | |
3. Additive Manufacturing for Hydrogen Energy Components
Since the invention of the first 3D printing machine by Chuck Hull in 1986 [
42], additive manufacturing (AM) has evolved into multiple technologies marketed under various trade names. AM is a layer-by-layer fabrication process that enables the creation of complex geometries directly from digital models. Unlike traditional subtractive manufacturing, AM allows precise control over component structure, including internal features, porosity, and topology, which is valuable for hydrogen energy components requiring lightweight, high-strength, and corrosion-resistant designs. According to ISO/ASTM 52900:2021 [
43], key AM methods includes Powder Bed Fusion (PBF), Material Extrusion (ME), Vat Photopolymerization (VP), Directed Energy Deposition (DED), Material Jetting (MJ), Binder Jetting (BJ), and Sheet Lamination (SL), based on their operational mechanisms and material feedstocks (
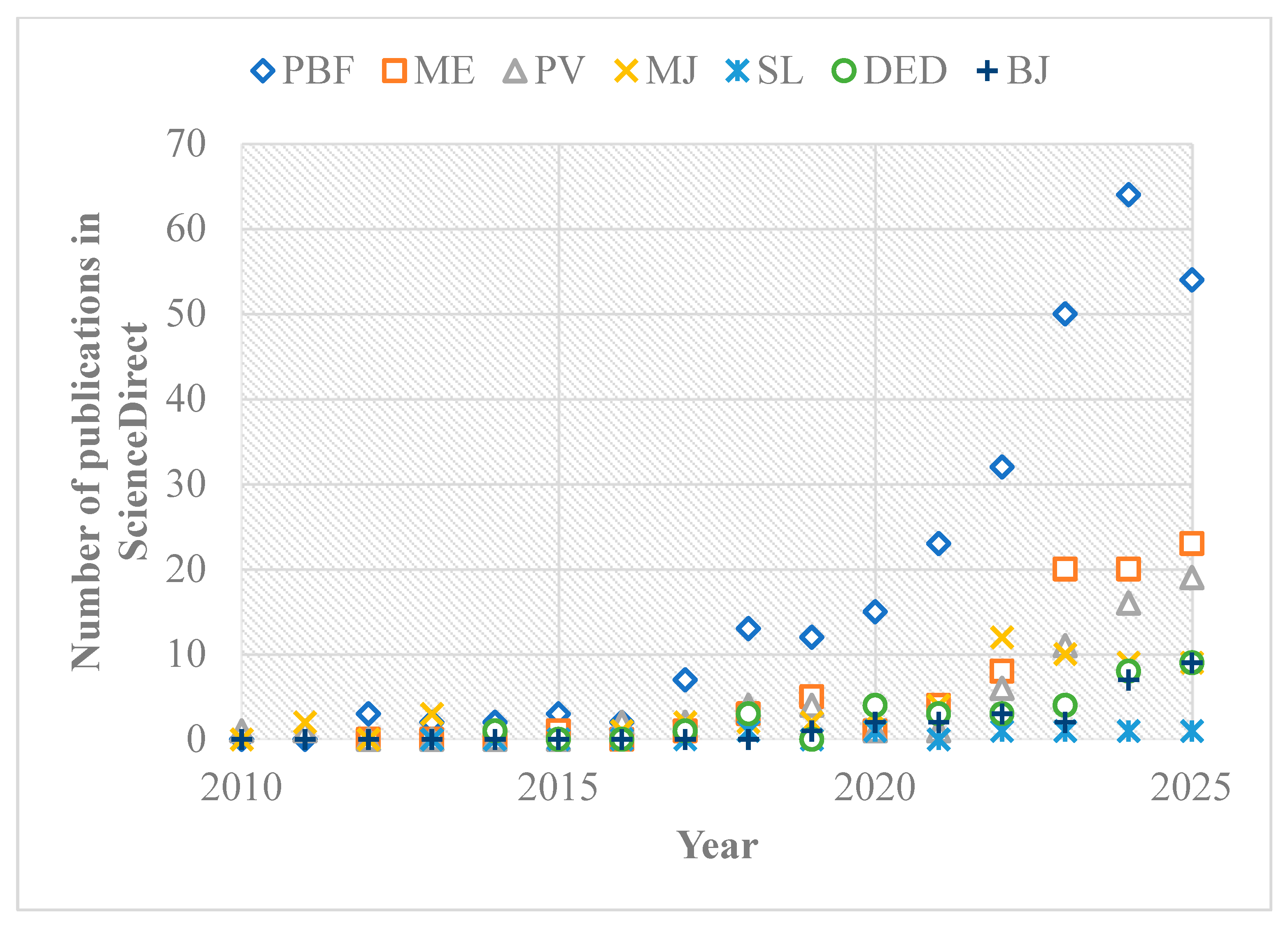
Table 2). A review of the literature indicates that Powder Bed Fusion (PBF) has emerged as the most widely adopted AM process for hydrogen energy components (
Figure 3), owing to its ability to process a broad range of material powders with high geometrical precision and reproducibility. Material Extrusion (ME) has also gained attention due to its operational simplicity and relatively low equipment and feedstock costs [
39]. Vat Photopolymerization (VP), one of the earliest AM technologies, has been applied to hydrogen energy components because of its high resolution and mature process capabilities. Directed Energy Deposition (DED) and Binder Jetting (BJ) offer promising applications but remain limited in scope. In contrast, Sheet Lamination (SL) has seen limited use, likely because of its dependence on adhesives for bonding layers, restricting complex geometry fabrication. The different contributions of AM to hydrogen energy components can be summarized visually in
Figure 4. This flowchart highlights how AM enables design freedom, multi-material integration, lightweighting, and functional feature incorporation across production, storage, and utilization systems, providing pathways to enhance efficiency, durability, and cost-effectiveness.
Research on AM for hydrogen energy components has accelerated significantly after 2015, despite AM being established for over three decades and widely adopted in medical and aerospace industries since the early 2000s [
44]. This growing interest aligns with global decarbonization efforts and the transition toward sustainable energy systems, where hydrogen plays a central role. The urgent need to reduce greenhouse gas emissions has motivated researchers and industry practitioners to explore AM-enabled innovations for critical components, particularly electrolysers, which are pivotal for green hydrogen production from renewable energy sources. AM utilizes metals and alloys for electrolysers, fuel cell plates, and storage tanks; polymers/composites for housing and seals; and ceramics for high-temperature applications. Multi-material AM allows functional integration, combining mechanical, thermal, and catalytic properties in a single part. These capabilities support lightweighting, enhanced durability, optimized mass/heat transport, and rapid prototyping, improving the performance, efficiency, and cost-effectiveness of hydrogen production, storage, and utilization systems [
10,
11,
12,
39,
41,
43,
44,
45,
46,
47].
In commercial electrolyzers and fuel cells, bipolar and flow plates are typically fabricated from stainless steel using conventional CNC machining due to its high strength, thermal stability, and established reliability [
48,
49,
50]. However, additive manufacturing (AM) offers several advantages over CNC methods. AM enables the fabrication of complex geometries, internal cooling channels, and porous structures that are challenging to produce with subtractive techniques [
18,
34,
51]. Near-net-shape AM processes reduce material waste, while multi-material printing allows functional integration of catalytic layers and cooling pathways within a single build, minimizing assembly steps [
24,
41,
47]. Furthermore, AM supports rapid prototyping and design customization, enabling faster iteration of electrolyzer and fuel cell components to meet specific performance requirements [
35,
40,
51]. This comparison highlights how AM complements traditional manufacturing by offering enhanced design flexibility, material efficiency, and functional integration [
31,
32,
39].
One critical consideration in applying AM to hydrogen energy components is the resolution limit of each AM technology, particularly when fabricating serpentine or other intricate flow channels. Different AM processes offer varying levels of precision. PBF and VP can achieve fine resolutions between 25 and 100 µm, enabling highly complex and microfluidic channel geometries, while material extrusion (ME/FDM) is limited to 200–500 µm and is more suitable for larger channels with post-processing for smoother surfaces. DED offers coarser resolutions of 100–300 µm, making it better suited for larger structural channels, whereas BJ can produce complex geometries with resolutions of 50–200 µm, although post-sintering may affect channel accuracy.
Table 3 summarizes the typical resolution ranges and practical implications for each AM technology, highlighting the trade-offs between design flexibility and manufacturing feature size [
34,
39,
43,
51]. Understanding these limitations is essential for optimizing component performance while maintaining manufacturability in electrolyzers, fuel cells, and other hydrogen energy systems.
Table 2.
Classification of AM Technologies and Their Relevance to Hydrogen Energy Components based on ISO/ASTM 52,900:2021 [
48].
Table 2.
Classification of AM Technologies and Their Relevance to Hydrogen Energy Components based on ISO/ASTM 52,900:2021 [
48].
| AM Technology | Machine Types | Printable Materials | Mechanism | Potential Applications in Hydrogen Energy | Ref. |
|---|
| Powder Bed Fusion (PBF) | SLS, SLM, DMLS, EBM | Metals, ceramics, polymers, composites | Selective fusion via laser/electron beams | Electrolyser and fuel cell components (bipolar plates, gas diffusion layers); high-density, corrosion-resistant parts | [47,52] |
| Material Extrusion (ME) | FFF, FDM, CFF | Polymers, composites, food, living cells | Heated filament deposition through nozzle | Low-cost hydrogen storage vessels; polymer membranes; composite structures for embrittlement resistance | [22,23] |
| Vat Photopolymerization (VP) | SLA, DLP, DLS | Photopolymers | UV/light curing of photosensitive resins | Micro-feature fabrication for flow channels, membranes, sensor housing in electrochemical devices | [22,24] |
| Directed Energy Deposition (DED) | LENS, WLAM, DLF, DP | Metals, ceramics, polymers | Deposition with laser/plasma/e-beam fusion | Repair and remanufacturing of electrolyser stacks; multimaterial graded electrodes; reformer components | [22,25] |
| Material Jetting (MJ) | DOD, PJ, NPJ | Polymers, ceramics, composites | UV curing of droplets from movable head | Prototyping of porous/lattice storage media; functional lightweight parts | [22,26] |
| Binder Jetting (BJ) | PB, WB, SB, FB | Metals, ceramics, polymers, sand | Binder selectively joins particles | Porous hydrogen storage structures; casting molds for fuel cell parts | [28,29] |
| Sheet Lamination (SL) | UAM, SDL, LOM, PSL | Metals, plastics, paper | Welding/adhesive/thermal bonding of sheets | Laminated housing for lightweight storage vessels; compact system enclosures | [22,27] |
Table 3.
Resolution Limits of AM Technologies and Implications for Hydrogen Energy Components.
Table 3.
Resolution Limits of AM Technologies and Implications for Hydrogen Energy Components.
| AM Technology | Typical Resolution | Suitable Channel Features | Practical Implications | Ref. |
|---|
| PBF | 25–100 µm | Microfluidic channels, serpentine flow paths | Enables precise, high-complexity flow designs; suitable for dense metallic bipolar plates and optimized flow fields | [8,43,53] |
| VP | 25–100 µm | Fine flow channels, small diameter piping | High-resolution polymer structures; excellent for complex electrode or flow field prototypes | [9,11] |
| ME/FDM | 200–500 µm | Larger channels, low-complexity paths | Suitable for structural or prototype components; post-processing may improve surface smoothness | [8,12] |
| DED | 100–300 µm | Large channels, structural features | Best for metallic structural components; limited for microfluidics | [43,53] |
| BJ | 50–200 µm | Complex geometries with moderate channel detail | Post-sintering may alter channel accuracy; suitable for metallic or ceramic lattice structures | [8,9] |
4. Hydrogen Production Components
To fully appreciate the role of additive manufacturing (AM) in advancing hydrogen technologies, it is essential to evaluate its specific contributions across different hydrogen production systems. AM improves key electrolyser types, including Proton Exchange Membrane Electrolyser Cells (PEMECs), Solid Oxide Electrolyser Cells (SOECs), Microbial Electrolyser Cells (MECs), and Alkaline Electrolysers (AELs), as well as catalytic reforming systems by enabling customized geometries, integrated functionalities, and improved material performance (
Table 4).
Table 4 provides a structured overview of how AM improves components, mitigates challenges, and identifies research needs for large-scale industrial deployment. For instance, in PEMECs, AM enables production of dense, corrosion-resistant bipolar plates with engineered flow fields and integrated coatings to resist acidic environments and hydrogen embrittlement [
18,
49]. SOECs benefit from thermally stable cermet parts, which reduce interfacial degradation under high-temperature operation (800–1100 °C) [
18,
39]. MEC electrodes and flow channels can be fabricated with porous structures that support biofilm growth and efficient electron transfer [
18,
40], while AEL components are optimized for reactant distribution with reduced assembly steps [
18,
35]. AM also allows integration of catalytic layers and optimized pore morphology in reforming and photoelectrochemical systems, enhancing reaction rates and operational stability [
18,
35,
40]. Although AM offers improved geometrical precision, monolithic fabrication, and reduced assembly complexity for electrolysers (PEMECs, SOECs, MECs, and AELs), few challenges or limitations exist. These limitations include material susceptibility to corrosion and hydrogen embrittlement, thermal instability under high-temperature operation, and scalability constraints for industrial deployment. Additionally, process standardization and reproducibility remain barriers for large-scale adoption. To address these challenges, potential solutions include deploying corrosion-resistant and high-temperature-capable alloys, hybrid AM approaches for site-specific material deposition, functional integration of coatings or self-catalytic layers, and AI-assisted optimization of component designs. Coupled with lifecycle cost assessment, these strategies can improve durability, reduce operational risks, and facilitate industrial-scale adoption.
Table 4.
AM’s contribution to hydrogen production technologies, key components and research needs.
Table 4.
AM’s contribution to hydrogen production technologies, key components and research needs.
| Hydrogen Production Technology | Key Components | AM Contributions | Challenges/Research Needs |
|---|
| Proton Exchange Membrane Electrolyser Cells (PEMECs) | Bipolar plates, membranes, gas diffusion layers, end plates | PBF produces dense, corrosion-resistant metallic bipolar plates with engineered flow fields [35]; AM enables integration of coatings/alloys to resist acidic environments & hydrogen embrittlement [18] | Validation under high-pressure acidic conditions; long-term durability testing [34] |
| Solid Oxide Electrolyser Cells (SOECs) | Ceramic–metal (cermet) electrodes, interconnects, seals | AM enables thermally stable cermet parts; reduces interfacial degradation [39]; supports high-temp operation (~800–1100 °C) [18] | Stability under repeated thermal cycling; mitigation of crack propagation [34] |
| Microbial Electrolyser Cells (MECs) | Electrodes, flow channels | AM reduces material usage and fabrication cost [18]; enables porous structures for biofilm growth & efficient electron transfer [40] | Scale-up to industrial size; cost–benefit analysis [34] |
| Alkaline Electrolysers (AELs) | Electrodes, separators, bipolar plates | AM reduces assembly steps with monolithic builds [35]; optimizes flow-field channels for efficient reactant distribution [18] | Performance validation under fluctuating load conditions [34] |
| Catalytic reforming & photoelectrochemical systems | Catalysts, flow channels, membranes | AM integrates catalytic layers with flow fields [40]; increases active surface area & optimizes pore morphology [35]; enhances reaction rates and stability [18] | Demonstration of efficiency gains in industrial-scale systems [34] |
5. Hydrogen Storage Components
Hydrogen storage presents a substantial technical challenge due to volumetric density, high diffusivity, and susceptibility to embrittlement. Conventional storage methods include compression, liquefaction, adsorption, and chemical bonding, which face limitations that impede large-scale adoption. AM offers innovative solutions through lightweight lattice structures, hierarchical porosity, multi-material integration, and embedded functional features, offering safer, more efficient, and cost-effective storage systems (
Table 5). For physical storage, AM enables lightweight lattice-optimized tanks and topology-optimized walls to reduce mass while maintaining strength and safety [PBF, SL]. Adsorption storage benefits from hierarchically porous scaffolds and optimized interconnectivity to improve gas diffusion and storage density [BJ, ME]. Chemical storage methods, such as metal hydrides or liquid organic hydrogen carriers (LOHCs), are enhanced through multi-material AM printing and tailored alloy deposition for improved hydrogen uptake/release and service life [DED, MJ]. Cross-cutting AM advantages include increased surface-to-volume ratios, embedded thermal management channels, and integrated multifunctional designs that reduce assembly requirements and overall cost.
Hydrogen storage poses significant technical hurdles, including low volumetric density, embrittlement, diffusion losses, and cyclic degradation. Although AM enables lightweight lattice tanks, porous adsorption scaffolds, and integrated multi-material designs, long-term durability, safety validation, and performance under repeated cycling remain unresolved. Addressing these issues requires innovative material strategies such as high-entropy alloys, multi-material printing for integrated thermal pathways, and hierarchical structural designs to optimize hydrogen retention and flow. Future research should combine AM-enabled design with AI-driven simulations to predict mechanical and thermal performance, alongside techno-economic evaluation to assess cost-effectiveness and scalability.
6. Hydrogen Utilization Components
Hydrogen utilization systems, such as fuel cells, burners, reformers, and turbines, are critical for converting chemical energy into electricity and driving industrial and distributed energy applications. These components operate under extreme conditions of pressure, temperature, and chemical stress, where design and materials critically affect performance. AM offers lightweight, topology-optimized components, monolithic integration of multiple functions, and tailored porosity for improved mass transport and thermal management (
Table 6).
Fuel cells, reformers, and turbines require high thermal and chemical stability, precise flow-field designs, and efficient mass transport. Conventional components face limitations in electrical conductivity, corrosion resistance, and integration of catalytic structures. Although AM provides opportunities for topology-optimized lightweight designs and embedded functional channels, challenges remain in meeting industrial safety standards, ensuring long-term stability under extreme operating conditions, and reducing production costs. Potential solutions involve the use of multi-material AM, lattice-structured catalytic supports, internal cooling channels, and machine learning-assisted process monitoring to enhance performance consistency and predict failure modes.
7. Printing Hydrogen Energy Components via PBF
The versatility of powder-bed fusion (PBF) has driven extensive research in fabricating hydrogen energy components, with numerous studies showing significant performance gains (
Table 7). For example, PBF-produced liquid–gas diffusion layers (LGDLs) for PEMECs offered precisely controlled pore morphology (
Figure 5a), reducing ohmic losses and boosting current density compared with woven or sintered layers [
55]. Laube et al. [
56] printed porous tubular electrodes for PEMECs that achieved 450 mA/cm
2 at 2 V—exceeding most conventional hollow-electrode designs (
Figure 5c). Márquez et al. [
57] fabricated nylon polymer electrodes for water splitting, where the large catalytic surface area enhanced overall performance.
In PEMFCs, bio-inspired graphite bipolar plates improved performance by 20–25% while lowering costs [
58]. For PEMECs, PBF enabled monolithic printing of cathode bipolar plates and current distributors in stainless steel (
Figure 5b), eliminating assembly steps [
59]. Yang et al. [
60] advanced this further, printing an integrated unit that combined a bipolar plate, LGDL, current distributor, and gasket (
Figure 5f). This design reduced interfacial resistance, increased hydrogen output by 61.8%, and achieved 91% efficiency at 1.62 V and 1000 mA/cm
2. The all-in-one structure amplified component performance 14-fold and lowered costs by removing assembly [
61].
In SOFCs, the electrolyte, anode, and cathode were printed in a single PBF cycle. A three-cell stack with integrated interconnects (
Figure 5k) delivered 0.36 W/cm
2—double the output of previous assemblies—and demonstrated scalability for higher volumetric power density [
62]. Beyond fuel cells, bio-inspired flow-field plates with interconnected narrow channels outperformed grooved designs [
63,
64]. Lei et al. [
65] fabricated triply periodic minimal surface catalyst supports, enhancing methanol steam reforming efficiency (
Figure 5d). Similarly, Zheng et al. [
66] reported higher hydrogen output and system efficiency through increased catalyst loading in PBF-fabricated chambers.
For hydrogen storage, Bürger et al. [
67] printed metal-hydride structures with a large internal surface area, achieving 2.1 kW/kgMH at −20 °C and 8 bar. Inconel 625 displayed twice the corrosion resistance of hot-rolled samples [
68], attributed to AM-specific microstructures resistant to corrosion and hydrogen embrittlement. Heat-resistant FeCrAl reformers with integrated Ni/Al
2O
3 catalytic layers (
Figure 5j,k) reduced structural volume 3.6-fold and improved reforming efficiency at 800 °C [
69]. Across fuel cells and reformers, PBF’s ability to generate complex channels and maximize surface area consistently enhanced operational performance while meeting corrosion and temperature resistance standards.
Ndoye et al. [
70] demonstrated lattice-structured electrodes for photocatalytic electrolysis, achieving a hydrogen generation rate of 1.15 sccm. Agudelo et al. [
71] increased the porosity of 3D-printed high-temperature anodes from 14% to 16%, raising peak power density to 329.25 W/m
2 at 0.61 V and 0.1 A, outperforming less porous electrodes. In summary, as detailed in
Table 7 and the examples above, PBF enables hydrogen energy devices with optimized geometries, superior performance, and improved economic viability.
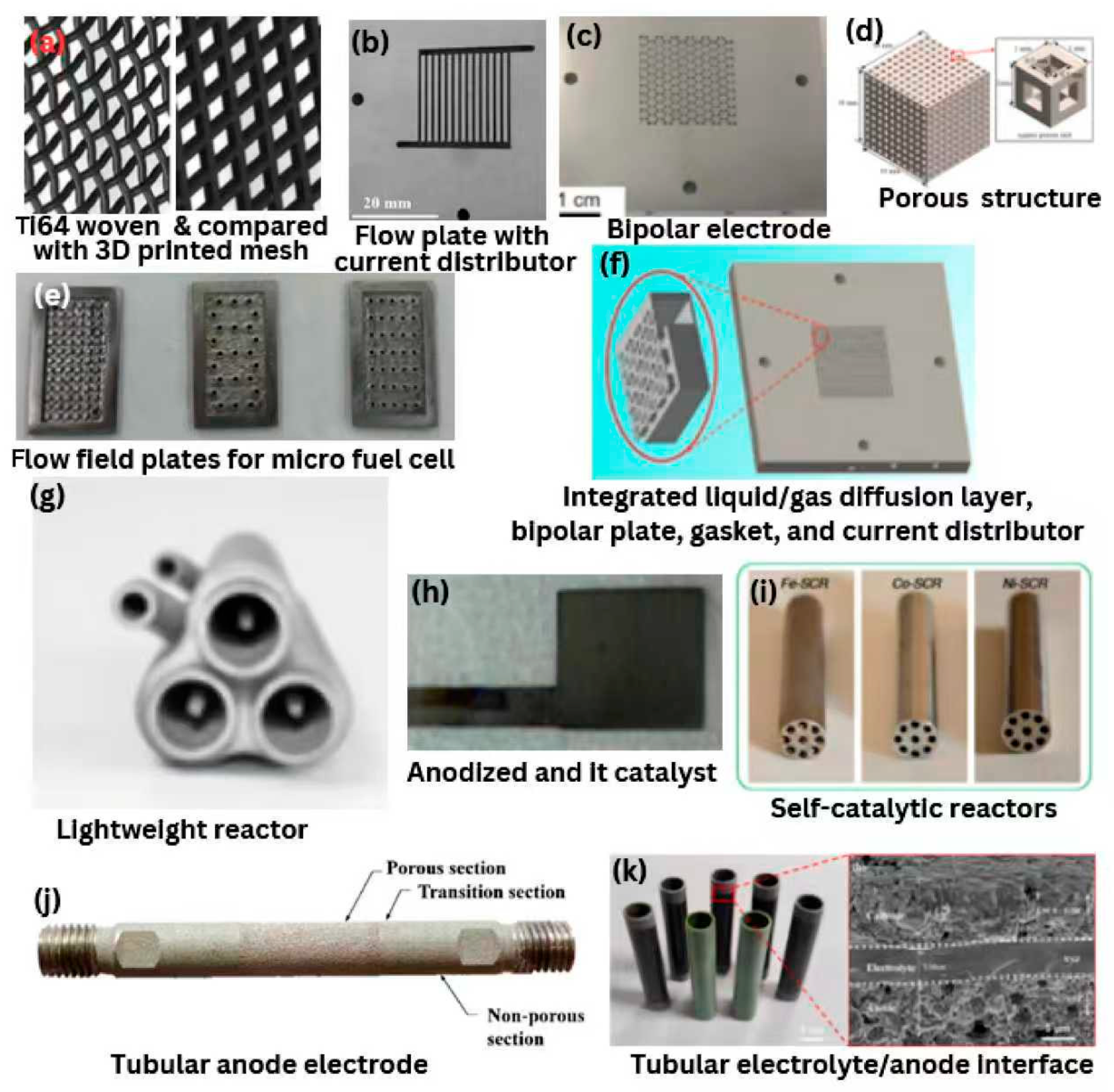
Figure 5.
Hydrogen energy components printed via PBF: (
a) Comparison between Ti64 woven and printed mesh [
55]; (
b) a parallel flow field current distributor [
59]; (
c) 3D-printed all in on bipolar electrode [
61]; (
d) ordered and porous stainless steel structure [
72]; (
e) flow field plates for micro fuel cell [
63]; (
f) AM integrated current distributor, bipolar plate, liquid/gas diffusion layer and gasket [
60]; (
g) lightweight reactor [
67]; (
h) anode fabricated by AM, anodized and it catalyst coated by ALD [
56]; (
i) the physical Self-catalytic reactors after polishing the outer surfaces [
73]; (
j) tubular anode electrode [
71]; (
k) electrolyte/anode interface cross-section of the bamboo-like tubular SOFCs stacks [
62].
Figure 5.
Hydrogen energy components printed via PBF: (
a) Comparison between Ti64 woven and printed mesh [
55]; (
b) a parallel flow field current distributor [
59]; (
c) 3D-printed all in on bipolar electrode [
61]; (
d) ordered and porous stainless steel structure [
72]; (
e) flow field plates for micro fuel cell [
63]; (
f) AM integrated current distributor, bipolar plate, liquid/gas diffusion layer and gasket [
60]; (
g) lightweight reactor [
67]; (
h) anode fabricated by AM, anodized and it catalyst coated by ALD [
56]; (
i) the physical Self-catalytic reactors after polishing the outer surfaces [
73]; (
j) tubular anode electrode [
71]; (
k) electrolyte/anode interface cross-section of the bamboo-like tubular SOFCs stacks [
62].
Table 7.
Hydrogen energy components produced via PBF.
Table 7.
Hydrogen energy components produced via PBF.
| Performance Outcome | Material & Justification | Remarks | Ref. |
|---|
| Corrosion potential of −61.5 mV; current density 300 μA/cm2; penetration rate 1.76–5.09 mm/yr | Inconel 625—selected for high mechanical strength and anti-corrosive properties in HT-PEMFC | Corrosion rates of bipolar plates are governed by microstructures from the AM process | [68] |
| Peak power density 329.25 W/m2; voltage 0.61 V at 0.1 A | 316L stainless steel (austenitic)—excellent chemical and corrosion resistance | Gas diffusion layer (GDL) with 16% porosity exhibited superior performance | [71] |
| Photoelectrochemical H2 generation: 11.15 sccm | Maraging steel—use nickel-coated 3D-printed electrodes | Demonstrated efficient hydrogen evolution | [70] |
| Hydrogen production: 1.779 V at 2.0 A/cm2 | Stainless steel—high strength, corrosion resistance, conductivity | Intricate 3D-printed parallel flow fields acted as bipolar plates & current collectors, reducing assembly cost | [59] |
| Operational thermal power: 2.1 kW/kgMH | Aluminum—chosen for low component weight | Demonstrated potential for lightweight AM metal hydrides (ratio 0.97–1.56) | [67] |
| 86.5% performance increase at 2 A/cm2, 1.716 V, 80 °C | Stainless steel—durable under high T/P, corrosion-resistant | Monolithic fabrication reduced overall weight and improved efficiency | [60] |
| Methanol steam reforming: 12 mL/h at 250 °C, 64.3% efficiency | Stainless steel—resistant to high T/P and corrosion | Ordered-porous AM parts improved catalytic hydrogen generation efficiency | [72] |
| 91% energy efficiency at 1.62 V, 1000 mA/cm2 | Stainless steel—high durability and conductivity | AM enabled monolithic bipolar electrodes with superior operational efficiency | [61] |
| CO2 conversion: 71%; CH4 conversion: 65% at 1073 °C | Fe-based powders—allotropic catalysts for Fischer–Tropsch synthesis | AM metallic parts served as both reactor and catalyst | [73] |
| Power density: 0.36 W/cm2 at 700 °C | Ti-containing 316 stainless steel—improved corrosion resistance | Interconnected SOFC cells delivered higher power density | [62] |
| H2 evolution: 200 mL/h | Cu—excellent electrical conductivity | Small channels caused high pressure drops, limiting efficiency | [74] |
| High H2 yield: 7.6 wt% biomass at 800 °C | Kanthal® AM100 Bethel, USA (FeCrAl alloy)—high heat resistance | 3D-printed Ni/Al2O3/FeCrAl catalyst with open cellular structure improved pyrolysis reforming | [69] |
| Current density: 1.515 A/cm2; power density: 363 mW/cm2 | Stainless steel—resistant to high T/P, durable | Enclosed channels in flow fields showed better performance than open grooves | [63] |
| Methanol conversion: 91.5% (BCCS) & 95% (FCCS) | Stainless steel—high strength and corrosion resistance | AM-printed BCCS/FCCS catalysts outperformed conventional counterparts | [66] |
| — | Stainless steel—reliable under harsh conditions | Triply periodic minimal surface structures provided high surface area, improving H2 yield over sintered felt | [65] |
| Current density: 1.19 A/cm2; power density: 238 mW/cm2 | Stainless steel—good corrosion resistance & conductivity | AM micro fuel cells achieved higher operational capacity | [64] |
| Operational voltage: 2.18 V; current 1.5 A/cm2 at 20 °C | Ti6Al4V alloy—corrosion resistant in acidic & humid environments | EBM-produced diffusion layers showed customizable pore design, lower cost for Ti parts | [55] |
| — | Ti6Al4V alloy—high strength-to-weight ratio, conductivity | AM topology-optimized end plates reduced mass & volume for high-pressure electrolysis | [75] |
| Current density: 450 mA/cm2 at 2 V, 60 °C | Ti Grade 5—strong, corrosion-resistant, low density | AM enabled advanced electrode design with higher conductivity & surface area | [56] |
| Current density: 15 mA/cm2 at 60 °C; degradation 76 μV/h for 500 h | Ti6Al4V alloy—strength, corrosion resistance, conductivity | Performance limited by ionic conductivity of electrodes; requires improvement | [76] |
| — | Ni-based & high-entropy alloys (HEAs)—superior strength, corrosion resistance, tunable properties | HEAs offer resistance to hydrogen embrittlement, enabling high-temperature applications | [77] |
8. Printing Hydrogen Energy Components via ME
Most standard filaments, such as PLA (polylactic acid) and ABS (acrylonitrile butadiene styrene), can be used to print hydrogen energy components when catalytic properties are not required [
78]. In many cases, coatings are applied to meet corrosion and temperature resistance requirements [
79]. Chisholm et al. [
80] fabricated bio-inspired flow plates for PEMECs using polypropylene via ME and coated them with silver. The coating preserved the lightweight, low-cost structure without altering its topography. ME-produced components weighed seven times less than their metallic counterparts [
81], while improved electrode wettability enhanced hydrogen and oxygen evolution. The ME process thus reduces electrode mass and provides components with broader compatibility in harsh environments compared to metals [
82]. Ahn et al. [
83] used electrodeposition to coat a graphene-based pyramid electrode (
Figure 6b), achieving a photocurrent of −3.01 mA at 0.02 V—three times higher than planar electrodes (−0.91 mA). Transition metal carbides and nitrides (MXenes) were used to coat 3D-printed nanocarbon electrodes (
Figure 6c), yielding high conductivity for hydrogen generation [
84]. Given their effectiveness and low cost, MXenes are considered a promising route for hydrogen technologies. Similarly, Iffelsberger et al. [
85] electrodeposited MoSx on ME electrodes (
Figure 6i) for hydrogen evolution. A catalyst substrate printed from ABS enhanced thermal conductivity by 34% [
86], demonstrating the ability of ME to produce 3D structures with tailored heat and mass transfer properties that improve catalytic efficiency.
Qian et al. [
87] printed porous ceramic electrodes for a methanol steam reforming microreactor (
Figure 6e). The large surface area and optimized pore distribution increased methanol conversion by 13.3% compared to conventional supports, while ceramic loading ensured chemical stability. Diaz-Herrezuelo et al. [
88] fabricated porous activated carbon electrodes (
Figure 6e) impregnated with 5 wt% Pd for catalytic dehydrogenation of formic acid. The system produced CO-free hydrogen under ambient conditions with 81% FA conversion and a 6 mL/min hydrogen flow rate, demonstrating stability and recyclability as a potential hydrogen-carrier technology. Functionalized filaments can also be directly printed without coatings. Foster et al. [
89] fabricated HER electrodes using graphene-based PLA (
Figure 6b), which maintained stable voltage up to 1000 cycles, comparable to platinum electrodes. Research on functional filaments is ongoing. Kreider et al. [
90] developed processable metal–organic frameworks (MOFs), using ABS with ≤10% MOF-5 to print hydrogen storage structures (
Figure 6d), which exhibited extensive internal surface areas for adsorption. Ghosh et al. [
91] produced carbon-based active filaments with tunable flexibility, conductivity, and printability. Electrodes printed from these filaments delivered a current density of 1.82 mA/cm
2 for HER. Copper-based filaments were also developed to print spiral electrodes for microbial electrolysis cells (MECs). A single spiral achieved 1.5 mA/cm
2 at 2 V, though adding more spirals did not improve current density due to electrode–electrolyte resistance [
92]. Direct printing of functionalized catalytic filaments that eliminate coatings could enable electrodes with greater durability and performance under harsh conditions.
The ME process shows strong potential for producing hydrogen energy components at a lower cost than other AM methods (
Table 8). Although some instances reported lower efficiency, production rates per cost unit were superior to conventional techniques [
82]. Yang et al. [
93] printed PLA bipolar plates (
Figure 6e) with an electrochemical performance of 2.21 V at 1 A/cm
2. While performance was below that of conventional graphite plates, costs were reduced to one-tenth, suggesting a viable pathway for low-cost hydrogen production. Techno-economic analysis indicated conventional methods are nearly six times more expensive [
93]. Overall, ME demonstrates the ability to fabricate hydrogen energy components with reduced cost, improved resistance to harsh environments, and potential for future performance optimization.
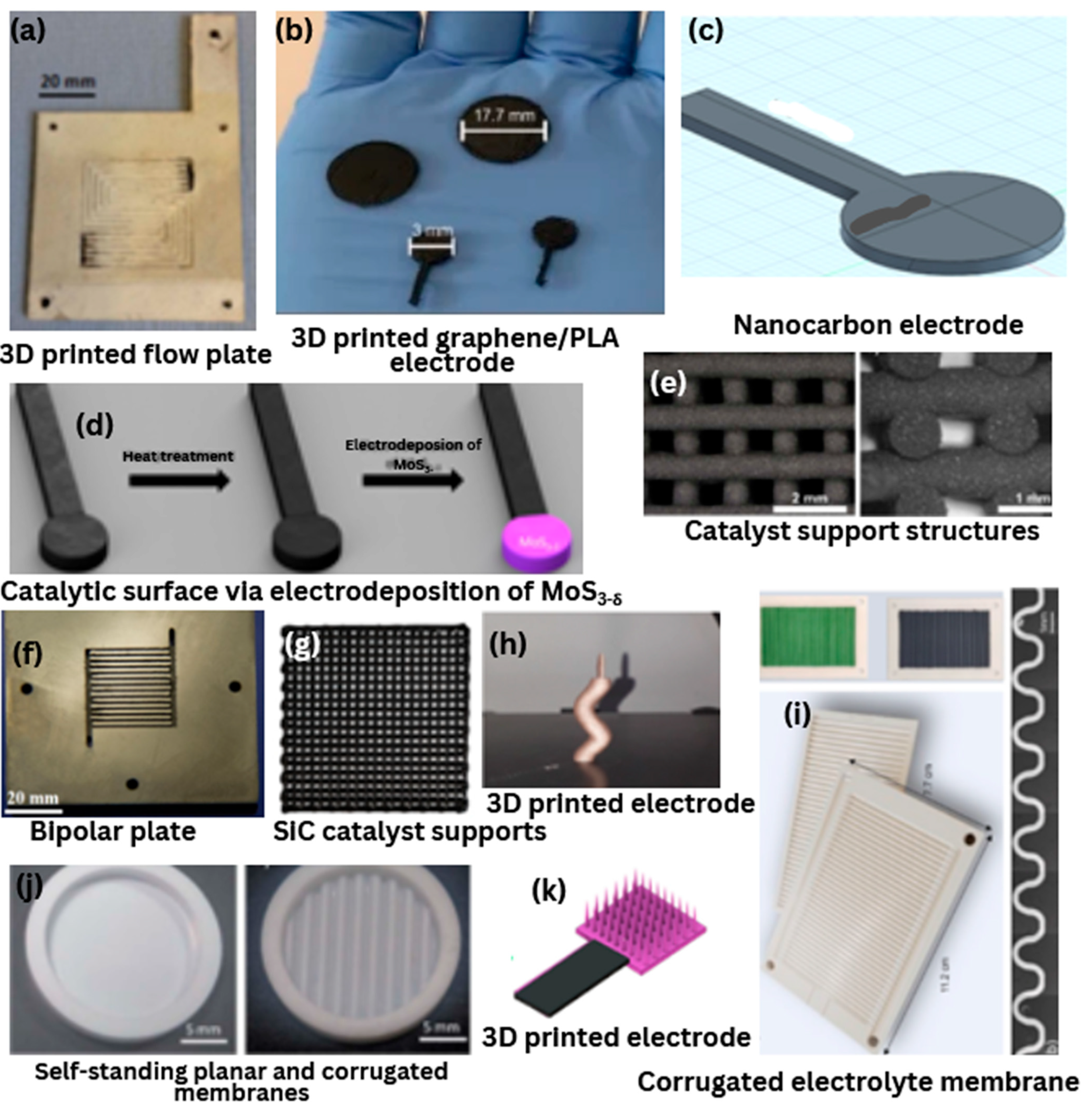
Figure 6.
Hydrogen energy components printed via ME: (
a) 3D-printed flow plate electrodeposited with silver [
80]; (
b) 3D-printed graphene/PLA electrode [
89]; (
c) 3D-printed nanocarbon electrode [
84]; (
d) 3D-printed catalytic surface via electrodeposition of MoS
3-δ from NH
4 MoS
4 carried out on conductive graphene electrode surface [
85]; (
e) Cross-section views of the 3D-printed catalyst support structures [
88]; (
f) 3D-printed bipolar plate and etched cross-section [
93]; (
g) 3D-printed SiC catalyst supports [
87]; (
h) 3D-printed electrode [
92]; (
i) 3D-printed sintered complex-shaped cells with embedded channels and the corrugated electrolyte membrane after sintering [
98]; (
j) 3D-printed self-standing 8YSZ planar and corrugated membranes [
99]; (
k) 3D-printed electrode for water splitting [
100].
Figure 6.
Hydrogen energy components printed via ME: (
a) 3D-printed flow plate electrodeposited with silver [
80]; (
b) 3D-printed graphene/PLA electrode [
89]; (
c) 3D-printed nanocarbon electrode [
84]; (
d) 3D-printed catalytic surface via electrodeposition of MoS
3-δ from NH
4 MoS
4 carried out on conductive graphene electrode surface [
85]; (
e) Cross-section views of the 3D-printed catalyst support structures [
88]; (
f) 3D-printed bipolar plate and etched cross-section [
93]; (
g) 3D-printed SiC catalyst supports [
87]; (
h) 3D-printed electrode [
92]; (
i) 3D-printed sintered complex-shaped cells with embedded channels and the corrugated electrolyte membrane after sintering [
98]; (
j) 3D-printed self-standing 8YSZ planar and corrugated membranes [
99]; (
k) 3D-printed electrode for water splitting [
100].
9. Printing Hydrogen Energy Components via PV
The freedom of PV design enables fabrication of electrolyte supports with increased surface area, reduced cost, shorter turnaround time, and minimal waste. Lira et al. [
101] demonstrated this by printing yttria-stabilized zirconia electrodes (
Figure 6k) with a 16 cm
2 active area, achieving 340 mA/cm
2 at 1.3 V and a low degradation rate of 11 mV/kh over 1150 h. This value is seven times lower than the ~1.57 mV/kh reported by Batalla et al. [
76] for PBF porous transport electrodes. Corrugated yttria-stabilized zirconia electrodes with embedded interconnectors (
Figure 6j) further condensed SOC stacks, showing that electrolyte surface area directly correlates with SOC performance. Márquez et al. [
102] confirmed this by printing corrugated scandia-stabilized zirconia electrolytes (
Figure 6i) with a 3.2 cm
2 active area, achieving 500 mW/cm
2 at 900 °C for 350 h. Collectively, PV-enabled electrolytes have driven SOC performance from 340 mA/cm
2 reported in 2021 by Lira et al. [
101] to a peak of 1.3 V reported in 2024 by Kostretsova [
103].
PV has also been applied to fabricate SiCN ceramic monoliths via pyrolysis at 1000 °C from preceramic resin containing 10 wt% silica for ammonia cracking, achieving a 99% hydrogen conversion rate at 600–1000 °C [
104]. Wei et al. [
73] achieved 99.96% electrolyte density with 176 mW/cm
2 at 850 °C, noting that performance was limited by the oxide-ion conductivity of YSZ and recommending ScSZ as a higher-performing alternative [
102]. PV has further been used to produce gas diffusion layers for PEMFCs by pyrolyzing printed structures into conductive carbon. These achieved 205 mW/cm
2 power density, below the ~543 mW/cm
2 typically reported for commercial PEMFC electrodes [
104]. Optimization of the pyrolysis process is therefore required to improve the performance of PV-derived components.
10. MJ, BJ, SL, DED for Printing Hydrogen Energy Components and Hybrid Printing
AM processes such as MJ, BJ, SL, and DED have received limited research and industrial attention for hydrogen energy components (
Figure 4,
Table 9 and
Table 10). This may be due to their printing mechanisms, which are less suitable for fabricating intricate miniature structures. For instance, despite the five-axis deposition capability of DED, it cannot produce overhanging structures. Unlike powder bed systems with self-supporting mechanisms, DED lacks support structures to accommodate overhangs, limiting its ability to fabricate complex hierarchical geometries. Similarly, SL and BJ rely on binders and adhesives, which may not deliver the geometric precision or chemical stability required for harsh electrochemical environments. These factors likely explain the limited exploitation of MJ, BJ, SL, and DED in hydrogen energy applications, as also noted by Mesecke et al. [
8]. Nevertheless, some techno-economic studies suggest potential advantages. Dzogbewu and de Beer [
12] used DED to fabricate TiFe intermetallic hydrogen storage structures and reported lower cost, faster turnaround, and reduced energy consumption compared to PBF [
105]. Niedermeyer et al. [
106] demonstrated BJ by printing Al
2O
3 catalyst supports and microfluidic devices, indicating its potential for catalytically active components.
AM has also advanced from single-material to multi-material printing [
12]. Hybrid manufacturing, which integrates multiple AM techniques within a single component, has emerged to exploit the functional strengths of each process [
105]. For example, MJ and ME were combined to print porous electrodes for SOC, eliminating multiple production steps and achieving an area-specific resistance of 2.1 Ω·cm
2 at 750 °C, comparable to other SOC series [
101]. PV and ME have also been integrated to monolithically print an 8-cell YSZ SOFC stack in a single step, with PV used for the electrolyte and ME for the electrodes [
103]. Current literature primarily reports hybrid strategies involving BJ, MJ, or PV for SOCs. However, combining PBF and ME may offer complementary advantages for hydrogen energy components in the future.
Hybridization has also been applied to enhance electrode performance. A combination of ME and combustion synthesis produced nickel electrodes with engineered macropores, where ME generated macropores and combustion synthesis added nanopores, resulting in 131 mV for the hydrogen evolution reaction at 30 mA/cm
2 [
97]. In addition, AM processes are increasingly integrated with conventional methods to produce tailored structures for specific applications—for example, PBF-LB/M with CNC [
106], PV with CNC [
107], and DED with CNC [
108].
Table 9.
Hydrogen energy components produced via PV.
Table 9.
Hydrogen energy components produced via PV.
| Hydrogen Energy Component | Performance | Material Rationale | Remarks | Ref |
|---|
| Electrolyte for solid oxide cells | 340 mA/cm2 at 1.3 V with low degradation (11 mV/kh) | 8 mol% yttria-stabilized zirconia (8YSZ): high oxygen ion conductivity | AM enables fabrication of large active areas (16 cm2) for enhanced hydrogen generation | [109] |
| Electrolyte for solid oxide cells | Power density of 500 mW/cm2 at 900 °C | Scandia-stabilized zirconia (ScSZ): high ionic conductivity and structural stability at high T | Stereolithography allows planar and corrugated SOCs with >350 h stable operation | [102] |
| Microreactor for ammonia cracking | 99% conversion efficiency for H2 production | SiCN: robust against corrosive processes and high T | Preceramic resin + AM enables non-oxide ceramic microreactor with excellent thermal and chemical resistance | [110] |
| Electrolyte for solid oxide cells | 600 mA/cm2 at 1.3 V and 900 °C | 8YSZ: high oxygen ion conductivity | Corrugated SOCs via AM outperform planar designs | [99] |
| Electrolyte for solid oxide cells | 200 mW/cm2 at 900 °C | 3 mol% YSZ: good ionic conductivity, flexibility, and stability at high T | AM enhances SOC stacks with volumetric and gravimetric power densities 3–4× higher than planar equivalents | [98] |
| Electrode for water splitting | Cell voltage of 1.80 V at 10 mA/cm2 | Conductive carbon: high conductivity, surface area, stability, low cost, printable | SLA enables high-resolution needle arrays with superior geometry compared to FFF | [100] |
| Electrolyte for solid oxide cells | 1.04 V, peak power density of 176 mW/cm2 at 850 °C | 8YSZ: high oxygen ion conductivity | SLA-based printing achieves electrolyte density of 99.96% | [73] |
| Gas diffusion layer for PEMEC | Power density of 205 mW/cm2 | UV-curing resin (material unspecified) | Printed microstructures improve advective-diffusive oxygen transport over conventional carbon paper | [104] |
Table 10.
Technological readiness level (TRL) of AM hydrogen energy components.
Table 10.
Technological readiness level (TRL) of AM hydrogen energy components.
| AM Technology | Innovation/Contribution | TRL * | Ref |
|---|
| PBF | Monolithic catalyst with periodic open-cellular geometry, increasing active surface area and achieving 7.6 wt% H2 yield from biomass at 800 °C—higher than recent literature reports; validated through lab-scale experiments | 4 | [69] |
| | Self-catalytic 3D-printed reactor functioning as both reactor and catalyst for direct CO, CO2, and CH4 conversion to syngas; high surface area enhances efficiency | 4 | [73] |
| | Reactor with high weight-specific thermal power for fuel cell preheating, attributed to near-ideal thermodynamic properties of the AM structure | 4 | [67] |
| | Novel 3D-printed injector geometries designed to stabilize methane–hydrogen fuel mixtures at reduced cost | 4 | [111] |
| ME | Triply periodic minimal surface (TPMS) geometry improved reactor thermal efficiency and resistance to high temperatures | 3 | [96] |
| | 3D-printed nanocarbon and screen-printed carbon electrodes with enhanced temperature and corrosion resistance | 4 | [84] |
| | Carbon-based electrodes with improved corrosion resistance | 3 | [82] |
| | Graphene–PLA electrodes offering high resistance to corrosion and oxidation | 4 | [89] |
| | 3D-printed carbon/polymer filament electrodes with high corrosion resistance | 4 | [91] |
| PV | Planar and corrugated solid oxide cells with scandia-stabilized zirconia, demonstrating high electrochemical performance | 5 | [102] |
| | Nickel/lanthanum strontium manganite composite electrodes with complex hierarchical geometries and large active surface area, yielding high performance | 5 | [109] |
| | Electrically conductive carbon gas diffusion layer for PEMECs with micro- and macro-scale features enhancing catalytic activity | 4 | [104] |
| | Dense yttria-stabilized zirconia electrolytes reducing cost of complex solid oxide fuel cells | 5 | [99] |
| | Flexible stack designs enabled by AM improve cost-competitiveness of solid oxide fuel cells | 5 | [98] |
11. Technological Readiness and Future Directions of AM Hydrogen Components
Additive manufacturing (AM), or 3D printing, has demonstrated substantial potential for improving hydrogen energy components across production, storage, and utilization systems. By enabling design freedom, AM allows precise control over geometry, porosity, and thermal pathways, supporting the fabrication of hydride modules, electrolyzer components, and fuel-cell parts with optimized mass transport, thermal management, and structural integrity [
8,
9,
51]. These capabilities facilitate reductions in weight and volume, improved geometric precision, and functional integration, such as internal cooling channels, hierarchical porosity, and topology-optimized lightweight structures, which collectively enhance efficiency, durability, and operational performance [
9,
11,
12,
51]. Monolithic builds reduce assembly complexity, part counts, and production steps, translating into lower material usage, decreased costs, and greater flexibility for large-scale deployment [
1,
13].
Laboratory- and pilot-scale studies indicate that AM-fabricated hydrogen components can achieve performance comparable to, or exceeding, conventionally manufactured equivalents, while significantly reducing material costs, production steps, and waste [
114,
115,
116]. Self-catalytic AM reactors, for example, eliminate the need for post-fabrication coatings, addressing uneven deposition and degradation issues and enhancing long-term reliability [
114,
115,
116]. Furthermore, the integration of high-entropy alloys (HEAs) and other advanced materials enhances resistance to hydrogen embrittlement, corrosion, and high-temperature operation, addressing critical limitations for industrial deployment [
117,
118,
119].
Despite these advancements, several technical and industrial challenges remain. Operational stresses, high temperatures, corrosive environments, hydrogen embrittlement, and multi-cycle degradation limit the long-term performance and industrial applicability of AM components [
4,
5,
53]. Cost-intensive materials, complex process parameters, and limited industrial-scale demonstrations further constrain widespread adoption [
8,
120,
121,
122,
123,
124,
125,
126]. Addressing these gaps requires innovations in functional design, multi-material integration, and predictive modeling, alongside robust lifecycle cost analyses to quantify economic and environmental benefits.
High-build-rate AM techniques offer potential cost reductions at industrial scales, while low-cost methods enhance accessibility and broader adoption. Coupled with AI-driven materials design, AM enables predictive modeling of hydrogen absorption/desorption kinetics, structural resilience, thermal transport efficiency, and multi-cycle stability, allowing systematic optimization of HEAs and hydride composites for PEMEC, SOEC, and AEL systems [
127]. This integration of computational intelligence and AM fabrication creates a closed-loop workflow in which predictive design informs fabrication, experimental validation refines AI models, and iterative optimization accelerates the development of scalable, reliable, and high-performance hydrogen storage components.
From a techno-economic perspective, AM enables lightweight, topology-optimized, and material-efficient designs that reduce both material and assembly costs compared to conventional manufacturing techniques such as CNC machining or casting [
7,
53]. Rapid prototyping and on-demand manufacturing facilitate shorter production cycles, reduced inventory requirements, and faster deployment of hydrogen infrastructure. Life-cycle assessments indicate that when combined with lightweighting, local production, and functional integration, AM-enabled hydrogen systems can significantly reduce greenhouse gas emissions, energy consumption, and operational costs relative to conventional approaches [
2].
Looking forward, several opportunities can further advance the field and bridge laboratory-scale innovation with industrial-scale adoption. These include hybrid AM processes for site-specific deposition of functional materials, AI-assisted generative design for predictive optimization, multi-material integration for enhanced structural and functional performance, and comprehensive techno-economic assessments to guide industrial deployment and policy decisions [
2,
7,
13,
53]. Innovations in materials capable of withstanding extreme pressures (~700 bar for storage, ~50 bar for production/use) and high temperatures (up to 1100 °C), combined with predictive modeling of degradation and performance, will be crucial for transitioning AM hydrogen components from TRL 4–5 laboratory prototypes to TRL 6–7 industrial demonstrations.
By addressing these technical, economic, and environmental challenges, AM provides a transformative pathway for the hydrogen economy. It offers scalable, durable, and cost-effective solutions for high-performance hydrogen storage and energy conversion systems, supporting climate mitigation, renewable energy integration, and industrial innovation. The integration of AI-driven design, advanced materials, and AM fabrication establishes a replicable framework that can guide policy, foster workforce development, and accelerate the global transition toward a sustainable hydrogen-based energy infrastructure.
12. Conclusions
Additive manufacturing (AM) has demonstrated hydrogen energy components with transformative potential through design freedom, precise geometry control, and the ability to fabricate porous structures that significantly increase catalytic surface area and potential efficiency. AM addresses critical challenges in hydrogen production, storage and utilization, including resistance to harsh environments, material inefficiencies, and component complexity. Multi-material and hybrid AM strategies enable selective deposition of functional materials, optimizing durability, performance, and integration across complex hydrogen systems.
The integration of artificial intelligence (AI) and machine learning (ML) significantly enhances the capabilities of additive manufacturing (AM) in hydrogen energy applications. These technologies enable generative design, real-time process monitoring, defect detection, multi-material optimization, and predictive assessments of cost and energy efficiency, collectively allowing for more precise, efficient, and sustainable component fabrication. By leveraging AI-driven design, AM can reduce material waste, simplify assembly processes, and optimize operational energy usage, thereby increasing the overall efficiency of hydrogen storage, production, and utilization systems.
From a techno-economic perspective, AM presents promising cost advantages through reduced material consumption, assembly simplification, and localized production. However, comprehensive economic validation remains limited, particularly for large-scale deployment. Bridging technical innovation with robust cost–benefit analyses is essential to demonstrate the feasibility of industrial adoption and justify public and private investment in hydrogen infrastructure. Current technological readiness levels indicate that most AM hydrogen components reside at NASA TRL 4–5, requiring further validation under industrial conditions (TRL 6) to realize their full potential. Achieving this transition is critical for enabling scalable, cost-effective, and high-performance hydrogen systems that can meaningfully contribute to global decarbonization and net-zero objectives.
AM, when combined with AI-driven design and informed by rigorous techno-economic evaluation, provides a clear pathway toward durable, efficient, and environmentally sustainable hydrogen energy solutions. This integrated approach not only advances technical performance but also supports policy objectives and climate-compatible energy strategies by demonstrating the feasibility of large-scale, industrial hydrogen infrastructure.
Despite these advantages, challenges remain in ensuring material durability, resistance to high temperatures and corrosive environments, and industrial scalability. By employing hybrid AM strategies, multi-material printing, AI-guided process optimization, and lifecycle techno-economic analyses, these challenges can be systematically addressed. This integrated perspective establishes a roadmap for future research and innovation, guiding both technical advancement and strategic policy planning.
Moreover, critical opportunities exist for expanding AM capabilities in hydrogen technologies, including the development of hybrid and multi-material components, AI-assisted design and predictive modeling, and comprehensive techno-economic evaluation frameworks. By pursuing these avenues, this project not only strengthens the technical foundation of next-generation hydrogen storage but also facilitates rapid industrial adoption, workforce development, and policy alignment, ensuring that AM-enabled hydrogen systems transition from laboratory success to widespread, real-world deployment.
Overall, the integration of AI, AM, and rigorous economic and environmental assessment positions this approach as a transformative enabler of the hydrogen economy, providing scalable, high-performance, and climate-resilient solutions for a sustainable energy future.