Non-Fuel Carbon-Neutral Use of Lignite: Mechanism of Bitumen and Humic Acid Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
3. Results
- The bitumen chemically interacts with the humic acids, forming new compounds with a denser structure that are more heat resistant than unmodified bitumen.
- As a result of intensive dispersion of solid humic acid particles within the bitumen, through intermolecular interactions of a physical nature, complex structural units (micelles) with a denser structure are formed with some of the bitumen’s hydrocarbons or their groups, which are more heat resistant than unmodified bitumen.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hridoy, S.M.; Protyai, M.I.H.; Rashid, A.B.; Sayeed, M.A. Marshall property analysis of an improved bituminous mix obtained by mixing plastic waste with bitumen. S. Afr. J. Chem. Eng. 2025, 53, 40–48. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, C.; Weng, Y.; Wang, T.; Yang, S.; Ma, S.; Chen, Y. Investigating the anti-ageing property of SBS copolymer modified bitumen incorporating surface organic layered double hydroxides. Constr. Build. Mater. 2025, 463, 140034. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Luo, R.; Zhang, L.; Wang, F.; Liu, J.; Liang, Y. Influence of surface layer bitumen on the performance evolution of the base layer over service time. Case Stud. Constr. Mater. 2024, 21, 3560. [Google Scholar] [CrossRef]
- Pyshyev, S.; Prysiazhnyi, Y.; Demchuk, Y.; Borbeyiyong, G.I.; Vytrykush, N. Adhesive modifiers for bitumen obtained from coumarone-indene fractions of liquid coal coking products. Int. J. Adhes. Adhes. 2025, 138, 103933. [Google Scholar] [CrossRef]
- Jizhe, Z.; Weidong, C.; Weihua, L.; Shurong, L.; Wenwu, Z.; Zongliang, H. Performance assessment of composite modified bitumen integrated with desulfurized crumb rubber and SBS modifier as waterproofing adhesive layer. Int. J. Adhes. Adhes. 2024, 134, 103802. [Google Scholar] [CrossRef]
- Hu, T.; Luo, Y.; Zhu, Y.; Chu, Y.; Hu, G.; Xu, X. Mechanochemical preparation and performance evaluations of bitumen-used waste polypropylene modifiers. Case Stud. Constr. Mater. 2024, 21, e03471. [Google Scholar] [CrossRef]
- Sabzoi, N.; Muhammad, J.; Rebecca, G.; Filippo, G. Recycled plastic as bitumen modifier: The role of recycled linear low-density polyethylene in the modification of physical, chemical and rheological properties of bitumen. J. Clean. Prod. 2020, 266, 121988. [Google Scholar] [CrossRef]
- Jexembayeva, A.; Konkanov, M.; Aruova, L.; Zhaksylykova, L.; Baidaulet, Z. Preparation of polymer bitumen binder in the presence of a stabilizer. Polym. Eng. Sci. 2024, 65, 6–13. [Google Scholar] [CrossRef]
- Xiao, N.; Zhang, Y.; Xia, H.; Lei, Y.; Luo, Y. Effects of organic nano calcium carbonate on aging resistance of bio-asphalt. Adv. Mater. Sci. Eng. 2022, 2022, 6043030. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, F.; Li, Y.; Liu, K.; Zhu, J.; Gong, G. Study on the Properties of Graphene Oxide–Wood Tar-Based Composite Rejuvenated Asphalt. Coatings. 2024, 14, 1081. [Google Scholar] [CrossRef]
- Zhou, S.; Long, K.; Zhang, Z.; Li, S.; Ai, C.; Yan, C. Development of sustainable lignin-based coatings for layered double hydroxides: Enhancing synergistic anti-aging properties in bitumen. Fuel 2025, 380, 133166. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, C.; Sun, G.; Ma, X.; Du, H. An Investigation of the Effects of Thermo-Oxidative Aging and the Freeze–Thaw Cycle on the Performance of Polyester-Based, Self-Adhesive Asphalt Waterproofing Membranes. Appl. Sci. 2024, 14, 8237. [Google Scholar] [CrossRef]
- Koyun, A.N.; Büchner, J.; Wistuba, M.P.; Grothe, H. Laboratory and field ageing of SBS modified bitumen: Chemical properties and microstructural characterization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126856. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Oliviero Rossi, C. Bitumen and bitumen modification: A review on latest advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef]
- Pensini, E.; Tchoukov, P.; Yang, F.; Xu, Z. Effect of humic acids on bitumen films at the oil-water interface and on emulsion stability: Potential implications for groundwater remediation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 53–59. [Google Scholar] [CrossRef]
- Gutierrez, L.; Pawlik, M. Influence of humic acids on oil sand processing. Part I: Detection and quantification of humic acids in oil sand ores and their effect on bitumen wettability. Int. J. Miner. Process. 2014, 126, 117–125. [Google Scholar] [CrossRef]
- Oliviero, R.C.; Teltayev, B.; Angelico, R. Adhesion promoters in bituminous road materials: A review. Appl. Sci. 2017, 7, 524. [Google Scholar] [CrossRef]
- Singh, S.K.; Pandey, A.; Ravindranath, S.S. Effect of additives on the thermal stability of SBS modified binders during storage at elevated temperatures. Constr. Build. Mater. 2022, 314, 125609. [Google Scholar] [CrossRef]
- Hunter, R.N.; Self, A.; Read, J.; Hobson, E. The Shell Bitumen Handbook; Thomas Telford Ltd.: London, UK, 2003. [Google Scholar]
- Donchenko, M.; Grynyshyn, O.; Prysiazhnyi, Y.; Pyshyev, S.; Kohut, A. The Problem of Road Bitumen Technological Aging and Ways to Solve It: A Review. Chem. Chem. Technol. 2024, 18, 284–294. [Google Scholar] [CrossRef]
- Pyshyev, S.; Miroshnichenko, D.; Chipko, T.; Donchenko, M.; Bogoyavlenska, O.; Lysenko, L.; Miroshnichenko, M.; Prysiazhnyi, Y. Use of Lignite Processing Products as Additives to Road Petroleum Bitumen. ChemEngineering 2024, 8, 27. [Google Scholar] [CrossRef]
- Crude Oil Prices Today. Oilprice. Available online: https://oilprice.com/ (accessed on 10 June 2025).
- Lignite Coal Price Index. Business Analytiq. Available online: https://businessanalytiq.com/procurementanalytics/index/lignite-coal-price-index/ (accessed on 1 May 2025).
- IMARC Group. Lignite Coal Price Trend. Available online: https://www.imarcgroup.com/lignite-coal-price-trend (accessed on 1 June 2025).
- BGR—Federal Institute for Geosciences and Natural Resources. BGR Energy Study 2023—Data and Developments in German and Global Energy Supply; BGR: Hannover, Germany, 2024; 154 p. [Google Scholar]
- Miroshnichenko, D.V.; Pyshyev, S.V.; Lebedev, V.V.; Bilets, D.Y. Deposits And Quality Indicators Of Brown Coal In Ukraine. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2022, 3, 5–10. [Google Scholar] [CrossRef]
- Brown Coal. Genesis and Distribution. Institute of Geology. Available online: https://insgeo.com.ua/brown_coal/ (accessed on 20 June 2022).
- Skybová, M.; Turčániová, Ľ.; Čuvanová, S.; Zubrik, A.; Hredzák, S.; Hudymáčová, Ľ. Mechanochemical activation of humic acids in the brown coal. J. Alloys Compd. 2007, 434–435, 842–845. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, C.; Zhao, X.Y.; Cao, J.P. Highly efficient conversion of low-rank coals to high-quality humic acids via ozone oxidation depolymerization at room temperature. Fuel 2025, 395, 135209. [Google Scholar] [CrossRef]
- Francioso, O.; Ciavatta, C.; Montecchio, D.; Tugnoli, V.; Sánchez-Cortés, S.; Gessa, C. Quantitative estimation of peat, brown coal and lignite humic acids using chemical parameters, 1H-NMR and DTA analyses. Bioresour. Technol. 2003, 88, 189–195. [Google Scholar] [CrossRef]
- Sarlaki, E.; Hossein, K.M.; Marzban, N.; Shafizadeh, A.; Ahmad, S.F.; Hu, S.; Tabatabaei, M.; Aghbashlo, M. Advances and challenges in humic acid production technologies from natural carbonaceous material wastes. Chem. Eng. J. 2024, 498, 155521. [Google Scholar] [CrossRef]
- Aftab, K.; Javed, J.; Habibhah, S.U.; Malik, A.; Hassan, A.; Rizwan, M.K.; Busquets, R.; Ahmad, N.; Haque, A. Process optimization and method validation for efficient valorization of low- grade coal into humic substances. Fuel 2024, 369, 131796. [Google Scholar] [CrossRef]
- Poljansek, I.; Krajnc, M. Characterization of Phenol-Formaldehyde Prepolymer Resins by In Line FT-IR Spectroscopy. Acta Chim. Slov. 2005, 52, 238–244. [Google Scholar]
- Ke, Y.; Zhu, X.; Wang, K.; Wang, L.; Zhou, S.; Zhang, Z. Role of humic acid on benzo[a]anthracene: Insights from aging on adsorption, speciation distribution and bioavailability. Environ. Pollut. 2025, 368, 125723. [Google Scholar] [CrossRef]
- Niu, H.Q.; Zhao, R.X.; Yang, H.Y.; Tong, L.L.; Zhou, Y.Q. Variations in structure and adsorption characteristics of humic acid during pressure oxidation process. Trans. Nonferrous Met. Soc. China. 2024, 34, 1694–1709. [Google Scholar] [CrossRef]
- Lebedev, V.; Miroshnichenko, D.; Pyshyev, S.; Kohut, A. Study of hybrid humic acids modification of environmentally safe biodegradable films based on hydroxypropyl methyl cellulose. Chem. Chem. Technol. 2023, 17, 357–364. [Google Scholar] [CrossRef]
- ISO 5073:2021; Brown Coals and Lignites—Determination of Humic Acids. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/79744.html (accessed on 15 December 2021).
- Lebedev, V.; Miroshnichenko, D.; Xiaobin, Z.; Pyshyev, S.; Dmytro, S.; Nikolaichuk, Y. Use of Humic Acids from Low-Grade Metamorphism Coal for the Modification of Biofilms Based on Polyvinyl Alcohol. Pet. Coal 2021, 63, 953–962. [Google Scholar]
- DSTU 8995:2020; Lignite, Hard Coal, Anthracite and Oil Shale. Accelerated Methods for Moisture Determination. TK 92: Kyiv, Ukraine, 2020. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=89550 (accessed on 1 July 2021).
- DSTU GOST 11022-95; Solid Mineral Fuel. Methods for Ash Determination (ISO 1171-81), with Amendment No. 1. CIS: Minsk, Belarus, 2001. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=71354 (accessed on 1 January 1997).
- DSTU 9220:2023; Solid Mineral Fuel. Methods for Determination of Volatile Matter Yield. TK 92: Kyiv, Ukraine, 2023. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=103115 (accessed on 1 February 2024).
- DSTU 3528-97; Solid Mineral Fuels. Determination of Total Sulfur. Esch Method (ISO 334-92). Держстандарт України: Kyiv, Ukraine, 1997. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=78878 (accessed on 1 July 1998).
- DSTU ISO 17247:2010; Coal. Elemental Analysis (ISO 17247:2006, IDT). Держспoживстандарту України: Kyiv, Ukraine, 2010. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=55150 (accessed on 1 July 2012).
- DSTU EN 1426:2018; Bitumen and Bituminous Binders. Determination of Needle Penetration Depth (EN 1426:2015, IDT). TK 92: Kyiv, Ukraine, 2018. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=78299 (accessed on 1 June 2019).
- DSTU EN 1427:2018; Bitumen and Bituminous Binders—Determination of Softening Point by the Ring and Ball Method (EN 1427:2015, IDT). TK 92: Kyiv, Ukraine, 2018. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=78301 (accessed on 1 June 2019).
- DSTU 8825:2019; Bitumen and Bituminous Binders. Method for Determination of Ductility. TK 92: Kyiv, Ukraine, 2019. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=82135 (accessed on 1 January 2020).
- EN 13398:2018; Bitumen and Bituminous Binders. Method for Determining Elasticity (EN 13398:2017, IDT). CEN: Brussels, Belgium, 2019. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=79270 (accessed on 1 December 2019).
- DSTU EN 12592:2018; Bitumen and Bituminous Binders. Determination of Solubility (EN 12592:2014, IDT). TK 92: Kyiv, Ukraine, 2018. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=78551 (accessed on 1 June 2019).
- DSTU 9169:2021; Bitumen and Bituminous Binders. Determination of Adhesivity to Mineral Aggregate. TK 92: Kyiv, Ukraine, 2021. Available online: https://online.budstandart.com/ua/catalog/doc-page?id_doc=97049 (accessed on 1 August 2019).
- DSTU B EN 12607-1:2015; Bitumen and Bituminous Binders. Determination of Resistance to Hardening Under the Influence of Heat and Air. Part 1. RTFOT Method (EN 12607-1:2014, IDT). Мінрегіoн України: Kyiv, Ukraine, 2015. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=63683 (accessed on 1 July 2016).
- DSTU 9116:2021; Bitumen and Bituminous Binders. Polymer Modified Road Bitumens. Specifications. TK 92: Kyiv, Ukraine, 2021. Available online: https://online.budstandart.com/ua/catalog/doc-page?id_doc=96264 (accessed on 1 March 2022).
- Huculak-Mączka, M.; Hoffmann, J.; Hoffmann, K. Evaluation of the possibilities of using humic acids obtained from lignite in the production of commercial fertilizers. J. Soils Sediments 2018, 18, 2868–2880. [Google Scholar] [CrossRef]
- Nouali, M.; Derriche, Z.; Ghorbel, E. Effect of clay materials on phase separation in plastic bag waste-modified bitumen during high-temperature storage. Transp. Eng. 2025, 19, 100306. [Google Scholar] [CrossRef]
- Kar, S.; Maity, J.P.; Jean, J.S.; Liu, C.C.; Nath, B.; Lee, Y.C.; Bundschuh, J.; Chen, C.Y.; Li, Z. Role of organic matter and humic substances in the binding and mobility of arsenic in a Gangetic aquifer. J. Environ. Sci. Health Part A. 2011, 46, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, D.; Lebedeva, K.; Cherkashina, A.; Lebedev, V.; Tsereniuk, O.; Krygina, N. Study of Hybrid modification with humic acids of environmentally safe biodegradable hydrogel films based on hydroxypropyl methylcellulose. C-J. Carbon Res. 2022, 8, 71. [Google Scholar] [CrossRef]
- Pyshyev, S.V.; Miroshnychenko, D.V.; Shved, M.Y.; Korchak, B.O.; Lebedev, V.V. Register of Lignite Deposits in Ukraine Recommended for Use in “Green” Technologies, 1st ed.; Publishing House “Spolom”: Lviv, Ukraine, 2024; © Lviv Polytechnic National University; pp. 1–147. [Google Scholar]
- International Bitumen Emulsion Federation (IBEF). Available online: https://www.ibef.net/en/emulsions-2/key-figures/ (accessed on 1 November 2024).
- UNdata. A World of Information. Available online: http://data.un.org/Data.aspx?q=bitumen&d=EDATA&f=cmID%3aBT (accessed on 11 December 2024).
- Bitumen Price. Available online: https://bitumenprice.org (accessed on 14 July 2025).
- Basekim. Daily Bitumen Price on 2025. Available online: https://carbokim.com/bitumen-price/ (accessed on 15 July 2025).
- Made-in-China. GT Humic. Available online: https://gthumic.en.made-in-china.com (accessed on 15 July 2025).







| No. | Index | Unit of Measurement | Value | Methods and Standards |
|---|---|---|---|---|
| 1 | Moisture content (Wa) | wt.% | 9.60 | [39] |
| 2 | Ash content (Ad) | wt.% | 7.90 | [40] |
| 3 | Volatile matter yield (Vdaf) | wt.% | 52.30 | [41] |
| 4 | Total sulfur content (Std) | wt.% | 3.90 | [42] |
| 5 | Carbon content (Cdaf) | wt.% | 62.34 | [43] |
| 6 | Hydrogen content (Hdaf) | wt.% | 4.63 | |
| 7 | Nitrogen content (Ndaf) | wt.% | 0.77 | |
| 8 | Oxygen content (Oddaf) | wt.% | 28.36 |
| № з/п | Index | Value | ||
|---|---|---|---|---|
| BND 70/100 | BND 70/100 + Humic Acids | Methods and Standards | ||
| 1 | Penetration at 25 °C, dmm | 78 | 72 | [44] |
| 2 | Softening point (SP), °C | 52.6 | 54 | [45] |
| 3 | Ductility at 25 °C, cm | 58 | 15 | [46] |
| 4 | Elastic recovery at 25 °C, % | 17.5 | 28.7 | [47] |
| 5 | Solubility in organic solvent, % | 99.95 | – | [48] |
| 6 | Adhesion to gravel, mark | 3.5 | 3.5 | [49] |
| 7 | Adhesion to glass, % | 65 | 60 | [49] |
| 8 | Resistance to hardening at 163 °C (RTFOT method): | [50] | ||
| Mass change, wt.% | 0.086 | 0.156 | ||
| Softening point after RTFOT, °C | 59.6 | 59.4 | ||
| Penetration at 25 °C after RTFOT, dmm | 39 | 48 | ||
| Softening point change, °C | 6.8 | 5.4 | ||
| Retained penetration, % | 50.0 | 68.6 | ||
| 9 | Homogeneity | – | No clumps or particles of humic acids are observed | [51] |
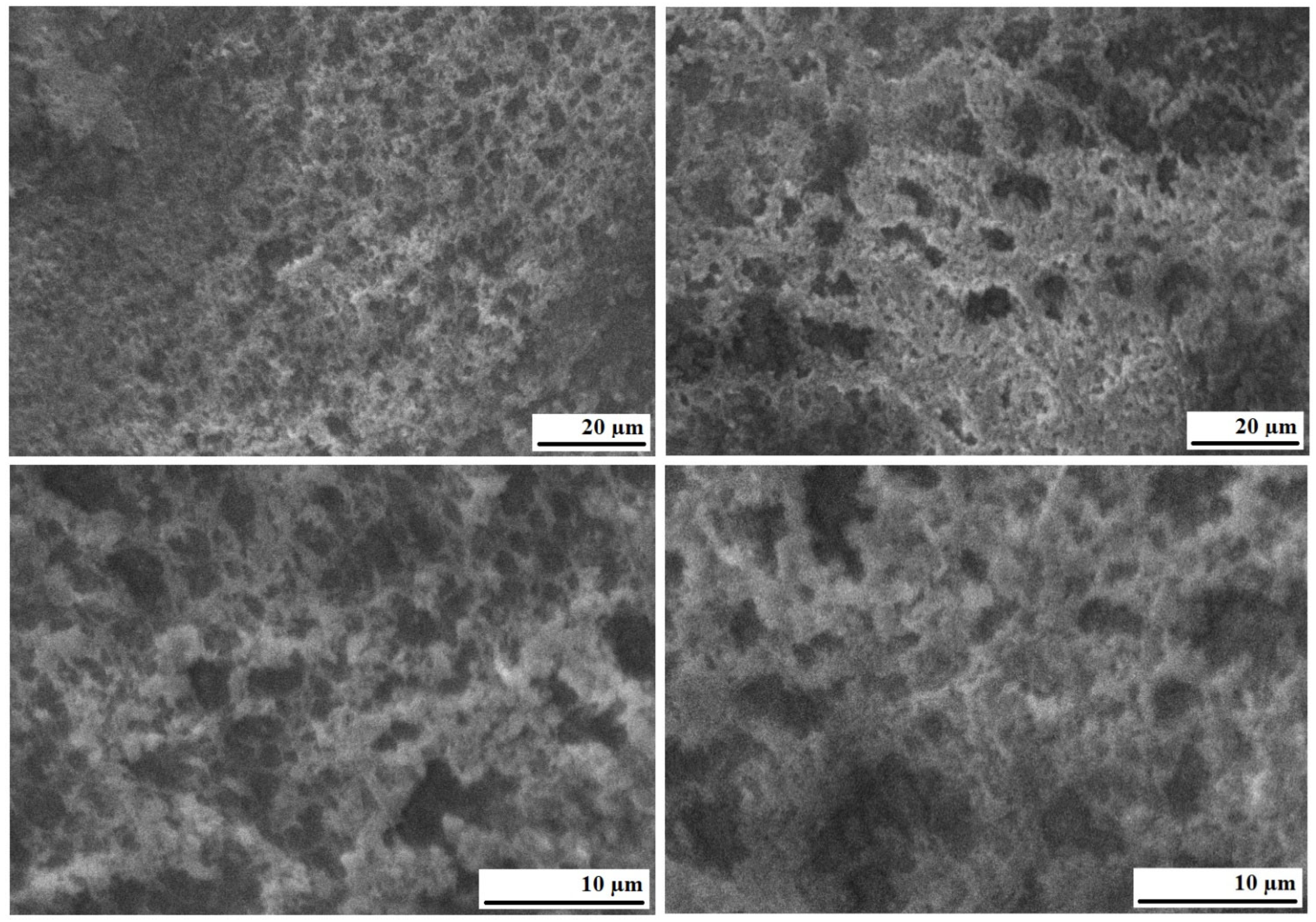
| Sample Name | Research Methods |
|---|---|
| Humic acids | DTA/DTG, FTIR, SEM |
| Bitumen BND 70/100 | DTA/DTG, FTIR, SEM |
| Bitumen BND 70/100 + 2.0 wt.% humic acids | DTA/DTG, FTIR |
| Bitumen BND 70/100 + 12.0 wt.% humic acids | SEM |
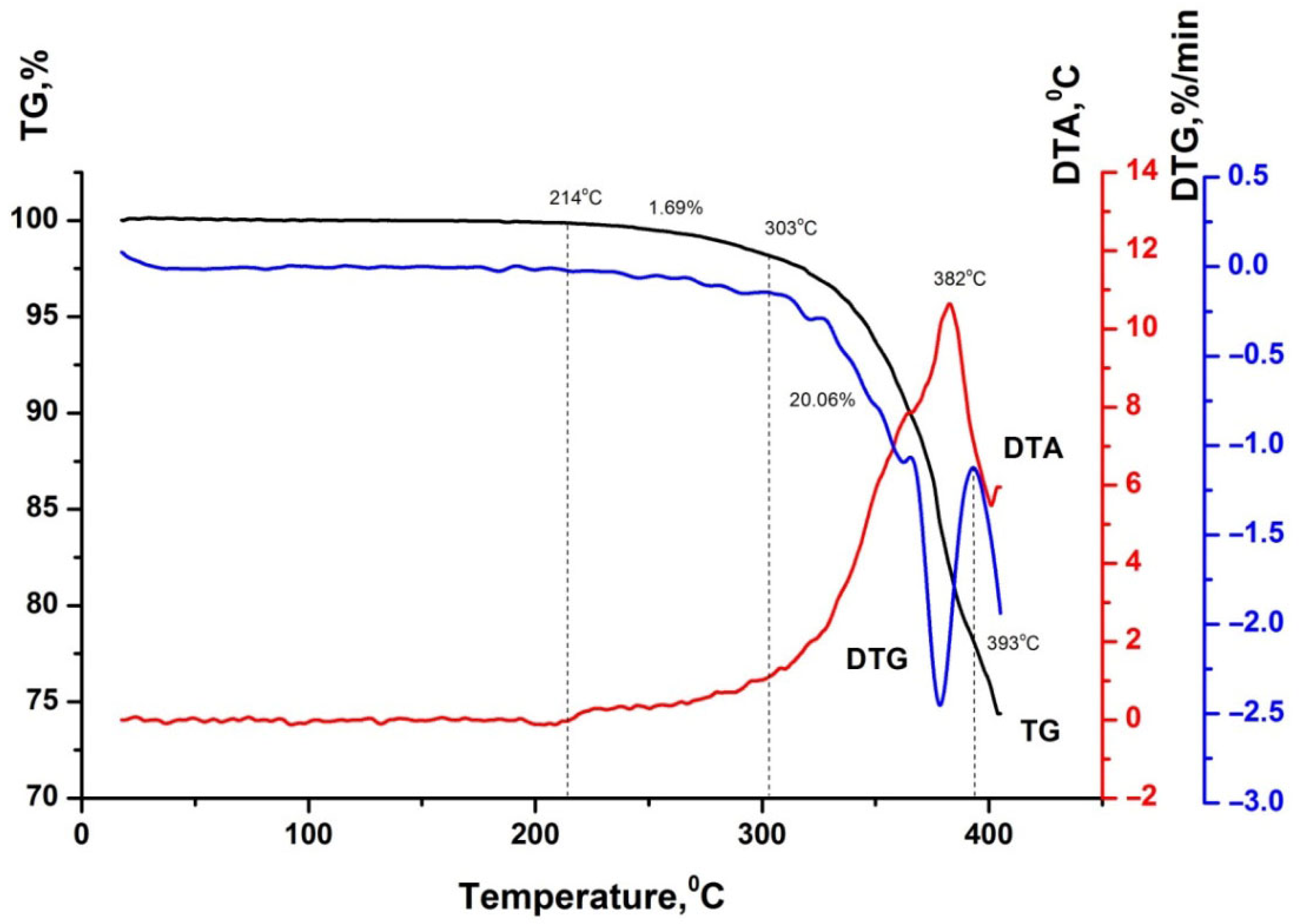
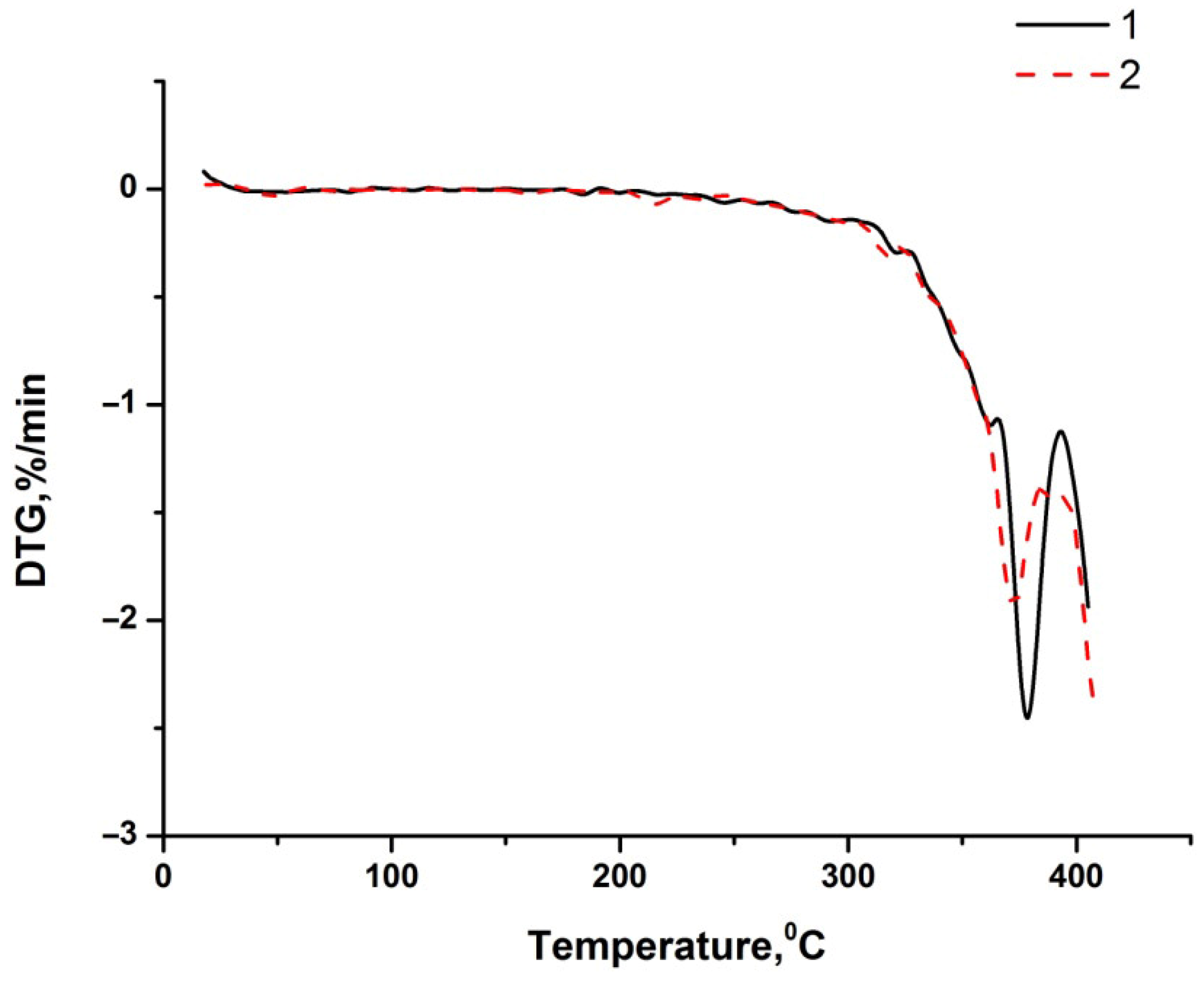
| Sample | Temperature Range, °C | Mass Loss, % |
|---|---|---|
| Humic acids | 20–170 | 11.48 |
| 170–334 | 11.84 | |
| Bitumen BND 70/100 | 20–214 | – |
| 214–303 | 1.69 | |
| 303–393 | 20.06 | |
| Bitumen BND 70/100 + 2.0 wt.% humic acids | 20–237 | - |
| 237–305 | 1.50 | |
| 305–389 | 17.83 |
| № | Wavenumber, cm−1 | Bitumen | Humic Acids | Bitumen+ 2.0 wt.% Humic Acids | Structural Fragment of the Molecule | Atom Groups | |||
|---|---|---|---|---|---|---|---|---|---|
| Absorbance, a.u. * | Relative, % | Absorbance, a.u. * | Relative, % | Absorbance, a.u. * | Relative, % | ||||
| 1 | 3047 | 0.0051 | 0.53 | - | - | - | - | CH3 | ν(C–H) asymmetric |
| 2 | 2919–2917 | 0.2897 | 29.92 | 0.0128 | 2.78 | 0.2855 | 30.94 | –CH2– | ν(C–H) asymmetric |
| 3 | 2850–2849 | 0.2055 | 21.22 | 0.0090 | 1.95 | 0.2029 | 21.99 | CH3 and CH2 or −O−CH3 | ν(C–H) symmetric |
| 4 | 1603–1602 | 0.0102 | 1.05 | 0.0340 | 7.38 | 0.0139 | 1.51 | Ar or –COO- | ν (C=C) aromatic ring or ν (C–O) asymmetric, symmetric |
| 5 | 1461–1430 | 0.0950 | 9.81 | 0.0118 | 2.55 | 0.0995 | 10.78 | –C–H (CH3) –C–H(CH2) or Ar | δ (CH3) asymmetric δ (CH2) asymmetric or ν (C=C) aromatic ring |
| 6 | 1376–1375 | 0.0446 | 4.61 | 0.0099 | 2.15 | 0.0521 | 5.64 | –C–H (CH3) | δ (CH3) symmetric |
| 7 | 1169 | - | - | 0.0096 | 2.08 | - | - | R–COO–R` | – |
| 8 | 1035–1029 | 0.0074 | 0.77 | 0.0532 | 11.56 | 0.0144 | 1.56 | −O−CH3 | δ (C–O) methoxyl group |
| 9 | 1007 | - | - | 0.0553 | 12.01 | - | - | O-containing compounds | ν (C–O–C, O–O, etc.) asymmetric, symmetric |
| 10 | 912 | - | - | 0.0178 | 3.87 | - | - | ||
| 11 | 872–871 | 0.0174 | 1.80 | - | - | 0.0173 | 1.87 | Ar | δ (C–H) in aromatic ring |
| 12 | 819–810 | 0.0226 | 2.33 | 0.0008 | 0.17 | 0.0260 | 2.82 | Ar (–CH=CH–) | δ (C–H) outside of the area of aromatic ring (mainly in the presence of alkyl substituents) |
| 13 | 749–745 | 0.0305 | 3.15 | 0.0035 | 0.76 | 0.0289 | 3.13 | Ar (–CH=CH–) | δ (C–H) outside of the area of aromatic ring (mainly in the presence of alkyl substituents) |
| 14 | 720 | 0.0427 | 4.41 | - | - | 0.0415 | 4.49 | Ar (–CH=CH–) | δ (C–H) outside of the area of aromatic ring (mainly in the presence of alkyl substituents) |
| 15 | 532 | - | - | 0.0532 | 11.56 | - | - | Me(CH3)x | ρ (Me–C) |
| 16 | 465 | - | - | 0.0709 | 15.40 | - | - | ||
| Total | 0.7707 | 79.60 | 0.3417 | 74.22 | 0.7820 | 84.73 | – | – | |
| The rest of the IR bands | 0.1976 | 20.40 | 0.1187 | 25.78 | 0.1410 | 15.27 | – | – | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prysiazhnyi, Y.; Lypko, Y.; Chipko, T.; Miroshnichenko, D.; Zhylina, M.; Miroshnychenko, M.; Omelianchuk, H.; Pyshyev, S. Non-Fuel Carbon-Neutral Use of Lignite: Mechanism of Bitumen and Humic Acid Interaction. Clean Technol. 2025, 7, 81. https://doi.org/10.3390/cleantechnol7030081
Prysiazhnyi Y, Lypko Y, Chipko T, Miroshnichenko D, Zhylina M, Miroshnychenko M, Omelianchuk H, Pyshyev S. Non-Fuel Carbon-Neutral Use of Lignite: Mechanism of Bitumen and Humic Acid Interaction. Clean Technologies. 2025; 7(3):81. https://doi.org/10.3390/cleantechnol7030081
Chicago/Turabian StylePrysiazhnyi, Yuriy, Yurii Lypko, Taras Chipko, Denis Miroshnichenko, Maryna Zhylina, Mykhailo Miroshnychenko, Hennadii Omelianchuk, and Serhiy Pyshyev. 2025. "Non-Fuel Carbon-Neutral Use of Lignite: Mechanism of Bitumen and Humic Acid Interaction" Clean Technologies 7, no. 3: 81. https://doi.org/10.3390/cleantechnol7030081
APA StylePrysiazhnyi, Y., Lypko, Y., Chipko, T., Miroshnichenko, D., Zhylina, M., Miroshnychenko, M., Omelianchuk, H., & Pyshyev, S. (2025). Non-Fuel Carbon-Neutral Use of Lignite: Mechanism of Bitumen and Humic Acid Interaction. Clean Technologies, 7(3), 81. https://doi.org/10.3390/cleantechnol7030081











