Abstract
Municipal solid waste (MSW) and refuse-derived fuel (RDF) incineration generate over 20 million tons of residues annually in the EU. These include bottom ash (IBA), fly ash (FA), and air pollution control residues (APCr), which pose significant environmental challenges due to their leaching potential and hazardous properties. While these residues contain valuable metals and reactive mineral phases suitable for carbonation or alkaline activation, chemical, techno-economic, and policy barriers have hindered the implementation of sustainable, full-scale management solutions. Accelerated carbonation technology (ACT) offers a promising approach to simultaneously sequester CO2 and enhance residue stability. This review provides a comprehensive assessment of waste incineration residue carbonation, covering 227 documents ranging from laboratory studies to field applications. The analysis examines reactor designs and process layouts, with a detailed classification based on material characteristics, operating conditions, investigated parameters, and the resulting pollutant stabilization, CO2 uptake, or product performance. In conclusion, carbonation-based approaches must be seamlessly integrated into broader waste management strategies, including metal recovery and material repurposing. Carbonation should be recognized not only as a CO2 sequestration process, but also as a binding and stabilization strategy. The most critical barrier remains chemical: the persistent leaching of sulfates, chromium(VI), and antimony(V). We highlight what we refer to as the antimony problem, as this element can become mobilized by up to three orders of magnitude in leachate concentrations. The most pressing research gap hindering industrial deployment is the need to design stabilization approaches specifically tailored to critical anionic species, particularly Sb(V), Cr(VI), and SO42−.
1. Introduction
About 103 Mt of residual waste are thermally treated in the European Union (EU) each year across 499 waste-to-energy (WtE) plants [1]. Overall, these figures have steadily increased over the past decades [2]. WtE thermal treatment effectively reduces waste volume by 80–90% while recovering energy in the form of electricity and heat, thereby contributing to the energy supply and decreasing reliance on landfills [3]. However, approximately 20–25% of incinerated wastes results in bottom ash (IBA), while 2–4% constitutes fly ash (FA) and air pollution control residues (APCr). Figure 1 illustrates the municipal solid waste (MSW) incineration process diagram for a typical electricity and heat cogeneration plant, highlighting the collection points for BA, FA, and APCr. Annually, about 20 million tons of IBA and 2.5–3 million tons of FA/APCr are produced in the EU. France, Italy, and Germany alone generate 2.9, 1.03, and 4.8 million tons of IBA, respectively [4]. Natural aging of IBA, followed by metal recovery using magnetic separation for ferrous metals and eddy current separation for non-ferrous metals from coarse fractions (>3–6 mm), is the most widely applied treatment method [5,6]. Eddy current separation relies on the induction of repulsive forces in non-ferrous metals when exposed to a rapidly changing magnetic field, enabling their separation from the waste stream. Following the removal of recoverable metals, the remaining mineral fraction, commonly referred to as bulk residues, typically follow two main paths: disposal in landfills or repurposing as secondary raw materials, primarily for road construction and other civil engineering applications [7,8,9,10,11]. Managing these residues poses environmental challenges in both reuse and landfilling due to their long-term leachability and hazardous properties [12,13,14,15,16]. However, IBA is a significant resource reservoir [17,18].
In particular, fine IBA fractions (<2 mm), accounting for 35–40 wt.% of total IBA, present both a challenge and an opportunity. They contain the highest concentrations of heavy metals (Cu, Zn, Cd, Pb, etc.), posing the greatest leaching risk while also offering potential for critical metal recovery to support the circular economy [19,20,21,22,23,24,25,26]. Additionally, IBA fines host most of the calcium-leaching mineral phases, enabling carbonation [27]. Carbonation involves the reaction of gaseous carbon dioxide with metal-bearing oxides in the presence of moisture, leading to the precipitation of carbonate minerals [28,29,30]. Engineering this naturally occurring process accelerates it, reducing the timescale to hours or even minutes. Despite decades of intensive research, no advanced or sustainable solution for harnessing the resource potential of IBA fines has proven viable at a full scale. This results in long-term risks and the loss of valuable metals and CO2 mitigation potential.
Although IBA is not the most abundant waste type, IBA’s heterogeneity, toxicity, and variability over time and location add significant complexity to the management process [31]. In contrast, accelerated carbonation technology (ACT) or enhanced metal recovery (EMR) solutions are already established for other industrial solid waste streams such as black mass (Mecaware, France), steel slags and refractory (Orbix, Belgium and Mineral Carbonation International, Australia, or RHI Magnesita) [32], cement kiln dusts (Carbon8, UK), construction and demolition wastes (Neustark, Switzerland or Carborok, France), and even FA and APCr (ACT: O.C.O Technology, UK and EMR: AIK Technik AG, Switzerland) [33]. Boskalis (Netherlands) and AIK Technik AG are among the most advanced companies in the processing of bottom ash fines, employing liquid-phase treatments mainly based on density separation and hydrometallurgy, respectively.
Thus, the rise in metal prices, coupled with incentives for circular economy practices and CO2 sequestration, have created an unprecedented opportunity to bridge the gap in IBA management. Furthermore, despite valorization solutions like ACT-based lightweight aggregates, cements, and geopolymers, as well as EMR processes such as FLUWA and FLUREC acid leaching, FA and APCr are still predominantly landfilled, including in underground repositories like salt mines [34,35,36]. Similarly, the remaining mineral fraction of processed IBA fines is still commonly landfilled. While the adoption of advanced waste management practices is increasing, logistical challenges, as well as technological and chemical barriers, continue to limit the adoption of these alternatives [37,38]. Yet, accelerated carbonation of IBA, FA, and APCr can simultaneously sequester CO2 and improve the leaching behavior of these residues, facilitating their reuse [39,40,41]. The techno-economic viability of this recycling approach depends on four key factors [42]:
- Landfilling diversion through reduced leaching, as increasing hazardous waste disposal fees range from EUR 150 to EUR 300 per ton in the EU and East Asia.
- Carbon credit certification and mitigation of projected carbon tax liabilities, with durable CO2 removal via mineralization expected to be valued at EUR 220–380 per ton by 2030 under the EU’s extended regulations (Oil Price Information Service, LLC & CDR.fyi Inc., Wilmington, DE, USA, 2025).
- Synergistic integration with other treatments, such as metal recovery, to generate co-revenue [43,44,45].
- Additionally, selling inert aggregate for civil engineering applications offers a minor revenue stream.
Overall, the technical challenge lies in balancing pollutant retention and mineral dissolution to supply divalent cations for carbonation while maintaining low water and energy consumption. Furthermore, ref. [46] reported that transitioning from laboratory-scale to pilot-scale testing can present challenges regarding reproducibility, as the operating conditions in pilot plant units inherently exhibit variability. Moreover, increased material usage in pilot-scale testing amplifies the significance of variations in the residue composition, potentially resulting in markedly different outcomes despite similar operating conditions during testing. Hence, gaining insight into system behavior under different operating conditions is essential for fine-tuning process parameters and assessing feasibility before scaling up for commercial deployment.
Earlier literature reviews have established the foundation, with studies focusing on ACT of cement-based materials [47] and MSW residues [48], as well as broader reviews covering industrial alkaline waste ACT and by-product utilization [49,50,51,52]. Further studies examining holistically mineral carbonation have also been conducted [53,54]. Additionally, the legal situation and current practice of BA utilization in Europe was recently reviewed by [4,55], while the study from [56] summarized the legal framework for FA/APCr. Finally, to effectively impact IBA treatment processes, it is important to consider current practices, particularly those related to aging and metal recovery, as extensively reviewed by [57]. However, no comprehensive review has specifically addressed accelerated carbonation of waste incineration residues since 2007, despite significant advancements in the field. Thus, the review focused primarily on the literature published after 2007.
This review builds on previous research by examining the carbonation of waste incineration residues from laboratory to field scale. A comprehensive bibliographic search was conducted using Scopus and Google Scholar, while patent searches were performed on Espacenet. The literature review incorporated various combinations of the following keywords: bottom ash, fly ash, air pollution control residues, MSW and RDF incineration, carbonation, pilot-scale, field-scale, and full-scale. The research primarily focused on accelerated carbonation of waste incineration residues, with particular emphasis on pilot- and field-scale data. A total of 227 documents were reviewed, comprising 205 research articles, 10 review papers, three conference proceedings, one PhD thesis, one book, one book chapter, and six website documents. Recent document, posterior to 2007, were privileged. A meticulous classification was performed based on the type of waste incineration residues, material characteristics, operating conditions, investigated parameters, and the resulting pollutant stabilization, CO2 uptake, or product performance. The review is subject to limitations, including the possibility that some relevant studies were missed and variability in study methods and reporting that may affect the completeness of the findings.
The main objectives of this review are as follows:
- Providing a comprehensive understanding of the reactivity of waste incineration residues during carbonation and the key operational parameters influencing this reactivity.
- Assessing comprehensively the impact of carbonation on the leaching behavior of potentially toxic elements (PTEs).
- Examining the diverse laboratory-scale applications of accelerated carbonation technology, focusing on reactor designs, operational conditions, associated performances in terms of CO2 uptake and reaction kinetics, and potential integration into broader waste treatment processes.
- Reviewing case studies that have implemented accelerated carbonation from pilot scale to full-scale applications.
- Establishing guidelines for current and future research aimed at advancing waste incineration residue treatments, with a particular emphasis on processes incorporating carbonation.
This review aims to serve as a high-quality reference for researchers and industry stakeholders seeking to optimize accelerated carbonation strategies for alkaline waste valorization. By bridging laboratory research to field-scale applications, it supports the development of sustainable solutions for waste incineration residue management within a circular economy, integrating concepts such as urban mining and industrial symbiosis. The broad and exploratory objectives of this review, covering reactivity, leaching behavior, reactor design, and case studies, lend themselves to a review approach, which is well suited to mapping heterogeneous research outputs, identifying knowledge gaps, and guiding future work.
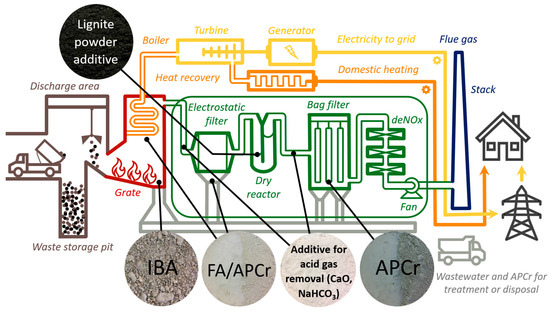
Figure 1.
Municipal solid waste incineration process diagram of a typical electricity and heat cogeneration plant, showing the collection points of the solid residues IBA, FA, APCr, as well as the most common solid additives (lignite powder, lime, sodium bicarbonate). Not to scale. Note that electrofilter residues can be classified as either FA or APCr, depending on whether additives were used upstream. Reprinted from Wehrung [58].
2. Evaluating the Suitability of Waste Incineration Residues for Carbonation
2.1. Core Physicochemical Properties of Waste Incineration Residues
While a detailed characterization of waste incineration residues is beyond the scope of this review, it is essential to outline their key physicochemical properties, as these directly influence their environmental behavior and potential for valorization. Waste incineration residues present a considerable challenge in waste management due to their heterogeneity across time and space. They exhibit wide variability in chemical composition, mineralogy, particle size distribution, and morphology, all of which impact their stability, leachability, and reusability.
This variability arises from multiple interconnected factors. Most notably, the nature of the waste input, typically MSW or RDF, is itself highly heterogeneous and subject to spatial and seasonal fluctuations. For example, the organic content and moisture levels of the waste stream often increase during the summer months in regions with high fruit and vegetable consumption, which in turn influences combustion behavior and residue formation.
The type of incineration technology used also plays a critical role in shaping residue characteristics. Differences in furnace design (e.g., moving grate, fluidized bed, rotary kiln), operating temperatures, and residence times all contribute to the composition of the resulting ashes. These operational factors directly affect the formation of mineral phases, the concentration and speciation of metals, and the residual carbon content in the ashes.
Moreover, the broad legal admissibility of non-hazardous waste types in European incinerators contributes significantly to input variability. The European Waste Catalogue lists numerous categories of non-hazardous waste permitted for incineration, including vegetal residues (e.g., bark, sawdust, fruit waste), plastics and composites (e.g., packaging materials, offcuts, tires), wood waste, paper and cardboard, textiles, digestates, and non-infectious healthcare waste. It also includes residual fractions from waste treatment processes, such as unsorted municipal fractions, sorting residues, and rejected compost, as well as bulky household waste, market waste, and street-cleaning residues. This broad spectrum of admissible inputs further amplifies the heterogeneity of incineration residues.
As a consequence of this variability, post-treatment strategies must be carefully tailored. The choice and dosage of chemical additives in stabilization processes, for example, often require site-specific optimization to ensure consistent treatment performance and reliable environmental outcomes.
This section provides an overview of the core physicochemical characteristics of the main incineration residues: IBA, FA, and APCr. This foundation will support the following sections, where we assess the feasibility of carbonation-based treatments to stabilize these residues, mitigate their environmental impact, and enhance their potential for reuse.
Table 1 summarizes the generation, collection methods, chemical composition, pH, mineralogy, and particle size of IBA, FA, and APCr.
2.1.1. Bottom Ash (IBA)
IBA is the most abundant residue from waste incineration, collected at the bottom of the incineration chamber after combustion. It typically comprises about 80–90 wt.% of the total ash output. According to the German DIN 38414-S4 procedure test, the release of Pb, Zn, and Cu is the main hazard associated with using IBA as a secondary building material [59]. Immediately after collection, IBA is quenched with water to rapidly lower its temperature, which promotes natural carbonation during aging with exposure to atmospheric CO2. This process occurs predominantly within the first 48 h and slows down, with few improvements expected beyond 3 months [60]. Natural carbonation lowers the pH from 11–12 to 8–9 and reduces the release of HMs such as Pb, Zn, and Cu, while mobilizing space for storage.
The chemical composition of IBA is rich in silica (SiO2: 20–50%), calcium (CaO: 10–25%), iron (Fe2O3: 4–10%), and aluminum (Al2O3: 5–20%) [61]. IBA also contain a considerable amount of HMs such as As, Cd, Cr, Cu, Hg, Mo, Pb, Sb, V, and Zn in variable quantities. Average concentration ranges reported for base and precious metals in IBA fractions are typically as follows: Cu (1000–5000 mg/kg), Zn (1500–6000 mg/kg), Pb (200–1500 mg/kg), Sn (10–500 mg/kg), Cr (50–1000 mg/kg), Au (<6 mg/kg), Ag (<10 mg/kg), and Sb (5–500 mg/kg) [62,63].
The dominant minerals in IBA include quartz (SiO2), calcite (Cal, CaCO3), and various silicates and aluminosilicates such as wollastonite (CaSiO3) and gehlenite (Ca2Al2SiO7) [64]. IBA contains a slag fraction, formed by the solidification of molten silicates, metal oxides, and salts during cooling [65]. IBA particles typically range from coarse sand to small gravel, with a mean particle size between 1 mm and 10 mm. Notably, calcium content tends to increase with decreasing particle size, making the finer fractions richer in Ca and poorer in Si [20,22,66]. Leachability also increases significantly with decreasing particle size [20]. Due to the relatively inert nature of the coarser fractions, IBA can be used as a construction material, particularly in road bases and as aggregate in concrete production [19]. However, managing the finer fraction (<2 mm) remains a major hurdle, despite recently implemented innovative treatment sequences at full scale [5,67].
Notably, the mineralogy of IBA is also distinguished by the presence of various calcium aluminate phases, which typically include hydrocalumite (Hcl, Ca2Al(OH)6(Cl,OH)·2H2O), katoite (Kto, Ca3Al2(OH)12), and ettringite (Ett, Ca6Al2(SO4)3(OH)12·26H2O). These phases are hydration products resulting from the aqueous reaction between Ca(OH)2 and Al(OH)3 consequently to quenching at the incineration chamber outlet [68,69]. Finally, ultrafine IBA fraction (<0.25 mm) typically contains a significant proportion of amorphous reactive pozzolans (i.e., siliceous and aluminous materials) [70].
While the total content of major components in IBA remains fairly consistent across space and time, reflecting the characteristics of the incineration feedstock, trace metal and salt contents vary significantly. These differences are largely attributed to the varying operating conditions (e.g., temperature) used by thermal treatment plants [71].
2.1.2. Fly Ash (FA)
In addition to IBA, Europe annually produces about 2.5 million tons of waste incineration FA and APCr, while globally, the production reaches approximately 10 million tons [56]. These represent a relatively small fraction, about 2%, of the total global IAW production, which is around 500 million tons per year [72]. Notably, when considering the combined outputs from waste, coal, and biomass combustion sectors, FA is recognized as the world’s fifth largest material resource [73].
FA is a fine, potentially hazardous by-product collected from flue gases after combustion. It is typically retrieved from deposits beneath the boiler and from electrostatic precipitators. The International Ash Working Group defines FA as “the particulate matter carried over from the combustion chamber and removed from the flue gas stream prior to addition of any type of sorbent material” [74]. FA typically constitutes about 10–20% of the total ash produced in WtE gas cleaning systems and is most often mixed with APCr for landfilling [75]. In Western Europe, FA and APCr are typically landfilled in repositories such as salt mines, including the Monte Kali salt mine in Philippsthal, southern Germany, where waste is imported from neighboring countries like France. Tens of thousands of tons per year are transported by truck from locations up to 1000 km away.
FA is typically characterized by high levels of calcium (CaO: 20–35%), silicon (SiO2: 10–30%), aluminum (Al2O3: 5–20%), as well as HMs such as Zn, Pb, and Cd in significant amounts [61]. FA contains minerals such as portlandite (Por, Ca(OH)2), anhydrite (CaSO4), and various silicates, chlorides, and sulfates. It also contains amorphous glassy phases and fine particles of unburned carbon [72]. In other cases, sodium and potassium chlorides can constitute the major phase, as at the WtE plant in Gerbido, Turin [76,77]. FA particles are typically very fine, often with a mean diameter less than 100 µm, and include a significant fraction of respirable particles (<10 µm) [66]. FA’s hazardous nature limits its reuse, but it can be stabilized through processes like carbonation or solidification and used in cement production or as filler in building materials [78].
Fabricius [79] conducted comprehensive analyses for a wide range of elements present in FA, time-dependent fluctuations (daily, monthly, and annually), and leaching characteristics, including implications for the market potential of recovering elements. Bi, Cr, and W (in the order of magnitude of hundreds of mg/kg) and Mg, P, and Sb (g/kg range) were classified as worth to recover in this study.
2.1.3. Air Pollution Control Residues (APCr)
APCr are collected downstream in the gas cleaning system in the bag filters [80]. APCr are residues from flue gas cleaning systems that capture fine particulates and acidic gases (HCl, SO2). Similar to FA, these residues are highly variable in composition and are often considered hazardous waste due to their leachable content of HMs, dioxins, and soluble salts [56]. APCr originating purely from bag house filters typically contains high levels of soluble salts like chlorides (Cl: 20–40%), sulfates, and toxic metals. The calcium content is high if lime is used for acid gas scrubbing, while the sodium content is elevated when sodium bicarbonate is employed. APCr contains soluble salts (e.g., CaCl2, KCl), amorphous phases, and complex oxides of metals and metalloids. APCr particles are typically fine, with a significant portion under 50 µm, resulting in high leachability and reactivity [61]. This fine particle size and high reactivity pose challenges for safe disposal [66]. As with FA, APCr can be treated through stabilization and solidification processes to reduce their hazard potential. They are increasingly used in the production of secondary raw materials like lightweight aggregates (LWAs) or in cementitious applications [56].

Table 1.
Generation, collection area, chemical composition (wt.%), pH, mineralogy, and particle size of IBA, FA, and APCr.
Table 1.
Generation, collection area, chemical composition (wt.%), pH, mineralogy, and particle size of IBA, FA, and APCr.
| Residue Type | Generation and Collection | Chemical Composition (wt.%), pH | Mineralogy and Phases | Particle Size | Ref. |
|---|---|---|---|---|---|
| Bottom Ash (IBA) | Collected at the incinerator bottom after combustion; usually quenched with water, ~200–250 kg/t of MSW. | SiO2: 20–50; CaO: 10–25; Al2O3: 5–20; Fe2O3: 4–10; SO3, Na2O, K2O, MgO, TiO2, P2O5: 1–5; MnO, BaO, CuO, ZnO, Cr2O3: 0.1–1; trace constituents: Ni, Sn, Pb, Cd, Sb, Sr, Zr, As, Hg, Ag, Au, …; pH: 11–12 (fresh), 8–9 (aged). | Quartz, calcite, wollastonite, gehlenite, hydrocalumite, katoite, ettringite, mayenite, apatite, ilmenite, perovskite, amorphous pozzolans, slag phases. | 8–12% <0.25 mm; 30–40% <2 mm; Si increases with particle size, Ca decreases. | [4,5,6,7,14,17,18,19,20,21,22,23,24,25,26,27,31,37,42,57,58,62,63,64,65,71] |
| Fly Ash (FA) | Collected from boilers and electrostatic precipitators, upstream of sorbent injection, ~10–30 kg/t of MSW. | CaO: 15–30; Cl: 10–25; SO3: 10–20; SiO2: 4–10; Na2O, K2O: 5–20; ZnO: 2–8; Al2O3, MgO, Fe2O3, PbO, Cr2O3: 0.05–2; trace: Cu, Mn, Ba, Cd, Sb, Sr, Zr, As, Hg; pH: 8–12. | Halite, sylvite, portlandite, chlorides, anhydrite, bassanite, sulfates, silicates, amorphous glass, unburned carbon. | Fine (<100 µm); respirable fraction <10 µm. | [8,11,15,34,35,36,56,58,66,72,73,75,76,77,79] |
| Air Pollution Control Residues (APCr) | Collected in baghouse filters or upstream, after flue gas treatment (post-sorbent), ~10–30 kg/t of MSW. | Major constituents: 10–25: Cl, 7–15: SO3; CaO (lime use), Na2O (NaHCO3 use), minor: SiO2, Al2O3, Fe2O3, Na2O, K2O, MgO, ZnO; trace: Mn, Ba, Pb, Cd, Sb, Sr, Zr, As, Hg; pH: 8–13.5. | Acid gas scrubbing product (CaClOH, NaCl, CaSO4, Ca(SO3)·0.5H2O) and residual additive (Ca(OH)2). | Ultrafine (<50 µm); highly reactive. | [16,41,58,66,72,75] |
2.2. Advantages and Drawbacks of Using Waste Incineration Residues for Carbonation
Table 2 compares the advantages and drawbacks of using waste incineration residues for carbonation.

Table 2.
Advantages and drawbacks of using waste incineration residues for carbonation.
2.3. Reactivity of Mineral Phases upon Carbonation
The reactivity of waste incineration residues depends on their physicochemical and mineralogical composition. For APCr, it is primarily governed by the type of acid gas-scrubbing additive used. When lime is employed, the residues are suitable due to their free CaO content. Conversely, if sodium bicarbonate (NaHCO3) is used without any lime input, the residues will most likely be unsuitable, as Na-carbonates are highly soluble. In APCr, most reactive minerals are calcium (chloro)(hydr)oxides (CCHO), including lime (Lm), portlandite (Por), and sinjarite (Snj) [81]. Other reactive minerals identified in waste incineration residues include Ca-silicates such as larnite (Lrn), hatrurite (Hrr), wollastonite (Wo), and rankinite (Rnk) [24], as well as Mg-(hydr)oxides like periclase (Per) and brucite (Brc). In IBA, Ca-aluminates such as Hcl, Kto, and Ett can be the main Ca-providing minerals for carbonation [82,83]. The overall model reactions involving the major Ca/Mg-bearing reactive phases present in waste incinerator residues are listed in Table 3.

Table 3.
Carbonation reactions involving major Ca/Mg-bearing reactive minerals in waste incineration residues. Adapted from Wehrung [58].
The carbonation capacity of waste incineration residues and other alkaline waste can usually range from 10 to 400 gCO2/kg, depending on the Ca and Mg content that is not already bound to carbonates, sulfates, or phosphates, i.e., more stable phases [84]. In terms of reaction kinetics, while CCHO and Ca-aluminates readily dissolve in the presence of dissolved CO2, supplying Ca for carbonation, the dissolution of Ca-silicates, including Lrn and Hrr, is significantly slower and thus becomes the rate-controlling step when they are the only remaining Ca sources. It is important to bear in mind that kinetics aspects should also be considered when estimating the potential reactivity of the residue in an applied context. A primary rate-controlling step of aqueous carbonation reactions is the Ca-bearing phase dissolution, along with CO2(g) dissolution, as the carbonate precipitation occurs almost immediately once the supersaturation conditions are reached, in the typical pH values (i.e., pH 8–14) [85]. Under near-ambient pressure and temperature conditions, the final pH of Ca-based systems undergoing aqueous carbonation decreases and stabilizes around 6.33, in pseudo-equilibrium with Cal. In contrast, Mg-based systems tend toward a final pH of approximately 7.15, reflecting pseudo-equilibrium with either hydromagnesite or nesquehonite. For example, CCHO and Ca-aluminates readily dissolve in water in the presence of dissolved CO2, thus supplying Ca ions for carbonate precipitation, while the direct effect of CO2 on basic silicates dissolution rates is very weak [86]. Thus, the dissolution of Ca-silicates present in waste incineration residues, particularly larnite (Lrn) and hatrurite (Hrr), is significantly slower, requiring a longer residence time in the reactor if they are to be converted into carbonates. However, their intrinsic carbonation capacities are high, which positively affects the overall process efficiency.
2.4. Quantification Methods
Among the most common methods used to quantify alkaline waste carbonation efficiency (), many authors have employed thermogravimetric analysis (TGA) as follows [87,88,89]:
where = amount of carbon dioxide gained by carbonation in weight percent; = total Ca content in weight percent; and = molecular weight of Ca and CO2.
An advanced TGA-based method for determining the CaCO3 content in alkaline wastes was also proposed by [90].
Another common method for quantifying CO2 absorption during carbonation, based on the pressure drop in closed reactors, is defined by the following equation [91]:
where (kPa) = atmospheric pressure measured during the calcimetry test; is the compressibility factor of CO2, depending of and , which can be calculated or retrieved from databases such as Aspen; (m3) = reactor volume occupied with gas; ([kPadm3]/[molK]) = 8.3145 is the ideal gas constant; (K) = temperature of reaction; (g) = mass of the tested sample.
Additionally, calcimetry can be used to quantify the calcium carbonate content (wt.%) in both the initial (raw) and final (carbonated) samples, as it is commonly applied to soils [92], as follows:
where (kPa) = A\atmospheric pressure measured during the calcimetry test; (m3) = V\volume of CO2 released; (g/mol) = M\molar mass of CaCO3; ([kPa dm3]/[molK]) = 8.3145 is the ideal gas constant; (K) = temperature measured during the calcimetry test; (g) = mass of the tested sample.
The Steinour equation represents the state of the art to evaluate the maximum theoretical amount (%) of CO2 that can be stored in a cement or waste material [93]. Based on this, Renforth [83] proposed a modified Steinour equation better suited for industrial alkaline waste carbonation. By subtracting the mass of CO2 already present as CaCO3 from this modified Steinour equation, the maximum theoretical amount of CO2 that can be stored by carbonation can be calculated as follows:
where , , and (wt.%) = elemental concentrations of Ca, Mg, S, and P, expressed as oxides, obtained by XRF; Mx (g/mol) = molar mass of those oxides; = pH-dependent coefficients factoring the relative contribution of each oxide, such as in a waste incineration residues; appear to be reasonable choices based on waste incineration residues alkalinity [84]; = mass of CO2 inherently present as CaCO3, quantified using XRPD Rietveld refinement or calcimetry.
3. Impacting Variables
The effect of carbonation reaction variables on cement-based materials and CO2 uptake was first described by [94] and later expanded by Gunning [95]. Their studies primarily focused on cement-based material production and the curing of fresh aggregates (pellets) using carbonation as a binding process. This section aims to build upon their work by incorporating more recent findings on advanced reactor designs, addressing the slow diffusion of CO2 in water, thereby enhancing the efficiency of the carbonation process.
Ex situ ACT is a thermo-hydro-mechanical–chemical process with a complex interplay of influencing variables. Figure 2 provides an overview of those variables impacting carbonation. This section reviews the impact of reagent composition and operative parameters—temperature, pressure, L/S ratio, particle size, and mixing system—as the most influential factors. In the context of waste incineration residue carbonation, the effects of operative parameters primarily influence CO2 diffusion in the liquid phase, Ca(OH)2 and Ca-aluminate dissolution kinetics and solubilities, and to a lesser extent, MgO and Ca-bearing silicates (Lrn and Hrr).
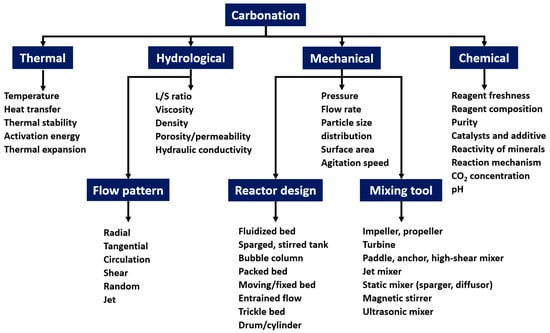
Figure 2.
Thermo-hydro-mechanical–chemical parameters impacting carbonation.
3.1. Temperature
Temperatures between 323 and 343 K optimize the CO2 absorption rate in Ca(OH)2 and APCr aqueous carbonation [96]. Meanwhile, the efficiency of Ca(OH)2 conversion to CaCO3 remains stable across temperatures from 283 to 363 K [58,97,98]. These temperatures are typical inside field-scale carbonation reactors, influenced by the gas stream temperature, incoming solid and liquid reagent properties, and insulation effectiveness [99]. Notably, the exothermic nature of carbonation reactions can raise the reactor interior temperature by up to 10–20 K. Additionally, increasing the temperature from 283 to 363 K in high-calcium content APCr aqueous carbonation gradually increases the final calcite/vaterite (Cal/Vtr) ratio. Tian [100] propose an optimal temperature near 680 K for dry, gas–solid waste incineration residue carbonation.
3.2. CO2 Pressure
Elevated CO2 partial pressures (PCO2) facilitate more rapid and extensive carbonation reactions, as described by Henry’s law, increasing the CO2 solubility in water. This increased pushing force induces carbonation of FA in slurries [101]. Studies demonstrate that CO2 uptake in high-calcium fly ash increases markedly under pressures ranging from 1 to 6 bar [88]. However, effective carbonation can still be achieved at the lower PCO2 found in flue gases from incineration plants, typically between 0.04 and 0.2 bar, making the direct utilization of flue gas feasible for ex situ carbonation without the need for prior separation or pressurization. Montes-Hernandez [102] reported that the carbonation efficiency of CaO by aqueous carbonation of coal combustion fly-ash was independent on the initial PCO2, tested from 10 to 40 bar. Ahmad [103] found that an increase in pressure from 0.7 to 4 bar had no significant effect on the rate of compressive strength evolution of concrete subjected to ACT curing. Xian [89] highlighted that ambient pressure carbonation provides a uniform and effective reaction, making it a viable alternative to high-pressure methods in early carbonation curing for converting industrial waste slag into cement material through carbonation activation.
3.3. Liquid-to-Solid Ratio and Relative Humidity
L/S ratio refers to the dimensionless mass ratio between water and solid material in a system undergoing carbonation or washing treatment. The higher the L/S, the lower the viscosity of the mixture. Low L/S ratios are preferred to limit water consumption. The optimum carbonation L/S ratio is traditionally considered to be between 0.2 and 0.3, especially for IAW case studies [104]. This is due to CO2 diffusion in water being approximately 105 times slower than in air; excess water in saturated material thus slows down carbonation. Moreover, low L/S values during CO2-curing can significantly enhance the performance of waste incinerator residue pelletization processes by refining porosity [105,106].
However, these typical conditions overlook the faster carbonation kinetics recently reported in well-designed open systems where CO2 flows continuously [107,108]. As developed in the following subsection, the reduction in internal CO2 mobility within the reactor, caused by water slowing CO2 diffusion compared to air, can be easily countered by employing a proper combination of static and dynamic mixing system. Notably, high L/S ratio carbonation (L/S > 2–3) may be considered as a washing process, enhanced by carbonation. Indeed, Um [109] evidenced that the carbonation-induced chloride removal effect surpassed that of conventional water-washing treatments of IBA, due to the decomposition of Hcl (, the primary insoluble chloride). Additionally, these dilute conditions inhibit the potential reaction of HMs (Cu2+, Pb2+, Zn2+) with SO42− and NO3− in the APCr solution. These reactions produce highly soluble compounds (CuSO4, PbSO4, ZnSO4, Cu(NO3)2, Pb(NO3)2, Zn(NO3)2), which, if formed under conditions of low L/S ratio or dry carbonation, would increase the solubility of HMs, thus elevating their leaching risks [110]. On the other hand, the relatively high volume of the liquid phase implies that wastewater treatment and recycling processes must be considered to make this approach feasible
In conclusion, the optimal L/S ratio for carbonation of waste incineration residues mainly depends on the particle size and desired outcomes, such as the removal of PTEs or the strength of the final aggregates in curing under CO2-rich conditions. A low L/S ratio (below 0.5) appears as a better option for waste stream having coarser grains such as IBA, allowing for continuous-feed processes using conveyor belts and tilted rotary drums [111]. In contrast, a higher L/S ratio (above 5–10) is better suited for batch treatments of FA and APCr, maximizing CO2 uptake and reaction kinetics while enhancing the dissolution of soluble PTEs through carbonation washing.
3.4. Mixing System
A mixing system is a mechanical or geometrical arrangement designed to enhance mass transfer and phase interaction by promoting contact and homogenization between different phases of a system, such as gases, liquids, and solids. In the context of carbonation processes, mixing systems play a crucial role in optimizing reaction kinetics, ensuring uniform reactant distribution, and improving overall process efficiency. Figure 3 illustrates the most common mixing systems used for ex situ accelerated carbonation.
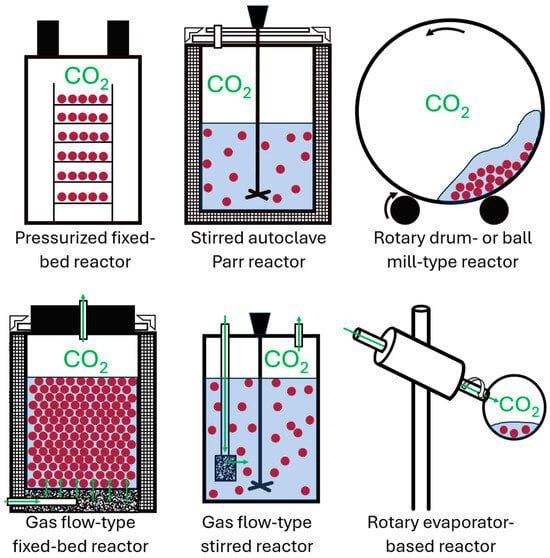
Figure 3.
Common reactor types used in laboratory-scale studies on waste incineration residues, as reported in the literature. Green arrows indicate CO2 flow, dotted rectangles represent porous and permeable media, red points denote waste incineration residues, and the blue shading represents water.
In aqueous carbonation, it is known that the uptake of CO2 by metal (hydr)oxide suspension increased with higher turbulence due to better gas/water exchange [112]. Static gas–liquid mixers such as sparger and diffusor combined with impellers enable fast CO2 absorption rates during aqueous Ca(OH)2 carbonation and therefore in the carbonation of high-calcium-(chloro)(hydr)oxide-content industrial alkaline waste. Hence, improving aqueous carbonation kinetics with CO2 micro- and nano-bubbles has recently been reported as a highly efficient approach [113,114,115]. A diffusor placed at the bottom of the reactor can eliminate energy consumption required to power mixing tools such as impellers by effectively harnessing the turbulence induced by the bubbles. Yet, care should be paid to prevent the blocking of the sparger holes when using such mixing tools in a gas–solid–liquid system. Said [116] proposed as a potential solution a sparger pointing downwards, positioned 5 cm above the bottom of the reactor, in the case of a pilot-scale steelmaking slag carbonation system. In general, promoting the dissolution of calcium ion-bearing phases, facilitating CO2 diffusion, and generating more nucleation sites are beneficial for achieving a higher carbonation degree [83].
In low L/S ratio carbonation systems, rotary drum reactors are extensively utilized across laboratory and industrial scales due to their high efficiency in promoting both mixing and carbonation [117]. Their continuous motion ensures the constant exposure of fresh, wet solid reactant surfaces to the CO2-rich gas. These reactors typically operate with a L/S ratio < 0.5 and can handle various waste particle sizes. Gunning [118] has demonstrated their effectiveness in producing lightweight aggregates from industrial alkaline waste, including FA/APCr, via cold-bonding CO2 curing, a process that hardens materials together at room temperature to form solid blocks, thanks to the binding properties of carbonates. This method does not require high temperatures, making it energy efficient. Similarly, Schnabel [111] successfully carbonated IBA in continuous-feed conditions, using a perforated mixing cage for optimal internal mixing.
3.5. Particle Size and Surface Area
The small particle size of waste incineration residues, particularly FA/APCr and the IBA fine fraction (approximately 10–12 wt.% < 250 μm), provides a significant advantage for reactivity to carbonation compared to other solid waste streams. Coarse particles have low surface area and slow dissolution rates, which favor the formation of a carbonate passivation layer, potentially leading to incomplete carbonation. Chen [119] highlighted that reducing particle size through mechanical ball milling significantly enhances the carbonation efficiency of FA by increasing pore channels, reaction sites, and improving mass transfer rates of CO2 and calcium ions, thereby deepening FA carbonation and enhancing the stabilization efficiency of HMs. However, mechanically crushing waste incineration residues to enhance cation leaching may be counterproductive due to substantial energy consumption and the release of encapsulated PTEs [120]. Said [116] estimates that the energy consumption for reducing grain size in steel slags increases exponentially for particles under 200 µm. Additionally, natural feedstocks like olivine require crushing for applications in ocean alkalinity enhancement (e.g., the French company Carbon Time) or engineered reactors (e.g., the company Paebbl in the Netherlands). Efficient crushing technologies are therefore essential in ACT. The company Paebbl has patented a proprietary ‘high-temperature and high-pressure milling reactor’ to optimize its process.
3.6. Gas Flow Rate and Velocity
The gas flow rate (GFR) is the volume of gas introduced into the system per unit of time (L/min), while velocity refers to the speed of gas movement through the system, typically measured in (m/s). Higher GFR increases the gas–liquid interfacial area and turbulence in a slurry column, positively affecting the overall carbonation kinetics. However, excessive GFR and velocity can result in more gas being lost to the atmosphere if the reactor cannot absorb it quickly enough due to insufficient gas-residence time. Proper dimensioning of the carbonation system is essential when adjusting GFR, and velocity is not feasible. Additionally, high GFR can elevate system pressure, necessitating more robust equipment and safety measures.
3.7. Chemical Impurities
Potential chemical impurities significantly impact carbonation systems. Gaseous impurities, such as sulfur oxides (SOx) and nitrogen oxides (NOx) in flue gas, and solid impurities, like HMs or organic compounds in feedstocks, can accumulate, particularly in systems with water reuse cycles [121]. These impurities interfere with key components of the carbonation process, affecting CO2 and solid reagent dissolution, calcium ions availability, and carbonate formation. Each impurity’s impact is highly dependent on the prevailing THMC conditions. For instance, ions like chloride, sulfate, and sodium may either accelerate or decelerate the CO2 absorption rate, contingent on their concentrations and temperature, as developed later in the experimental section. Furthermore, the presence of impurities often reduces the quality and market value of the carbonated products, highlighting the importance of effective impurity management to optimize the overall economic viability of the process [122].
3.8. Reagents Freshness
Reagents freshness promotes reactivity by avoiding natural weathering and pre-existing carbonate coatings. Moreover, using fresh reagents helps maintain consistency in the carbonation process results, leading to predictable and reliable results.
3.9. Reaction Completion Rate
As carbonation reactions progress, reactants are consumed, the reactive solid–liquid interfacial area decreases, and pH gradually drops. Consequently, the kinetics of carbonation, including CO2 absorption and carbonate precipitation, slow down. The rate of this slowdown depends on the specific chemical nature of the reactants (e.g., Ca(OH)2, MgO). Stopping the carbonation process before complete consumption of reactants affects the final pH and the stabilization of PTEs.
4. Elements’ Behavior upon Carbonation
This section, along with Table 4, provides a summary of the leaching behavior and key characteristics of typical elements observed in waste incineration during carbonation. The accelerated carbonation processes applied to waste incineration residues can only partially stabilize metal contaminants [123]. Metals forming divalent cations such as Ba, Pb, Cu, Hg, Sr, and Zn may be stabilized into carbonates, while oxyanionic metalloid and metal species (As, Sb, V, Mo, Cr), chlorides, and sulfates typically remain mobile. A previous review by [124] provides a comprehensive geochemical overview of the leaching behavior of oxyanionic species in alkaline waste, focusing on more oxidized species (As(V), As(III), Cr(VI), Se(IV), Se(VI), Sb(V), Mo(VI), V, and W(VI)), as they are more commonly found in waste leachates. In contrast, elements in their elemental state, along with hydroxides and oxides formed by reduced species at high pH (Cr(III), Sb(III), V(IV), and VIII), show only limited solubility. Overall, pH and particle size are the most crucial factors impacting the leaching of elements from waste incineration residues [125].
Besides carbonate precipitation, the leaching of heavy metals, sulfates, and chlorides from waste incineration residues is governed by various chemical and physical mechanisms, including the precipitation of other stable phases (e.g., iron antimonate for Sb or barium sulfate), HMs’ chemical incorporation into minerals (e.g., Sb and Cr oxyanions into ettringite), and adsorption onto reactive surfaces (e.g., Fe/Al oxy-hydroxides) [14,126,127]. While the literature extensively reviews the leaching behavior of waste incineration residues [66,128,129,130,131,132], this section specifically examines the impact of carbonation.

Table 4.
Advantages and drawbacks of using waste incineration residues for carbonation. Leaching and geochemical behavior upon carbonation of typical elements observed in waste incineration residue systems. ●: adverse, potentially critical—leaching typically increases; ○: beneficial—leaching consistently decreases to acceptable levels; □: contrasted—stabilization varying significantly depending on the physicochemical conditions.
Table 4.
Advantages and drawbacks of using waste incineration residues for carbonation. Leaching and geochemical behavior upon carbonation of typical elements observed in waste incineration residue systems. ●: adverse, potentially critical—leaching typically increases; ○: beneficial—leaching consistently decreases to acceptable levels; □: contrasted—stabilization varying significantly depending on the physicochemical conditions.
| Element | General Impact | Behavior upon Carbonation |
|---|---|---|
| As | □ | During waste combustion, arsenic (As) oxidation is incomplete and continues during flue gas cooling. Its adsorption onto ash particles is enhanced at low temperatures, particularly with CaO injection. Reactions with Ca, Fe, and Al compounds form various arsenates, some of which decompose at 1323 K. However, part of the arsenic, including stable As(III), remains embedded in the ash matrix even at high temperatures [133]. Montes-Hernandez [134] reported a 78% arsenate removal efficiency during Ca(OH)2 carbonation from synthetic wastewater, and up to 85–88% in the study from Thriveni [135]. Ni [110] reported a 21% decrease in the leachate. Khan [136] observed significant As immobilization efficiencies, with As(V) reduced from 97.3% to 86.0% and As(III) from 97.3% to 86.0% during CaO carbonation. As(V) favored Vtr formation, while As(III) promoted Cal formation. K-edge XANES confirmed the incorporation of arsenic oxyanions within Cal crystals obtained post-treatment. Garcíia [137] attributed arsenate retention in carbonated soil to the neoformation of iron hydroxysulfates and iron oxyhydroxides, such as goethite or ferrihydrite, rather than to carbonate precipitation. |
| Al | ○ | Chimenos [59] explained that in IBA, aluminum (Al) solubility is controlled by the precipitation of gibbsite or Al-sulfate neoformations, which may help immobilize HMs. In APCr, geochemical modeling from Astrup [138] confirms that the leaching of Al is influenced by solubility control from Al2O3 and Al(OH)3. Um [109] evidenced that Al was released by Ett and Friedel’s salts decomposition and reprecipitated as Al-amorphous phases during IBA carbonation. It is well established that carbonation-induced neutralization at pH 7–8 leads to the formation of Al(OH)3, either as gibbsite or an amorphous phase. |
| Ba | ○ | Astrup [138] suggested that the leaching of barium (Ba) is influenced by solubility control from Ba(S,Cr)O4 solid solutions. BaCO3 may form, as solubility at 298 K is about 0.025 mg/L. Ba was effectively stabilized in wastepaper fly ash at pilot-scale natural carbonation [139]. |
| Cu | □ | In IBA, the carbonate bound fraction of copper (Cu) showed an increasing trend with the increase of the particle size [140]. Meima [141] attributed a reduction of more than 50% in Cu leaching to sorption onto neoformed amorphous Al-minerals following carbonation. Arickx [142] reported that Cu leaching in a specific fraction (Ø 0.1–2 mm) of IBA decreased to approximately 13% of its initial value, attributed to pH decrease and neoformation of Al (hydr)oxides, affecting the adsorption of fulvic acids and humic acids (Hy). Ni [110] document a 10–40% decrease in the leachate. |
| Cd | ○ | In IBA, the carbonate-bound fraction of cadmium (Cd) showed a decreasing trend with the increase of the particle size [140]. The WATEQ.4F database indicates that CdCO3 (otavite) aqueous solubility is 0.9 mg/L at 25 °C in pure water. Li [143] found no obvious relationship between FA carbonation and Cd mobility. Wang [144] reported that highly active porous materials composed of Cal and periclase nanoparticles from calcined clayey dolomite carbonation was an ideal adsorbent for removal of Cd from aqueous solutions. Habte [145] demonstrated that Ca(OH)2 carbonation could remove 99.9% of Cd from wastewaters. Kim [146] showed that Cd coprecipitates with Cal. However, Cd was not effectively immobilized by FA carbonation [147]. |
| Cr | ● | Chromium (Cr) mobility is regularly reported as critical during carbonation. Todorovic [148] found that the release of Cr decreased by 97% using IBA, while it remains critical using APCr. Cr leaching increased during basic oxygen furnace slag carbonation [149]. Um [150] found that Cr leaching increases due to the decomposition of Ett and Hcl but then decreases as Cr ions become adsorbed on the resulting amorphous Al-phase. Employing FA, Qin [147] concluded that Cr leaching increased after carbonation. Lapp [151] found that carbonation was a key process exacerbating Cr(VI) leaching during chromite ore processing residue aging, mainly due to the decomposition of Cr-Hcl and Kto. Wehrung [58] found that Cr leaching increased during IBA carbonation, attributed to the dissolution of Ca-aluminate coatings on Fe-Mn-Cr particles, which triggered Cr mobility. Astrup [138] suggested that Ba(S,Cr)O4 phases may be suitable candidates for Cr solubility control, further supported by the fact that Ba concentrations were close to saturation with Ba(S,Cr)O4 in their geochemical modeling. Paul [152] found that spent Cl−-bearing Hcl is recyclable for CrO42− removal. Hunter [153] observed that the extent of Cr incorporation within calcium carbonate exceeded predictions by a thermodynamic model, being 3000 times higher than anticipated. Maftei [154] showed that Cr(VI) can be sequestered by iron oxyhydroxides through coprecipitation and adsorption. In conclusion, chromium leaching remains a persistent issue hindering the industrial deployment of waste incineration residue treatment strategies, requiring stabilization approaches specifically tailored to its behavior. |
| Fe | □ | Iron (Fe) is substantially released during raw waste incineration residues carbonation. Aqueous Ca(OH)2 carbonation is known as a powerful method to remove Fe(II) from water [155]. Nilsson [156] concluded that the addition of zero valent iron (Fe(0)) in combination with carbonation, by increasing the number of adsorption sites for HMs, could be an effective pre-treatment method for decreasing their mobility in IBA. |
| Hg | □ | Guha [157] observed that cement mixtures that were carbonated to a higher degree had lower levels of mercury (Hg) leaching. Reynolds [99] presented evidence for mercury mineralization during field-scale coal FA carbonation, suggesting mercuric carbonate (HgCO3) formation. Ni [110] reported a 33% decrease in the leachate. Wenyi [158] reported that flue gas desulphurization gypsum carbonation effectively reduces Hg leaching, influenced by process conditions and byproduct compositions, with wet flue gas desulphurization gypsum showing particularly favorable decreases. |
| Mn | ● | Manganese (Mn) is mobilized after carbonation [147]. Mn is largely present as Fe-Mn-Cr oxide, originating from the oxidation of stainless steel [58]. Mn leaching was found to be highly pH-dependent, steadily increasing as the pH decreases from 12–13 to near-neutral during carbonation [98]. |
| Mo | □ | Molybdenum (Mo) is relatively involatile, with a thermodynamically stable hexavalent state in wastes and leachates [124]. During thermal treatment of solid waste, Mo leaching increases up to 600 °C due to oxidation to molybdate but decreases at higher temperatures as molybdate is incorporated into an amorphous phase, reducing its mobility [159]. Mo is also likely to exist as Ca-molybdate in incineration bottom ash, which can dissolve during carbonation, with calcium reprecipitating as carbonates. Dijkstra [160] found that accelerated carbonation reduced the leaching of Mo, which was attributed to enhanced adsorption onto Fe/Al (oxy-hydr)oxides. Meima [141] showed that Mo was poorly affected during IBA carbonation, with a reduction of less than 3% detected. |
| Ni | ● | Nickel (Ni) leaching increases after carbonation [147]. In IBA, Ni [110] was found both in Fe-Ni-Cr oxides from stainless steel oxidation and as Ni0. |
| Pb | ○ | Lead (Pb) is the most positively affected critical toxic element during carbonation, with reported concentration decreasing from one to several order of magnitude leachates [110,161,162]. Optimum pH range for the immobilization of Pb is 10–11, with an increase in leached concentration with the decrement of pH values [147]. Cerussite (PbCO3) appeared to act as a solubility controlling mineral in the pH range 6–12 [48]. |
| Sb | ● | Municipal solid waste contains approximately 10–60 ppm of antimony (Sb). Above 500 °C, nearly all Sb volatilizes from IBA. Despite its predicted volatility, mass balance analyses show that about 50% remains in the grate ashes [163]. Any remaining Sb is oxidized by metal oxides and absorbed as involatile metal antimonate [124]. Cornelis [164,165] documented that acidification and carbonation increased antimonate leaching, controlled by Ca-antimonate (romeite) dissolution in IBA at pH 8–11. Sb exhibits the most critical leaching behavior during carbonation, being mobilized by up to three orders of magnitude in leachate concentrations. This may constitute what could be called the antimony problem in the field of alkaline waste carbonation, particularly concerning waste incineration residues. Sb mobility in IBA may be governed by four mechanisms: (a) calcium antimonate formation, (b) adsorption onto iron (hydr)oxides, (c) iron antimonate formation, and (d) incorporation into or adsorption onto ettringite. Thus, Sb solubility primarily depends on the pH, Ca2+, and OH− availability in the leaching solution, which are critical for the formation and stability of Ca–Sb precipitates [11,166]. Sb is known to be mobilized by carbonation in both APCr and FA [104,167,168]. Sb could be a critical potential toxic elements in secondary building materials containing waste incineration residues, and long-term monitoring of the release should be taken into account in the future [169]. In conclusion, the antimony problem remains one of the most pressing research gaps hindering the industrial deployment of waste incineration residues treatment strategies, requiring stabilization approaches specifically tailored to its behavior. |
| Se | □ | Selenium (Se) is almost completely volatilized from IBA [124]. Se in FA is mainly present as SeO2 [170]. Montes-Hernandez [134] reported a 90% selenite removal efficiency during Ca(OH)2 carbonation from synthetic wastewater, while selenite did not have any physicochemical affinity/effect, remaining unaffected or increasing, during APCr carbonation [148]. In the presence of Ca(OH)2, selenium is primarily retained by forming selenite on ash particles and through adsorption, though this process is inhibited by HCl, SO2, and H2O, as these molecules may compete with SeO2 for adsorption on Ca(OH)2 sites [171]. Chen [172] found that carbonation is beneficial by neutralizing the pH for stabilization of Se. |
| Sr | ○ | Strontium (Sr) concentrations in ash generated from mixed municipal waste were found to range between 237 and 825 mg/kg [173]. Erdemoğlu [174] found that SrCO3 readily precipitates in water sparged with CO2, as Sr behaves biogeochemically very similarly to Ca. Boukobza’s modeling study [175] corroborated the precipitation of SrCO3 in the presence of CO2 and leached Sr2+ ions. |
| V | ● | Vanadium (V) is one of the most critical unstabilized HMs. Using APCr, Astrup [138] suggests that V and Pb have a geochemical affinity, as their leaching is influenced by the solubility control of Pb2V2O7, Pb3(VO4)2. V leaching increased during basic oxygen furnace slag carbonation [149]. Vogel [169] concluded that the coarse and magnetic fraction of the bottom ashes contain larger amounts of V(III) and V(IV) compounds, which might enter the WtE plants from vanadium carbide-containing steel tools. V could therefore potentially be a critical toxic element in secondary building materials containing IBA, and long-term monitoring of its release should be taken into account in the future. |
| Zn | ○ | The WATEQ.4F database indicates that ZnCO3 (smithsonite) aqueous solubility is 7.6 mg/L at 25 °C in pure water. Zinc (Zn) is one of the most positively affected HMs during carbonation. For both fresh and carbonated APCr, the leaching of Zn was found to be influenced by solubility control from CaZn2(OH)6 · 2H2O, zincite (ZnO), Zn2SiO4, and ZnSiO3, while smithsonite (ZnCO3) was undersaturated by more than one order of magnitude in the eluate [48,138]. Zn is systematically reported as significatively affected by carbonation, with leaching reduction factors ranging between 3 and 10 [110]. Zn leachability depends strongly on pH, following a U-shape trend [138,147]. Kim [146] showed that Zn coprecipitates with Cal. |
| Na | □ | Sodium (Na) is released during aqueous carbonation primarily due to the decomposition of chlorides and sulfates, while NaHCO3 may precipitate in dry or low L/S ratio carbonation. Due to their high solubility, both NaHCO3 and Na2CO3 typically do not precipitate in most FA carbonation systems. |
| K | □ | Similar to Na, potassium (K) is released during aqueous carbonation primarily due to the decomposition of chlorides and sulfates. K2CO3 does not precipitate due to its high solubility. |
| Cl | ● | Chloride (Cl−) remains mobile after carbonation both in IBA and APCr [148]. Mayenite in IBA removes Cl− from water, forming Hcl [152]. The efficiency of chloride removal during aqueous carbonation is primarily controlled by the complete dissolution of calcium (hydroxi)chlorides and alkali-chlorine (CaCl2.xH2O, CaClOH and NaCl, KCl, respectively) [176]. Employing IBA, [109] evidenced that the carbonation-induced chloride removal effect surpassed that of conventional water-washing treatments due to the decomposition of Friedel’s salt (the primary insoluble chloride). |
| S | ● | Sulfite (SO3−) and sulfate (SO42−) typically form gypsum and hannebachite, respectively, in FA and APCr aqueous carbonation. SO42− remains mobile after carbonation both in IBA and APCr [148]. Using APCr, Astrup [138] showed that gypsum solubility matched the measured data at pH values below 9–10. At higher pH, the near-saturation levels of Al, Ca, and S with Ett strongly support Ett as the controlling mineral. In addition, SO2 in flue gas can react with water to form H2SO4, which then reacts with calcium in waste incineration residues to form gypsum. This process reduces the availability of calcium for CO2 uptake, as verified experimentally by Jiang [177]. In conclusion, similarly to Cr(VI) and Sb(V), SO42− leaching remains a persistent issue hindering the industrial deployment of waste incineration residue treatment strategies, requiring stabilization approaches specifically tailored to its behavior. |
5. Reactor Design and Process Layout from Laboratory to Field Scales
In this section, data extracted from the included sources reporting carbonation experiments were systematically organized into tables summarizing key characteristics such as reactor types, operational parameters, CO2 uptake, and leaching behavior. These data were then qualitatively analyzed to identify patterns, gaps, and opportunities within accelerated carbonation research and applications.
5.1. Reactor Design for Laboratory-Scale Carbonation
Figure 3 summarizes the reactor types used in laboratory-scale studies on waste incineration residues, highlighting their advantages and drawbacks based on the critical assessment of this section. Table 5 summarizes of the experimental conditions, investigated parameters, CO2 uptake achieved, and results and conclusions for laboratory-scale investigations of IBA carbonation reported in the reference literature. The evaluation considers suitability and scalability, assessing the reactor’s ability to accommodate different grain sizes and its representativeness of real-world conditions to ensure that laboratory findings can be effectively scaled up to industrial applications. Performance and durability are also key factors, including resistance to corrosion, chemical attacks, and mechanical impacts, particularly in high-pH environments or when handling abrasive residues. Additional considerations include pressure tolerance, clogging susceptibility, ease of operation, and stirring efficiency. Process efficiency is evaluated based on the reactor’s capacity to enhance CO2 diffusion, optimize material dissolution, and minimize energy consumption to ensure effective reaction kinetics. Reactor geometry and flow dynamics are examined for their influence on mixing efficiency, residence time, and uniform material exposure to reactive conditions. Experimental flexibility and monitoring capabilities are also considered, including whether the reactor is transparent or opaque for flow pattern observation, its ability to test multiple samples simultaneously for consistent investigations, and the integration of automation tools such as sensors, pH monitoring, temperature control, and real-time data acquisition. Finally, adaptability and operational control are assessed, focusing on the reactor’s flexibility in adjusting key parameters such as temperature, pressure, and agitation speed to accommodate various reaction conditions, including gas–liquid–solid interactions.
5.1.1. Pressurized Fixed-Bed Reactor
The pressurized fixed-bed reactor, as used by [178], consists of a gas-tight glass vessel equipped with stainless steel sample racks covered with nylon grids to enhance CO2 access. This design offers several advantages: it is transparent, allows simultaneous testing of multiple samples, requires no energy input for mixing, and accommodates all grain sizes. However, it is not optimized for enhancing carbonation kinetics due to the absence of a solid mixing system. Additionally, it requires external CO2 pressurization, exhibits a low CO2 absorption rate, and operates only under mild pressure conditions.
5.1.2. Stirred Autoclave Paar Reactor
The stirrer-type autoclave Parr reactor made of Hastelloy alloy and equipped with an overhead stirrer, as employed by [91], offers strong mechanical stirring. It is impact- and corrosion-resistant, with a modular design suitable for aqueous carbonation in both flow-through and pressurized modes. This design enables fast CO2 absorption and high uptake efficiency, making it optimal for small particles. However, its limitations include opacity, energy consumption for mixing, water usage, and reduced suitability for large grain sizes.
5.1.3. Rotary Drum- or Ball Mill-Type Reactor
The rotary drum- or ball mill-type reactor typically operates through rotary motion driven by rollers and a belt transmission. It is impact-resistant and highly modular, accommodating various mixing tools such as cages and abrasive materials. This design is suitable for both flow-through and pressurized conditions, making it ideal for processing bulky materials and producing pelletized aggregates. However, its limitations include opacity, energy consumption for mixing, and reduced suitability for powdery materials [179,180,181,182].
5.1.4. Gas Flow-Type Fixed-Bed Reactor
The gas flow-type fixed-bed reactor, as described by [117], consists of a stainless-steel cylindrical vessel with a continuous gas flow entering from the lower side and exiting at the upper outlet. The ashes are placed on a gravel layer covered by a geotextile fabric, which retains fine particles while allowing gas passage. This reactor is impact-resistant, insulated, and optimized for both dry and wet carbonation. It is particularly suitable for large grain sizes and requires no energy input for mixing. However, its limitations include opacity, lack of solid mixing, potential material compaction, unsuitability for fine powders, and a low CO2 absorption rate.
5.1.5. Gas Flow-Type Stirred Reactor
The stirring-type reactor, as described by Wehrung [97] and Um [183], consists of a modular, double-wall Pyrex vessel equipped with static gas–liquid mixers, an overhead stirrer for suspensions, and gas flow sensors. Its advantages include transparency, high modularity, and the ability to generate ultrafine CO2 bubbles, resulting in a fast absorption rate and high CO2 uptake efficiency. This design is optimal for aqueous carbonation, particularly with small particles. However, it has several limitations: its Pyrex construction makes it fragile, and it requires energy input for mixing, consumes water, is unsuitable for large grain sizes, has porous mixers prone to clogging, operates only under mild pressure conditions, and has relatively low mechanical stirring strength.
5.1.6. Rotary Evaporator-Based Reactor
The rotoevaporator-type reactor consists of an inclined glass flask with a single rotating neck. It is transparent, modular, and suitable for both aqueous and semi-dry carbonation, offering fast CO2 absorption and high CO2 uptake efficiency. However, it has several limitations: it is fragile, requires energy for mixing, consumes high amounts of water, and cannot sustain elevated pressures [184].

Table 5.
Summary of the experimental conditions, investigated parameters, CO2 uptake achieved, results, and conclusions for laboratory-scale investigations of IBA carbonation reported in the reference literature. PS = particle size, MS = mixing speed, WC = water content, RH = relative humidity, P = pressure, T = temperature, RT = reaction time, GFR = gas flow rate, HM = heavy metal, TOC = total organic carbon, TIC = total inorganic carbon, SCM = shrinking core model, RSM = response surface methodology, CRW = cold-rolling wastewater.
Table 5.
Summary of the experimental conditions, investigated parameters, CO2 uptake achieved, results, and conclusions for laboratory-scale investigations of IBA carbonation reported in the reference literature. PS = particle size, MS = mixing speed, WC = water content, RH = relative humidity, P = pressure, T = temperature, RT = reaction time, GFR = gas flow rate, HM = heavy metal, TOC = total organic carbon, TIC = total inorganic carbon, SCM = shrinking core model, RSM = response surface methodology, CRW = cold-rolling wastewater.
| Reactor Characteristics | Operational Parameters | Investigated Parameters | CO2 Uptake | Results | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| Stainless steel pressure reactor with 150 mL Teflon inner lining | P: 1–3–10 bar, T: 30-40-50 °C, L/S: 0–0.6, RT: 0.5–48 h, PS: 0.15–12 mm. | Effect of PS, carbonation kinetics, mineralogical transformation, metal leaching (Pb, Cr, Cu, Zn, Mo). | PS [0.425–12 mm]: 4.0–4.2%; PS [0.425–0.15 mm]: 9.0%; PS < 0.15 mm: 14.0%. | Leaching: Cu, Zn, Pb ↓ 99%, but Cr, Mo ↑; mineralogy: dissolution of portlandite and formation of calcite and quartz; pH: decrease from 12.8 to 10.5. | Accelerated carbonation improves BA stability and sequesters CO2, but efficiency depends on particle size and operational conditions. | [27] |
| Closed CO2 incubator (Sanyo MCO-18AIC) | T: 35 °C and 50 °C, WC: 15%, RT: 2 h, P: 1 atm, CO2 20%. | Effect of PS, degree of carbonation, HM leaching (Pb, Zn, Cu, Cr) and soluble components (DOC, Cl−, SO42−), mineralogy. | CO2 uptake ↓ when PS ↑. | Leaching: Pb, Zn, Cu, Cr ↓, SO42− ↑, Cl− stable; pH drop: 12 → 9; mineralogy: portlandite converted to calcite. | Grinding to 425 µm minimizes morphological differences and enhances carbonation efficiency. PS separation benefits carbonation and ↓ the volume of IBA to be treated. | [185] |
| Gas flow-type fixed-bed reactor | T: 20 °C, GFR: 20–500 mL/min, RT: 2 h (rapid), 14 days (slow) WC: 30%. | HM leaching (Pb, Zn, Cd, Cr, Cu, Ca) under various conditions: untreated, carbonated, and ground after carbonation. | 0.68-1.05-1.06-2.22 mmol-CO2/g-IBA. | Leaching: Pb ↓ up to 99%, Cu ↓ up to 49%, Cr ↓ up to 99.7%; grinding: increase in HM concentrations due to destruction of carbonate coating. | Physical confinement from carbonation helps immobilize HMs. | [120] |
| Closed bottle, OxiTop control system (1 L) | T: 30 °C, RT: 1–3 weeks, WC: 23%, 13%, 7%. | O2 consumption and CO2 production during microbial respiration; leaching of TOC and TIC; pH monitoring. | Non-specified | CO2 production: 0.86 L/kg of BA (at 23% WC); pH drop: 10.7 → 8.2 in 3 weeks; TOC reduction: 30%; TIC increase: formation of CaCO3 carbonates. | The biodegradation of organic matter significantly contributes to BA stabilization. | [186] |
| Closed CO2 incubator (Sanyo MCO-18AIC) | T: 35-42.5-50 °C, WC: 5-15-18.8-25%, RT: 2-4-6-8-24-168 h, IBA layer thickness = max 2 mm. | PS, IBA source plant, effect of WC and T on CO2 uptake; Cu and Cr leaching. | 3.8% CO2 uptake after 2 h (T = 50 °C, WC = 15%). | Leaching reduction: Cu (↓ 90% linked to carbonation and DOC decrease), Cr (reduction pH-dependent); optimal conditions: WC = 15%, T = 35 °C or 50 °C. | WC and T significantly impact carbonation efficiency and HM leaching, requiring careful optimization. | [187] |
| Gas flow-type slurry reactor (glass column 0.55 m × 0.065 m) | PS (<125, 125–350, 350–500 μm), CO2 100% or diluted (10–30%), P: 1 atm, RT: 120 min, L/S: 5–40, CO2 flow rate: 0.2–1.5 L/min. | Effects of RT, CO2 concentration, L/S ratio, PS, and CO2 flow rate on CO2 uptake. | 102 gCO2/kg-IBA (90.7% conversion); Theoretical capacity: 127 gCO2/kg-BA for PS < 125 μm. | Optimal conditions: L/S = 10, CO2 flow rate = 1.15 L/min, PS: 125–350 μm. | Coupling IBA with CRW enhances carbonation reactions; SCM analysis: reaction kinetics controlled by diffusion through IBA layer; RSM used to optimize operational parameters for maximum carbonation conversion. | [188] |
| Gas flow-type fixed-bed stainless steel reactor (15 L, 28 cm × 35 cm) | Simulated gas flue gas: 10% CO2, 90% N2; GFR: 4 L/(kg·h); T: 20 °C; P: atm; RT: 60 h. | Metal leaching before and after treatment volume and GFR. | 37 gCO2/kg-IBA | Leaching: Pb (↓99.9%), Ba (↓94.5%), Zn (↓99%), Cu (↑15%), Sb (↑25%); Cost: 16–20 €/t IBA vs. 45–70 €/t for conventional landfilling. | Using incineration gas (10% CO2) instead of pure CO2 is viable. | [117] |
5.2. Competence Center for Sustainable Engineering and Environmental Systems (ZEuUS), THM University of Applied Sciences, Gießen, Germany
The research reviewed in this section is the outcome of a collaboration between the THM University of Applied Sciences in Gießen and the Department of Geosciences in Cologne, led by Prof. Harald Weigand. Since 2018, the researchers have developed multiple ACT systems for IBA, leading to field-scale testing of the technology. This innovative approach leverages a rotary drum reactor to optimize the carbonation reactivity of freshly produced IBA under continuous-feed conditions, previously proposed by [180]. Indeed, rotating drum reactors applied to IBA carbonation are known to provide better performances than the fixed bed ones [117].
5.2.1. Laboratory-Scale Tests
The investigations began by developing a method to monitor the carbonation of IBA under both static and dynamic conditions. Batch tests were conducted at near-atmospheric pressure in both a fixed-bed setup and a lab-scale rotary drum reactor (Figure 4) [189]. In the preliminary study, CO2 uptakes from 15 to 26 gCO2/kg IBA (static and dynamic tests, respectively) were achieved, with Ca(OH)2 presumed as the main reactive phase during carbonation. The second step consisted in studying the effect of rotation speed and reactor fill level on the solids’ motion, as well as the effects of CO2-concentration, fill level, and moisture on IBA carbonation [190]. Increasing the CO2 concentration and the self-heating in response to the CO2 uptake significantly enhanced IBA carbonation and reduced the mobility of Pb and Zn, with the fill level being non-limiting within the tested range. A correspondence was established between bed behavior (slipping, slumping, rolling) and the combinations of fill level and rotation speed. The optimum moisture content for the tested IBA particle size was 21 wt.%. The experiments were then refined to mimic real-field conditions with simulated flue gas, assessing the effects of CO2 supply, mixing tools, and reactor loadings on IBA leaching behavior [191]. When specific CO2 supply exceeded 100 gCO2/kg IBA, the leachate tests reached non-hazardous waste thresholds. Among the tested mixing tools, the perforated mixing cage performed best, even at 50% fill levels, and minimized IBA incrustations. A laboratory scale-up introduced an IBA feeding system to experiment with continuous reactor feed, investigating IBA residence time, gas temperature, and humidity [181]. An 80-min residence time effectively reduced IBA leachability to meet German non-hazardous waste standards, decreasing amphoteric trace metal concentrations significantly, while slightly increasing the mobility of oxyanonic metals.
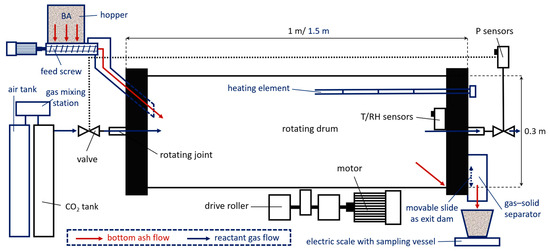
Figure 4.
Set-up of the laboratory-scale BA carbonation experiments under dynamic conditions. The blue sections represent the second scaled-up setup designed for testing under continuous BA feed conditions. Adapted with permission from Elsevier [181].
Recently, the team published a study on the impact of aging on chromite ore processing residues (COPR) and Cr(VI) mobility in an open landfill [151]. This study is relevant to our review, as COPR, like IBA, contain calcium aluminate phases (Hcl, Ett) influencing Cr mobility during carbonation. Results show that carbonation during aging coincided with Cr(VI) translocation to the landfill subsurface, where leachate exceeded 1.6 g·L−1, and CaCrO4 precipitated. These findings highlight carbonation as a key aging process that may worsen Cr(VI) leaching by dissolving Cr-bearing Hcl.
5.2.2. Field-Scale Implementation
The full-scale study utilized a fine fraction of IBA (<20 mm), from a refuse-derived fuel incinerator, with exhaust from a combined heat and power unit as the reactant gas [111]. Results indicated that reactor loading depended on the rotation-normalized mass flow rate of IBA, with characteristics varying based on reactor discharge design and mixing tool usage [178]. Leaching tests demonstrated that IBA residence times as short as 60 min were adequate to convert carbonated IBA into nonhazardous waste suitable for geotechnical applications, surpassing previous laboratory findings and suggesting the potential for ACT implementation at incinerator sites. Figure 5 represents the process flow diagram and reactor design, while Table 6 summarizes the operational conditions and investigated parameters for the test campaign.
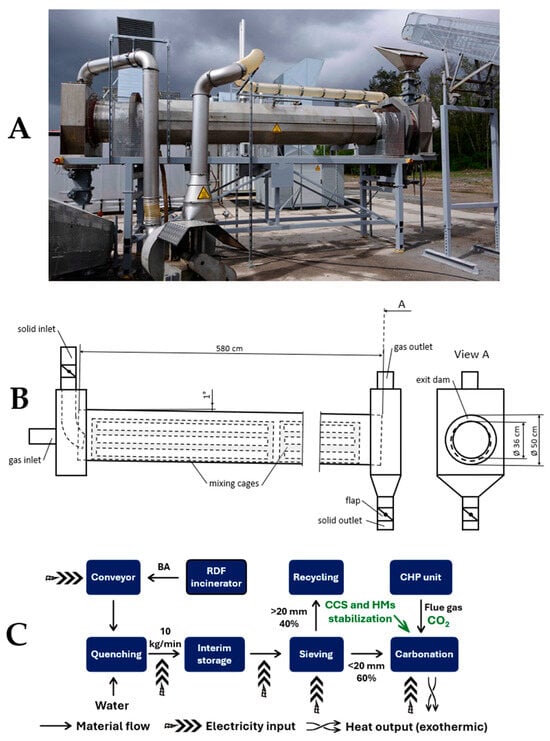
Figure 5.
(A) Side view of the full-scale process setup, reprinted from Schnabel [111]; (B) schematic of the carbonation reactor with annular exit dam and mixing cages, adapted from Schnabel [111]; (C) process flow diagram leading to the full-scale refuse-derived fuel IBA ACT.

Table 6.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory-scale investigations and the full-scale campaign. PS = particle size, MS = mixing speed, WC = water content, RH = relative humidity, P = pressure, T = temperature, FL = fill level, RT = reaction time, GFR = gas flow rate, MFR = mass flow rate.
5.3. Faculty of Engineering and Science, University of Greenwich, United Kingdom
The research reviewed in this section has been conducted across multiple institutions, including the Department of Civil Engineering at Imperial College of Science and the Centre for CO2 Technology at University College London, under the direction of Prof. Colin D. Hills and Dr. Paula J. Carey [192]. Both researchers were pioneers in the field of IAW carbonation, starting their work on ACT as soon as in the 1990s. In the early 2000s, they created Carbon8, collaborating with the English Environment Agency to develop treatment solutions for IBA, FA, and APCr. They patented their ACT technology under patent number US10343199B2 for the production of secondary aggregates and founded Carbon8 Systems Ltd. in 2006. A second company, O.C.O Technology, was founded by Dr. Peter J. Gunning in 2010 [193]. In both cases, an innovative approach integrates carbonation into the processing sequence, enabling the production of LWAs labeled as ‘end-of-waste’, used in construction, thereby eliminating the need for FA/APCr landfill disposal. The Carbon8 team also operates in sectors beyond waste incineration, including cement (cement kiln dusts), steel (slags), paper/pulp (paper ash), mining (tailings), construction/demolition (concrete fines), and biomass (ash).
5.3.1. Laboratory-Scale Tests
The Carbon8 team, early on, understood the economic implications of stricter waste management regulations beyond their environmental impact. Their initial research explored the effects of carbonation on various cement-waste mixes, using ordinary Portland cement, white Portland cement, rapid hardening Portland cement, sulphate-resisting Portland cement, and calcium aluminate cement, combined with pozzolans like pulverized fuel ash and silica fume. They found that curing in a CO2-rich environment significantly improved the strength and microstructure of the samples while enhancing metal fixation. Silica fume blended samples demonstrated higher strength compared to those with pulverized fuel ash, due to differences in hydration reactions and reduced CO2 permeability. Additionally, carbonation decreased the concentration of leachable metals across all cement types, notably enhancing chromium fixation in white Portland cement and silica fume mixes. Overall, the binder choice critically influenced the effectiveness of waste metal immobilization in carbonated cement-waste systems [127,194].
Subsequently, the research shifted towards the waste incineration sector. Table 7 summarizes the waste incineration residue carbonation investigations conducted by the team. Bertos [94] studied the ACT of IBA, FA, and APCr, identifying three key parameters: reaction time, particle size, and L/S ratio. Their work found that optimal carbonation occurs with smaller particles, specifically those below 710 µm for IBA and below 212 µm for FA, and with a L/S ratio of 0.3–0.4 for IBA and 0.2–0.3 for APCr. The process also reduces ash porosity, enhances the formation of calcium-metal salts and metal silicate complexes, and lowers pH to near neutral. CaCO3 gain (wt.%) were observed for both IBA (1.34 wt.%) and APCr (4.96 wt.%). The C8S team conducted multiple studies on the interactions between carbonated cement mixtures and HMs like Hg [157], Cd, Co, Cu, Ni, and Zn [195]. Despite promising stabilization results, especially for divalent, cation-forming HMs, these studies highlighted that carbonation alone is insufficient to reduce the release of soluble salts, such as SO42− and Cl−, to levels below the landfill acceptance limits for hazardous waste [143].
Additional studies, such as those conducted by Chen [196] and Araizi [197], explored various optimization routes for cement-based solidification/stabilization during carbonation, such as utilizing Na2CO3 and ultrasound enhancement, respectively. Notably, Gunning [118] explored diverse combustion residues as CO2-reactive binders (biomass, wood and paper ash, waste incineration IBA/FA, cement bypass dust, cement kiln dust), combined with quarry fines, to produce ACT aggregates with properties akin to commercially available LWAs. These aggregates, exclusively composed of waste, were formed through a cold-bonding process, imbuing CO2 gas during production, contrasting with energy intensive firing or sintering aggregates production processes.

Table 7.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory-scale investigations. PS = particle size, L/S = liquid-to-solid ratio, RH = relative humidity, P = pressure, T = temperature, RT = reaction time.
Table 7.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory-scale investigations. PS = particle size, L/S = liquid-to-solid ratio, RH = relative humidity, P = pressure, T = temperature, RT = reaction time.
| Reactor Characteristics | Operational Parameters | Investigated Parameters | CO2 Uptake | Ref. |
|---|---|---|---|---|
| Stainless steel chambers | IBA/FA/APCr, PS FA/APCr: <500 μm; IBA < 2800 μm, RH 65%, L/S 0.2, CO2 100%, P 3 bar, room T, RT 20 min–24 h. | PS, RT, WC | CaCO3 gain (wt.%): IBA: 1.34 gCO2/kg; APCr: 4.96 gCO2/kg | [47] |
| Two reactors: flow-through/closed stainless steel chamber with a cooling plate. | FA, CaO: 36.3 wt.%, mean PS 66 μm, L/S 0.1–0.8, CO2 100%, P 3 bar, room T, RT 180 min–3 days. | RH, T | 70–100 gCO2/kg | [143] |
| Sealed 0.5 L polyethylene vessels | CFA: CaO 7.5 wt.% + CaO additive, PS CFA < 45 μm, SSA 1.2 m2/g, high L/S, wet, CO2 100%, P 3 bar, room T, RT > 30 min. | HMs precipitation from wastewater | - | [198] |
| Pressurized reaction vessel with plastic containers (50 mL) | IBA, FA, CaO: 32–33 wt.%, PS < 250 μm, RH 75%, L/S to form smooth paste, P 2 bar, room T, RT 72 h. | IAW type | 4–7 wt.% | [33] |
| Plastic containers (50 mL) | APCr, CaO: 65 wt.%, PS mean 10.7 μm, L/S 0.2–100, CO2 100%, P 3 bar, room T, RT 180 min. | L/S ratio, sonication effect (ultrasound) | up to 16 wt.% | [197] |
5.3.2. From Pilot to Field-Scale Implementation—Production of Lightweight Aggregate Using Carbonation
Lab-scale findings indicated that the strength of quarry fines pelletized with waste incineration FA binder was lower than with paper ash and cement kiln dusts, leading to the selection of paper ash for the initial LWAs production in the pilot-scale carbonation reactor (see Figure 6, Figure 7 and Figure 8) [118]. The study found that increasing the reactive binder content in aggregates and accelerating the carbonation process led to improved compressive strengths and resistance to freezing and thawing. While compressive strengths were generally higher than those of LWAs like lightweight expanded clay aggregate and Aardelite (blockwork LWA) but lower than Lytag (structural LWA), ACT resulted in denser structures due to carbonate formation, potentially enhancing strength and durability. The carbonated aggregates showed satisfactory fracture behavior in concrete cubes, with fractures propagating through aggregate particles, indicating good bonding with the cement paste. Moreover, aggregates bound with 50% ash were comparable to lightweight, expanded clay aggregate-based concrete. Pilot-scale production confirmed that carbonated aggregates exhibited properties comparable to or better than commercially available LWAs in the UK, suggesting potential for practical implementation.
Figure 6.
Flowchart of the pilot scale trials to process APCr. Adapted from Gunning [199].
Figure 7.
Raw materials: (A) paper ash, (B) quarry fines, and (C) pelletized product (upper section) as well as pilot-scale rotary carbonation reactor (lower section). Reprinted with permission from Elsevier [118].
Figure 8.
Aggregate manufacturing plant using alkaline waste residues. Reprinted from Gunning [193].
Antemir [12] assessed the long-term performance of aged waste forms treated by stabilization/solidification up to 16 years old at private sites in the UK and USA. The study found that the S/S materials met their acceptance criteria for physical performance, exhibiting properties similar to cement-bound materials rather than concrete. Contaminant release from the S/S soils remained within acceptable limits, indicating effective long-term immobilization and continued protection of groundwater, identified as the primary contamination risk.
Figure 7 and Figure 8 show the components of the aggregate manufacturing pilot plant using alkaline waste residues. The process begins with waste silos feeding a first-stage carbonation reactor. An elevator then conveys the material to a batch mixer, where a reagent (binder) is added. The mixture is subsequently transferred to a second-stage rotary drum carbonation reactor to produce aggregates, which are finally stored at the exit.
5.3.3. First Field-Scale Implementation: Carbon8 Systems
Carbon8, through ACT, manufactures the CircaBuild aggregates and the CircaGrow fertilizers, which find applications in concrete blocks, ready-mix concrete, precast concrete, pipe bedding, road filler, and green roofing substrate. The company has consistently prioritized research and development over rapid market expansion. In 2020, Carbon8 deployed its Plug’n Play solution, the CO2ntainer, at Vicat Group’s cement plant in Montalieu, France. This marked the first commercial use of the containerized system. The CO2ntainer is a compact, modular version of the ACT housed in two 40 FT containers. Each CO2ntainer can treat up to 12 kt of residues and between 1.5 and 4 kt of CO2 annually. Figure 9 retraces the development stages from laboratory to full scale proposed by [199].

Figure 9.
Development stages from laboratory to full scale. Adapted from Gunning [199].
5.3.4. Second Field-Scale Implementation: O.C.O Technology
O.C.O Technology Ltd. was founded by Peter J. Gunning in 2010 and is a subsidiary of Grundon Waste Management Ltd. The company produces Manufactured Limestone (M-LS) LWAs from APCr through three operational facilities across the UK in Brandon (East), Avonmouth (South-West) and Leeds (North). In 2023, the company declared a waste-processing capacity of 200,000 tons per year, generating more than 500,000 tons of M-LS. In 2022, the M-LS 6F Series factsheet [200] stated that one ton of M-LS aggregate results in approximately 49.1 kgCO2 per ton of CO2 negative emissions. The activity of the company results in an increasing net carbon capture of approximately 25,000 tCO2-eq per year.
The LWAs manufacturing process involves blending, carbonation, granulation into pellets using binders, and curing under a CO2 atmosphere. In the UK, pure CO2 is used in the process. Figure 10 summarizes the full-stage process to stabilize and valorize APCr as useful construction products. Formulations are tailored for specific applications, including End of Waste, earthworks, masonry, concrete, asphalt (cold foam), and pavements (eco blends).
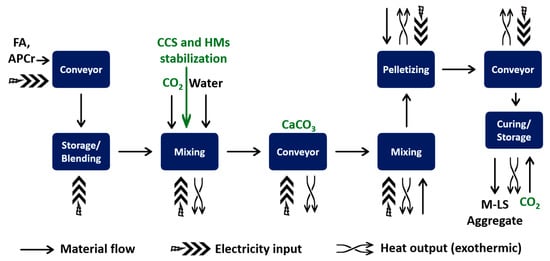
Figure 10.
Multiple, full-stage process to stabilize and valorize waste incineration residues as useful construction products. Adapted from O.C.O Technology, M-LS environmental product declaration [201].
The primary technical challenge is reported to be the inherent variability in composition of APCr, which necessitates continuous monitoring and adequate adjustment of the treatment process to ensure consistency in the final product.
Since 2021, O.C.O Technology has partnered with the Japanese company Kobelco Eco-Solutions Co., Ltd. to deploy ACT at the Fukui WtE plant. Figure 11 shows the mixer/CO2 reactor installed at the Kobelco WtE plant in Japan. For the first time, CO2 was sourced directly from the flue gas of the plant. O.C.O Technology has won the prestigious King’s Award 2024 (UK) for Enterprise in Sustainable Development.

Figure 11.
Image of the mixer/CO2 reactor installed at O.C.O Technology’s first overseas trial CO2 treatment facility at the Kobelco WtE plant in Japan [202].
5.4. Department of Mechanical and Industrial Engineering, University of Brescia, Italy
Various institutions have conducted the research reviewed in this section, including the Department of Mechanical and Industrial Engineering in Brescia, the Department of Industrial Chemistry in Bologna, Italy, and the Department of Chemical Engineering in Coimbra, Portugal, under the direction of Prof. Elsa Bontempi. This research group employs a distinctive approach, blending FA/APCr with various waste materials to embody the “waste treated by waste” paradigm. A key aspect of their method involves the use of colloidal silica to produce a range of treated fillers, known as COSMOS (COlloidal Silica Medium to Obtain Safe inert), as a glass–ceramic powder [203]. Moreover, in Italian APC technologies, not only lime but also NaHCO3 additives are commonly used for acid gas scrubbing, sometimes resulting in case studies of FA/APCr with low free-lime content, which limits their reactivity upon carbonation. To enhance their reactivity, blends have included lime-rich sources such as flue gas desulphurization gypsum or wood ash. This addition facilitates carbonation reactions and improves the entrapment of HMs [204]. Table 8 summarizes the experimental conditions, investigated parameters, and CO2 uptake achieved during laboratory carbonation investigations.
5.4.1. Laboratory-Scale Tests
The FA/APCr treatment technology employs both amorphous silica and calcium hydroxide sources as stabilizing agents to form Zn and Pb silicates (PbSiO3 and Zn2SiO4) and carbonates while lowering pH [204]. The colloidal silica initially proposed has been replaced by more sustainable sources: rice husk ash, silica fume [205], and coal fly ash to a lesser extent, which act as physical adsorbents for metal ions by reacting with silanol groups on the silica surface [56]. Flue gas desulphurization gypsum residue (FGDGr), or alternatively wood ash, provide calcium hydroxide for carbonation reactions, completing the stabilization process. This approach is not purely a carbonation process; it integrates FGDGr-promoted carbonation with amorphous silica to entrap HMs. This one-step stabilization process achieves optimal results through a balanced dosage of reactants. The reactivity of Ca(OH)2 and Mg(OH)2 were later investigated as substitutes to FGDGr for HM immobilization. Data revealed that mixing FA with Mg(OH)2 significantly decreased Pb and Zn leachability after two months [206].
Additionally, Assi [207] investigated the effect of aging on various formulations of waste incineration FA with FGDGr, CFA, and IBA on Zn and Pb leaching reduction. They concluded that the material could uptake up to 90 gCO2/kg FA, significantly reducing leaching [78]. The COSMOS products were incorporated into polypropylene composites to replace Cal [208]. Since the aging process is slow, often taking months, ACT tests have aimed to identify the optimal mix formulations, L/S ratio, and the effect of PCO2, reducing the reaction time to less than 72 h [101]. Overall, the stabilization of Zn and Pb was attributed to pozzolanic reactions, forming C–S–H and C–A(–S)–H, which react with CO2 to form CaCO3, sequestering up to 165 gCO2/kg of ash [81]. Finally, an industrial symbiosis using cork fly ash and waste incineration FA/IBA was shown to sequester up to 77.38 gCO2/kg of mixture, potentially stabilizing HMs enough to enable the reuse of this material as a substitute for natural resources [209].

Table 8.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory-scale investigations and the full-scale campaign. PS = particle size, P = pressure, T = temperature, RT = reaction time, FGD = flue gas desulphurization gypsum, CFA = coal fly ash.
Table 8.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory-scale investigations and the full-scale campaign. PS = particle size, P = pressure, T = temperature, RT = reaction time, FGD = flue gas desulphurization gypsum, CFA = coal fly ash.
| Reactor Characteristics | Operational Parameters | Investigated Parameters | CO2 Uptake (gCO2/kg of Ash) | Ref. |
|---|---|---|---|---|
| Closed cylinder | Blends of waste incineration FA/IBA + FGD + CFA + Silica Fume, L/S 1, P 1 bar, CO2 100 vol.%, room T, RT 3 months. | Mix formulations | up to 90 | [207] |
| Closed cylinder (75 mL) | CaClOH 1–26 wt.%; Ca(OH)2 1–23 wt.%. Blends of FA/IBA + FGD + CFA, L/S 0.7/0.9/1.2, P 15 bar, CO2 100 vol.%, room T, RT 17 h to 12 days | L/S ratio, P | up to 152 | [101] |
| Closed cylinder (75 mL) | FA: CaClOH 30 wt.%; Ca(OH)2 5 wt.%. Blends of FA/IBA + FGD + CFA, PS < 106 μm, L/S 0.91, P 15 bar, CO2 100 vol.%, room T, RT 5 to 72 h. | Mix formulations | 55–165 | [81] |
5.4.2. The COSMOS Washing Technology—Pilot Plant Testing
The pilot plant (Figure 12) comprises four distinct parts: a storage and handling unit for powders and liquids, a mixing unit, dosing units for powders and liquids with a control panel, and a sludge washing unit. The heart of the plant is the mixer, a frustum-conical steel container (AISI 304) with a 200 L load capacity. It can prepare up to 100 kg of product and features a heating system, enabling mixing tests from room temperature to 70 °C [210].
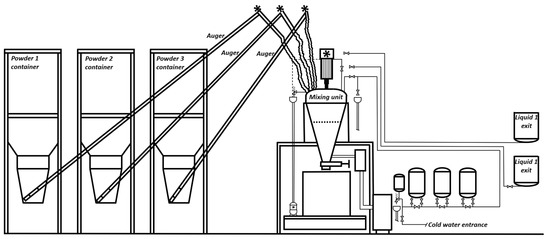
Figure 12.
Diagram of the pilot plant designed in the context of the COSMOS-RICE project. Adapted with permission from American Chemical Society [210].
To prepare the COSMOS-RICE product, the team blended 65% FA, 20% FGDGr, 15% coal fly ash, and 20% rice husk ash of the total ash weight. Tap water heated to 60 °C was added at a L/S 1. The mixture was stirred for 30 min in the plant, then poured into containers. It was left to mature at room temperature until fully dry, forming the COSMOS-RICE product.
5.5. Korea Institute of Geosciences and Mineral Resources (KIGAM), South Korea
The research reviewed in this section was conducted at the Korea Institute of Geoscience and Mineral Resources (KIGAM) in South Korea under the direction of Prof. Ji Whan Ahn. The work has extensively focused on the valorization of IBA and slag kiln residue (KR). This includes identifying optimal operational parameters for HM stabilization, dechlorination, and ferromagnetic separation, as well as designing reactors and processes, modeling carbonation mechanisms, and producing aggregates, concrete, and pure Cal for paper-making. By addressing these fundamental aspects of IBA treatment, the team has successfully developed pilot and full-scale IBA treatment plants.
5.5.1. Laboratory-Scale Tests
The KIGAM team recognized early on the carbon mitigation potential and secondary material valorization of IBA. Table 9 summarizes the experimental conditions, parameters investigated, and CO2 uptake achieved during laboratory and field-scale carbonation investigations. Ahn [211] demonstrated that washing IBA and substituting it up to 10% nearly matched the compressive strength of mortar using only Portland cement. You [212] observed that the carbonation potential of IBA was concentrated in the fine fraction where Ca(OH)2 content was higher. Buffering of CO2 in larger particles was attributed to Ca(OH)2 adhering to these particles rather than the particles themselves, suggesting that effective reduction of aging and valorization of IBA could be achieved by separating small particles from large ones using methods such as vibrating screens.
Han [213] underscored the recovery potential of ferromagnetic materials through wet magnetic separation in coal IBA. Nam [214] modeled that the carbonation reaction in IBA was predominantly governed by phase-boundary control initially, transitioning to product-layer-diffusion control as the reaction progressed, resulting in a decreased overall reaction rate. Um [150] found that initial carbonation of IBA increased chromium leaching due to the dissolution of crystalline phases like Ett and Hcl. However, subsequent formation of amorphous aluminum phases adsorbed chromium ions, effectively stabilizing chromium and sequestering up to 34 gCO2/kg of IBA in the <0.15 mm grain fraction.
Um [183] reported that under submerged conditions (L/S 10), IBA exhibited negligible formation of a carbonated layer, achieving a maximum CO2 reaction value of 38.5 g/kg. Conversely, the wet condition (L/S 0.3) showed a lower degree of carbonation due to internal CO2 diffusion. They also investigated how these conditions affected the leaching behavior of HMs, noting that the wet condition, with its carbonated-layer effect, provided more effective stabilization against HM leaching compared to the submerged condition. The KIGAM team further demonstrated that Ca(OH)2 carbonation could remove 85–88% of arsenate (As) [135] and nearly 100% of Cd and Pb from wastewaters [145].

Table 9.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory and the field-scale scale investigations. PS = particle size, MS = mixing speed, L/S = liquid-to-solid ratio, P = pressure, T = temperature, RT = reaction time, GFR = gas flow rate, MFR = mass flow rate.
Table 9.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory and the field-scale scale investigations. PS = particle size, MS = mixing speed, L/S = liquid-to-solid ratio, P = pressure, T = temperature, RT = reaction time, GFR = gas flow rate, MFR = mass flow rate.
| Reactor Characteristics | Operational Parameters | Investigated Parameters | CO2 Uptake | Ref. |
|---|---|---|---|---|
| Closed, acrylic double-walled sealed cylinder-shaped 2 L reactor | IBA, CaO 11.97–36.95 wt.%, PS < 0.15 mm to >4.75 mm, L/S 0.1/0.2/0.3, CO2 10/20/30 vol.%, P 1 bar, T 20/40/60 °C, RT 20 min | L/S, CO2 concentration, T, PS | 21–35 wt.% | [214] |
| CO2 incubation chamber | IBA, CaO 28.1 wt.%, PS < 0.15 mm, L/S 0.3, CO2 30 vol.%, P 1 bar, T 20/40/60 °C, RT up to 240 min | Cr leaching behavior | 10–30 gCO2/kg | [150] |
| Field-scale, mix-tank for slurry mixing and loop reactor for carbonation | IBA/FA/APCr mix, FA/KR slag, CaO 39.4 wt.%, PS < 0.15 mm, L/S 0.3/10, CO2 30 vol.%, P 10–40 bar, room T, MFR 15–20 t/hour | T, L/S, CO2 concentration | 26–35 gCO2/kg | [215] |
| Two reactors: gas flow-type (500 mL) and stirring-type reactor (2 L) | IBA, PS < 0.15 mm, L/S 0.3/10, CO2 30 vol.%, P 1.1 bar, 25 °C, loading 100 g | Reactor type, L/S ratio | 38.5 gCO2/kg | [183] |
| Closed stainless steel reactor with heating jacket | Ca(OH)2 15 g/L, 400 rpm, V solution 1 L, CO2 100 vol.%, P 20 bar, 30 °C, RT 80 min | As removal from wastewater | - | [135] |
| 1 L glass reactor with porous CO2 ceramic bubbler | Ca(OH)2 1–3 g/L, 300–400 rpm, V solution 1 L, CO2 100 vol.%, 1 L/min, P 1 bar, 25 °C, RT 15–30 min | Ca(OH)2 dosage, Cd and Pb concentration and removal, GFR | - | [145] |
5.5.2. Pilot-Scale Demonstration Plant
The KIGAM team’s pilot plant in South Korea focuses on treating IBA and kiln residue slag from the steelmaking process. Initially, the waste undergoes impurity removal, particle separation, and iron separation. Following these steps, it is sorted into cyclic aggregates suitable for manufacturing and carbonation processes. Fine powders (<0.15 mm) are mixed into a slurry and carbonated in a loop reactor with simulated flue gas, then processed through dehydration. This facility integrates various processes, including HM stabilization, dechlorination, and the production of aggregates and concrete, with a capacity of 200 kg/h. Additionally, the plant features a precipitated calcium carbonate (PCC) unit designed to produce PCC of 99.9% purity and particle sizes ranging from 8−20 μm to 0.3−0.5 μm. This high-purity PCC is used in recycled paper-making [215,216]. Figure 13 illustrates the multi-processing workflow.
Figure 13.
IBA processing workflow. Adapted from Lee [215].
5.6. Earth Sciences Department, University of Turin, Italy
The research reviewed in this section was conducted at the Earth Sciences Department of the University of Turin under the supervision of Prof. Alessandro Pavese and Prof. Linda Pastero since 2018. The studies cover both fundamental characterization and stabilization/solidification strategies of waste incineration residues, including IBA, FA, and APCr, with a particular focus on ash washing and carbonation experiments. Additionally, various investigations have assessed the potential for repurposing these residues as secondary raw materials for construction applications. Laboratory research led to the creation of the company Alkaline Technologies SAS in 2025, founded by Fabien Michel and Quentin Wehrung. Based in Nantes, France, the company aims to develop a full-scale advanced management strategy for IBA fine fractions by integrating EMR with ACT through its R&D program. The Earth Sciences Department and the company established an academia–industry research partnership to conduct the ASHES program, which this section presents, and which takes place in Turin.
5.6.1. Laboratory-Scale Tests
The groundwork began with an in-depth characterization of IBA and FA to assess their speciation distribution and properties as a function of particle size, with a particular focus on the leachability of heavy metals [20,77]. Multiple washing treatments were carried out to reduce residue leachability, including conventional water-washing, as well as the more innovative steam washing and carbonation washing [63,76,217,218]. The repurposing of FA in phosphate-based geopolymers and cements was investigated, with a focus on their impact on structure and compressive strength [219,220,221].
Carbonation experiments were performed in 1.5 to 5 L Pyrex reactors equipped with a thermostatic bath, stirrer, and CO2 spargers. Table 10 summarizes the carbonation studies. A novel approach used high-precision gas flow sensors (GFS) to monitor CO2 absorption rates over time, enabling in situ, real-time kinetic and stoichiometric analysis under continuous CO2 flow.

Table 10.
Summary of the experimental conditions, investigated parameters, and CO2 uptake achieved for the laboratory investigations. MS = mixing speed, L/S = liquid-to-solid ratio, PCO2= CO2 partial pressure, T = temperature, RT = reaction time, GFR = gas flow rate.
Studies on the impact of operational parameters on CO2 absorption during Ca(OH)2 aqueous carbonation showed that increasing the CO2–water interfacial surface area was key to accelerating dissolution kinetics, triggering a cascade of coupled dissolution (CO2 and Ca(OH)2) and precipitation (CaCO3). The effects of Na and K chlorides and sulfates were also assessed, with 3.5 wt% NaCl (seawater level) optimizing CO2 absorption rates [98].
Investigations comparing the reactivity of various waste incineration IBA, FA, and APCr showed that bag filter residues from plants using lime as a sorbent are the most reactive. All residues with high calcium (chloro)(hydr)oxides content exhibited similar kinetic behavior during carbonation, as shown by the comparable trends and intensities in their CO2 absorption rate patterns. However, IBA carbonation was primarily driven by Ca-aluminates—specifically Hcl and Kto—rather than by lime, while Ett could also be the main Ca-providing mineral, depending on the IBA source.
Aqueous carbonation notably increases the release of HMs into the water, while significantly reducing their release during subsequent leaching tests. Samples with the highest CCHO contents, the APCr, showed the most substantial improvements, with marked reductions in the leaching of Cr, Fe, Co, Ni, Cu, Zn, Cd, and Pb post-carbonation. However, Sb exhibited a critical leaching increase across all samples, both in wastewater and leachates, while Cr mobility was critical only in IBA. Therefore, additional separation and/or solidification are needed for oxyanion-forming elements like Sb. Notably, a novel Cr leaching mechanism during IBA carbonation was proposed, based on the decomposition of Cr-Mn-Fe oxide particles, a process driven by pH-lowering and the breakdown of Ca-aluminate coatings on slag particles. These findings suggest that to sustainably enhance the stabilization of fine IBA fractions, metal separation techniques should be implemented prior to carbonation [58,218].
5.6.2. The ASHES Program—Toward a Pilot Plant for Accelerated IBA Carbonation: Advancing Alkaline Technologies R&D
The Advanced Sampling for a Harmonized Environmental Solution (ASHES) program is an academia-industry R&D framework guiding the design of reactors and process layouts for IBA treatment through two complementary approaches:
- Developing an extensive geochemical database for IBA, covering both temporal and spatial variations.
- Conducting test campaigns that integrate EMR with ACT at the laboratory and pilot scales, within an urban mining and industrial symbiosis framework. The co-processing of IBA with other industrial alkaline wastes (e.g., steel slags, CDW) is also being considered.
The program is currently underway at the laboratory scale, generating data to guide the design of an initial pilot-scale unit for future deployment. The technological building blocks of the process are being tested individually, with parameters adjusted to ensure they work synergistically and achieve maximum environmental and cost efficiency.
IBA is being sampled from multiple incineration plants following a standardized procedure, with some sites undergoing continuous sampling over an extended period. Additionally, refuse-derived fuel incineration facilities are being considered to ensure all relevant IBA production scenarios are accounted for.
Liquid-phase magnetic separation was chosen for its ability to capture even the ultrafine magnetic particles present in IBA [222]. Wet shaking tables (WSTs) were chosen for density separation from particle sizes > 0.2 mm due to their ability to produce high-quality concentrate products, effectively harnessing the resource potential of IBA [57,223]. Additionally, WSTs offer ease of operation, along with minimal maintenance and operational costs. Key operational parameters, such as desk design, tilt, shaking frequency and amplitude, washing water flow rate, and feed ratio, must be optimized through a trial-and-error approach to achieve the desired performance [224]. Additional density separation methods, including centrifugation adapted for the ultrafine fraction (<0.2 mm), are currently under investigation. Aqueous carbonation was chosen because it requires milder conditions than gas–solid carbonation and allows further intensification using additives, process integration, alternative energy sources, and innovative reactor designs [225]. Additionally, metal separation in the liquid phase and aqueous carbonation can work seamlessly as part of a sequential full-treatment process.
Figure 14 presents a schematic of the projected IBA fines treatment unit, which integrates EMR and ACT. It should be noted that the invention outlined here is currently under patent application under the reference FR2415447. Liquid-phase magnetic and density separation work synergistically with aqueous carbonation, creating a fully liquid-phase process with water recirculation to minimize consumption and simplify operations. Raw IBA fines serve as feedstock and are treated immediately after production through a sequence of processes, including sieving, ultrafine fraction separation, mixing, magnetic separation, density separation, and carbonation. Ferrous and non-ferrous metal concentrates are collected for recycling by smelters, while the demetallized IBA is transferred to the carbonation unit. This unit consists of two batch-operated carbonation reactors that run in parallel and alternately. While one reactor is being emptied or recharged, CO2 is injected into the other, ensuring continuous CO2 absorption. As CO2 is absorbed by the suspension, the pH decreases from an initial alkaline range of 11.5–12.5 to a final reference value between 7 and 8. Once the pH reaches this level, the reactor is emptied, and the cycle begins anew. The suspension is separated by filtration, and the carbonated product is collected for reuse as mineral additives in aggregate production and other civil engineering applications [226].
Figure 14.
Schematic representation of the projected IBA fines treatment unit, integrating liquid-phase EMR and ACT, comprising the following steps: mixing, hydrocyclone separation, sieving, magnetic separation, density separation, carbonation washing, filtration, aggregate manufacturing, and wastewater regeneration.
6. Conclusions
This review highlights the significant progress made in the accelerated carbonation technology (ACT) of waste incineration residues, from laboratory-scale research to field-scale applications. Waste incineration residues, including incineration bottom ash (IBA), fly ash (FA), and air pollution control residues (APCr), are highly reactive materials that present both challenges and opportunities in terms of management and resource recovery. The following points summarize key findings, technological challenges, and future directions:
Technology status:
- ACT has reached full-scale application for FA and APCr.
- No commercial-scale ACT process currently exists for IBA, despite extensive laboratory research.
Reactor design insights:
- Gas-flow stirred reactors are optimal for achieving fast carbonation kinetics.
- Rotary drum reactors are better suited for producing lightweight aggregates via CO2 curing.
Stabilization and mobilization of metals:
- Carbonation effectively can stabilize divalent metals (Pb, Zn, Cd, Cu, Sr).
- Oxyanionic species (Sb, Cr, V, Mo, S) can become mobilized during treatment.
- We highlight what we refer to as the antimony problem, as this element can become mobilized by up to three orders of magnitude in leachate concentrations.
Research gaps:
- The most pressing research gap hindering industrial deployment is the need to design stabilization approaches specifically tailored to critical anionic species, particularly Sb(V), Cr(VI), and SO42−.
Status of enhanced metal recovery (EMR):
- EMR technologies are implemented at full scale for FA, APCr, and coarse IBA.
- Fine IBA fractions remain underexploited industrially, although promising technologies are emerging in the EU (e.g., AIK Technik AG leaching reactor, Boskalis density separation process).
Need for integrated treatment trains:
- Innovative combinations of ACT, EMR, and mineral additive production are needed, moving beyond conventional bulk separation and backfilling strategies.
- Such treatment trains could reduce landfilling and support the circular economy.
Key technical challenges to address:
- Managing feedstock variability to ensure consistent treatment outcomes, including the development of a database to map compositional heterogeneity and identify the most suitable sites for full-scale deployment.
- Developing proprietary metal recovery, carbonation, and aggregate manufacturing formulations.
- Optimizing reactor configurations and scalable process layouts.
- Enhancing leachate recycling strategies to minimize water use.
- Improving solidification techniques for construction-grade applications.
In conclusion, this review fulfills its objective of providing a high-quality reference for researchers and industry stakeholders aiming to optimize accelerated carbonation strategies for alkaline waste valorization. By bridging laboratory research and field-scale applications, it supports the development of sustainable solutions for incineration residue management within a circular economy framework, integrating key concepts such as urban mining and industrial symbiosis.
Author Contributions
Conceptualization, Q.W.; methodology, Q.W.; software, Q.W.; validation, D.B., F.M., E.D., C.C., N.C., M.M., A.P., and L.P.; formal analysis, Q.W. and D.B.; investigation, Q.W., D.B., M.M.; resources, E.D., A.P., and L.P.; data curation, not applicable; writing—original draft preparation, Q.W.; writing—review and editing, Q.W., D.B., F.M., E.D., C.C., N.C., M.M., A.P., and L.P.; visualization, Q.W., D.B., F.M., E.D., C.C., N.C., M.M., A.P., and L.P.; supervision, E.D., A.P., and L.P.; project administration, A.P. and L.P.; funding acquisition, A.P. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
Quentin Wehrung and Fabien Michel are associate directors of ALKALINE TECHNOLOGIES, a company operating in the field of alkaline waste management, including carbonation and metal separation process development. The authors declare that this review was conducted independently and objectively as part of their academic activities. The other authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACT | Accelerated Carbonation Technology |
| APCr | Air Pollution Control residues |
| Cal | Calcite |
| CCHO | Calcium (chloro)(hydr)oxides |
| CDW | Construction and Demolition Waste |
| CRW | Cold-Rolling Wastewater |
| EMR | Enhanced Metal Recovery |
| Ett | Ettringite |
| FA | Fly Ash |
| FGDGr | Flue Gas Desulphurization Gypsum residue |
| GFR | Gas flow rate |
| GFS | Gas Flow Sensors |
| Hrr | Hatrurite |
| Hcl | Hydrocalumite |
| HM(s) | Heavy Metal(s) |
| IAW | Industrial Alkaline Waste |
| IBA | Incineration Bottom Ash |
| Kto | Katoite |
| kgw | Kilogram of water |
| L/S | Liquid-to-solid ratio |
| LCA | Lifecycle assessment |
| Lrn | Larnite |
| Mt | Million tons |
| MFR | Mass flow rate |
| MS | Mixing Speed |
| MSW | Municipal Solid Waste |
| Per | Periclase |
| ppm | parts per million |
| PTEs | Potentially Toxic Elements |
| RDF | Refuse-Derived Fuels |
| RH | Relative Humidity |
| Rnk | Rankinite |
| RSM | Response Surface Methodology |
| SCM | Shrinking Core Model |
| SSA | Specific Surface Area |
| TCLP | Toxicity Characteristic Leaching Procedure |
| TGA | Thermal Gravimetric Analysis |
| TIC | Total Inorganic Carbon |
| TOC | Total Organic Carbon |
| Vtr | Vaterite |
| Wo | Wollastonite |
| WST | Wet Shaking Tables |
| WtE | Waste-to-Energy |
| XRPD | X-Ray Powder Diffraction |
References
- CEWEP, Waste-to-Energy Plants in Europe in 2021. Available online: https://www.cewep.eu/waste-to-energy-plants-in-europe-in-2021/ (accessed on 20 April 2025).
- Eurostats, Municipal Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics (accessed on 20 April 2025).
- Assamoi, B.; Lawryshyn, Y. The environmental comparison of landfilling vs. incineration of MSW accounting for waste diversion. Waste Manag. 2012, 32, 1019–1030. [Google Scholar] [CrossRef]
- Blasenbauer, D.; Huber, F.; Lederer, J.; Quina, M.J.; Blanc-Biscarat, D.; Bogush, A.; Fellner, J. Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Manag. 2020, 102, 868–883. [Google Scholar] [CrossRef] [PubMed]
- Holm, O.; Simon, F.G. Innovative treatment trains of bottom ash (BA) from municipal solid waste incineration (MSWI) in Germany. Waste Manag. 2017, 59, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Šyc, M.; Simon, F.G.; Hykš, J.; Braga, R.; Biganzoli, L.; Costa, G.; Grosso, M. Metal recovery from incineration bottom ash: State-of-the-art and recent developments. J. Hazard. Mater. 2020, 393, 122433. [Google Scholar] [CrossRef]
- Fang, P.F.; Zhu, X.R.; Chen, H.S.; Chen, W. Engineering characteristics of bottom ash in municipal solid waste incinerators. In Geoenvironmental Engineering and Geotechnics: Progress in Modeling and Applications; ASCE: Reston, VA, USA, 2010; pp. 95–102. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, K.P.; Hyks, J.; Mulvad, J.K.; Frederiksen, J.O.; Hjelmar, O. Optimizing large-scale ageing of municipal solid waste incinerator bottom ash prior to the advanced metal recovery: Phase I: Monitoring of temperature, moisture content, and CO2 level. Waste Manag. 2019, 85, 95–105. [Google Scholar] [CrossRef]
- Spreadbury, C.J.; McVay, M.; Laux, S.J.; Townsend, T.G. A field-scale evaluation of municipal solid waste incineration bottom ash as a road base material: Considerations for reuse practices. Resour. Conserv. Recycl. 2021, 168, 105264. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Li, W.; Nie, Y.; Wang, F.; Bian, R.; Lu, C. Solidification/stabilization pre-treatment coupled with landfill disposal of heavy metals in MSWI fly ash in China: A systematic review. J. Hazard. Mater. 2024, 478, 135479. [Google Scholar] [CrossRef]
- Antemir, A.; Hills, C.D.; Carey, P.J.; Gardner, K.H.; Bates, E.R.; Crumbie, A.K. Long-term performance of aged waste forms treated by stabilization/solidification. J. Hazard. Mater. 2010, 181, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.C.; Ma, H.W. Life cycle risk assessment of bottom ash reuse. J. Hazard. Mater. 2011, 190, 308–316. [Google Scholar] [CrossRef]
- Verbinnen, B.; Van Caneghem, J.; Billen, P.; Vandecasteele, C. Long term leaching behavior of antimony from MSWI bottom ash: Influence of mineral additives and of organic acids. Waste Biomass Valorization 2017, 8, 2545–2552. [Google Scholar] [CrossRef]
- Wang, P.; Hu, Y.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Bisinella, V.; Astrup, T.F. Life cycle assessment of air-pollution-control residues from waste incineration in Europe: Importance of composition, technology and long-term leaching. Waste Manag. 2022, 144, 336–348. [Google Scholar] [CrossRef]
- Allegrini, E.; Maresca, A.; Olsson, M.E.; Holtze, M.S.; Boldrin, A.; Astrup, T.F. Quantification of the resource recovery potential of municipal solid waste incineration bottom ashes. Waste Manag. 2014, 34, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Allegrini, E.; Vadenbo, C.; Boldrin, A.; Astrup, T.F. Life cycle assessment of resource recovery from municipal solid waste incineration bottom ash. J. Environ. Manag. 2015, 151, 132–143. [Google Scholar] [CrossRef]
- del Valle-Zermeño, R.; Gómez-Manrique, J.; Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Material characterization of the MSWI bottom ash as a function of particle size. Effects of glass recycling over time. Sci. Total Environ. 2017, 581, 897–905. [Google Scholar] [CrossRef]
- Caviglia, C.; Confalonieri, G.; Corazzari, I.; Destefanis, E.; Mandrone, G.; Pastero, L.; Pavese, A. Effects of particle size on properties and thermal inertization of bottom ashes (MSW of Turin’s incinerator). Waste Manag. 2019, 84, 340–354. [Google Scholar] [CrossRef]
- Alam, Q.; Schollbach, K.; Rijnders, M.; van Hoek, C.; van der Laan, S.; Brouwers, H.J.H. The immobilization of potentially toxic elements due to incineration and weathering of bottom ash fines. J. Hazard. Mater. 2019, 379, 120798. [Google Scholar] [CrossRef]
- Loginova, E.; Volkov, D.S.; Van De Wouw, P.M.F.; Florea, M.V.A.; Brouwers, H.J.H. Detailed characterization of particle size fractions of municipal solid waste incineration bottom ash. J. Clean. Prod. 2019, 207, 866–874. [Google Scholar] [CrossRef]
- Huber, F.; Blasenbauer, D.; Aschenbrenner, P.; Fellner, J. Chemical composition and leachability of differently sized material fractions of municipal solid waste incineration bottom ash. Waste Manag. 2019, 95, 593–603. [Google Scholar] [CrossRef]
- Mantovani, L.; De Matteis, C.; Tribaudino, M.; Boschetti, T.; Funari, V.; Dinelli, E.; Pelagatti, P. Grain size and mineralogical constraints on leaching in the bottom ashes from municipal solid waste incineration: A comparison of five plants in northern Italy. Front. Environ. Sci. 2023, 11, 1179272. [Google Scholar] [CrossRef]
- Mantovani, L.; Tribaudino, M.; De Matteis, C.; Funari, V. Particle size and potential toxic element speciation in municipal solid waste incineration (MSWI) bottom ash. Sustainability 2021, 13, 1911. [Google Scholar] [CrossRef]
- Beikmohammadi, M.; Yaghmaeian, K.; Nabizadeh, R.; Mahvi, A.H. Analysis of heavy metal, rare, precious, and metallic element content in bottom ash from municipal solid waste incineration in Tehran based on particle size. Sci. Rep. 2023, 13, 16044. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Lategano, E.; Marini, C.; Polettini, A.; Pomi, R.; Rocca, S. Accelerated carbonation of different size fractions of bottom ash from RDF incineration. Waste Manag. 2010, 30, 1310–1317. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R. Carbon Capture and Storage: From Global Cycles to Global Solutions; Geochemical Perspectives: Houten, The Netherlands, 2023; pp. 179–180. [Google Scholar] [CrossRef]
- Delerce, S.; Marieni, C.; Oelkers, E.H. Carbonate geochemistry and its role in geologic carbon storage. Surf. Process. Transp. Storage 2022, 4, 423–477. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R.; Matter, J. Mineral carbonation of CO2. Elements 2008, 4, 333–337. [Google Scholar] [CrossRef]
- Alam, Q.; Schollbach, K.; van Hoek, C.; van der Laan, S.; de Wolf, T.; Brouwers, H.J.H. In-depth mineralogical quantification of MSWI bottom ash phases and their association with potentially toxic elements. Waste Manag. 2019, 87, 1–12. [Google Scholar] [CrossRef]
- Di Maria, A.; Snellings, R.; Alaerts, L.; Quaghebeur, M.; Van Acker, K. Environmental assessment of CO2 mineralisation for sustainable construction materials. Int. J. Greenh. Gas Control 2020, 93, 102882. [Google Scholar] [CrossRef]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef]
- Huang, T.Y.; Chiueh, P.T.; Lo, S.L. Life-cycle environmental and cost impacts of reusing fly ash. Resour. Conserv. Recycl. 2017, 123, 255–260. [Google Scholar] [CrossRef]
- Huber, F.; Laner, D.; Fellner, J. Comparative life cycle assessment of MSWI fly ash treatment and disposal. Waste Manag. 2018, 73, 392–403. [Google Scholar] [CrossRef]
- Wolffers, M.; Eggenberger, U.; Schlumberger, S.; Churakov, S.V. Characterization of MSWI fly ashes along the flue gas cooling path and implications on heavy metal recovery through acid leaching. Waste Manag. 2021, 134, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Verbinnen, B.; Billen, P.; Van Caneghem, J.; Vandecasteele, C. Recycling of MSWI bottom ash: A review of chemical barriers, engineering applications and treatment technologies. Waste Biomass Valorization 2017, 8, 1453–1466. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, Z.; Luo, Z.; Yu, L.; Yin, K. Phase changes during various treatment processes for incineration bottom ash from municipal solid wastes: A review in the application-environment nexus. Environ. Pollut. 2021, 117618, 287. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R. The leaching behavior of incinerator bottom ash as affected by accelerated ageing. J. Hazard. Mater. 2004, 113, 209–215. [Google Scholar] [CrossRef]
- Arickx, S.; Van Gerven, T.; Vandecasteele, C. Accelerated carbonation for treatment of MSWI bottom ash. J. Hazard. Mater. 2006, 137, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Polettini, A.; Pomi, R.; Prigiobbe, V.; Von Zedwitz, V.N.; Steinfeld, A. CO2 sequestration by direct gas–solid carbonation of air pollution control (APC) residues. Energy Fuels 2006, 20, 1933–1940. [Google Scholar] [CrossRef]
- Bruno, M.; Abis, M.; Kuchta, K.; Simon, F.G.; Grönholm, R.; Hoppe, M.; Fiore, S. Material flow, economic and environmental assessment of municipal solid waste incineration bottom ash recycling potential in Europe. J. Clean. Prod. 2021, 317, 128511. [Google Scholar] [CrossRef]
- Kashefi, K.; Pardakhti, A.; Shafiepour, M.; Hemmati, A. Process optimization for integrated mineralization of carbon dioxide and metal recovery of red mud. J. Environ. Chem. Eng. 2020, 8, 103638. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Xiao, Y. Accelerated CO2 mineralization and utilization for selective battery metals recovery from olivine and laterites. J. Clean. Prod. 2023, 393, 136345. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.B. Acceleration of iron-rich olivine CO2 mineral carbonation and utilization for simultaneous critical nickel and cobalt recovery. Minerals 2024, 14, 766. [Google Scholar] [CrossRef]
- Librandi, P.; Costa, G.; de Souza, A.C.B.; Stendardo, S.; Luna, A.S.; Baciocchi, R. Carbonation of steel slag: Testing of the wet route in a pilot-scale reactor. Energy Procedia 2017, 114, 5381–5392. [Google Scholar] [CrossRef]
- Bertos, M.F.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Costa, G.; Baciocchi, R.; Polettini, A.; Pomi, R.; Hills, C.D.; Carey, P.J. Current status and perspectives of accelerated carbonation processes on municipal waste combustion residues. Environ. Monit. Assess. 2007, 135, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chang, E.E.; Chiang, P.C. CO2 capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Bodor, M.; Santos, R.M.; Van Gerven, T.; Vlad, M. Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation—A review. Cent. Eur. J. Eng. 2013, 3, 566–584. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G. CO2 utilization and long-term storage in useful mineral products by carbonation of alkaline feedstocks. Front. Energy Res. 2021, 9, 592600. [Google Scholar] [CrossRef]
- Stokreef, S.; Sadri, F.; Stokreef, A.; Ghahreman, A. Mineral carbonation of ultramafic tailings: A review of reaction mechanisms and kinetics, industry case studies, and modelling. Cleaner Eng. Technol. 2022, 8, 100491. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Petrol. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- Bandarra, B.S.; Silva, S.; Pereira, J.L.; Martins, R.C.; Quina, M.J. A study on the classification of a mirror entry in the European List of Waste: Incineration bottom ash from municipal solid waste. Sustainability 2022, 14, 10352. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Šyc, M.; Výravský, J.; Sierra, H.M.; Korotenko, E.; Kameníková, P. Resource recovery potential of incineration bottom ash fine fraction. Waste Manag. 2024, 190, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Wehrung, Q.; Bernasconi, D.; Destefanis, E.; Caviglia, C.; Curetti, N.; Di Felice, S.; Bicchi, E.; Pavese, A.; Pastero, L. Aqueous carbonation of waste incineration residues: Comparing BA, FA, and APCr across production scenarios. Minerals 2024, 14, 1269. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Fernández, A.I.; Nadal, R.; Espiell, F. Short-term natural weathering of MSWI bottom ash. J. Hazard. Mater. 2000, 79, 287–299. [Google Scholar] [CrossRef]
- Santos, R.M.; Mertens, G.; Salman, M.; Cizer, Ö.; Van Gerven, T. Comparative study of ageing, heat treatment and accelerated carbonation for stabilization of municipal solid waste incineration bottom ash in view of reducing regulated heavy metal/metalloid leaching. J. Environ. Manag. 2013, 128, 807–821. [Google Scholar] [CrossRef]
- Margallo, M.; Taddei, M.B.; Hernández-Pellón, R.; Aldaco, R.; Irabien, Á. Environmental sustainability assessment of the management of municipal solid waste incineration residues: A review of the current situation. Clean Technol. Environ. Policy 2015, 17, 1333–1353. [Google Scholar] [CrossRef]
- Wei, Y.; Shimaoka, T.; Saffarzadeh, A.; Takahashi, F. Mineralogical characterization of municipal solid waste incineration bottom ash with an emphasis on heavy metal-bearing phases. J. Hazard. Mater. 2011, 187, 534–543. [Google Scholar] [CrossRef]
- Destefanis, E.; Caviglia, C.; Bernasconi, D.; Bicchi, E.; Boero, R.; Bonadiman, C.; Confalonieri, G.; Corazzari, I.; Mandrone, G.; Pastero, L.; et al. Valorization of MSWI bottom ash as a function of particle size distribution, using steam washing. Sustainability 2020, 12, 9461. [Google Scholar] [CrossRef]
- Forteza, R.; Far, M.; Seguı, C.; Cerdá, V. Characterization of bottom ash in municipal solid waste incinerators for its use in road base. Waste Manag. 2004, 24, 899–909. [Google Scholar] [CrossRef]
- Van De Wouw, P.M.F.; Loginova, E.; Florea, M.V.A.; Brouwers, H.J.H. Compositional modelling and crushing behaviour of MSWI bottom ash material classes. Waste Manag. 2020, 101, 268–282. [Google Scholar] [CrossRef]
- Nikravan, M.; Ramezanianpour, A.A.; Maknoon, R. Study on physiochemical properties and leaching behavior of residual ash fractions from a municipal solid waste incinerator (MSWI) plant. J. Environ. Manag. 2020, 260, 110042. [Google Scholar] [CrossRef]
- Alam, Q.; Florea, M.V.A.; Schollbach, K.; Brouwers, H.J.H. A two-stage treatment for Municipal Solid Waste Incineration (MSWI) bottom ash to remove agglomerated fine particles and leachable contaminants. Waste Manag. 2017, 67, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Gevers, B.R.; Labuschagné, F.J. Green synthesis of hydrocalumite (CaAl-OH-LDH) from Ca(OH)2 and Al(OH)3 and the parameters that influence its formation and speciation. Crystals 2020, 10, 672. [Google Scholar] [CrossRef]
- Jiménez, A.; Rives, V.; Vicente, M.A. Thermal study of the hydrocalumite–katoite–calcite system. Thermochim. Acta 2022, 713, 179242. [Google Scholar] [CrossRef]
- Nag, M.; Labira, D.; Shimaoka, T.; Nakayama, H.; Komiya, T. Utilization of size-fractionated pozzolanic bottom ash to immobilize heavy metals in MSW incinerator fly ash. J. Environ. Chem. Eng. 2023, 11, 110394. [Google Scholar] [CrossRef]
- Rocca, S.; van Zomeren, A.; Costa, G.; Dijkstra, J.J.; Comans, R.N.; Lombardi, F. Characterisation of major component leaching and buffering capacity of RDF incineration and gasification bottom ash in relation to reuse or disposal scenarios. Waste Manag. 2012, 32, 759–768. [Google Scholar] [CrossRef]
- Phua, Z.; Giannis, A.; Dong, G.; Lisak, D.; Ng, W.J. Characteristics of incineration ash for sustainable treatment and reutilization. Environ. Sci. Pollut. Res. 2019, 26, 16974–16997. [Google Scholar] [CrossRef]
- Zacco, A.; Borgese, L.; Gianoncelli, A.; Struis, R.P.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Chandler, A.J.; Eighmy, T.T.; Hjelmar, O.; Kosson, D.S.; Sawell, S.E.; Vehlow, J.; Hartlén, J. Municipal Solid Waste Incinerator Residues; Elsevier: Amsterdam, The Netherlands, 1997; Available online: https://www.elsevier.com/books/municipal-solid-waste-incinerator-residues/chandler/978-0-444-82563-6 (accessed on 20 April 2025).
- De Boom, A.; Degrez, M. Belgian MSWI fly ashes and APC residues: A characterisation study. Waste Manag. 2012, 32, 1163–1170. [Google Scholar] [CrossRef]
- Caviglia, C.; Destefanis, E.; Pastero, L.; Bernasconi, D.; Bonadiman, C.; Pavese, A. MSWI fly ash multiple washing: Kinetics of dissolution in water, as function of time, temperature and dilution. Minerals 2022, 12, 742. [Google Scholar] [CrossRef]
- Bernasconi, D.; Caviglia, C.; Destefanis, E.; Agostino, A.; Boero, R.; Marinoni, N.; Pavese, A. Influence of speciation distribution and particle size on heavy metal leaching from MSWI fly ash. Waste Manag. 2022, 138, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2020, 245, 118779. [Google Scholar] [CrossRef]
- Fabricius, A.L.; Renner, M.; Voss, M.; Funk, M.; Perfoll, A.; Gehring, F.; Duester, L. Municipal waste incineration fly ashes: From a multi-element approach to market potential evaluation. Environ. Sci. Eur. 2020, 32, 88. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Di Bartolomeo, E.; Polettini, A.; Pomi, R. The effects of accelerated carbonation on CO2 uptake and metal release from incineration APC residues. Waste Manag. 2009, 29, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.P.; Zanoletti, A.; Ducoli, S.; Zacco, A.; Iora, P.; Invernizzi, C.M.; Bontempi, E. Accelerated and natural carbonation of a municipal solid waste incineration (MSWI) fly ash mixture: Basic strategies for higher carbon dioxide sequestration and reliable mass quantification. Environ. Res. 2023, 217, 114805. [Google Scholar] [CrossRef]
- Goni, S.; Guerrero, A. Accelerated carbonation of Friedel’s salt in calcium aluminate cement paste. Cem. Concr. Res. 2003, 33, 21–26. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, Y.; Jin, Y.; Wang, X.; Li, J.; Yang, S.; Wang, W. Highly efficient carbonation and dechlorination using flue gas micro-nano bubble for municipal solid waste incineration fly ash pretreatment and its applicability to sulfoaluminate cementitious materials. J. Environ. Manag. 2024, 353, 120163. [Google Scholar] [CrossRef]
- Renforth, P. The negative emission potential of alkaline materials. Nat. Commun. 2019, 10, 1401. [Google Scholar] [CrossRef]
- Van Balen, K. Carbonation reaction of lime, kinetics at ambient temperature. Cem. Concr. Res. 2005, 35, 647–657. [Google Scholar] [CrossRef]
- Golubev, S.V.; Pokrovsky, O.S.; Schott, J. Experimental determination of the effect of dissolved CO2 on the dissolution kinetics of Mg and Ca silicates at 25 °C. Chem. Geol. 2005, 217, 227–238. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Sutter, L.L.; Kawatra, S.K.; Eisele, T.C.; Gierke, J.S. Mineral carbonation for carbon sequestration in cement kiln dust from waste piles. J. Hazard. Mater. 2009, 168, 31–37. [Google Scholar] [CrossRef]
- Ćwik, A.; Casanova, I.; Rausis, K.; Koukouzas, N.; Zarębska, K. Carbonation of high-calcium fly ashes and its potential for carbon dioxide removal in coal fired power plants. J. Clean. Prod. 2018, 202, 1026–1034. [Google Scholar] [CrossRef]
- Xian, X.; Mahoutian, M.; Zhang, S.; Shao, Y.; Zhang, D.; Liu, J. Converting industrial waste into a value-added cement material through ambient pressure carbonation. J. Environ. Manag. 2023, 325, 116603. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chang, E.E.; Kim, H.; Chen, Y.H.; Chiang, P.C. Validating carbonation parameters of alkaline solid wastes via integrated thermal analyses: Principles and applications. J. Hazard. Mater. 2016, 307, 253–262. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Zhang, R.; French, D.; Grigore, M.; Yu, B.; Zhao, S. Effects of fly ash properties on carbonation efficiency in CO2 mineralisation. Fuel Process. Technol. 2019, 188, 79–88. [Google Scholar] [CrossRef]
- Silva, J.M.; Bomio, M.R.; Motta, F.V.; Santos, R.M. Assessment of calcimetry as a reliable method for monitoring soil inorganic carbon stocks. ACS Agric. Sci. Technol. 2024, 4, 781–790. [Google Scholar] [CrossRef]
- Tominc, S.; Ducman, V. Methodology for evaluating the CO2 sequestration capacity of waste ashes. Materials 2023, 16, 5284. [Google Scholar] [CrossRef]
- Bertos, M.F.; Li, X.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. Investigation of accelerated carbonation for the stabilisation of MSW incinerator ashes and the sequestration of CO2. Green Chem. 2004, 6, 428–436. [Google Scholar] [CrossRef]
- Gunning, P.J. Accelerated Carbonation of Hazardous Wastes. Ph.D. Thesis, University of Greenwich, London, UK, 2011. [Google Scholar]
- Montes-Hernández, G.; Renard, F.; Geoffroy, N.; Charlet, L.; Pironon, J. Calcite precipitation from CO2–H2O–Ca(OH)2 slurry under high pressure of CO2. J. Cryst. Growth 2007, 308, 228–236. [Google Scholar] [CrossRef]
- Falzone, G.; Mehdipour, I.; Neithalath, N.; Bauchy, M.; Simonetti, D.; Sant, G. New insights into the mechanisms of carbon dioxide mineralization by portlandite. AIChE J. 2021, 67, e17160. [Google Scholar] [CrossRef]
- Wehrung, Q.; Pastero, L.; Bernasconi, D.; Cotellucci, A.; Bruno, M.; Cavagna, S.; Pavese, A. Impact of operational parameters on the CO2 absorption rate in Ca(OH)2 aqueous carbonation—Implications for process efficiency. Energy Fuels 2024, 38, 16678–16691. [Google Scholar] [CrossRef]
- Reynolds, B.; Reddy, K.J.; Argyle, M.D. Field application of accelerated mineral carbonation. Minerals 2014, 4, 191–207. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J. Sequestration of flue gas CO2 by direct gas–solid carbonation of air pollution control system residues. Environ. Sci. Technol. 2012, 46, 13545–13551. [Google Scholar] [CrossRef]
- Sorrentino, G.P.; Guimarães, R.; Valentim, B.; Bontempi, E. The influence of liquid/solid ratio and pressure on the natural and accelerated carbonation of alkaline wastes. Minerals 2023, 13, 1080. [Google Scholar] [CrossRef]
- Montes-Hernández, G.; Pérez-López, R.; Renard, F.; Nieto, J.M.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef]
- Ahmad, S.; Assaggaf, R.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Adekunle, S.K.; Ali, S.I. Effects of carbonation pressure and duration on strength evolution of concrete subjected to accelerated carbonation curing. Constr. Build. Mater. 2017, 136, 565–573. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Y.; Nie, Y. Investigation of accelerated and natural carbonation of MSWI fly ash with a high content of Ca. J. Hazard. Mater. 2010, 174, 334–343. [Google Scholar] [CrossRef]
- Tang, P.; Xuan, D.; Poon, C.S.; Tsang, D.C. Valorization of concrete slurry waste (CSW) and fine incineration bottom ash (IBA) into cold bonded lightweight aggregates (CBLAs): Feasibility and influence of binder types. J. Hazard. Mater. 2019, 368, 689–697. [Google Scholar] [CrossRef]
- Ferraro, A.; Ducman, V.; Colangelo, F.; Korat, L.; Spasiano, D.; Farina, I. Production and characterization of lightweight aggregates from municipal solid waste incineration fly-ash through single- and double-step pelletization process. J. Clean. Prod. 2023, 383, 135275. [Google Scholar] [CrossRef]
- Jiang, J.G.; Du, X.J.; Chen, M.Z.; Zhang, C. Continuous CO2 capture and MSWI fly ash stabilization, utilizing novel dynamic equipment. Environ. Pollut. 2009, 157, 2933–2938. [Google Scholar] [CrossRef]
- Tiefenthaler, J.; Mazzotti, M. Experimental investigation of a continuous reactor for CO2 capture and CaCO3 precipitation. Front. Chem. Eng. 2022, 4, 830284. [Google Scholar] [CrossRef]
- Um, N. Effect of Cl removal in MSWI bottom ash via carbonation with CO2 and decomposition kinetics of Friedel’s salt. Mater. Trans. 2019, 60, 837–844. [Google Scholar] [CrossRef]
- Ni, P.; Xiong, Z.; Tian, C.; Li, H.; Zhao, Y.; Zhang, J.; Zheng, C. Influence of carbonation under oxy-fuel combustion flue gas on the leachability of heavy metals in MSWI fly ash. Waste Manag. 2017, 67, 171–180. [Google Scholar] [CrossRef]
- Schnabel, K.; Brück, F.; Mansfeldt, T.; Weigand, H. Full-scale accelerated carbonation of waste incinerator bottom ash under continuous-feed conditions. Waste Manag. 2021, 125, 40–48. [Google Scholar] [CrossRef]
- Back, M.; Bauer, M.; Stanjek, H.; Peiffer, S. Sequestration of CO2 after reaction with alkaline earth metal oxides CaO and MgO. Appl. Geochem. 2011, 26, 1097–1107. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, K.; Chen, L.; Yuan, Q. Review on accelerated carbonation of calcium-bearing minerals: Carbonation behaviors, reaction kinetics, and carbonation efficiency improvement. J. Build. Eng. 2024, 86, 108826. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawai, T.; Kamijima, Y.; Shinohara, S.; Tanaka, M. Application of ultrafine bubbles for enhanced carbonation of municipal solid waste incineration ash during direct aqueous carbonation. Next Sustain. 2024, 3, 100020. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, Z.; Gu, Z.; Liu, F.; Shen, P.; Poon, C.S. A novel approach for improving aqueous carbonation kinetics with CO2 micro- and nano-bubbles. Chem. Eng. J. 2024, 500, 157363. [Google Scholar] [CrossRef]
- Said, A.; Laukkanen, T.; Järvinen, M. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl. Energy 2016, 177, 602–611. [Google Scholar] [CrossRef]
- Lombardi, L.; Carnevale, E.A.; Pecorini, I. Experimental evaluation of two different types of reactors for CO2 removal from gaseous stream by bottom ash accelerated carbonation. Waste Manag. 2016, 58, 287–298. [Google Scholar] [CrossRef]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Production of lightweight aggregate from industrial waste and carbon dioxide. Waste Manag. 2009, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, Y.; Chen, Z.; Fu, C.; Li, M.; Mao, T.; Yan, J. Accelerated carbonation of ball-milling modified MSWI fly ash: Migration and stabilization of heavy metals. J. Environ. Chem. Eng. 2023, 11, 109396. [Google Scholar] [CrossRef]
- Sakita, S.; Nishimura, K. Physical containment of municipal solid waste incineration bottom ash by accelerated carbonation. J. Mater. Cycles Waste Manag. 2016, 18, 687–694. [Google Scholar] [CrossRef]
- Porter, R.T.; Fairweather, M.; Pourkashanian, M.; Woolley, R.M. The range and level of impurities in CO2 streams from different carbon capture sources. Int. J. Greenh. Gas Control 2015, 36, 161–174. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Hou, L.; Deng, D. Effect of carbonation on physical sulfate attack on concrete by Na2SO4. Constr. Build. Mater. 2018, 193, 211–220. [Google Scholar] [CrossRef]
- Van Gerven, T.; Van Keer, E.; Arickx, S.; Jaspers, M.; Wauters, G.; Vandecasteele, C. Carbonation of MSWI-bottom ash to decrease heavy metal leaching, in view of recycling. Waste Manag. 2005, 25, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Zhang, Y.; Cetin, B.; Likos, W.J.; Edil, T.B. Impacts of pH on leaching potential of elements from MSW incineration fly ash. Fuel 2016, 184, 815–825. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: Literature review and experimental study. J. Hazard. Mater. 2006, 136, 20–33. [Google Scholar] [CrossRef]
- Lange, L.C.; Hills, C.D.; Poole, A.B. The effect of accelerated carbonation on the properties of cement-solidified waste forms. Waste Manag. 1996, 16, 757–763. [Google Scholar] [CrossRef]
- Buchholz, B.A.; Landsberger, S. Leaching dynamics studies of municipal solid waste incinerator ash. J. Air Waste Manag. Assoc. 1995, 45, 579–590. [Google Scholar] [CrossRef]
- Di Gianfilippo, M.; Costa, G.; Pantini, S.; Allegrini, E.; Lombardi, F.; Astrup, T.F. LCA of management strategies for RDF incineration and gasification bottom ash based on experimental leaching data. Waste Manag. 2016, 47, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Steenari, B.M. Leaching optimization of municipal solid waste incineration ash for resource recovery: A case study of Cu, Zn, Pb and Cd. Waste Manag. 2016, 48, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; He, Y.; Cheng, D.; Yang, E.H. Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef]
- Alam, Q.; Lazaro, A.; Schollbach, K.; Brouwers, H.J.H. Chemical speciation, distribution and leaching behavior of chlorides from municipal solid waste incineration bottom ash. Chemosphere 2020, 241, 124985. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Chen, J.; Li, A.; Yao, H.; Low, F.; Zhang, L. Speciation transformation of arsenic during municipal solid waste incineration. Proc. Combust. Inst. 2015, 35, 2883–2890. [Google Scholar] [CrossRef]
- Montes-Hernández, G.; Concha-Lozano, N.; Renard, F.; Quirico, E. Removal of oxyanions from synthetic wastewater via carbonation process of calcium hydroxide: Applied and fundamental aspects. J. Hazard. Mater. 2009, 166, 788–795. [Google Scholar] [CrossRef]
- Thriveni, T.; Ahn, J.W.; Ramakrisna, C. Arsenic oxyanions removal from wastewater by accelerated carbonation. J. MMIJ 2017, 133, 1–3. [Google Scholar] [CrossRef]
- Khan, M.D.; Song, Y.; Shakya, S.; Lim, C.; Ahn, J.W. In situ carbonation mediated immobilization of arsenic oxyanions. J. Mol. Liq. 2023, 383, 121911. [Google Scholar] [CrossRef]
- García, I.; Diez, M.; Martín, F.; Simón, M.; Dorronsoro, C. Mobility of arsenic and heavy metals in a sandy-loam textured and carbonated soil. Pedosphere 2009, 19, 166–175. [Google Scholar] [CrossRef]
- Astrup, T.; Dijkstra, J.J.; Comans, R.N.; Van der Sloot, H.A.; Christensen, T.H. Geochemical modeling of leaching from MSWI air-pollution-control residues. Environ. Sci. Technol. 2006, 40, 3551–3557. [Google Scholar] [CrossRef] [PubMed]
- Bouzar, B.; Mamindy-Pajany, Y.; Mkahal, Z.; Benzerzour, M.; Abriak, N.E. Pilot-scale natural carbonation of waste paper fly ash for stabilization of Ba and Pb. Green Technol. Sustain. 2024, 2, 100075. [Google Scholar] [CrossRef]
- Yao, J.; Kong, Q.; Zhu, H.; Long, Y.; Shen, D. Content and fractionation of Cu, Zn, and Cd in size fractionated municipal solid waste incineration bottom ash. Ecotoxicol. Environ. Saf. 2013, 94, 131–137. [Google Scholar] [CrossRef]
- Meima, J.A.; van der Weijden, R.D.; Eighmy, T.T.; Comans, R.N. Carbonation processes in municipal solid waste incinerator bottom ash and their effect on the leaching of copper and molybdenum. Appl. Geochem. 2002, 17, 1503–1513. [Google Scholar] [CrossRef]
- Arickx, S.; De Borger, V.; Van Gerven, T.; Vandecasteele, C. Effect of carbonation on the leaching of organic carbon and of copper from MSWI bottom ash. Waste Manag. 2010, 30, 1296–1302. [Google Scholar] [CrossRef]
- Li, X.; Bertos, M.F.; Hills, C.D.; Carey, P.J.; Simon, S. Accelerated carbonation of municipal solid waste incineration fly ashes. Waste Manag. 2007, 27, 1200–1206. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Xie, J.; Li, H.; Chen, T.; Chen, P.; Chen, D. An insight into the carbonation of calcined clayey dolomite and its performance to remove Cd (II). Appl. Clay Sci. 2017, 150, 63–70. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Thriveni, T.; Mulatu, D.; Lee, M.H.; Jung, S.H.; Ahn, J.W. Removal of Cd(II) and Pb(II) from wastewater via carbonation of aqueous Ca(OH)2 derived from eggshell. Process Saf. Environ. Prot. 2020, 141, 278–287. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, S.S.; Fenter, P.; Myneni, S.C.; Nikitin, V.; Peters, C.A. Carbonate coprecipitation for Cd and Zn treatment and evaluation of heavy metal stability under acidic conditions. Environ. Sci. Technol. 2023, 57, 3104–3113. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Yi, Y.; Fang, M. Carbonation of municipal solid waste gasification fly ash: Effects of pre-washing and treatment period on carbon capture and heavy metal immobilization. Environ. Pollut. 2022, 308, 119662. [Google Scholar] [CrossRef]
- Todorovic, J.; Ecke, H. Demobilisation of critical contaminants in four typical waste-to-energy ashes by carbonation. Waste Manag. 2006, 26, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Ling, D.; Sarvaramini, A.; Guo, M.; Elsen, J.; Larachi, F.; Van Gerven, T. Stabilization of basic oxygen furnace slag by hot-stage carbonation treatment. Chem. Eng. J. 2012, 203, 239–250. [Google Scholar] [CrossRef]
- Um, N.; Nam, S.Y.; Ahn, J.W. Effect of accelerated carbonation on the leaching behavior of Cr in municipal solid waste incinerator bottom ash and the carbonation kinetics. Mater. Trans. 2013, 54, 1510–1516. [Google Scholar] [CrossRef]
- Lapp, F.; Brück, F.; Göske, J.; Dohrmann, R.; Mansfeldt, T.; Weigand, H. Fresh and aged chromite ore processing residues (COPR): Weathering-induced alteration of chemical properties, Cr (VI) mobility and mineralogy at open dumpsites in Kanpur, India. Water Air Soil Pollut. 2025, 236, 121. [Google Scholar] [CrossRef]
- Paul, B.; Chang, W. Mayenite-to-hydrocalumite transformation for the removal of chloride from salinized groundwater and the recycling potential of spent hydrocalumite for chromate removal. Desalination 2020, 474, 114186. [Google Scholar] [CrossRef]
- Hunter, H.A.; Ling, F.T.; Peters, C.A. Coprecipitation of heavy metals in calcium carbonate from coal fly ash leachate. ACS EST Water 2020, 1, 339–347. [Google Scholar] [CrossRef]
- Maftei, A.E.; Lupu, A.; Rodriguez-Blanco, J.D.; Rateau, R.; Brinza, L. Chromium removal via coprecipitation with carbonates and iron oxyhydroxides minerals: The effect of organic complexing agents. Sci. Total Environ. 2025, 965, 178686. [Google Scholar] [CrossRef]
- Hamdouni, A.G.; Montes-Hernandez, G.; Tlili, M.; Findling, N.; Renard, F.; Putnis, C.V. Removal of Fe(II) from groundwater via aqueous portlandite carbonation and calcite-solution interactions. Chem. Eng. J. 2016, 283, 404–411. [Google Scholar] [CrossRef]
- Nilsson, M.; Andreas, L.; Lagerkvist, A. Effect of accelerated carbonation and zero valent iron on metal leaching from bottom ash. Waste Manag. 2016, 51, 97–104. [Google Scholar] [CrossRef]
- Guha, B.; Hills, C.D.; Carey, P.J.; Macleod, C.L. Leaching of mercury from carbonated and non-carbonated cement-solidified dredged sediments. Soil Sediment Contam. 2006, 15, 621–635. [Google Scholar] [CrossRef]
- Wenyi, T.; Suping, G.; Wei, X.; Youxu, L.; Zixin, Z. Feature changes of mercury during the carbonation of FGD gypsum from different sources. Fuel 2018, 212, 19–26. [Google Scholar] [CrossRef]
- Verbinnen, B.; Billen, P.; Vandecasteele, C. Thermal treatment of solid waste in view of recycling: Chromate and molybdate formation and leaching behavior. Waste Manag. Res. 2014, 32, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.J.; Van Zomeren, A.; Meeussen, J.C.; Comans, R.N. Effect of accelerated aging of MSWI bottom ash on the leaching mechanisms of copper and molybdenum. Environ. Sci. Technol. 2006, 40, 4481–4487. [Google Scholar] [CrossRef]
- Jianguo, J.; Maozhe, C.; Yan, Z.; Xin, X. Pb stabilization in fresh fly ash from municipal solid waste incinerator using accelerated carbonation technology. J. Hazard. Mater. 2009, 161, 1046–1051. [Google Scholar] [CrossRef]
- Bogush, A.A.; Stegemann, J.A.; Roy, A. Changes in composition and lead speciation due to water washing of air pollution control residue from municipal waste incineration. J. Hazard. Mater. 2019, 361, 187–199. [Google Scholar] [CrossRef]
- Paoletti, F.; Sirini, P.; Seifert, H.; Vehlow, J. Fate of antimony in municipal solid waste incineration. Chemosphere 2001, 42, 533–543. [Google Scholar] [CrossRef]
- Cornelis, G.; Van Gerven, T.; Snellings, R.; Verbinnen, B.; Elsen, J.; Vandecasteele, C. Stability of pyrochlores in alkaline matrices: Solubility of calcium antimonate. Appl. Geochem. 2011, 26, 809–817. [Google Scholar] [CrossRef]
- Cornelis, G.; Van Gerven, T.; Vandecasteele, C. Antimony leaching from MSWI bottom ash: Modelling of the effect of pH and carbonation. Waste Manag. 2012, 32, 278–286. [Google Scholar] [CrossRef]
- Li, J.; Fu, P.; Zhang, S.; Li, J.; Liu, Y.; Wu, C.; Ni, W. Enhanced leaching control of chromium, antimony, and chlorine utilizing CO2-curing slag-fly ash-based agent. J. Environ. Chem. Eng. 2024, 12, 114606. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R.; Prigiobbe, V. Comparison of different reaction routes for carbonation of APC residues. Energy Procedia 2009, 1, 4851–4858. [Google Scholar] [CrossRef]
- Cappai, G.; Cara, S.; Muntoni, A.; Piredda, M. Application of accelerated carbonation on MSW combustion APC residues for metal immobilization and CO2 sequestration. J. Hazard. Mater. 2012, 207–208, 159–164. [Google Scholar] [CrossRef]
- Vogel, C.; Scholz, P.; Kalbe, U.; Caliebe, W.; Tayal, A.; Vasala, S.J.; Simon, F.G. Speciation of antimony and vanadium in municipal solid waste incineration ashes analyzed by XANES spectroscopy. J. Mater. Cycles Waste Manag. 2024, 26, 2152–2158. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, L.; Guo, G.; Liu, H.; Li, S.; Dong, L.; Hu, H. Selenium leaching behaviors from MSWI fly ash and its control. J. Ind. Eng. Chem. 2024, 134, 163–168. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, H.; Huang, Y.; Guo, G.; Gong, L.; Zou, C.; Yao, H. Effect of hydrated lime on the migration behavior of selenium in the MSWI plants: Experimental and theoretical study. Chem. Eng. J. 2022, 433, 134603. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Brown, K.G.; van der Sloot, H.A.; Meeussen, J.C.; Garrabrants, A.C.; Kosson, D.S. Impact of oxidation and carbonation on the release rates of iodine, selenium, technetium, and nitrogen from a cementitious waste form. J. Hazard. Mater. 2023, 449, 131004. [Google Scholar] [CrossRef]
- Alicja, K.; Grzegorz, C. Strontium leaching from municipal waste subjected to incineration. Environ. Geochem. Health 2024, 46, 220. [Google Scholar] [CrossRef]
- Erdemoğlu, M.; Canbazoğlu, M. The leaching of SrS with water and the precipitation of SrCO3 from leach solution by different carbonating agents. Hydrometallurgy 1998, 49, 135–150. [Google Scholar] [CrossRef]
- Boukobza, E.; Bar-Nes, G.; Ben-David, O.K.; Carmeli, B. Modeling of strontium leaching from carbonated Portland cement pastes using a simplified diffusion-kinetic analytical model. Appl. Geochem. 2019, 100, 258–267. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Zhang, Y.; Yue, Y.; Zhang, J.; Qian, G. Controlling role of CaClOH in the process of dechlorination for municipal solid incineration fly ash utilization. ACS EST Eng. 2022, 2, 2150–2158. [Google Scholar] [CrossRef]
- Jiang, J.; Tian, S.; Zhang, C. Influence of SO2 in incineration flue gas on the sequestration of CO2 by municipal solid waste incinerator fly ash. J. Environ. Sci. 2013, 25, 735–740. [Google Scholar] [CrossRef]
- Schnabel, K.; Brück, F.; Mansfeldt, T.; Weigand, H. Putting accelerated carbonation of bottom ash into practice: Operation of a continuous-feed pilot scale reactor. In Proceedings of the 19th International Conference on Environmental Science and Technology, Rhodes, Greece, 4–7 September 2019; p. 2. [Google Scholar]
- Łączny, J.M.; Iwaszenko, S.; Gogola, K.; Bajerski, A.; Janoszek, T.; Klupa, A.; Cempa-Balewicz, M. Study on the possibilities of treatment of combustion by-products from fluidized bed boilers into a product devoid of free calcium oxide. J. Sustain. Min. 2015, 14, 164–172. [Google Scholar] [CrossRef]
- Zingaretti, D.; Costa, G.; Baciocchi, R. Assessment of the energy requirements for CO2 storage by carbonation of industrial residues. Part 1: Definition of the process layout. Energy Procedia 2013, 37, 5850–5857. [Google Scholar] [CrossRef][Green Version]
- Brück, F.; Ufer, K.; Mansfeldt, T.; Weigand, H. Continuous-feed carbonation of waste incinerator bottom ash in a rotating drum reactor. Waste Manag. 2019, 99, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, K.; Brück, F.; Pohl, S.; Weigand, H. Development and test of a rotating drum reactor for the simultaneous hydration and carbonation of dry biomass bottom ash. Waste Biomass Valorization 2022, 13, 4319–4330. [Google Scholar] [CrossRef]
- Um, N.; Ahn, J.W. Effects of two different accelerated carbonation processes on MSWI bottom ash. Process Saf. Environ. Prot. 2017, 111, 560–568. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, W.T. Semi-dry carbonation process using fly ash from solid refused fuel power plant. Sustainability 2019, 11, 908. [Google Scholar] [CrossRef]
- Lin, W.Y.; Heng, K.S.; Sun, X.; Wang, J.Y. Accelerated carbonation of different size fractions of MSW IBA and the effect on leaching. Waste Manag. 2015, 41, 75–84. [Google Scholar] [CrossRef]
- Rendek, E.; Ducom, G.; Germain, P. Carbon dioxide sequestration in municipal solid waste incinerator (MSWI) bottom ash. J. Hazard. Mater. 2006, 128, 73–79. [Google Scholar] [CrossRef]
- Lin, W.Y.; Heng, K.S.; Sun, X.; Wang, J.Y. Influence of moisture content and temperature on degree of carbonation and the effect on Cu and Cr leaching from incineration bottom ash. Waste Manag. 2015, 43, 264–272. [Google Scholar] [CrossRef]
- Chang, E.E.; Pan, S.Y.; Yang, L.; Chen, Y.H.; Kim, H.; Chiang, P.C. Accelerated carbonation using municipal solid waste incinerator bottom ash and cold-rolling wastewater: Performance evaluation and reaction kinetics. Waste Manag. 2015, 43, 283–292. [Google Scholar] [CrossRef]
- Brück, F.; Fröhlich, C.; Mansfeldt, T.; Weigand, H. A fast and simple method to monitor carbonation of MSWI bottom ash under static and dynamic conditions. Waste Manag. 2018, 78, 588–594. [Google Scholar] [CrossRef]
- Brück, F.; Schnabel, K.; Mansfeldt, T.; Weigand, H. Accelerated carbonation of waste incinerator bottom ash in a rotating drum batch reactor. J. Environ. Chem. Eng. 2018, 6, 5259–5268. [Google Scholar] [CrossRef]
- Brück, F.; Mansfeldt, T.; Weigand, H. Flow-through carbonation of waste incinerator bottom ash in a rotating drum batch reactor: Role of specific CO2 supply, mixing tools and fill level. J. Environ. Chem. Eng. 2019, 7, 102975. [Google Scholar] [CrossRef]
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization technology for carbon capture, utilization, and storage. Front. Energy Res. 2020, 8, 142. [Google Scholar] [CrossRef]
- Gunning, P.; Hills, C.; Antemir, A.; Carey, P. Novel approaches to the valorisation of ashes using aggregation by carbonation. In Proceedings of the 2nd International Slag Valorisation Symposium, Leuven, Belgium, 18–20 April 2011. [Google Scholar]
- Lange, L.C.; Hills, C.D.; Poole, A.B. Effect of carbonation on properties of blended and non-blended cement solidified waste forms. J. Hazard. Mater. 1997, 52, 193–212. [Google Scholar] [CrossRef]
- Pellegrini, F.; Hills, C.D.; Carey, P.J.; Gardner, K.H.; Maries, A. Sorption and desorption of Cd, Co, Cu, Ni and Zn from carbonated Portland cement. Adv. Appl. Ceram. 2006, 105, 185–190. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Araizi, P.K.; Hills, C.D.; Maries, A.; Gunning, P.J.; Wray, D.S. Enhancement of accelerated carbonation of alkaline waste residues by ultrasound. Waste Manag. 2016, 50, 121–129. [Google Scholar] [CrossRef]
- Chen, Q.; Ke, Y.; Zhang, L.; Tyrer, M.; Hills, C.D.; Xue, G. Application of accelerated carbonation with a combination of Na2CO3 and CO2 in cement-based solidification/stabilization of heavy metal-bearing sediment. J. Hazard. Mater. 2009, 166, 421–427. [Google Scholar] [CrossRef]
- Guning, P.; Hills, C. 2014 Carbon Negative: First Commercial Application of Accelerated Carbonation Technology. Available online: https://gala.gre.ac.uk/id/eprint/13580/1/13580_Hills_Carbon_negative_first_commercial_application_%28conf._paper%29_AAM__2014.pdf (accessed on 23 April 2025).
- O.C.O Technology, M-LS 6F Series Factsheet. Available online: https://oco.co.uk/wp-content/themes/crush-theme/assets/pdf/M-L-6F-Sales-Factsheets-2022.pdf (accessed on 20 April 2025).
- O.C.O Technology, M-LS Environmental Product Declaration, Manufacturing Process. Available online: https://oco.co.uk/wp-content/themes/crush-theme/assets/pdf/epd-april-2023.pdf (accessed on 20 April 2025).
- O.C.O Technology Trials First Overseas CO2 Treatment Facility at Japanese EfW Plant. Available online: https://oco.co.uk/o-c-o-technology-trials-first-overseas-co2-treatment-facility-at-japanese-efw-plant/ (accessed on 20 April 2025).
- Bontempi, E.; Zacco, A.; Borgese, L.; Gianoncelli, A.; Ardesi, R.; Depero, L.E. A new method for municipal solid waste incinerator (MSWI) fly ash inertization, based on colloidal silica. J. Environ. Monit. 2010, 12, 2093–2099. [Google Scholar] [CrossRef]
- Bosio, A.; Zacco, A.; Borgese, L.; Rodella, N.; Colombi, P.; Benassi, L.; Bontempi, E. A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: A green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem. Eng. J. 2014, 253, 377–384. [Google Scholar] [CrossRef]
- Rodella, N.; Bosio, A.; Dalipi, R.; Zacco, A.; Borgese, L.; Depero, L.E.; Bontempi, E. Waste silica sources as heavy metal stabilizers for municipal solid waste incineration fly ash. Arab. J. Chem. 2017, 10, S3676–S3681. [Google Scholar] [CrossRef]
- Bilo, F.; Assi, A.; Zanoletti, A.; Bontempi, E. Dataset on the use of metal hydroxides, instead of flue gas desulfurization residues, to stabilize fly ash by using bottom ash. Data Brief 2020, 28, 104970. [Google Scholar] [CrossRef]
- Assi, A.; Federici, S.; Bilo, F.; Zacco, A.; Depero, L.E.; Bontempi, E. Increased sustainability of carbon dioxide mineral sequestration by a technology involving fly ash stabilization. Materials 2019, 12, 2714. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ducoli, S.; Ramorino, G.; Gobetti, A.; Bontempi, E. A circular economy virtuous example—Use of a stabilized waste material instead of calcite to produce sustainable composites. Appl. Sci. 2020, 10, 754. [Google Scholar] [CrossRef]
- Sorrentino, G.P.; Guimaraes, R.; Cornelio, A.; Zanoletti, A.; Valentim, B.; Bontempi, E. Mitigating CO2 emissions through an industrial symbiosis approach: Leveraging cork ash carbonation. Heliyon 2024, 10, e32893. [Google Scholar] [CrossRef]
- Benassi, L.; Pasquali, M.; Zanoletti, A.; Dalipi, R.; Borgese, L.; Depero, L.E.; Bontempi, E. Chemical stabilization of municipal solid waste incineration fly ash without any commercial chemicals: First pilot-plant scaling up. ACS Sustain. Chem. Eng. 2016, 4, 5561–5569. [Google Scholar] [CrossRef]
- Ahn, J.W.; Cho, H.C.; Han, G.C.; You, G.S.; Um, N.I. Effects of leaching of heavy metals in cement-based recycling via carbonation of municipal solid waste incineration bottom ash. Geosyst. Eng. 2006, 9, 7–10. [Google Scholar] [CrossRef]
- You, G.S.; Ahn, J.W.; Han, G.C.; Cho, H.C. Neutralizing capacity of bottom ash from municipal solid waste incineration of different particle size. Korean J. Chem. Eng. 2006, 23, 237–240. [Google Scholar] [CrossRef]
- Han, G.C.; Um, N.I.; You, K.S.; Cho, H.C.; Ahn, J.W. Recovery of ferromagnetic material by wet magnetic separation in coal bottom ash. Geosyst. Eng. 2009, 12, 9–12. [Google Scholar] [CrossRef]
- Nam, S.Y.; Seo, J.; Thriveni, T.; Ahn, J.W. Accelerated carbonation of municipal solid waste incineration bottom ash for CO2 sequestration. Geosyst. Eng. 2012, 15, 305–311. [Google Scholar] [CrossRef]
- Lee, H.W.; Han, C.; Whan, A.J. CO2 sequestration of real flue gases from landfill municipal solid waste incineration (MSWI)—Pilot scale demonstration. Int. J. Phys. Appl. Sci. 2015, 2, 54–63. [Google Scholar]
- Lim, M.; Han, G.C.; Ahn, J.W.; You, K.S. Environmental remediation and conversion of carbon dioxide (CO2) into useful green products by accelerated carbonation technology. Int. J. Environ. Res. Public Health 2010, 7, 203–228. [Google Scholar] [CrossRef]
- Wehrung, Q.; Bernasconi, D.; Cotellucci, A.; Destefanis, E.; Caviglia, C.; Bicchi, E.; Pavese, A.; Pastero, L. Carbonation washing of waste incinerator air pollution control residues under wastewater reuse conditions. J. Environ. Chem. Eng. 2024, 13, 115272. [Google Scholar] [CrossRef]
- Bernasconi, D.; Caviglia, C.; Destefanis, E.; Bonadiman, C.; Brombin, V.; Mancinelli, M.; Tassinari, R.; Pavese, A. Steam washing for MSWI-FA treatment. Waste Manag. 2025, 195, 10–21. [Google Scholar] [CrossRef]
- Bernasconi, D.; Viani, A.; Zárybnická, L.; Mácová, P.; Bordignon, S.; Caviglia, C.; Destefanis, E.; Gobetto, R.; Pavese, A. Phosphate-based geopolymer: Influence of municipal solid waste fly ash introduction on structure and compressive strength. Ceram. Int. 2023, 49, 22149–22159. [Google Scholar] [CrossRef]
- Bernasconi, D.; Viani, A.; Zárybnická, L.; Mácová, P.; Bordignon, S.; Das, G.; Pavese, A. Reactivity of MSWI-fly ash in Mg-K-phosphate cement. Constr. Build. Mater. 2023, 409, 134082. [Google Scholar] [CrossRef]
- Bernasconi, D.; Viani, A.; Zárybnická, L.; Bordignon, S.; Godinho, J.R.; Maximenko, A.; Celikutku, C.; Jafri, S.F.; Borfecchia, E.; Wehrung, Q.; et al. Setting reaction of an olivine-based Mg-phosphate cement. Cem. Concr. Res. 2024, 186, 107694. [Google Scholar] [CrossRef]
- Luo, L.; Nguyen, A.V. A review of principles and applications of magnetic flocculation to separate ultrafine magnetic particles. Sep. Purif. Technol. 2017, 172, 85–99. [Google Scholar] [CrossRef]
- Pienkoß, F.; Abis, M.; Bruno, M.; Grönholm, R.; Hoppe, M.; Kuchta, K.; Simon, F.G. Heavy metal recovery from the fine fraction of solid waste incineration bottom ash by wet density separation. J. Mater. Cycles Waste Manag. 2022, 24, 364–377. [Google Scholar] [CrossRef]
- Sierra, H.M.; Šyc, M.; Korotenko, E. Wet shaking table operating parameters optimization for maximizing metal recovery from incineration bottom ash fine fraction. Waste Manag. 2024, 174, 539–548. [Google Scholar] [CrossRef]
- Zhang, N.; Chai, Y.E.; Santos, R.M.; Šiller, L. Advances in process development of aqueous CO2 mineralisation towards scalability. J. Environ. Chem. Eng. 2020, 8, 104453. [Google Scholar] [CrossRef]
- Wehrung, Q.; Bernasconi, D.; Michel, F.; Destefanis, E.; Caviglia, C.; Curetti, N.; Pasero, F.; Pavese, A.; Pastero, L. ASHES Program: An Academia-Industry Partnership Integrating Metal Recovery with Accelerated Carbonation from Fine Bottom Ashes Fractions. In Proceedings of the 86th EAGE Annual Conference & Exhibition, Toulouse, France, 5 June 2025; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2025; Volume 2025, pp. 1–5. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).