Membrane Technologies for Separating Volatile Fatty Acids Produced Through Arrested Anaerobic Digestion: A Review
Abstract
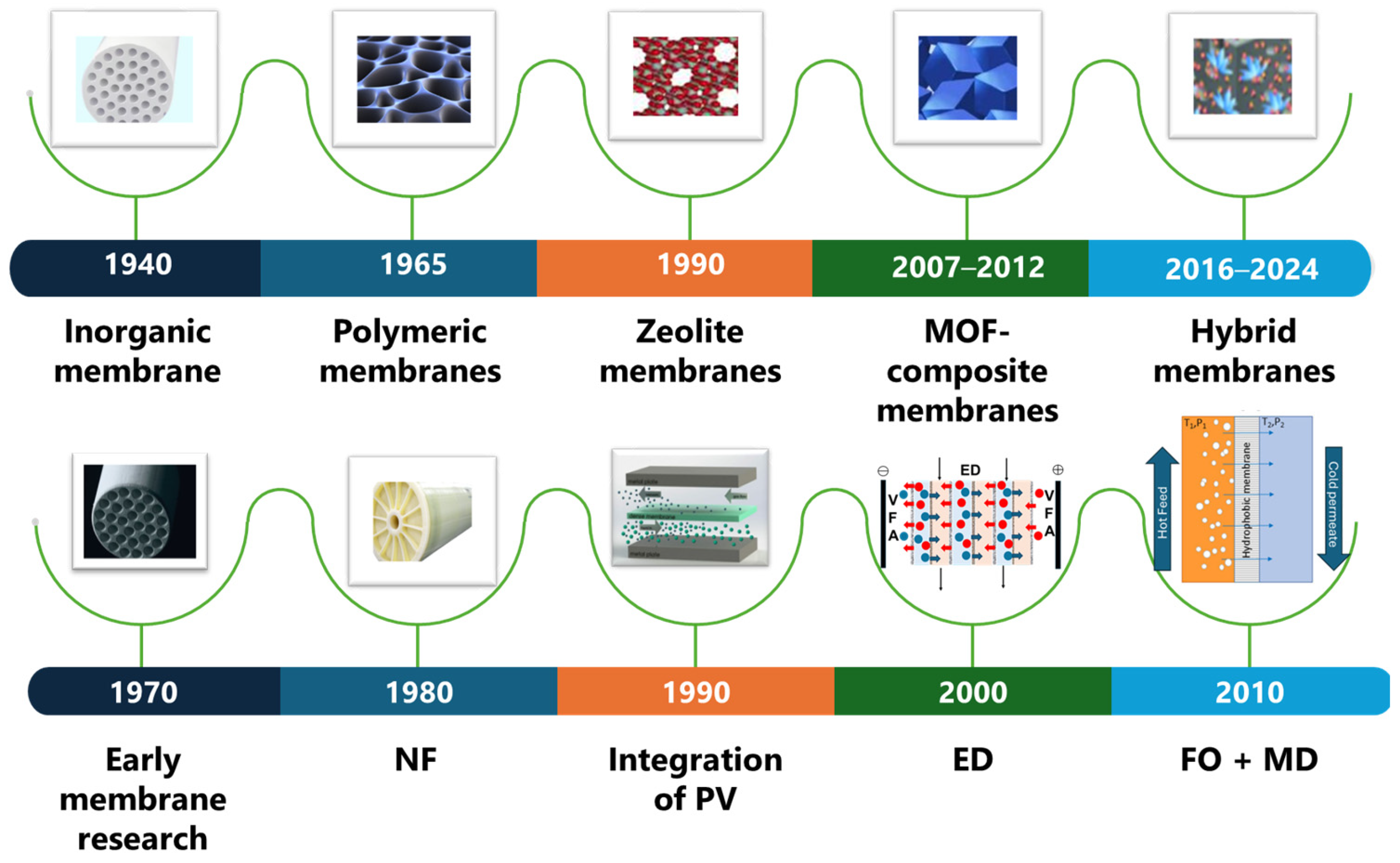
1. Introduction
2. Criteria for Choosing Specific Membrane Technologies
3. Developments in Membrane-Based Processes for VFA Separation and Purification
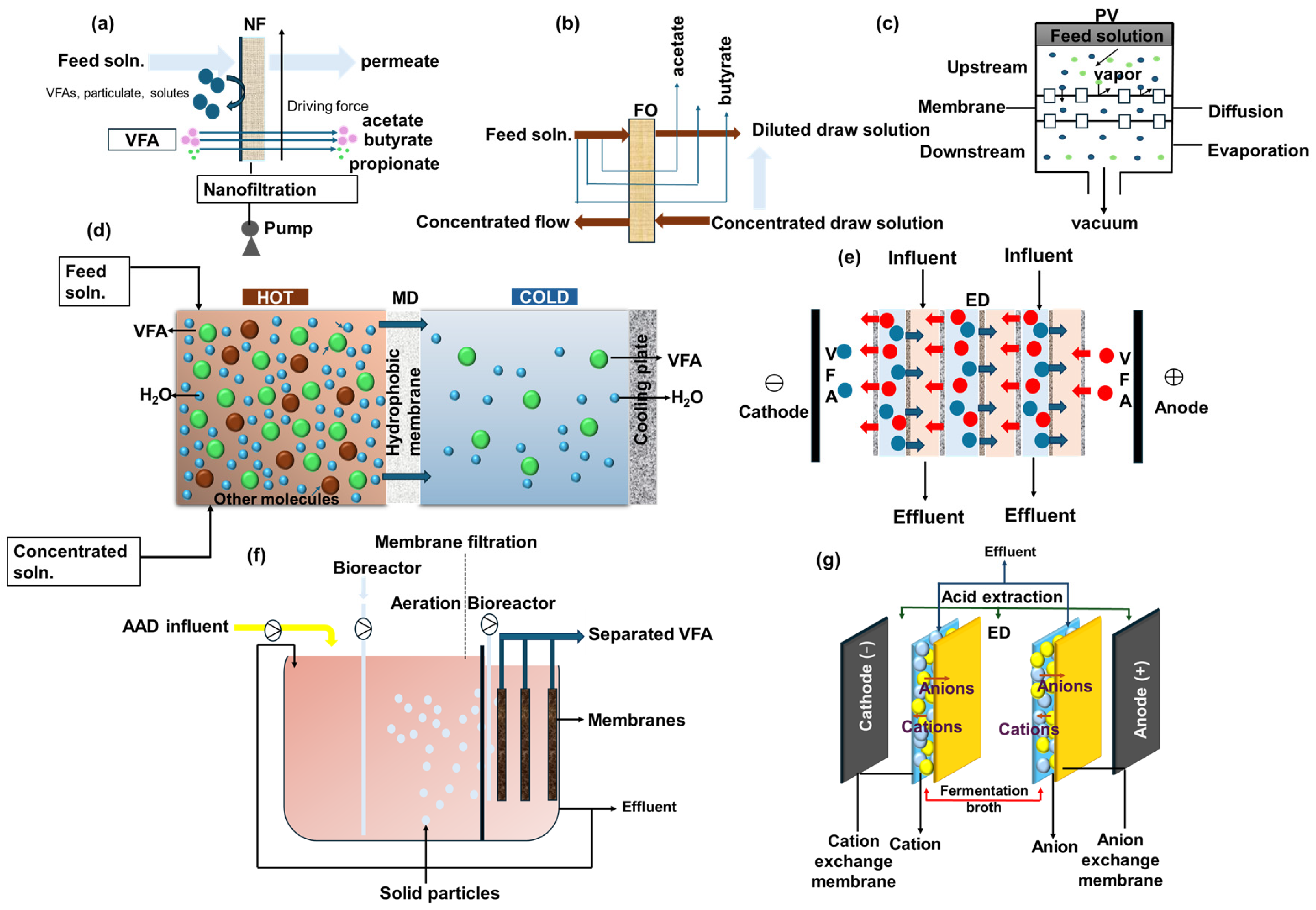
3.1. Pressure-Driven Membrane
| Raw Materials [-] | Total VFA Production [g/L] | Membrane-Based Process [-] | Membrane Materials [-] | Pressure [Bar] | Recovery [%] | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Butyric Acid | Other Acids | VFA | ||||||
| Straw | 2.9 | MF | Ceramic filter (α-Al2O3) | 1.03 | 60 | 40 | NA | 76.4 | [42] |
| Pretreated sludge | 8 | MF | Ceramic | 0.3–2 | NA | NA | NA | 87 | [43] |
| Food waste | 17 | NF | Cross flow filtration cell | 15 | 90 | 72 | 73 | 96 | [25] |
| Agriculture wastewater | 21 | NF | Composite polyamide | 10 | 65 | 34 | NA | 75 | [45] |
| Wastewater | 11 | UF | Polyvinyl fluoride | 0.95 | NA | NA | NA | 45 | [44] |
| VFA mixture | 13 | RO | Flat sheet membrane modules | 25 | 35 | NA | NA | NA | [49] |
| Fruits and vegetables | 8–20 | RO+NF | TFC polyamide | 30 | 40 | NA | NA | NA | [21] |
| Willow chips | 14 | NF | Cross flow filtration | 41 | NA | NA | 75 | 87 | [47] |
| Synthetic VFA soln. | 18 | NF | NF270 | 12 | 95 | NA | NA | 99 | [50] |
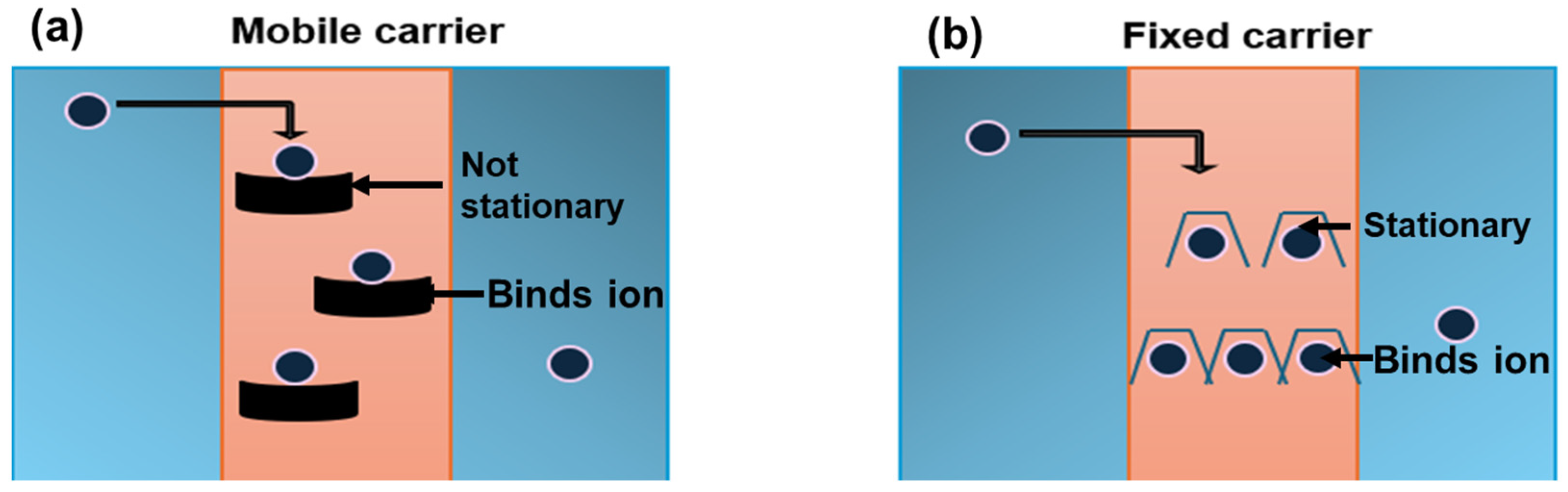
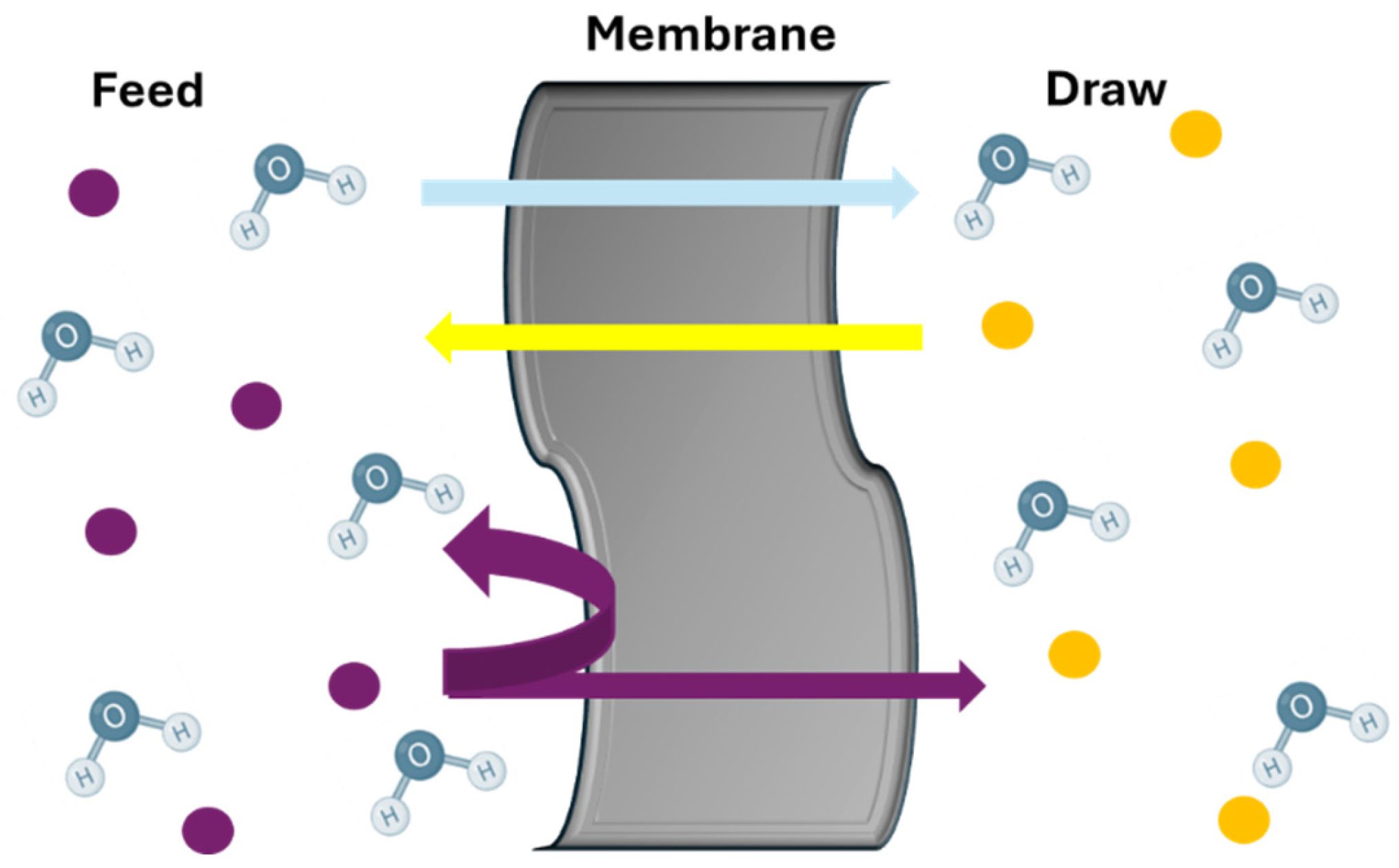
3.2. Concentration-Driven Membranes
3.3. Thermally Driven Membrane
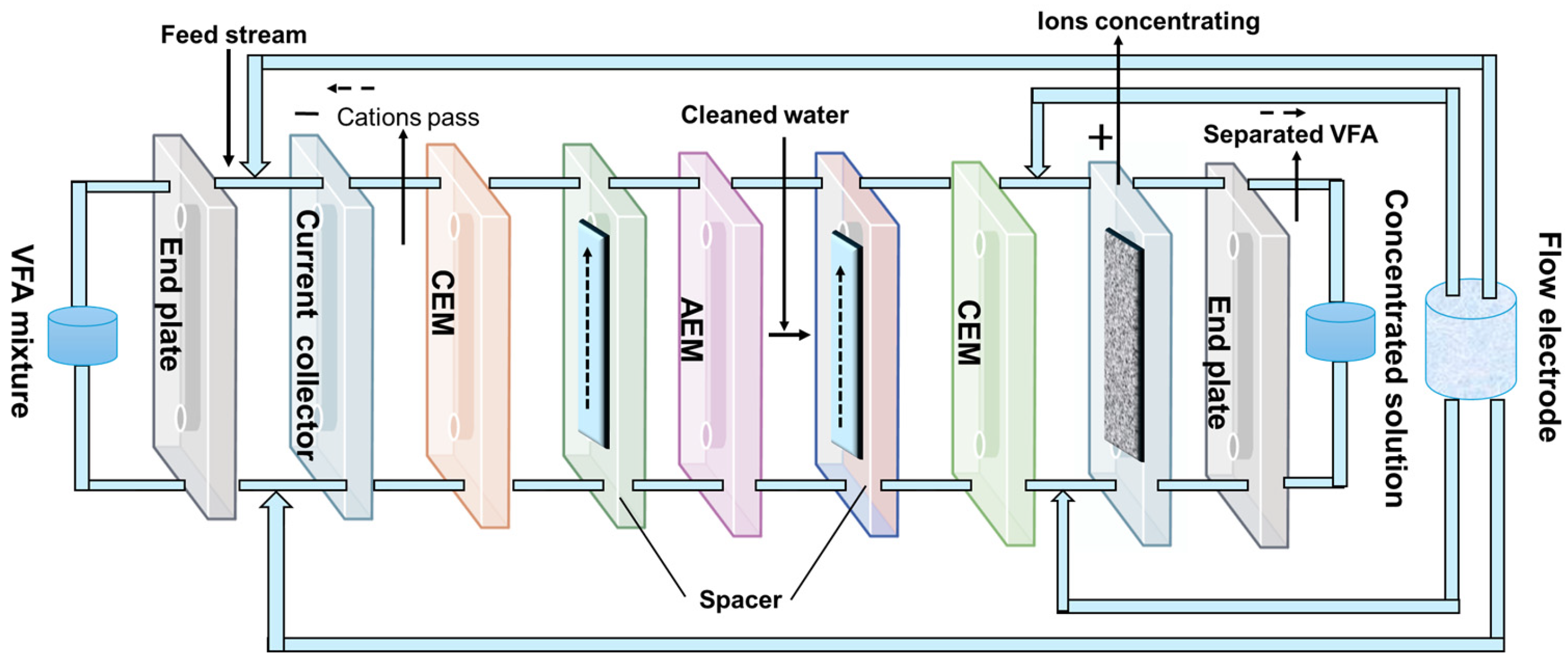
3.4. Electricity-Driven Membrane
| Raw Materials [-] | Total VFA Production [g/L] | Membrane-Based Process [-] | Membrane Materials [-] | Recovery [%] | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Butyric Acid | Total VFA | |||||
| Anaerobically digested grass | 16 | ED | PTFE Membrane | 54 | 45 | NA | 99 | [73] |
| Synthetic fermentation broth | 1.68 | MBEDC | Activated carbon with titanium nanowire | 99 | 95 | 97 | 91 | [74] |
| Wastewater | 2 | FCDI | Ion-exchange membranes | 83 | 87 | 66 | 99 | [75] |
| Grass | 4.5 | ED | PTFE Membrane | 68 | NA | NA | 78 | [32] |
| Cow manure | NA | ED | Polyelectrolyte | 60 | NA | NA | 80 | [76] |
| Wastewater | 10 | ED | Bipolar | 70 | NA | NA | 70 | [77] |
3.5. Membrane Contactor
3.6. Membrane Bioreactors
4. Challenges of VFA Separation and Purification by Membrane-Based Processes
4.1. Fouling
4.2. Energy Requirement Analysis
4.3. Selectivity and Permeability
4.4. Membrane Material and Its Stability
4.5. Environmental Challenges
4.6. Scale-Up
5. Advanced Membrane Technology
5.1. Forward Osmosis
5.2. Hybrid Membranes
6. Techno-Economic Analysis and Life Cycle Assessment
7. Future Direction
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Del Hierro, A.G.; Moreno-Cid, J.A.; Casey, E. Continuous Biomanufacturing for Sustainable Bioeconomy Applications. EFB Bioeconomy J. 2024, 4, 100071. [Google Scholar] [CrossRef]
- National Overview: Facts and Figures on Materials, Wastes and Recycling|US EPA. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 12 February 2025).
- Kim, N.J.; Lim, S.J.; Chang, H.N. Volatile Fatty Acid Platform: Concept and Application. Emerg. Areas Bioeng. 2017, 173–190. [Google Scholar] [CrossRef]
- Shetewi, T.; Finnegan, M.; Fitzgerald, S.; Xu, S.; Duffy, E.; Morrin, A. Investigation of the Relationship between Skin-Emitted Volatile Fatty Acids and Skin Surface Acidity in Healthy Participants—A Pilot Study. J. Breath Res. 2021, 15, 037101. [Google Scholar] [CrossRef]
- Vázquez-Fernández, A.; Suárez-Ojeda, M.E.; Carrera, J. Review about Bioproduction of Volatile Fatty Acids from Wastes and Wastewaters: Influence of Operating Conditions and Organic Composition of the Substrate. J. Environ. Chem. Eng. 2022, 10, 107917. [Google Scholar] [CrossRef]
- Giduthuri, A.T.; Ahring, B.K. Current Status and Prospects of Valorizing Organic Waste via Arrested Anaerobic Digestion: Production and Separation of Volatile Fatty Acids. Fermentation 2023, 9, 13. [Google Scholar] [CrossRef]
- Wu, H.; Dalke, R.; Mai, J.; Holtzapple, M.; Urgun-Demirtas, M. Arrested Methanogenesis Digestion of High-Strength Cheese Whey and Brewery Wastewater with Carboxylic Acid Production. Bioresour. Technol. 2021, 332, 125044. [Google Scholar] [CrossRef]
- Arrested Methanogenesis Technology for Sustainable Bioproducts and Biofuels Production from High-Strength Waste Streams-American Chemical Society. Available online: https://acs.digitellinc.com/p/s/arrested-methanogenesis-technology-for-sustainable-bioproducts-and-biofuels-production-from-high-strength-waste-streams-588409 (accessed on 12 February 2025).
- Si, B.; Li, J.; Li, B.; Zhu, Z.; Shen, R.; Zhang, Y.; Liu, Z. The Role of Hydraulic Retention Time on Controlling Methanogenesis and Homoacetogenesis in Biohydrogen Production Using Upflow Anaerobic Sludge Blanket (UASB) Reactor and Packed Bed Reactor (PBR). Int. J. Hydrogen Energy 2015, 40, 11414–11421. [Google Scholar] [CrossRef]
- Rughoonundun, H.; Holtzapple, M.T. Converting Wastewater Sludge and Lime-Treated Sugarcane Bagasse to Mixed Carboxylic Acids–A Potential Pathway to Ethanol Biofuel Production. Biomass Bioenergy 2017, 105, 73–82. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Behera, M. Comparative Evaluation of Methanogenesis Suppression Methods in Microbial Fuel Cell during Rice Mill Wastewater Treatment. Environ. Technol. Innov. 2020, 17, 100509. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Bedaso, B.; Laumeyer, C.; Pan, C.; Malovanyy, A.; Baresel, C.; Plaza, E.; Cetecioglu, Z. Volatile Fatty Acids Production from Municipal Waste Streams and Use as a Carbon Source for Denitrification: The Journey towards Full-Scale Application and Revealing Key Microbial Players. Renew. Sustain. Energy Rev. 2023, 175, 113163. [Google Scholar] [CrossRef]
- Fasahati, P.; Liu, J. Techno-Economic Analysis of Production and Recovery of Volatile Fatty Acids from Brown Algae Using Membrane Distillation. Comput. Aided Chem. Eng. 2014, 34, 303–308. [Google Scholar] [CrossRef]
- Ge, X.; Chen, Y.; Sànchez i Nogué, V.; Li, Y. Volatile Fatty Acid Recovery from Arrested Anaerobic Digestion for the Production of Sustainable Aviation Fuel: A Review. Fermentation 2023, 9, 821. [Google Scholar] [CrossRef]
- Lacroce, E.; Rossi, F.; Gianico, A.; Gallipoli, A.; Gelosa, S.; Busini, V.; Maria Braguglia, C.; Masi, M. On the Extraction of Volatile Fatty Acids from Food Waste Mixtures: Comparison between the Use of Liquid–Liquid and Magnetic Nanoparticle Technologies. Chem. Eng. Sci. 2024, 298, 120370. [Google Scholar] [CrossRef]
- Tharani, D.; Ananthasubramanian, M. Process Intensification in Separation and Recovery of Biogenic Volatile Fatty Acid Obtained through Acidogenic Fermentation of Organics-Rich Substrates. Chem. Eng. Process.-Process Intensif. 2021, 169, 108592. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Kersten, S.R.A.; Schuur, B. Recovery of Volatile Fatty Acids from Fermented Wastewater by Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 9176–9184. [Google Scholar] [CrossRef]
- Xing, T.; Yu, S.; Tang, J.; Liu, H.; Zhen, F.; Sun, Y.; Kong, X. Liquid–Liquid Extraction of Volatile Fatty Acids from Anaerobic Acidification Broth Using Ionic Liquids and Cosolvent. Energies 2023, 16, 785. [Google Scholar] [CrossRef]
- Shin, B.; Shin, J.; Chandra Wirasembada, Y.; Park, K.Y.; Cho, J. Modeling the Flux of Volatile Fatty Acid in a Membrane Distillation with the Effect of PH. J. Memb. Sci. 2024, 690, 122230. [Google Scholar] [CrossRef]
- Li, C.; Cao, J.; Ren, H.; Li, Y.; Tang, S. Comparison on Kinetics and Microbial Community among Denitrification Process Fed by Different Kinds of Volatile Fatty Acids. Process Biochem. 2015, 50, 447–455. [Google Scholar] [CrossRef]
- Domingos, J.M.B.; Martinez, G.A.; Morselli, E.; Bandini, S.; Bertin, L. Reverse Osmosis and Nanofiltration Opportunities to Concentrate Multicomponent Mixtures of Volatile Fatty Acids. Sep. Purif. Technol. 2022, 290, 120840. [Google Scholar] [CrossRef]
- Zhu, Y. Evaluation of Nanofiltration for the Extraction of Volatile Faffy Acids from Fermentation Broth. 2020. Available online: https://theses.hal.science/tel-03008506v1/file/2020TOU30033a.pdf (accessed on 4 June 2025).
- Bak, C.; Yun, Y.M.; Kim, J.H.; Kang, S. Electrodialytic Separation of Volatile Fatty Acids from Hydrogen Fermented Food Wastes. Int. J. Hydrogen Energy 2019, 44, 3356–3362. [Google Scholar] [CrossRef]
- Aghapour Aktij, S.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of Membrane Processes for the Recovery and Purification of Bio-Based Volatile Fatty Acids: A Comprehensive Review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Pervez, M.N.; Mahboubi, A.; Uwineza, C.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Taherzadeh, M.J. Factors Influencing Pressure-Driven Membrane-Assisted Volatile Fatty Acids Recovery and Purification-A Review. Sci. Total Environ. 2022, 817, 152993. [Google Scholar] [CrossRef] [PubMed]
- Asghar, N.; Lee, H.; Jang, D.; Jang, A. Recovery of Volatile Fatty Acids Using Forward Osmosis: Influence of Solution Chemistry, Temperature, and Membrane Orientation. Chemosphere 2022, 303, 134814. [Google Scholar] [CrossRef]
- Fatima, S.; Govardhan, B.; Kalyani, S.; Sridhar, S. Extraction of Volatile Organic Compounds from Water and Wastewater by Vacuum-Driven Membrane Process: A Comprehensive Review. Chem. Eng. J. 2022, 434, 134664. [Google Scholar] [CrossRef]
- Tugtas, A.E. Recovery of Volatile Fatty Acids via Membrane Contactor Using Flat Membranes: Experimental and Theoretical Analysis. Waste Manag. 2014, 34, 1171–1178. [Google Scholar] [CrossRef]
- Parchami, M.; Uwineza, C.; Ibeabuchi, O.H.; Rustas, B.O.; Taherzadeh, M.J.; Mahboubi, A. Membrane Bioreactor Assisted Volatile Fatty Acids Production from Agro-Industrial Residues for Ruminant Feed Application. Waste Manag. 2023, 170, 62–74. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Huang, L.Y.; Lee, D.J.; Lai, J.Y. Forward Osmosis Membrane Bioreactor for Wastewater Treatment with Phosphorus Recovery. Bioresour. Technol. 2015, 198, 418–423. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez–Feito, R.; Dinsdale, R.M.; Guwy, A.J. Fermentative Volatile Fatty Acid Production and Recovery from Grass Using a Novel Combination of Solids Separation, Pervaporation, and Electrodialysis Technologies. Bioresour. Technol. 2021, 342, 125926. [Google Scholar] [CrossRef]
- Mohshim, D.F.; Bin Mukhtar, H.; Man, Z.; Nasir, R. Latest Development on Membrane Fabrication for Natural Gas Purification: A Review. J. Eng. 2013, 2013, 101746. [Google Scholar] [CrossRef]
- Nambi Krishnan, J.; Venkatachalam, K.R.; Ghosh, O.; Jhaveri, K.; Palakodeti, A.; Nair, N. Review of Thin Film Nanocomposite Membranes and Their Applications in Desalination. Front. Chem. 2022, 10, 781372. [Google Scholar] [CrossRef] [PubMed]
- Bóna, Á.; Bakonyi, P.; Galambos, I.; Bélafi-Bakó, K.; Nemestóthy, N. Separation of Volatile Fatty Acids from Model Anaerobic Effluents Using Various Membrane Technologies. Membranes 2020, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Jänisch, T.; Reinhardt, S.; Pohsner, U.; Böringer, S.; Bolduan, R.; Steinbrenner, J.; Oechsner, H. Separation of Volatile Fatty Acids from Biogas Plant Hydrolysates. Sep. Purif. Technol. 2019, 223, 264–273. [Google Scholar] [CrossRef]
- (PDF) Use of Complex Effluent Streams as a Potential Source of Volatile Fatty Acids (VFA)—A Review Article. Available online: https://www.researchgate.net/publication/230855224_Use_of_complex_effluent_streams_as_a_potential_source_of_Volatile_Fatty_acids_VFA_-_A_review_article (accessed on 12 February 2025).
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-Scale Production of VFAs from Sewage Sludge by Anaerobic Alkaline Fermentation to Improve Biological Nutrients Removal in Domestic Wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef]
- Arslan, D.; Zhang, Y.; Steinbusch, K.J.J.; Diels, L.; Hamelers, H.V.M.; Buisman, C.J.N.; De Wever, H. In-Situ Carboxylate Recovery and Simultaneous PH Control with Tailor-Configured Bipolar Membrane Electrodialysis during Continuous Mixed Culture Fermentation. Sep. Purif. Technol. 2017, 175, 27–35. [Google Scholar] [CrossRef]
- Pervez, M.N.; Bilgiç, B.; Mahboubi, A.; Uwineza, C.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Taherzadeh, M.J. Double-Stage Membrane-Assisted Anaerobic Digestion Process Intensification for Production and Recovery of Volatile Fatty Acids from Food Waste. Sci. Total Environ. 2022, 825, 154084. [Google Scholar] [CrossRef]
- Wang, X.X.; Wu, Y.H.; Zhang, T.Y.; Xu, X.Q.; Dao, G.H.; Hu, H.Y. Simultaneous Nitrogen, Phosphorous, and Hardness Removal from Reverse Osmosis Concentrate by Microalgae Cultivation. Water Res. 2016, 94, 215–224. [Google Scholar] [CrossRef]
- Zacharof, M.-P.; Lovitt, R.W. The Filtration Characteristics of Anaerobic Digester Effluents Employing Cross Flow Ceramic Membrane Microfiltration for Nutrient Recovery. Desalination 2014, 341, 27–37. [Google Scholar] [CrossRef]
- Kim, J.O.; Kim, S.K.; Kim, R.H. Filtration Performance of Ceramic Membrane for the Recovery of Volatile Fatty Acids from Liquid Organic Sludge. Desalination 2005, 172, 119–127. [Google Scholar] [CrossRef]
- Mineo, A.; Cosenza, A.; Mannina, G. Sewage Sludge Acidogenic Fermentation for Organic Resource Recovery towards Carbon Neutrality: An Experimental Survey Testing the Headspace Influence. Bioresour. Technol. 2023, 367, 128217. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Mandale, S.J.; Williams, P.M.; Lovitt, R.W. Nanofiltration of Treated Digested Agricultural Wastewater for Recovery of Carboxylic Acids. J. Clean. Prod. 2016, 112, 4749–4761. [Google Scholar] [CrossRef]
- Beck, A.; Ernst, M. Kinetic Modeling and Selectivity of Anion Exchange in Donnan Dialysis. J. Memb. Sci. 2015, 479, 132–140. [Google Scholar] [CrossRef]
- Xiong, B.; Richard, T.L.; Kumar, M. Integrated Acidogenic Digestion and Carboxylic Acid Separation by Nanofiltration Membranes for the Lignocellulosic Carboxylate Platform. J. Memb. Sci. 2015, 489, 275–283. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Landaburu-Aguirre, J.; Senán-Salinas, J.; Ortiz, J.M.; Molina, S. Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy. Membranes 2022, 12, 864. [Google Scholar] [CrossRef]
- Pratofiorito, G.; Horn, H.; Saravia, F. Impact of the Recovery on Concentrating Acetic Acid with Low-Pressure Reverse-Osmosis Membranes. Membranes 2021, 11, 742. [Google Scholar] [CrossRef]
- Cairone, S.; Naddeo, V.; Belgiorno, V.; Taherzadeh, M.J.; Mahboubi, A. Evaluating the Impact of Membrane Properties and Feed PH on Concentration and Fractionation of Volatile Fatty Acid Using Nanofiltration. J. Water Process Eng. 2024, 65, 105793. [Google Scholar] [CrossRef]
- Bringas, E.; San-Román, M.F.; Urtiaga, A.M.; Ortiz, I. Concentration-Driven Membrane Processes for the Recovery of Valuable Compounds from Industrial Wastes. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 474–491. [Google Scholar] [CrossRef]
- Farhan, N.M.; Ibrahim, S.S.; Alsalhy, Q.F. Modeling and Simulation of Pervaporation (PV) Separation for Alcohol Dehydration. Heliyon 2023, 9, e13713. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Zheng, Z.; Ma, H.; Yang, M.; Liu, H. Continuous Liquid Fermentation of Pretreated Waste Activated Sludge for High Rate Volatile Fatty Acids Production and Online Nutrients Recovery. Bioresour. Technol. 2018, 249, 962–968. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Cerrone, F.; Woods, T.; Joyce, K.; O’Flaherty, V.; O’Connor, K.; Babu, R. Pervaporation Separation of Butyric Acid from Aqueous and Anaerobic Digestion (AD) Solutions Using PEBA Based Composite Membranes. J. Ind. Eng. Chem. 2015, 23, 163–170. [Google Scholar] [CrossRef]
- Wódzki, R.; Nowaczyk, J. Propionic and Acetic Acid Pertraction through a Multimembrane Hybrid System Containing TOPO or TBP. Sep. Purif. Technol. 2002, 26, 207–220. [Google Scholar] [CrossRef]
- Wódzki, R.; Nowaczyk, J.; Kujawski, M. Separation of Propionic and Acetic Acid by Pertraction in a Multimembrane Hybrid System. Sep. Purif. Technol. 2000, 21, 39–54. [Google Scholar] [CrossRef]
- Wang, Y.; Shung Chung, T.; Gruender, M. Sulfonated Polybenzimidazole Membranes for Pervaporation Dehydration of Acetic Acid. J. Memb. Sci. 2012, 415–416, 486–495. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, K.H. Pervaporation Separation of Water-Acetic Acid Mixtures through Poly(Vinyl Alcohol) Membranes Crosslinked with Glutaraldehyde. J. Memb. Sci. 1996, 109, 257–265. [Google Scholar] [CrossRef]
- Sano, T.; Ejiri, S.; Yamada, K.; Kawakami, Y.; Yanagishita, H. Separation of Acetic Acid-Water Mixtures by Pervaporation through Silicalite Membrane. J. Memb. Sci. 1997, 123, 225–233. [Google Scholar] [CrossRef]
- Cath, T.Y. Osmotically and Thermally Driven Membrane Processes for Enhancement of Water Recovery in Desalination Processes. Desalination Water Treat. 2010, 15, 279–286. [Google Scholar] [CrossRef]
- Zhu, X.; Leininger, A.; Jassby, D.; Tsesmetzis, N.; Ren, Z.J. Will Membranes Break Barriers on Volatile Fatty Acid Recovery from Anaerobic Digestion? ACS EST Eng. 2021, 1, 141–153. [Google Scholar] [CrossRef]
- Yao, M.; Woo, Y.C.; Ren, J.; Tijing, L.D.; Choi, J.S.; Kim, S.H.; Shon, H.K. Volatile Fatty Acids and Biogas Recovery Using Thermophilic Anaerobic Membrane Distillation Bioreactor for Wastewater Reclamation. J. Environ. Manag. 2019, 231, 833–842. [Google Scholar] [CrossRef]
- Gryta, M.; Markowska-Szczupak, A.; Bastrzyk, J.; Tomczak, W. The Study of Membrane Distillation Used for Separation of Fermenting Glycerol Solutions. J. Memb. Sci. 2013, 431, 1–8. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Eykens, L.; Hitsov, I.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Direct Contact and Air Gap Membrane Distillation: Differences and Similarities between Lab and Pilot Scale. Desalination 2017, 422, 91–100. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Shi, L.; Fane, A.G.; Debowski, M. Performance Improvement of PVDF Hollow Fiber-Based Membrane Distillation Process. J. Memb. Sci. 2011, 369, 437–447. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, W.; Shen, N.; Song, H.; Li, Y.; Wang, G.; Chen, Y. Selective Separation of Volatile Fatty Acids and Phosphorous Recovery from Fermented Broth Using Flow-Electrode Capacitive Deionization. Waste Manag. 2023, 165, 12–18. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Biotechnol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Deng, S.; Wang, C.; Ngo, H.H.; Guo, W.; You, N.; Tang, H.; Yu, H.; Tang, L.; Han, J. Comparative Review on Microbial Electrochemical Technologies for Resource Recovery from Wastewater towards Circular Economy and Carbon Neutrality. Bioresour. Technol. 2023, 376, 128906. [Google Scholar] [CrossRef]
- Nagendranatha Reddy, C.; Kondaveeti, S.; Mohanakrishna, G.; Min, B. Application of Bioelectrochemical Systems to Regulate and Accelerate the Anaerobic Digestion Processes. Chemosphere 2022, 287, 132299. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Bioelectrochemical Recovery of Waste-Derived Volatile Fatty Acids and Production of Hydrogen and Alkali. Water Res. 2015, 81, 188–195. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, Y.; Ge, Z.; Lu, J.; Zhang, Y.; Liu, Z.; Ren, Z.J. Microbial Electrolysis Treatment of Post-Hydrothermal Liquefaction Wastewater with Hydrogen Generation. Appl. Energy 2018, 212, 509–515. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, W.W.; Huang, L.; Liu, H.Q.; Wang, Y.K.; Geng, Y.K.; Kwan-Sing Lam, P.; Yu, H.Q. Recovery of High-Concentration Volatile Fatty Acids from Wastewater Using an Acidogenesis-Electrodialysis Integrated System. Bioresour. Technol. 2018, 260, 61–67. [Google Scholar] [CrossRef]
- Oh, W.; Kim, N.; Kim, H.; Mackie, R.I.; Su, X. Controlling Bicontinuous Polyelectrolyte Complexation for Membrane Selectivity: Redox-Mediated Electrochemical Separation of Volatile Fatty Acids. Adv. Funct. Mater. 2024, 35, 2410511. [Google Scholar] [CrossRef]
- Yu, L.; Guo, Q.; Hao, J.; Jiang, W. Recovery of Acetic Acid from Dilute Wastewater by Means of Bipolar Membrane Electrodialysis. Desalination 2000, 129, 283–288. [Google Scholar] [CrossRef]
- Ravishankar, H.; Dessì, P.; Trudu, S.; Asunis, F.; Lens, P.N.L. Silicone Membrane Contactor for Selective Volatile Fatty Acid and Alcohol Separation. Process Saf. Environ. Prot. 2021, 148, 125–136. [Google Scholar] [CrossRef]
- Outram, V.; Zhang, Y. Solvent-Free Membrane Extraction of Volatile Fatty Acids from Acidogenic Fermentation. Bioresour. Technol. 2018, 270, 400–408. [Google Scholar] [CrossRef]
- Aydin, S.; Yesil, H.; Tugtas, A.E. Recovery of Mixed Volatile Fatty Acids from Anaerobically Fermented Organic Wastes by Vapor Permeation Membrane Contactors. Bioresour. Technol. 2018, 250, 548–555. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Z.; Zhou, M.; Jie, X.; Wang, L.; Kang, G.; Cao, Y. Removal of CO2 from Biogas by Membrane Contactor Using PTFE Hollow Fibers with Smaller Diameter. J. Memb. Sci. 2021, 627, 119232. [Google Scholar] [CrossRef]
- Yesil, H.; Tugtas, A.E.; Bayrakdar, A.; Calli, B. Anaerobic Fermentation of Organic Solid Wastes: Volatile Fatty Acid Production and Separation. Water Sci. Technol. 2014, 69, 2132–2138. [Google Scholar] [CrossRef]
- Kaya, E.; Hasanoğlu, A. Removal of Acetic Acid from Aqueous Post-Fermentation Streams and Fermented Beverages Using Membrane Contactors. J. Chem. Technol. Biotechnol. 2022, 97, 2218–2230. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane Bioreactor-Assisted Volatile Fatty Acids Production and in Situ Recovery from Cow Manure. Bioresour. Technol. 2021, 321, 124456. [Google Scholar] [CrossRef]
- Lukitawesa; Eryildiz, B.; Mahboubi, A.; Millati, R.; Taherzadeh, M.J. Semi-Continuous Production of Volatile Fatty Acids from Citrus Waste Using Membrane Bioreactors. Innov. Food Sci. Emerg. Technol. 2021, 67, 102545. [Google Scholar] [CrossRef]
- Wainaina, S.; Parchami, M.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J. Food Waste-Derived Volatile Fatty Acids Platform Using an Immersed Membrane Bioreactor. Bioresour. Technol. 2019, 274, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, F.; Klement, T.; Büchs, J.; Melin, T.; Wessling, M. Continuous Production and Recovery of Itaconic Acid in a Membrane Bioreactor. Bioresour. Technol. 2013, 137, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, H.; Meng, X.; Huang, Z.; Feng, Y.; Gao, Q.; Ruan, W. Volatile Fatty Acids Production from Kitchen Waste Slurry Using Anaerobic Membrane Bioreactor via Alkaline Fermentation with High Salinity: Evaluation on Process Performance and Microbial Succession. Bioresour. Technol. 2024, 399, 130576. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, A.; Ylitervo, P.; Doyen, W.; De Wever, H.; Taherzadeh, M.J. Reverse Membrane Bioreactor: Introduction to a New Technology for Biofuel Production. Biotechnol. Adv. 2016, 34, 954–975. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, J.; Zhang, F. Membrane Fouling Control in a Submerged Membrane Bioreactor with Porous, Flexible Suspended Carriers. Desalination 2006, 189, 292–302. [Google Scholar] [CrossRef]
- Mineo, A.; Cosenza, A.; Ng, H.Y.; Mannina, G. Volatile Fatty Acids from Sewage Sludge by Anaerobic Membrane Bioreactors: Lesson Learned from Two-Year Experiments with Fouling Analysis by the Resistance in Series Model. Results Eng. 2024, 21, 101839. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G.A. A Review on Membrane Biofouling: Prediction, Characterization, and Mitigation. Membranes 2022, 12, 1271. [Google Scholar] [CrossRef]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Wu, Z.; Zhou, Q. Role of Dissolved Organic Matters (DOM) in Membrane Fouling of Membrane Bioreactors for Municipal Wastewater Treatment. J. Hazard. Mater. 2010, 178, 377–384. [Google Scholar] [CrossRef]
- Rolf, J.; Cao, T.; Huang, X.; Boo, C.; Li, Q.; Elimelech, M. Inorganic Scaling in Membrane Desalination: Models, Mechanisms, and Characterization Methods. Environ. Sci. Technol. 2022, 56, 7484–7511. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of Nanotechnology in the Removal of Heavy Metal From Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Elsevier: London, UK, 2018; pp. 83–147. [Google Scholar] [CrossRef]
- Pérez, G.; Gómez, P.; Ortiz, I.; Urtiaga, A. Techno-Economic Assessment of a Membrane-Based Wastewater Reclamation Process. Desalination 2022, 522, 115409. [Google Scholar] [CrossRef]
- Chavez Velasco, J.A.; Tawarmalani, M.; Agrawal, R. Which Separation Scenarios Are Advantageous for Membranes or Distillations? AIChE J. 2022, 68, e17839. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; Ng, K.M.D.; Lau, W.J.; Ismail, A.F. A Review on the Use of Membrane Technology Systems in Developing Countries. Membranes 2021, 12, 30. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Wu, H.; Valentino, L.; Riggio, S.; Holtzapple, M.; Urgun-Demirtas, M. Performance Characterization of Nanofiltration, Reverse Osmosis, and Ion Exchange Technologies for Acetic Acid Separation. Sep. Purif. Technol. 2021, 265, 118108. [Google Scholar] [CrossRef]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Degradation of Polyamide Nanofiltration and Reverse Osmosis Membranes by Hypochlorite. Environ. Sci. Technol. 2012, 46, 852–859. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; León, G.; Gómez, M.; Murcia, M.D.; Gómez, E.; Gómez, J.L. Behaviour of RO98pHt Polyamide Membrane in Reverse Osmosis and Low Reverse Osmosis Conditions for Phenol Removal. Environ. Technol. 2011, 32, 1497–1502. [Google Scholar] [CrossRef]
- Yesil, H.; Taner, H.; Ugur Nigiz, F.; Hilmioglu, N.; Tugtas, A.E. Pervaporative Separation of Mixed Volatile Fatty Acids: A Study Towards Integrated VFA Production and Separation. Waste Biomass Valori. 2020, 11, 1737–1753. [Google Scholar] [CrossRef]
- Cañas Kurz, E.E.; Hellriegel, U.; Figoli, A.; Gabriele, B.; Bundschuh, J.; Hoinkis, J. Small-Scale Membrane-Based Arsenic Removal for Decentralized Applications–Developing a Conceptual Approach for Future Utilization. Water Res. 2021, 196, 116978. [Google Scholar] [CrossRef]
- Chang, Q.; Zhou, J.E.; Wang, Y.; Wang, J.; Meng, G. Hydrophilic Modification of Al2O3 Microfiltration Membrane with Nano-Sized γ-Al2O3 Coating. Desalination 2010, 262, 110–114. [Google Scholar] [CrossRef]
- Artuǧ, G.; Roosmasari, I.; Richau, K.; Hapke, J. A Comprehensive Characterization of Commercial Nanofiltration Membranes. Sep. Sci. Technol. 2007, 42, 2947–2986. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.; Du, H.; Xu, S.; Du, Y. Study on the Concentration of Acrylic Acid and Acetic Acid by Reverse Osmosis. Membranes 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Floros, I.N.; Kouvelos, E.P.; Pilatos, G.I.; Hadjigeorgiou, E.P.; Gotzias, A.D.; Favvas, E.P.; Sapalidis, A.A. Enhancement of Flux Performance in PTFE Membranes for Direct Contact Membrane Distillation. Polymers 2020, 12, 345. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of Ceramic and Polymeric Membrane Permeability and Fouling Using Surface Water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Cui, Q.; Shang, Y.; Fei, Z.; Tu, T.; Yan, S. Hydrophobic-Hydrophilic Janus Ceramic Membrane for Enhancing the Waste Heat Recovery from the Stripped Gas in the Carbon Capture Process. ACS Sustain. Chem. Eng. 2022, 10, 3817–3828. [Google Scholar] [CrossRef]
- Oh, W.; Kim, N.; Kim, H.; Mackie, R.I.; Su, X. Controlling Bicontinuous Polyelectrolyte Complexation for Membrane Selectivity: Redox-Mediated Electrochemical Separation of Volatile Fatty Acids (Adv. Funct. Mater. 6/2025). Adv. Funct. Mater. 2025, 35, 2570032. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Islam, M.D.; Uddin, F.J.; Rashid, T.U.; Shahruzzaman, M. Cellulose Acetate-Based Membrane for Wastewater Treatment—A State-of-the-Art Review. Mater. Adv. 2023, 4, 4054–4102. [Google Scholar] [CrossRef]
- Merlet, R.B.; Pizzoccaro-Zilamy, M.A.; Nijmeijer, A.; Winnubst, L. Hybrid Ceramic Membranes for Organic Solvent Nanofiltration: State-of-the-Art and Challenges. J. Memb. Sci. 2020, 599, 117839. [Google Scholar] [CrossRef]
- Li, W.; Molina-Fernández, C.; Estager, J.; Monbaliu, J.C.M.; Debecker, D.P.; Luis, P. Supported Ionic Liquid Membranes for the Separation of Methanol/Dimethyl Carbonate Mixtures by Pervaporation. J. Memb. Sci. 2020, 598, 117790. [Google Scholar] [CrossRef]
- Rebecchi, S.; Pinelli, D.; Bertin, L.; Zama, F.; Fava, F.; Frascari, D. Volatile Fatty Acids Recovery from the Effluent of an Acidogenic Digestion Process Fed with Grape Pomace by Adsorption on Ion Exchange Resins. Chem. Eng. J. 2016, 306, 629–639. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Noskov, A.S. Biodegradable Biopolymers: Real Impact to Environment Pollution. Sci. Total Environ. 2024, 947, 174445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huo, B.; Xu, Z.; Qi, H.; Li, X.; Cui, P.; Zhu, Z.; Wang, Y.; Yang, J.; Gao, J. Energy-Saving and Environmentally Friendly Pervaporation-Distillation Hybrid Process for Alcohol and Ester Recovery from Wastewater Containing Three Binary Azeotropes. Sep. Purif. Technol. 2022, 281, 119889. [Google Scholar] [CrossRef]
- Chen, T.; Wei, X.; Chen, Z.; Morin, D.; Alvarez, S.V.; Yoon, Y.; Huang, Y. Designing Energy-Efficient Separation Membranes: Knowledge from Nature for a Sustainable Future. Adv. Membr. 2022, 2, 100031. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Wang, L.F.; Pan, X.R.; Zhang, F.; Huang, M.S.; Li, W.W.; Liu, H.Q. Selective Separation of Volatile Fatty Acids, Nitrogen and Phosphorus from Anaerobic Acidogenic Fermentation via Forward Osmosis Membrane Process. Chem. Eng. J. 2023, 453, 139871. [Google Scholar] [CrossRef]
- Nurjanah, I.; Chang, T.T.; You, S.J.; Huang, C.Y.; Sean, W.Y. Reverse Osmosis Integrated with Renewable Energy as Sustainable Technology: A Review. Desalination 2024, 581, 117590. [Google Scholar] [CrossRef]
- Da Ros, C.; Conca, V.; Eusebi, A.L.; Frison, N.; Fatone, F. Sieving of Municipal Wastewater and Recovery of Bio-Based Volatile Fatty Acids at Pilot Scale. Water Res. 2020, 174, 115633. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the Right Stuff: The Trade-off between Membrane Permeability and Selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Altaee, A.; Samal, A.K.; Zhou, J.L.; Nguyen, T.V.; Ganbat, N. Treatment of Biologically Treated Landfill Leachate with Forward Osmosis: Investigating Membrane Performance and Cleaning Protocols. Sci. Total Environ. 2020, 744, 140901. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, D.I.; Kim, J.; Zhan, M.; Hong, S. An Integrated System for CO2 Capture and Water Treatment by Forward Osmosis Driven by an Amine-Based Draw Solution. J. Memb. Sci. 2019, 581, 9–17. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.; Lee, J.S.; Yoon, S.; Lee, J.H. Biphenyl-Based Covalent Triazine Framework-Incorporated Polydimethylsiloxane Membranes with High Pervaporation Performance for n-Butanol Recovery. J. Memb. Sci. 2020, 598, 117654. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K. Use of Membrane Techniques for Removal and Recovery of Nutrients from Liquid Fraction of Anaerobic Digestate. Membranes 2025, 15, 45. [Google Scholar] [CrossRef]
- El Kik, O.; Lesage, G.; Zaviska, F.; Sauvêtre, A.; Heran, M.; Lestremau, F. Synergistic Approach for Enhanced Wastewater Treatment: Harnessing the Potential of Bioelectrochemical Systems in Integration with Anaerobic Membrane Bioreactors. J. Environ. Chem. Eng. 2024, 12, 113162. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Wang, H.; Han, L.; Tanis-Kanbur, M.B.; Pranav, M.V.; Chew, J.W. Photothermal-Enhanced and Fouling-Resistant Membrane for Solar-Assisted Membrane Distillation. J. Memb. Sci. 2018, 565, 254–265. [Google Scholar] [CrossRef]
- Dongare, P.D.; Alabastri, A.; Pedersen, S.; Zodrow, K.R.; Hogan, N.J.; Neumann, O.; Wud, J.; Wang, T.; Deshmukh, A.; Elimelech, M.; et al. Nanophotonics-Enabled Solar Membrane Distillation for off-Grid Water Purification. Proc. Natl. Acad. Sci. USA 2017, 114, 6936–6941. [Google Scholar] [CrossRef]
- Yesil, H.; Calli, B.; Tugtas, A.E. A Hybrid Dry-Fermentation and Membrane Contactor System: Enhanced Volatile Fatty Acid (VFA) Production and Recovery from Organic Solid Wastes. Water Res. 2021, 192, 116831. [Google Scholar] [CrossRef]
- Karp, E.M.; Sànchez Nogué, V. BETO 2021 Peer Review: Separations in Support of Arresting Anaerobic Digestion; National Renewable Energy Laboratory: Golden, CO, USA, 2021. [Google Scholar]
- Xu, Y.; Liu, R.; Liu, H.; Geng, H.; Dai, X. Novel Anaerobic Digestion of Waste Activated Sludge via Isoelectric-Point Pretreatment: Ultra-Short Solids Retention Time and High Methane Yield. Water Res. 2022, 220, 118657. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Yu, H.; Lee, S.H.; Wang, L.; Zheng, J.; Wang, T.; Yun, Y.; Lee, J.K. ZnO Nanorod Array Modified PVDF Membrane with Superhydrophobic Surface for Vacuum Membrane Distillation Application. ACS Appl. Mater. Interfaces 2018, 10, 13452–13461. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, Y.; Qing, W.; Lansing, S.; Shi, J.; Zhang, W.; Wang, Z.W. Anhydrous Volatile Fatty Acid Extraction through Omniphobic Membranes by Hydrophobic Deep Eutectic Solvents: Mechanistic Understanding and Future Perspective. Water Res. 2024, 257, 121654. [Google Scholar] [CrossRef]
- Generous, M.M.; Qasem, N.A.A.; Akbar, U.A.; Zubair, S.M. Techno-Economic Assessment of Electrodialysis and Reverse Osmosis Desalination Plants. Sep. Purif. Technol. 2021, 272, 118875. [Google Scholar] [CrossRef]
- Figueira, M.; López, J.; Reig, M.; Cortina, J.L.; Valderrama, C. Techno-Economic Analysis of Seawater Reverse Osmosis Brines Treatment Using Nanofiltration Modelling Tools. Desalination 2023, 568, 117013. [Google Scholar] [CrossRef]
- Felix, V.; Hardikar, M.; Hickenbottom, K.L. Concentrate Circularity: A Comparative Techno-Economic Analysis of Membrane Distillation and Conventional Inland Concentrate Management Technologies. Desalination 2024, 574, 117213. [Google Scholar] [CrossRef]


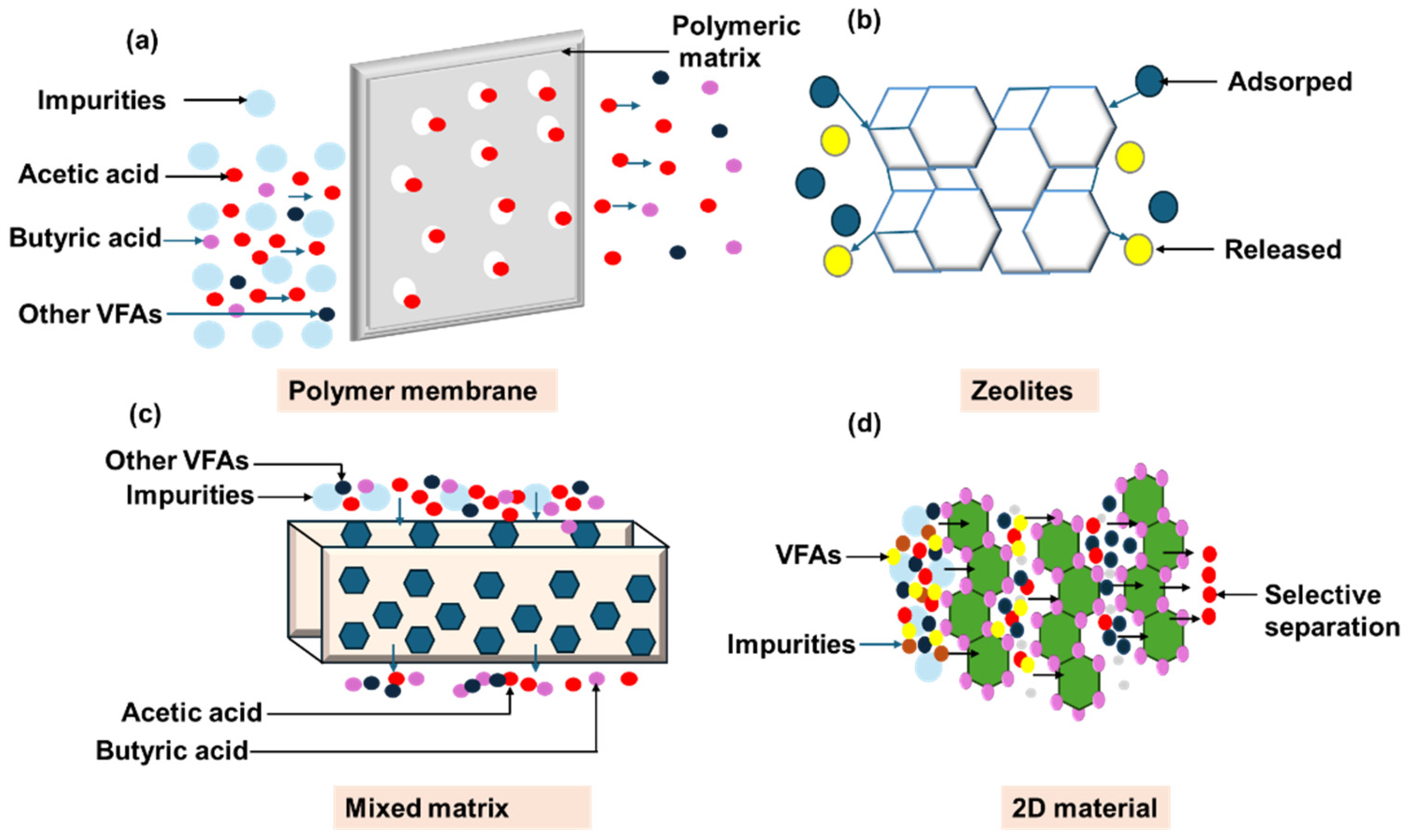
| Materials | Technology | Operating Conditions | VFA Recovered | VFA Recovery Rate (%) | Ref. |
|---|---|---|---|---|---|
| Synthetic VFA mixture | Reverse osmosis | Pressure 15 psi Temp 25 °C | Acetic acid | 44 | [38] |
| Anaerobic granular sludge | Electrodialysis | Temp: 30 °C pH: 5 | Acetic acid; propionic acid | 90 | [39] |
| Wastewater | Nanofiltration | pH: 2–11 Temp: 50 °C | Acetic acid; butyric acid | 75 | [35] |
| Food waste | Integrated MF-UF | pH: 5.4, HRT: 98 Temp: 37 °C | Acetic acid | 70–80 | [40] |
| Raw Materials [-] | VFA Production [g/L] | Membrane-Based Process [-] | Membrane Materials [-] | Recovery [%] | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Butyric Acid | Total VFA | |||||
| Anaerobic digestion of grass | 7.0 | PV | Sodium alginate membrane: polypropylene | NA | NA | 86.9 | 86.9 | [9,54] |
| Wastewater | 11 | Carrier-Mediated Separation | Polyelectrolytes and polymeric | 55 | 57 | NA | NA | [55] |
| Synthetic media | 12 | PV | Sulfonated polybenzimidazole | 50 | NA | NA | NA | [57] |
| Synthetic acetic acid | - | PV | Poly(vinyl alcohol) crosslinked with glutaraldehyde | 80–85 | NA | NA | 80–85 | [58] |
| Acetic acid— water mixture | 33 | PV | Silicate | 90 | NA | NA | NA | [59] |
| Raw Materials [-] | Total VFA Production [g/L] | Membrane-Based Process [-] | Membrane Materials [-] | Recovery [%] | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Valeric Acid | Butyric Acid | Total VFA | |||||
| Wastewater | 4 | MD | Hydrophobic | 53 | NA | NA | NA | [19] |
| Wastewater | - | Direct Contact | Hydrophobic porous | 50 | NA | NA | NA | [61] |
| Wastewater | - | Air Gap Membrane | Hydrophobic with an air gap | 10 | NA | NA | 95 | [19] |
| Wastewater | Vacuum MD | Polyvinylidene fluoride (PVDF) hollow fiber (HF) | 12 | NA | NA | 99 | [62] | |
| Synthetic media | 2 | MD | Polypropylene | NA | NA | NA | 87.5 | [63] |
| Raw Materials [-] | Total VFA Production [g/L] | Membrane-Based Process [-] | Membrane Materials [-] | Recovery [%] | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Other acids | Total VFA | |||||
| OFMSW leachate | 18.521 | MC | PTFE Membranes | 86–95 | NA | NA | NA | [28] |
| Municipal waste | 25 | MC | Modified PTFE/Silicone | NA | NA | Caproic acid: 41.5 | 86–95 | [78] |
| Real fermentate | NA | MC | Silicone Membranes | 72 | NA | Butyric acid: 21.5% | NA | [79] |
| Fermented beverages | 12–14 | MC | Polypropylene | 86 | NA | NA | 86 | [83] |
| Raw Materials [-] | MBR Configuration [-] | Membrane Materials [-] | Membrane Characteristics [-] | Operation Condition | VFA Produced [g/L] | VFA Recovered [%] | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp [°C] | pH [-] | OLR [g /L] | HRT [day] | |||||||
| Apple pomace | Integrated permeate channel flat sheet | PES membranes | Polyester spacer fabric coatings | 80 °C | 6 | 3.7 | 20–30 | 27–40 | 80 | [29] |
| Citrus waste | Tubular membrane | Polypropylene | Tubular membrane | 37 °C | 6 | 10 | 2.3–13.6 | 22 | 99 | [85] |
| Food waste | Submerged flat sheet | Flat sheet membrane | Pore size: 0.1–0.4 µm | 35–55 °C | 6.0 | 2 | 15 | 1.08 | NA | [86] |
| Chicken manure | Anaerobic membrane bioreactor (AnMBR) | Polyether sulfone (PES)/PVP | Pore size: 0.3 µm | 37 °C | 6.0 | 2–4 | 10 | 30 | 42% | [87] |
| Kitchen waste slurry | Anaerobic Membrane Bioreactors (AnMBRs) | Polyvinyl fluoride | Pore size: 0.2 µm | 38 | 6 | 2 | 45 | 14.6 | 32% | [88] |
| Membrane Process | Membrane Type/Material | VFA Targeted | Permeability (L/(m2·h·bar)) | Selectivity (LMH) | Ref. |
|---|---|---|---|---|---|
| Nanofiltration (NF) | NF90 (Polyamide) | Acetic acid, Propionic acid | 3.3 | 0.3 | [102] |
| Nanofiltration | NF270 (Polyamide) | Acetic acid, Butyric acid | 7.5 | 22 | [103] |
| Reverse Osmosis (RO) | RO 98pHt (Polyamide) | Acetic acid | 2.5 | 22 | [104] |
| Pervaporation (PV) | TDDA-PTFE (Composite) | Mixed VFAs | 13.12–14.21 (g/m2·h) | Acetic acid: 0.5 Propionic acid: 0.6 Butyric acid: 0.5 Valeric acid: 1.5 Caproic acid: 3 | [105] |
| Ultrafiltration (UF) | ES10 (Polyether sulfonate) | Acetic acid | 1.67 | Moderate | [106] |
| Microfiltration (MF) | Ceramic (Al2O3) | VFAs | Low | Low | [107] |
| Membrane Material | Stability Indicator | VFA Recovery/Performance | Conditions | Key Findings | Ref. |
|---|---|---|---|---|---|
| Polymeric | Moderate; sensitive to acidity | 65–75% recovery under mild acidic conditions | pH 5–7; HRT 1500 h; | Cost-effective, flexible | [115] |
| Ceramic | High; acid-resistant | 80–85% recovery | pH 3–7. lifetime > 2000 h; | Higher cost | [116] |
| Composite | Enhanced; oxidant resistant | 78–80% recovery | Lifetime 2500–3000 h; | High ion exclusion | [35] |
| Supported Ionic Liquid | Moderate; temperature sensitive | 70% recovery | Temperature range: 25–40 °C; | Selectivity driven by polarity | [117] |
| Ion Exchange Resins | High fouling resistance; moderate chemical stability; temperature-sensitive | PTFE: >75% recovery PVDF: ~70–80% recovery | Effective in acidic conditions; | Temperature variations affect performance | [118] |
| Technology /Study | CAPEX | OPEX | Revenue/Profit | Key Observations | Ref. |
|---|---|---|---|---|---|
| Membrane Distillation (Brown algae) | $21.8 M | $1.2 M/year (maintenance included); Utilities: $15.8 M/year | $50.6 M/year | High revenue: cost-effective | [13] |
| Electrodialysis (ED) | ED Unit: $12.89 M Stripping Column: $2.28 M Absorbing Column: $2.74 M | Energy: 5.43 kWh-kg/VFA ($0.695/kWh); Maintenance: 3% of CAPEX | MSP: $2.25/kg biobutanol | Effective VFA recovery | [138] |
| Reverse Osmosis (RO) | Direct Cost: $44.58 M Indirect Cost: $13.37 M | Power: $3.60 M/year Membrane Replacement: $1.74 M/year Total: $7.63 M/year | - | High initial costs: maintenance is expensive. | [139] |
| Nanofiltration (NF) | Direct Cost: $1.04 M Indirect Cost: $0.30 M Total: $1.34 M | $107,360.66 M/year | - | Lower CAPEX; Suitable for smaller-scale applications. | [24] |
| Hybrid (RO + MD) | RO: $5.90 M/year MD: $4.45 M/year Total: $5.02 M/year | RO: $7.63 M/year MD: $12.23 M/year Hybrid: $8.60 M/year | - | Combines benefits of RO and MD but increases complexity and cost. | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhuri, A.; Harahap, B.M.; Ahring, B.K. Membrane Technologies for Separating Volatile Fatty Acids Produced Through Arrested Anaerobic Digestion: A Review. Clean Technol. 2025, 7, 48. https://doi.org/10.3390/cleantechnol7020048
Chaudhuri A, Harahap BM, Ahring BK. Membrane Technologies for Separating Volatile Fatty Acids Produced Through Arrested Anaerobic Digestion: A Review. Clean Technologies. 2025; 7(2):48. https://doi.org/10.3390/cleantechnol7020048
Chicago/Turabian StyleChaudhuri, Angana, Budi Mandra Harahap, and Birgitte K. Ahring. 2025. "Membrane Technologies for Separating Volatile Fatty Acids Produced Through Arrested Anaerobic Digestion: A Review" Clean Technologies 7, no. 2: 48. https://doi.org/10.3390/cleantechnol7020048
APA StyleChaudhuri, A., Harahap, B. M., & Ahring, B. K. (2025). Membrane Technologies for Separating Volatile Fatty Acids Produced Through Arrested Anaerobic Digestion: A Review. Clean Technologies, 7(2), 48. https://doi.org/10.3390/cleantechnol7020048







