3.1. Water pH Dynamics During DCF Ozonation
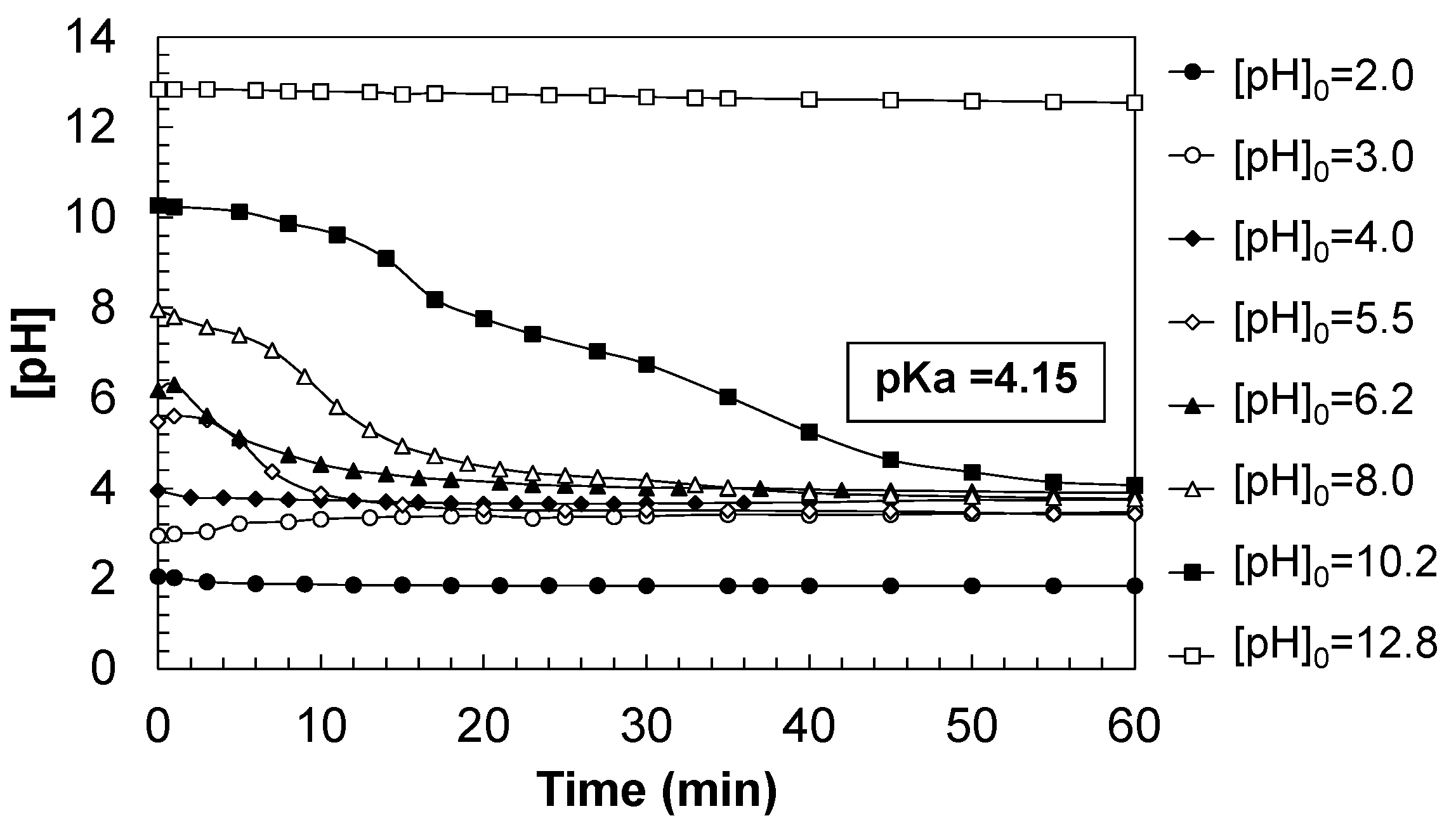
Figure 2 shows the evolution of water pH during the ozonation of diclofenac (DCF), under varying initial pH conditions ranging from highly acidic (pH
0 = 2.0) to strongly basic (pH
0 = 12.8). The pH behavior throughout the process is significantly influenced by the starting pH. At extreme acidic and basic conditions (pH
0 = 2.0 and 12.8), the pH remains practically constant during the entire treatment, indicating a buffering capacity at those extremes. In contrast, under mildly acidic to neutral conditions (pH
0 = 3.0–6.2), the pH tends to stabilize near the pKa value of DCF (≈4.15), suggesting equilibrium between the protonated and deprotonated species. Under moderately basic conditions (pH
0 = 8.0–10.2), a progressive pH decrease is observed as ozonation proceeds.
These pH variations are primarily due to redox reactions between ozone and DCF, as well as the formation of acidic species during the degradation of the contaminant. DCF is a weak acid with a pKa ≈ 4.15, and its acid-base dissociation is described as follows (Equation (1)):
At pH values above 4.15, the anionic form (DCF
−) predominates, while at lower pH values, the neutral (protonated) form is dominant. This dissociation affects both the reactivity of DCF with ozone and the stability of the pH of the system. During treatment, ozone reacts directly with the aromatic rings of the DCF molecule, generating oxygenated intermediates, such as iminoquinones and phenolic compounds, via reactions of the form (Equation (2)) [
17]:
These intermediates can undergo further oxidation, eventually forming low-molecular-weight carboxylic acids (e.g., oxalic or acetic acid), contributing to a decrease in pH (Equation (3)). This acid formation is especially evident under neutral or slightly basic conditions [
17]. Some of these intermediates, such as quinones or substituted aromatic acids, are known to interact with biomolecules such as proteins or DNA, potentially leading to oxidative stress or mutagenesis. Future studies should assess their persistence and toxicity using bioassays or molecular docking to evaluate potential interactions with key enzymes or receptors in aquatic organisms and humans.
In basic media, ozone decomposes more rapidly due to its reaction with hydroxide ions (OH
−), leading to the formation of highly reactive hydroxyl radicals through a radical chain mechanism typical of advanced oxidation processes (AOPs). This pathway can be summarized as follows [
24]:
Hydroxyl ion attack on ozone: the hydroxyl ion acts as a nucleophile and attacks ozone, generating a peroxide anion (
) and molecular oxygen (Equation (4)):
Acid–base equilibrium of the peroxide anion: the peroxide anion can be protonated, depending on the pH, forming the perhydroxyl radical (
), a precursor species of hydroxyl radicals (Equation (5)):
Formation of hydroxyl radicals: the perhydroxyl radical reacts with ozone, producing the superoxide radical (
) and the hydroxyl radical (
) (Equation (6))
Propagation reactions: the generated radicals (HO
•, O
2•−) continue to attack other ozone molecules and organic compounds such as DCF, amplifying radical production and promoting non-selective degradation (Equations (7) and (8)):
The radicals formed (HO•, O2•−) continue reacting with ozone and organic molecules such as DCF and its intermediates, promoting a non-selective oxidation. However, these reactions also lead to the generation of organic acids, which, over time, contribute to the gradual acidification of the solution, particularly evident at initial alkaline pH values such as 10.2.
Therefore, in view of the results obtained, it can be stated that the pH evolution during DCF ozonation reflects a complex balance between acid generation and the buffering capacity of the system. The pH stabilizes near the DCF pKa under mildly acidic or neutral conditions, while under alkaline conditions, progressive acidification occurs due to organic acid formation. In extreme media (strongly acidic or basic), the buffering capacity or limited reactivity results in a nearly constant pH. These findings highlight the critical role of pH in determining both the efficiency and the selectivity of the ozonation process.
3.2. First-Order Kinetics of DCF Ozonation
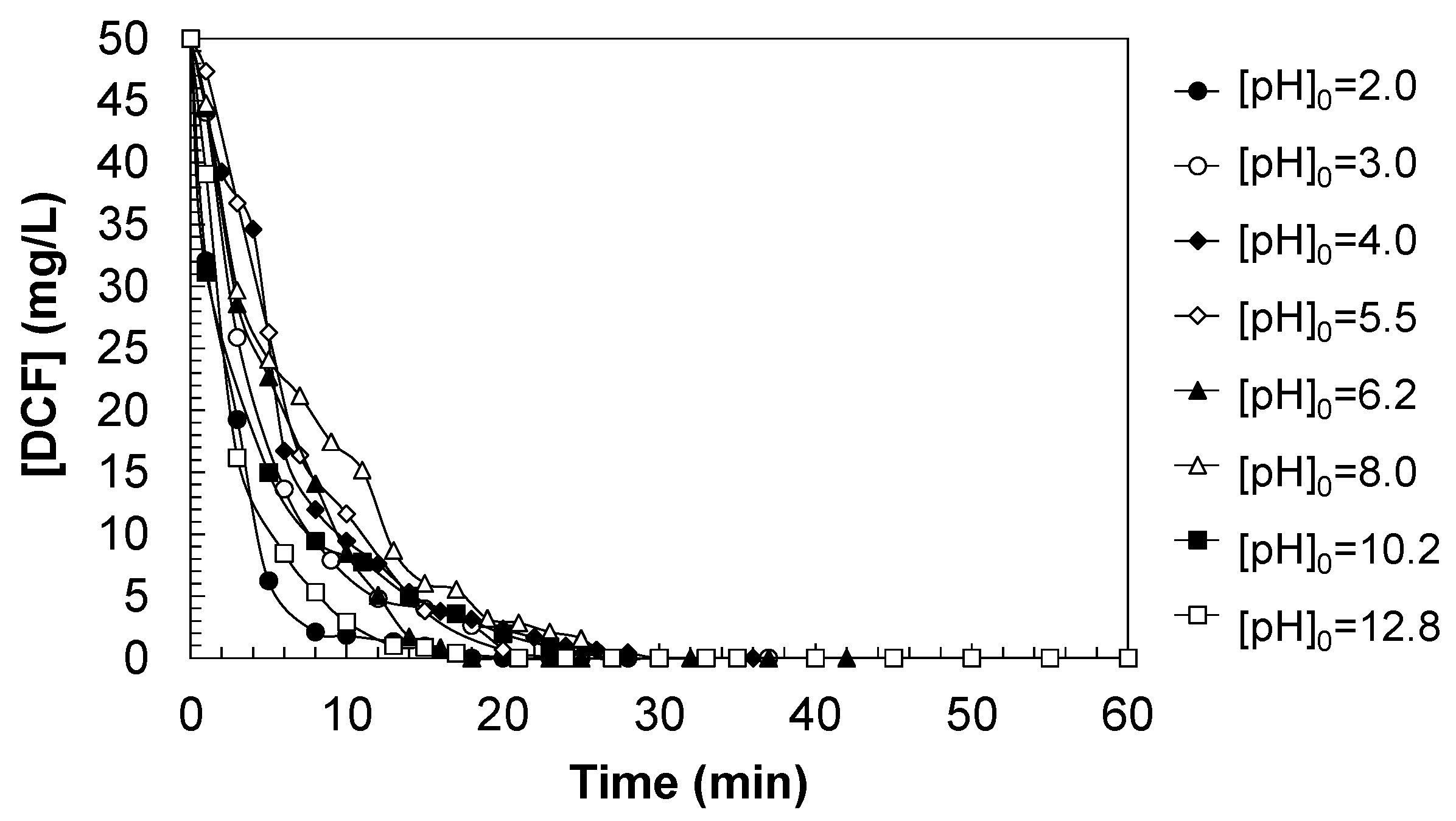
Figure 3 illustrates the effect of initial pH on the ozonation kinetics of DCF. The degradation profiles were evaluated under a wide pH range, from strongly acidic (pH
0 = 2.0) to strongly alkaline conditions (pH
0 = 12.8), maintaining a constant ozone dosage and temperature. Under highly acidic conditions (pH
0 = 3.0), DCF degradation proceeded rapidly during the first 10 min of reaction, achieving nearly complete removal within 20 min. This behavior may be attributed to the molecular (protonated) form of DCF, which exhibits higher reactivity towards molecular ozone (O
3), a selective electrophilic oxidant (see Equation (1)). Likewise, at mildly acidic-to-neutral pH values (pH
0 = 4.0–6.2), the degradation rate remained high, with the pH stabilizing near the pKa = 4.15, suggesting a dynamic equilibrium between protonated and deprotonated species that favors ozone attack. Molecular ozone can directly react with electron-rich aromatic moieties of DCF, leading to the formation of oxygenated intermediates and proton release (see Equation (2)).
As the initial pH increased to moderately alkaline values (pH0 = 8.0), a decrease in the degradation rate was observed. This effect is consistent with the increased prevalence of the anionic form of DCF (DCF−), which shows reduced reactivity towards ozone but can be attacked by hydroxyl radicals formed from ozone decomposition under alkaline conditions (see Equations (4)–(8)). Despite this, the competition between radical reactions and scavenging by carbonate/bicarbonate ions may hinder the overall efficiency of the process. At a strongly basic pH (pH0 = 12.8), the degradation rate was high in the initial stages. This behavior could be explained by the rapid ozone decomposition to radicals in the presence of hydroxide ions (OH−). Additionally, the system may become buffered, limiting further pH-induced reactivity changes.
Overall, the results demonstrate that ozonation of DCF is pH-dependent, with optimal degradation observed under acidic to mildly neutral conditions. The interplay between DCF speciation and ozone/radical reactivity governs the degradation kinetics and should be considered for the optimization of ozonation processes in wastewater treatment applications.
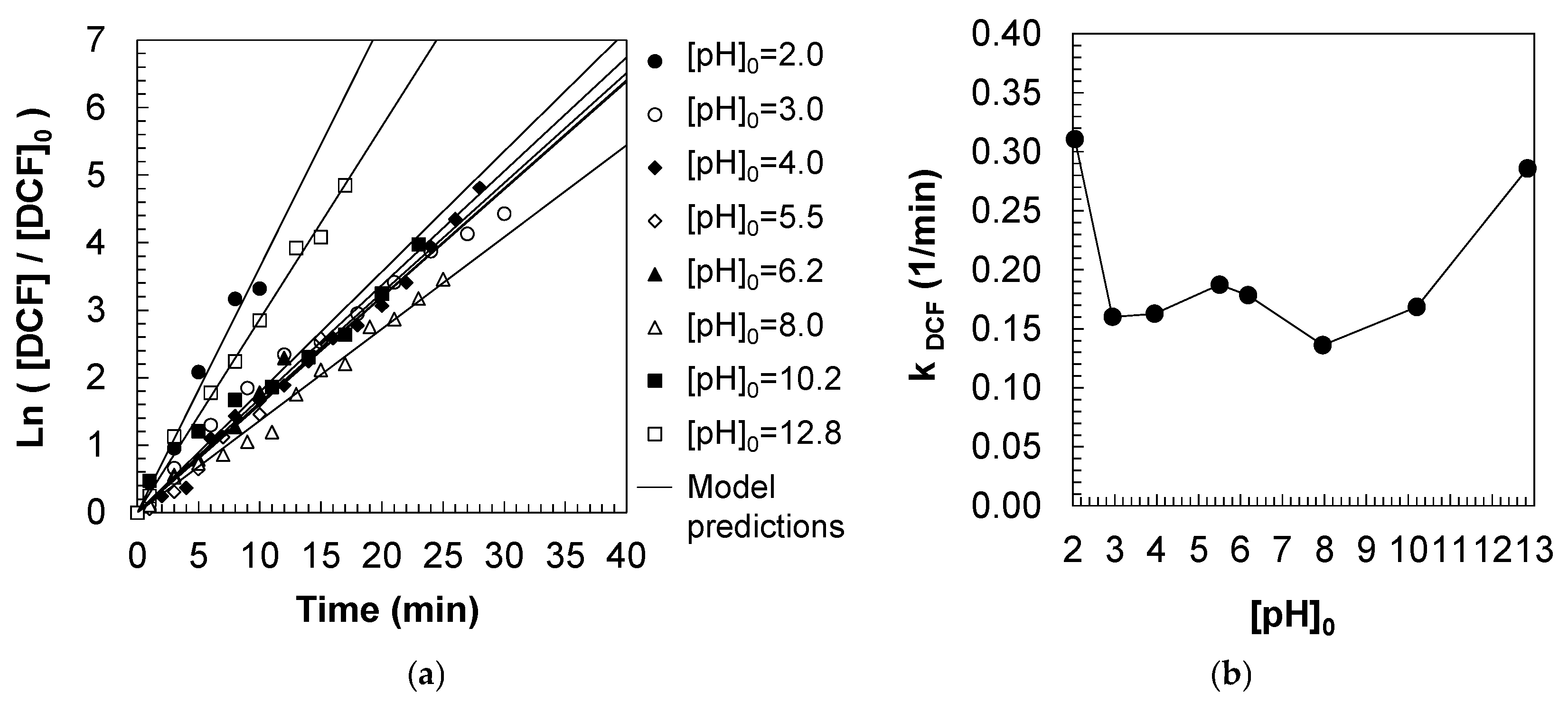
A pseudo-first-order kinetic model was employed to describe the degradation of DCF within the ozone contactor, based on the assumption that DCF is progressively converted into oxidized intermediates and by-products (Equation (9)). Integration of the corresponding mass balance (Equation (10)) yielded the first-order rate expression for DCF degradation (Equation (11)). Time-resolved concentration profiles of DCF were obtained experimentally, and the apparent pseudo-first-order rate constants (k
DCF, 1/min) were determined from the slopes of the linearized kinetic plots, as shown in
Figure 4a. These rate constants were subsequently correlated with the initial pH values ([pH]
0), as depicted in
Figure 4b.
Mass balance for the degradation of DCF:
First-order kinetic equation for degradation of DCF:
Figure 4b illustrates the variation in the apparent pseudo-first-order rate constant (k
DCF, 1/min) for the ozonation of DCF as a function of the initial pH (pH
0) of the aqueous solution. The degradation kinetics exhibit a pronounced pH dependence, delineating distinct oxidative regimes across the investigated range (pH 2.0–13.0). At strongly acidic conditions (pH ~ 2.5–3.0), the reaction rate is maximized, with k
DCF reaching approximately 0.30 1/min. This high efficiency is attributed to the greater stability of molecular ozone in acidic media and its enhanced selectivity toward electron-rich aromatic structures present in the DCF molecule. Ozone, in its molecular form, acts as the primary oxidant in this regime, facilitating rapid degradation. A notable decrease in the rate constant is observed between pH 3 and 4, with values declining to ~0.15 1/min. This local minimum suggests a reduction in ozone reactivity or possible changes in DCF speciation that decrease its susceptibility to oxidation. Between pH 4 and 6.5, the rate constant increases moderately, reaching a secondary local maximum around pH 6. This behavior may be related to a transitional regime where both molecular ozone and a limited generation of hydroxyl radicals coexist.
As the pH approaches neutral to slightly basic values (pH 7–8), a second decline in kDCF is observed (~0.13 1/min), indicating a decrease in overall oxidation efficiency. In this range, neither molecular ozone nor hydroxyl radicals are present at concentrations sufficient to sustain high degradation rates, possibly due to reduced ozone stability and limited radical generation. Above pH 9, a sharp increase in the rate constant is observed, culminating in values of ~0.30 1/min at pH 12.5. This marked enhancement is consistent with the decomposition of ozone to yield hydroxyl radicals, which dominate the oxidation mechanism under alkaline conditions. These radicals are highly reactive and non-selective, enabling an efficient breakdown of a wide range of organic moieties, including those found in DCF.
Overall, the degradation of DCF via ozonation is most efficient under strongly acidic and alkaline conditions, where molecular ozone and hydroxyl radicals, respectively, act as the primary oxidants. In contrast, ozonation efficiency is substantially reduced in the intermediate pH range, particularly near neutrality. These results underscore the critical role of pH in controlling the oxidation pathway and efficiency, and highlight the importance of pH optimization for minimizing residual DCF and potentially toxic transformation products in water treatment applications.
To complete this analysis, it is necessary to consider the solubility of DCF in water. As shown in
Figure 5, the aqueous solubility of DCF exhibits a strong dependence on pH, which, in turn, influences its availability for ozonation. At low pH values (1–3), DCF exists predominantly in its neutral (non-ionized) form, which is poorly soluble in water, with solubility levels below 5 mg/L. This low solubility limits the amount of DCF available in the aqueous phase, potentially restricting the extent of reaction despite the high oxidative efficiency of molecular ozone under acidic conditions. As the pH increases to around 4–5, DCF solubility rises sharply, reflecting the progressive ionization of the carboxylic acid group. At pH ≥ 6, solubility stabilizes near its maximum (~50 mg/L), indicating that DCF is predominantly present in its anionic form, which is significantly more hydrophilic.
The degradation rate constant (k
DCF) does not follow a linear relationship with solubility. This is due to the interplay between DCF speciation, oxidant reactivity, and the prevailing oxidation mechanism. In acidic media, although DCF solubility is limited, molecular ozone remains stable and reacts efficiently with the neutral DCF form. In alkaline conditions, where DCF is fully soluble and ionized, ozone decomposes into hydroxyl radicals, which exhibit higher reactivity but lower selectivity. Therefore, the overall degradation efficiency reflects not only the aqueous availability of DCF but also the chemical behavior and transformation pathways of the oxidants involved. Together, the trends in
Figure 4b and 4 highlight the multifaceted influence of pH on ozonation performance, affecting both contaminant solubility and oxidant speciation. The optimal conditions for DCF removal arise from a balance between its solubility, ionization state, and the nature of the oxidizing species, underscoring the importance of integrated pH control strategies in advanced water treatment processes.
3.3. UV/Vis Spectral Analysis of DCF During Ozonation: Reaction Dynamics
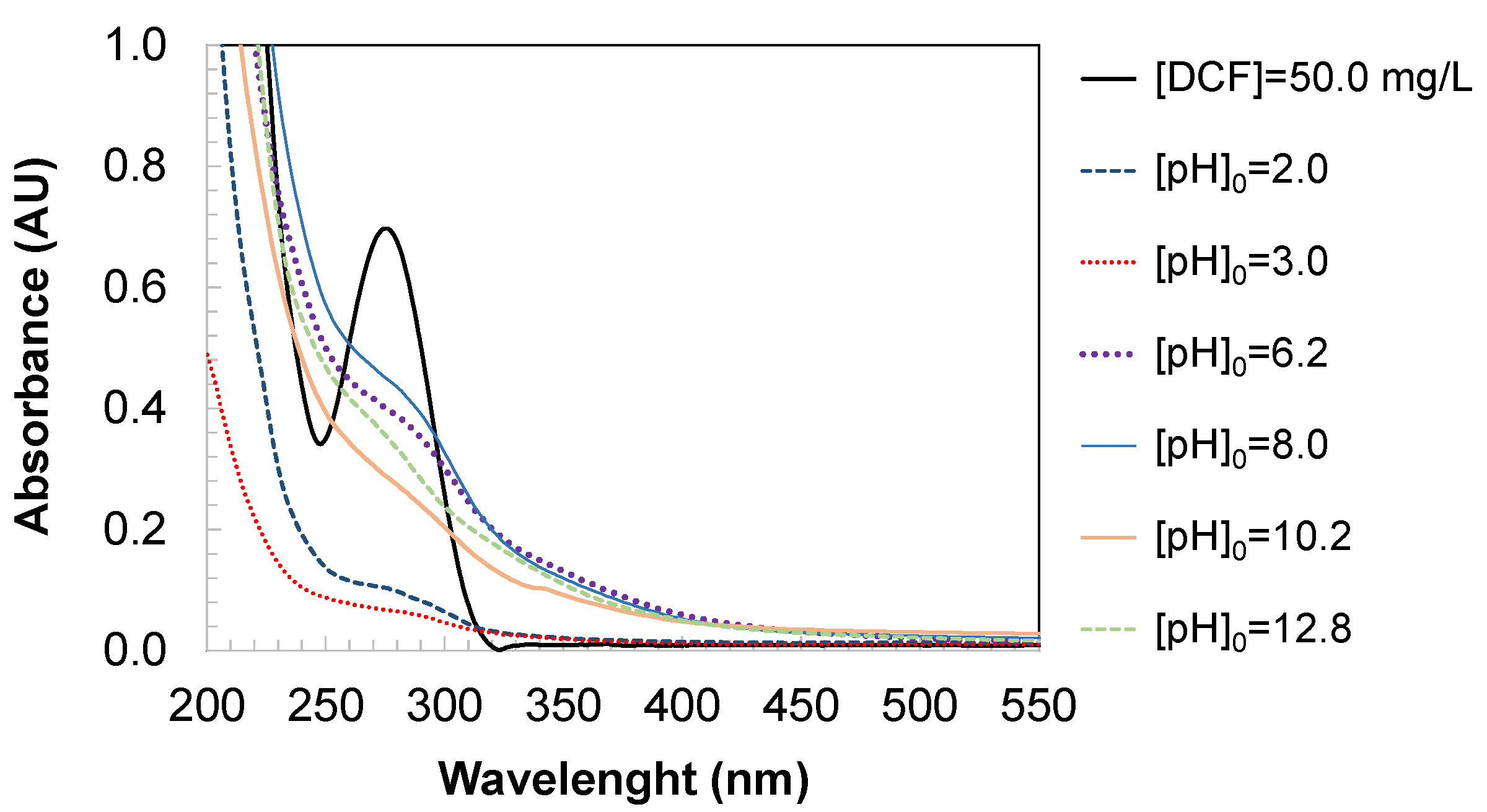
Figure 6 presents the UV/Vis absorption spectra obtained after the ozonation of aqueous DCF solutions at different initial pH values under controlled experimental conditions. The UV/Vis spectrum of DCF exhibits two characteristic absorption bands: a strong peak at 276 nm, attributed to the aromatic system, and a broader band in the deep UV region (<240 nm), corresponding to electronic transitions associated with the dichloroaniline ring and the conjugated carboxylic group. Following ozonation, a marked decrease in absorbance is observed in both spectral regions.
During the reaction, ozone attacks conjugated double bonds and aromatic rings. This leads to a reduction in the intensity of these bands as the DCF molecule undergoes degradation. The progressive breakdown of aromatic structures is reflected in a continuous decline in absorbance at these wavelengths.
Figure 7 shows the aromaticity of water analyzing during the DCF ozonation.
The aromaticity of aqueous solutions containing DCF when ozonized follows the next behavior: it increases in the first 10–20 min and then decreases until it stabilizes. This phenomenon can be explained by the intermediate reactions that occur during ozonation and the structural changes that diclofenac undergoes. The initial increase in aromaticity during ozonation of DCF is due to the formation of intermediates with greater conjugation or more aromatic rings. Subsequently, a more complete degradation of these structures by ozone leads to a decrease in aromaticity, until most of the aromatic compounds have been destroyed and the system reaches a stable value.
DCF is a compound that contains two aromatic (benzene) rings. These rings are responsible for its absorption characteristic in the UV range and, therefore, for its aromaticity. During the first minutes of ozonation, ozone reacts with DCF, but the first reactions are usually partial oxidations that can open or modify certain bonds of DCF, generating intermediate products that increase aromaticity. It is possible that these intermediates are compounds with additional or more conjugated aromatic structures, increasing the absorbance and thus the apparent aromaticity of the solution. This phenomenon has been observed in other AOPs, where partial fragmentation of compounds generates degradation products that still contain intact or even more reactive aromatic structures.
As ozone continues to react with DCF and its intermediates, ozone more completely attacks the aromatic rings. This leads to the opening of benzene rings and the formation of non-aromatic degradation products (such as carboxylic acids, ketones, or aliphatic compounds). During this process, aromaticity decreases because aromatic structures are destroyed or transformed into simpler, non-conjugated compounds. After a time, the degradation process reaches a point where most of the aromatic compounds have been transformed into non-aromatic products, such as low-molecular-weight acids. At this point, the aromaticity value stabilizes because the resulting products no longer contain aromatic structures or are minimally aromatic.
As ozonation proceeds, the original bands diminish and weaker new signals may emerge (
Figure 6), indicating the formation of degradation products. Over time, with sufficiently oxidation, the spectrum may reflect only the presence of inorganic species or final mineralization products such as nitrates or carboxylates, which typically absorb at shorter wavelengths and with significantly lower intensity than the parent compound (see
Figure 8).
It is important to note that during the ozonation of nitrogen-containing organic compounds, such as DCF, nitro compounds can form. These compounds may continue to oxidize and release nitrate into the aqueous solution. As DCF aromaticity decreases due to the breakdown of aromatic rings, nitrogen derivatives and eventually nitrates may be produced as degradation by-products. Nitrate concentration in the solution may increase as the ozonation process progresses, especially if nitrogen-containing products are generated and subsequently oxidized to nitrate.
On the other hand, oxygen scavengers (also called radical scavengers) are substances that react with free radicals, such as hydroxyl radicals, preventing their oxidative action. In aqueous ozone solutions, hydroxyl radicals are generated as part of secondary reactions of ozone. DCF can react directly with ozone but can also be degraded by hydroxyl radicals formed in the solution. Scavengers, such as bicarbonate (HCO3−) or alcohols, neutralize these radicals, limiting the oxidation of organic compounds by them. If scavenger concentration is high, DCF degradation by hydroxyl radicals will be lower, leading to a decrease in nitrate formation and other degradation products dependent on radical oxidation. Conversely, if scavenger concentration is low, hydroxyl radicals will be more available, increasing the degradation rate of DCF and the possible formation of nitro compounds and nitrates.
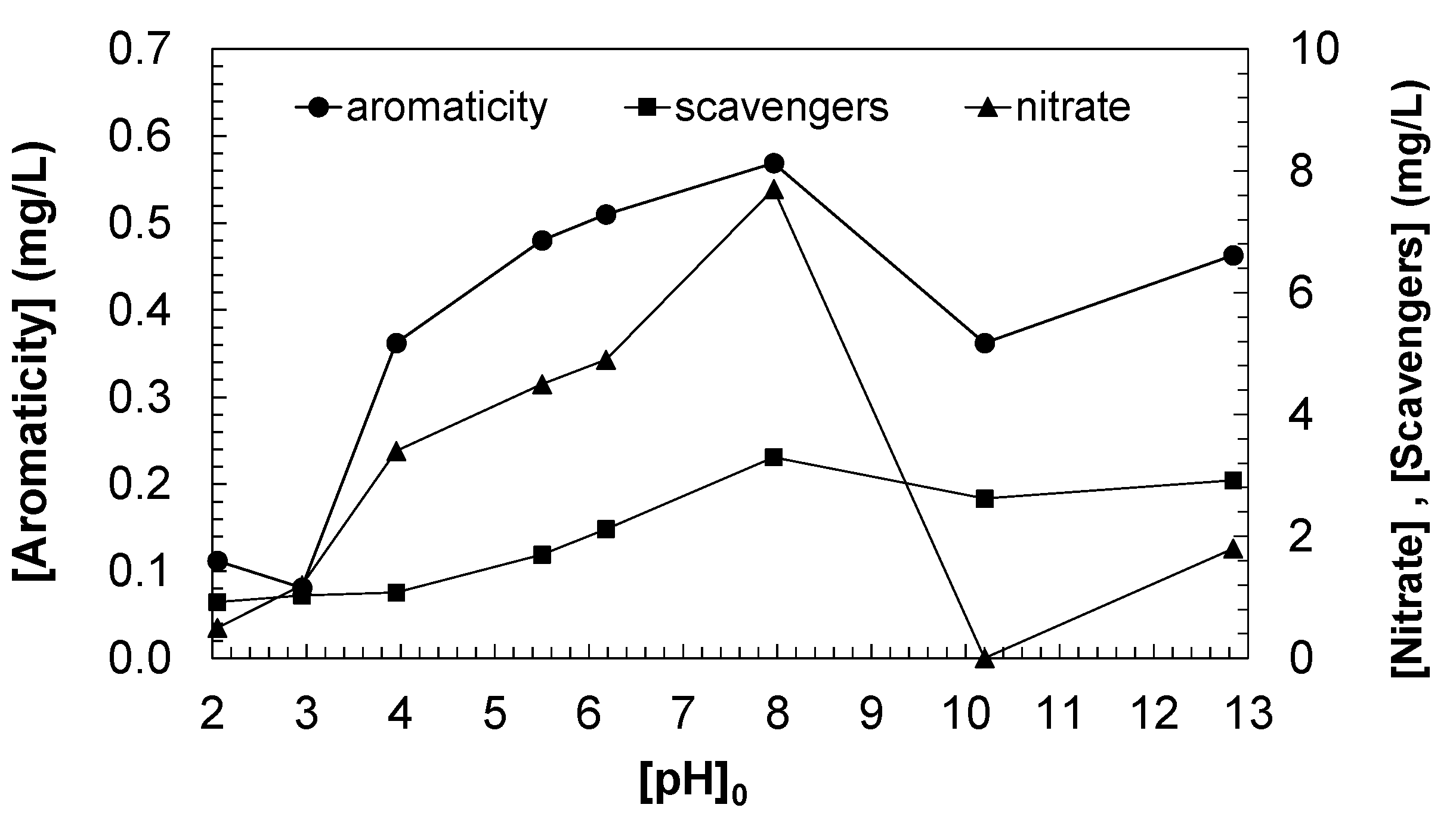
As shown in
Figure 8, there is a similarity among these parameters, confirming that the concentration of aromatic species, nitrate, and oxygen scavengers increases with the initial pH of the ozonated water, reaching maximum concentration when ozonizing DCF aqueous solutions with an initial pH of 8.0. However, when the treatment is carried out at initial pH values higher than 10, the concentration of aromatic species in the treated water, as well as the concentration of scavengers and nitrate, decreases. This behavior illustrates how pH strongly influences ozone degradation mechanisms and its secondary products, modifying ozone stability, radical production, and degradation efficiency of the species present in the solution.
To explain these results, it is necessary to first analyze the behavior of ozone as a function of pH, since ozone is a strong oxidant and its reactivity depends significantly on the pH of the solution. At low (acidic or neutral) pH, ozone mainly acts as a molecular species that selectively attacks double bonds and aromatic rings. At higher (basic) pH levels, ozone decomposes more rapidly into radicals, mainly hydroxyl radicals, which are much more reactive and less selective than molecular ozone. Hydroxyl radical production is favored by the presence of hydroxide ions (OH−) in basic solutions. This means that in high-pH solutions, the oxidation mechanism changes, molecular ozone becomes less stable, and more free radicals are generated, which affects DCF degradation and the formation of secondary products.
At pH levels around 8.0, ozone remains relatively stable, but an appreciable amount of hydroxyl radicals is already being produced. In this pH range, there is a balance between direct degradation by molecular ozone (which attacks aromatic rings) and degradation by hydroxyl radicals. In this situation, DCF degradation is significant, but a large number of intermediate products still containing aromatic rings (aromatic species) are also generated, which explains the increase in the concentration of these species. Furthermore, partial degradation of DCF can release nitrogen-containing groups that are later oxidized to nitrates, contributing to the increase in nitrate concentration. As for oxygen scavengers (radical scavengers) like bicarbonates and other species, they may be present in increasing concentrations, as pH 8.0 is ideal for maintaining a balance between hydroxyl radical formation and the scavengers’ ability to neutralize these radicals.
At very high pH levels (above 10), the system behavior changes drastically. Ozone decomposes rapidly into hydroxyl radicals due to the high concentration of hydroxide ions in the water. This means that the amount of molecular ozone available to directly attack aromatic species (like DCF) is very low. Hydroxyl radicals are extremely reactive but poorly selective. In this highly basic system, radicals attack not only aromatic rings but also any other available molecules, promoting complete oxidation of the intermediate products. This leads to a rapid destruction of the aromatic species formed in the initial degradation steps. The decrease in aromatic species is due to hydroxyl radicals efficiently destroying intermediates that still contain aromatic groups.
Regarding nitrates, the high concentration of radicals in highly basic solutions may promote the complete oxidation of nitrogen-containing compounds but may also cause more advanced degradation products to be nitrogen gas (N2) or nitrites (NO2−) instead of nitrates, which would explain the decrease in nitrate concentration at this pH range. As regards oxygen scavengers like bicarbonate, under very-high-pH conditions, bicarbonate ions tend to convert into carbonate (CO32−), which may reduce their ability to act as scavengers. Additionally, hydroxyl radicals are so abundant and reactive that they exceed the scavengers capacity to neutralize them, leading to a decrease in their effective concentration in the solution.
3.4. Effect of pH on Water Color Evolution During DCF Ozonation: Physical Transformations
The spectral changes observed not only indicate the structural decomposition of DCF, but also reveal the transient formation of conjugated intermediates and chromophores—molecular structures that are often linked to pharmacological activity or environmental toxicity.
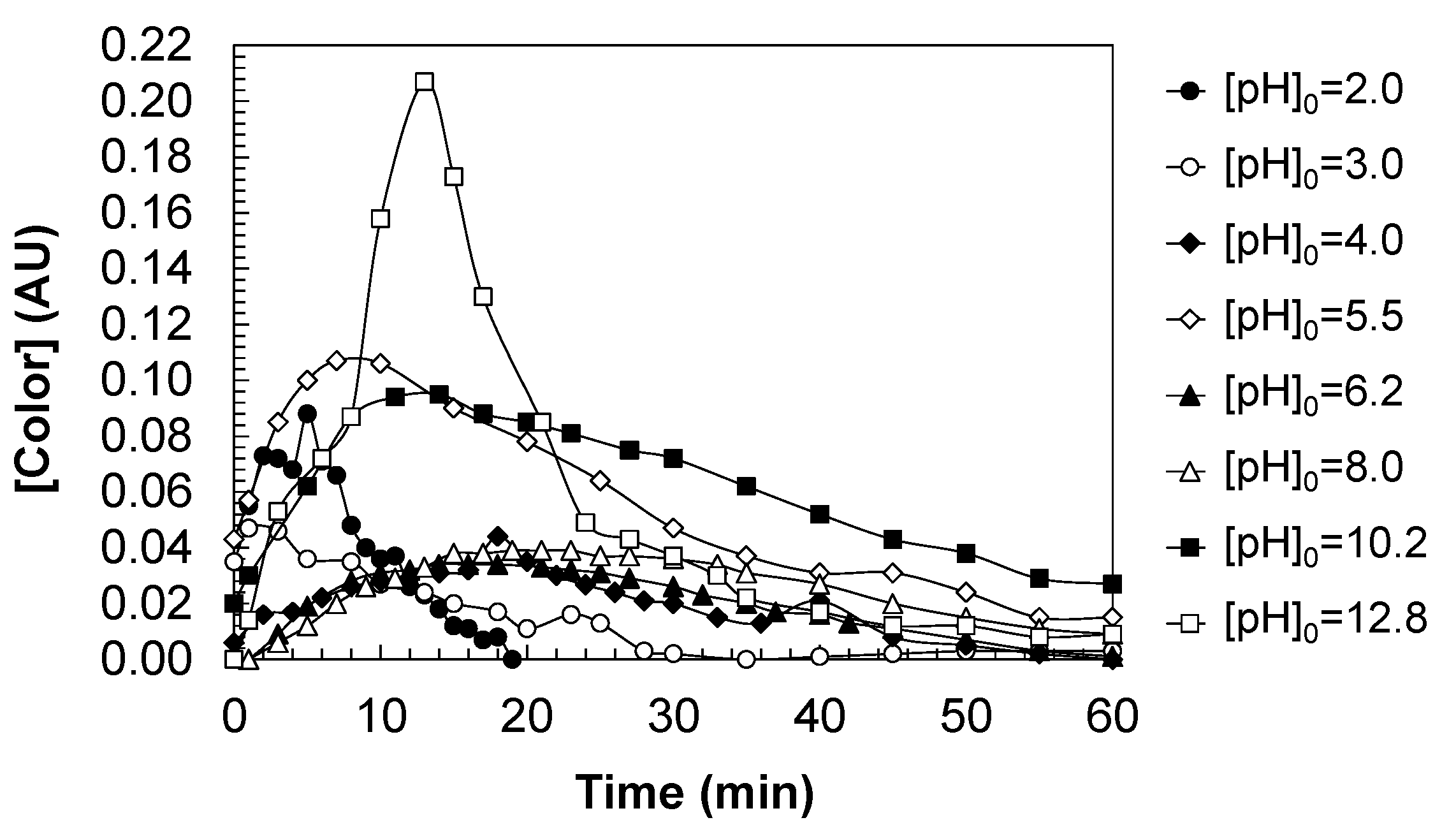
Figure 9 shows the formation of a yellow-brown color in the aqueous solutions during the DCF ozonation.
Aqueous solutions of DCF are initially colorless, but when DCF ozonized, the water turns a yellow color, the intensity of which depends on the operating conditions. Once a maximum value is reached, the color loses intensity until the treated water is colorless in appearance. Operating at [pH]0 = 12.8, the color forms abruptly, observing a pronounced peak because the ozonation conditions are strongly oxidizing and concentrate the formation of colored intermediates in a short period of time. Operating at lower pH values, the colored species’ generation is distributed throughout the reaction.
The formation of a yellow color in water during the ozonation of aqueous DCF solutions with ozone can be explained by the partial degradation of the diclofenac structure and the formation of decomposition intermediate compounds. This phenomenon occurs through the mechanisms of oxidation of DCF by ozone, where ozone attacks various parts of the DCF structure, including double bonds, aromatic rings, and functional groups. DCF contains aromatic rings (benzene-like) and functional groups susceptible to oxidation, such as the carboxylate group (-COO−) and chlorine atoms (-Cl). When ozone reacts with these parts of diclofenac, bonds are broken, leading to the formation of intermediate products like carboxylic acids, ketones, aldehydes, and quinones.
Next, the formation of chromophoric intermediate products occurs. During ozonation, intermediate products containing conjugated structures, that is, systems with alternating double bonds, such as oxidized aromatic compounds, quinones, or phenolic derivatives, are generated. These compounds are responsible for light absorption in the visible spectrum, which can result in a yellow coloration. A possible class of intermediate compounds are quinones, which are typical products of aromatic ring oxidation. Quinones, especially benzoquinones, are yellow compounds that can form when ozone oxidizes the aromatic system of DCF. Also, oxidized phenolic compounds can also produce a yellow color. Oxidation of the aromatic rings in DCF may lead to phenolic derivatives that exhibit coloration.
In addition, the ozonation process can generate low-molecular-weight products such as organic acids (e.g., oxalic acid, formic acid) or oxygenated fragments of the original DCF structures, which are more difficult to fully oxidize and may contribute to the yellow coloration. These by-products are hard to remove completely and may be responsible for the persistence of the yellow color in the solution. This color may gradually disappear as the oxidation process continues and the intermediate products degrade into simpler compounds, such as carbon dioxide and water, in the final stages of ozonation.
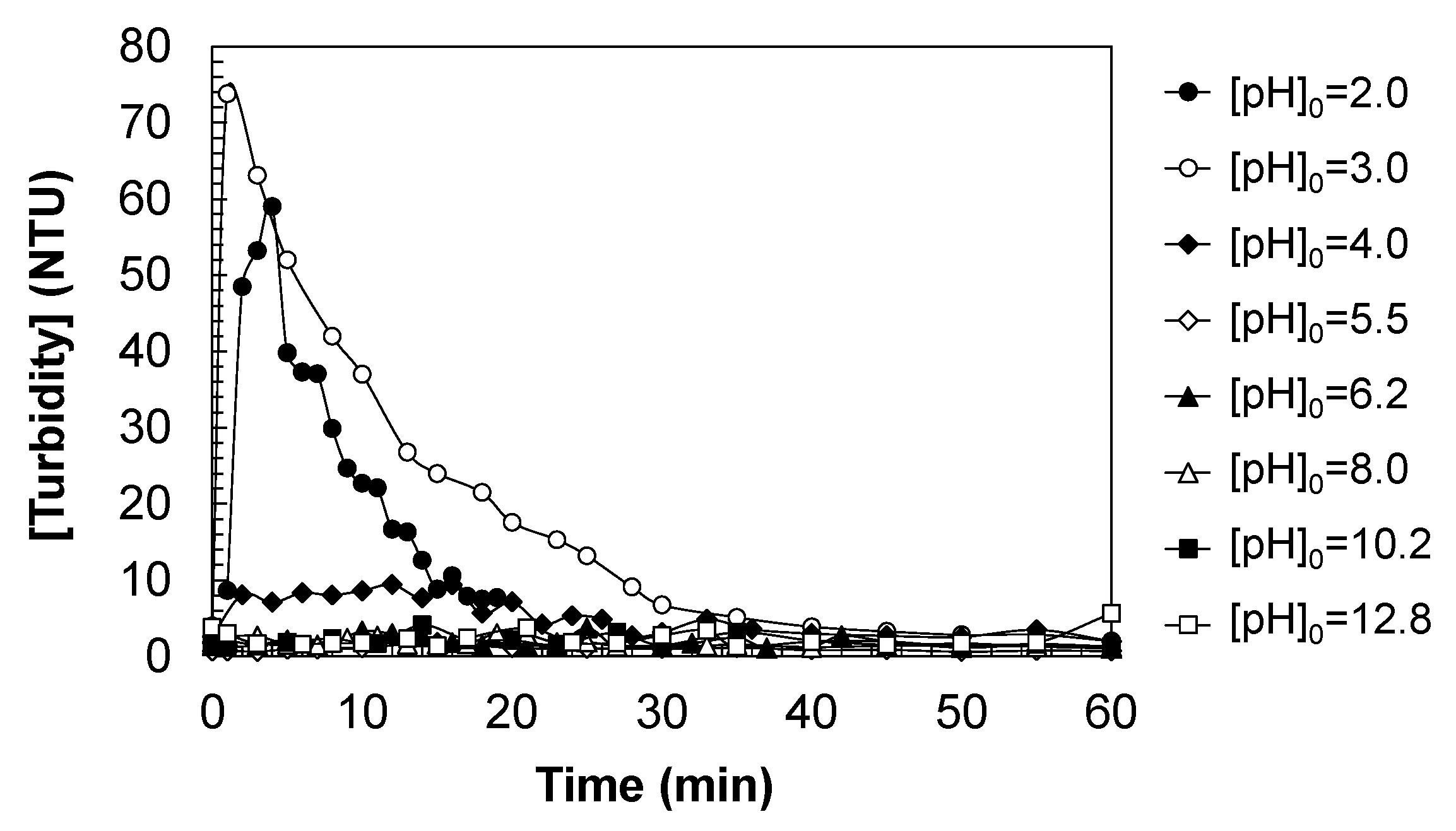
Beyond the progressive color changes observed during ozonation, additional physical transformations were also evident, particularly at low pH values, where a notable increase in turbidity was detected (
Figure 10). While color evolution is primarily attributed to the formation and subsequent degradation of soluble chromophoric by-products, the appearance of turbidity indicates the presence of suspended, insoluble phases. This phenomenon becomes especially relevant under acidic conditions, where the solubility of DCF (see
Figure 5) dramatically decreases, leading to visible precipitation.
Turbidity generation during DCF ozonation is strongly influenced by the initial pH of the solution, particularly under acidic conditions (pH0 < 4.0). As DCF is poorly soluble in water at low pH, the adjustment of the aqueous solution to acidic conditions results in the immediate formation of a white suspension. This turbidity corresponds to the precipitation of neutral DCF molecules, which are generated via protonation of the anionic carboxylate group (C14H10Cl2NO2−) into its non-ionized carboxylic acid form (-COOH). The loss of charge and increased hydrophobicity upon protonation significantly reduces solubility, leading to colloidal or particulate dispersion in the water matrix.
At an initial pH of 3.0, the maximum turbidity was recorded (~75 NTU), confirming that insolubilization of DCF is most pronounced under these conditions. This behavior aligns with the solubility profile previously discussed, where DCF remains largely non-ionized and poorly soluble below pH 4. Despite this turbidity, the ozonation process demonstrated high efficiency in degrading not only dissolved DCF but also the suspended fraction, ultimately yielding treated water with turbidity values below 1 NTU.
These results highlight the dual role of ozonation in both chemical oxidation and physical clarification, and emphasize the importance of pH control to minimize colloidal formation during treatment. Given the environmental persistence of DCF and the limitations of conventional treatment systems, the integration of ozonation as a pre-treatment stage prior to Nature-Based Solutions (NBSs) could be a promising strategy. The pre-oxidation of DCF at controlled pH values can reduce its toxicity and improve its biodegradability, potentially enhancing the performance of downstream biological systems such as constructed wetlands or soil aquifer treatment. Moreover, monitoring optical and physicochemical indicators during ozonation provides a practical tool for real-time optimization in decentralized water reuse schemes.