Influence of Fuel Types and Equivalence Ratios on NOx Emissions in Combustion: A Comparative Analysis of Methane, Methanol, Propane, and Hydrogen Blends
Abstract
1. Introduction
2. Literature Review
3. Model and Methods
4. Results
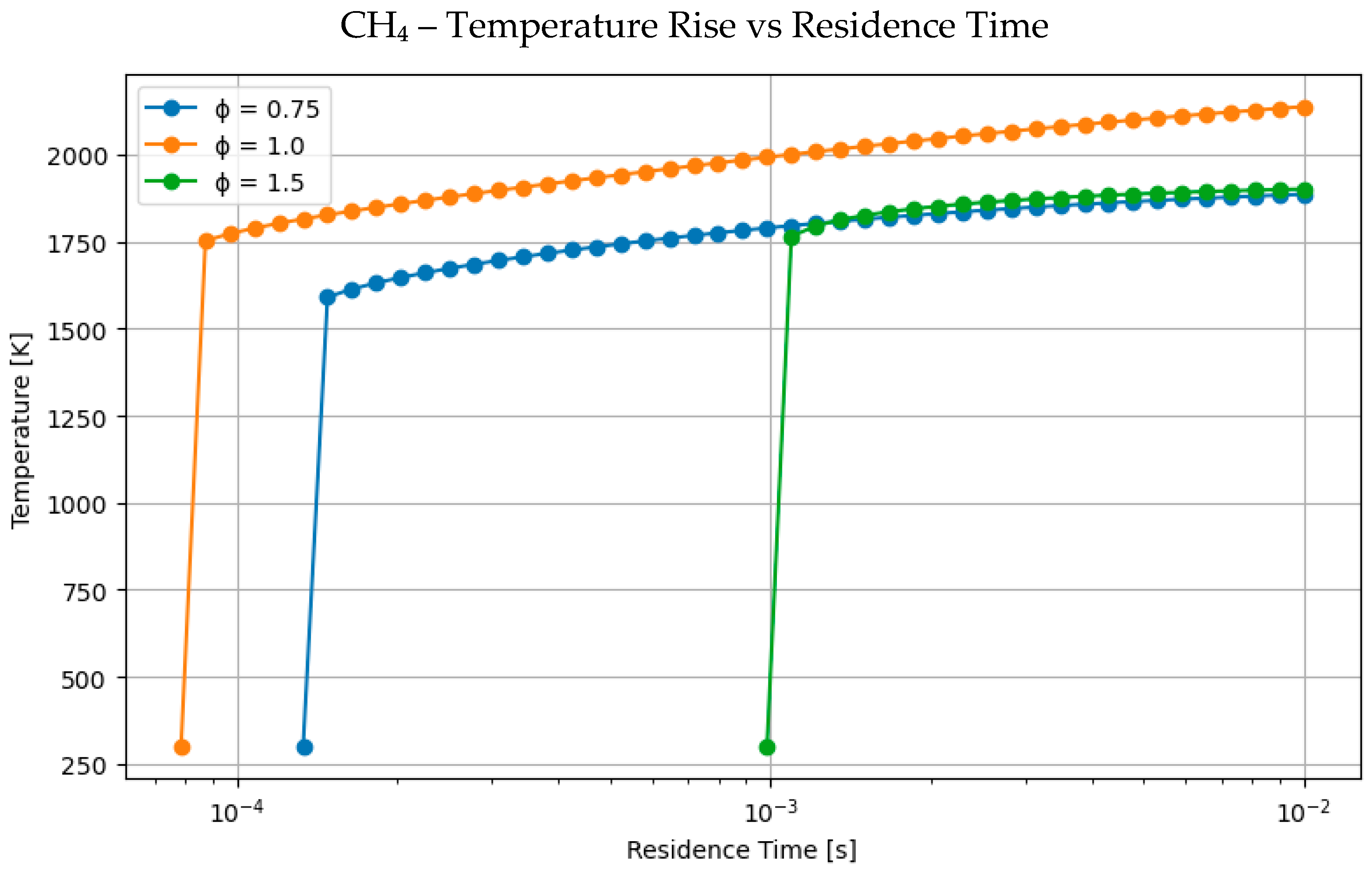
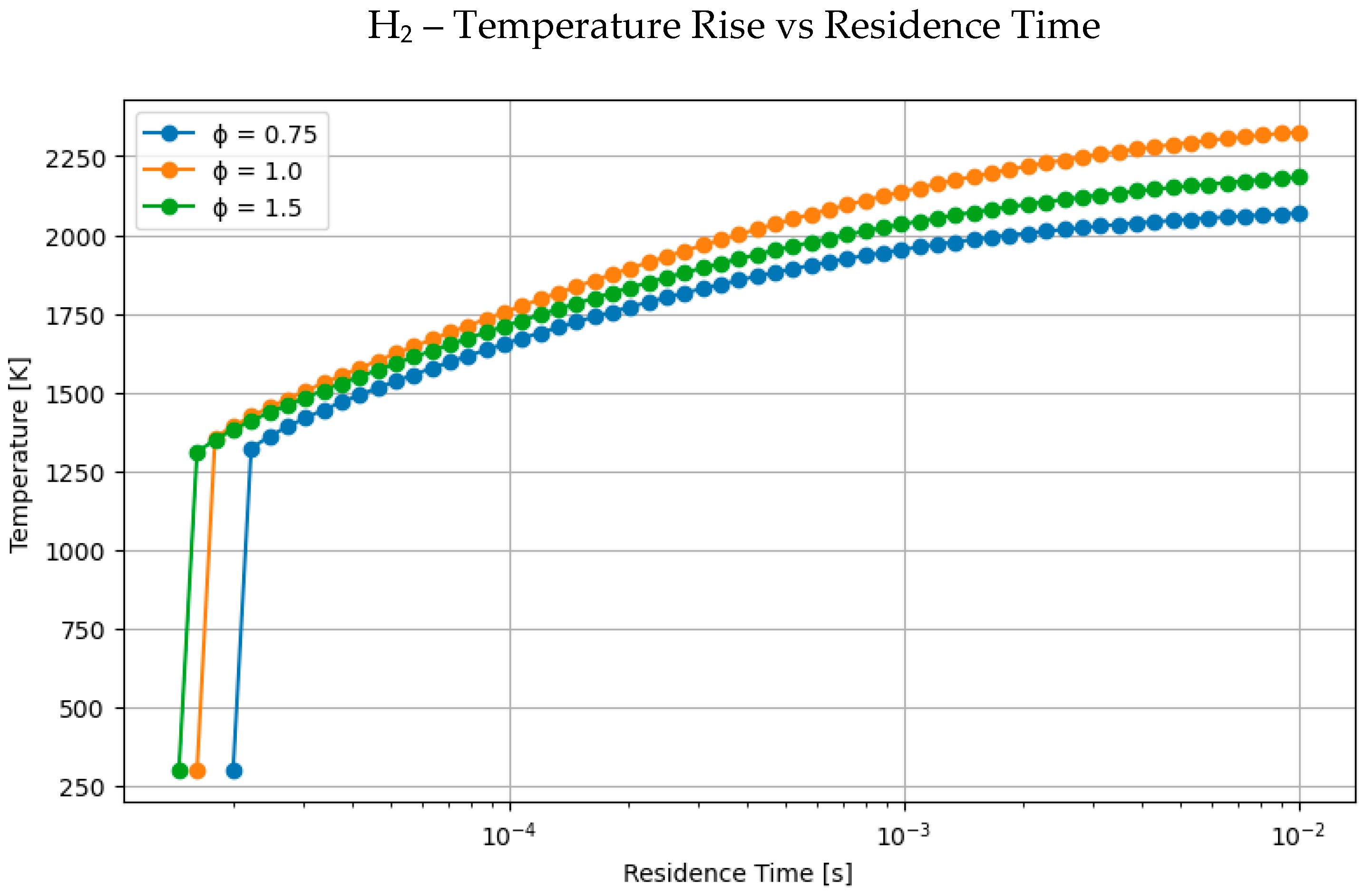
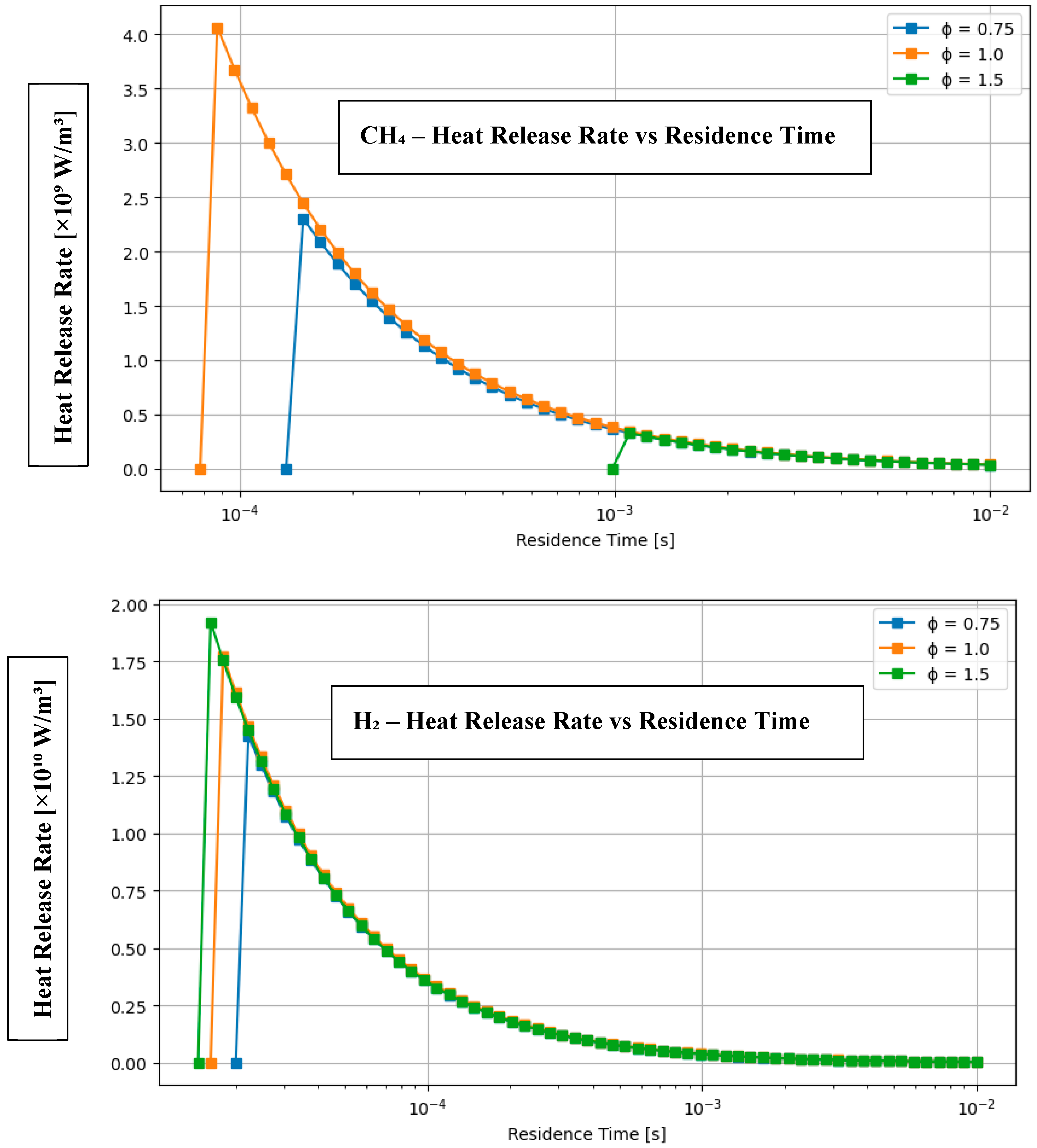
4.1. Comparative Analysis of Hydrogen and Methane Combustion: The Impact of Equivalence Ratio and Fuel Characteristics
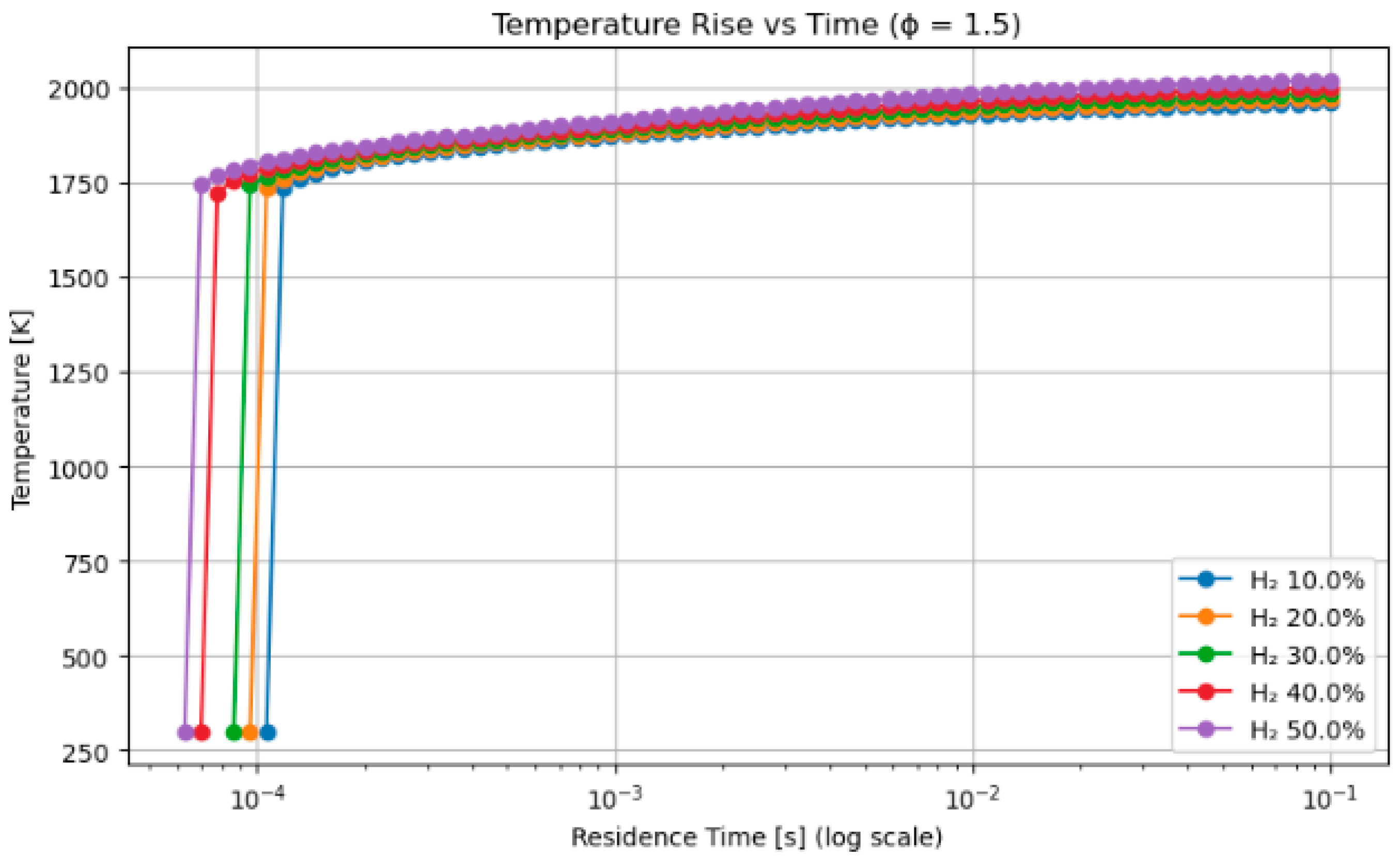
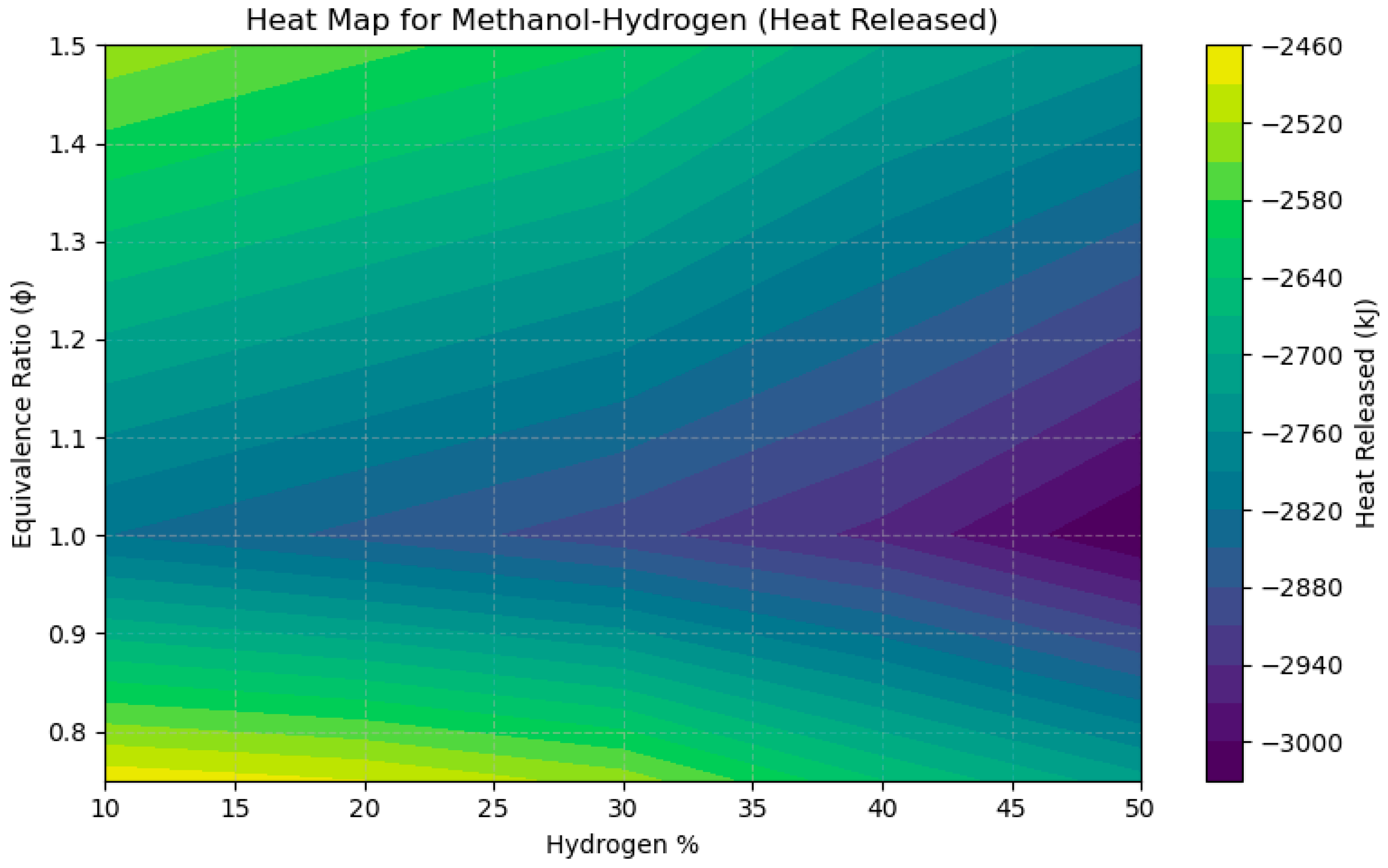
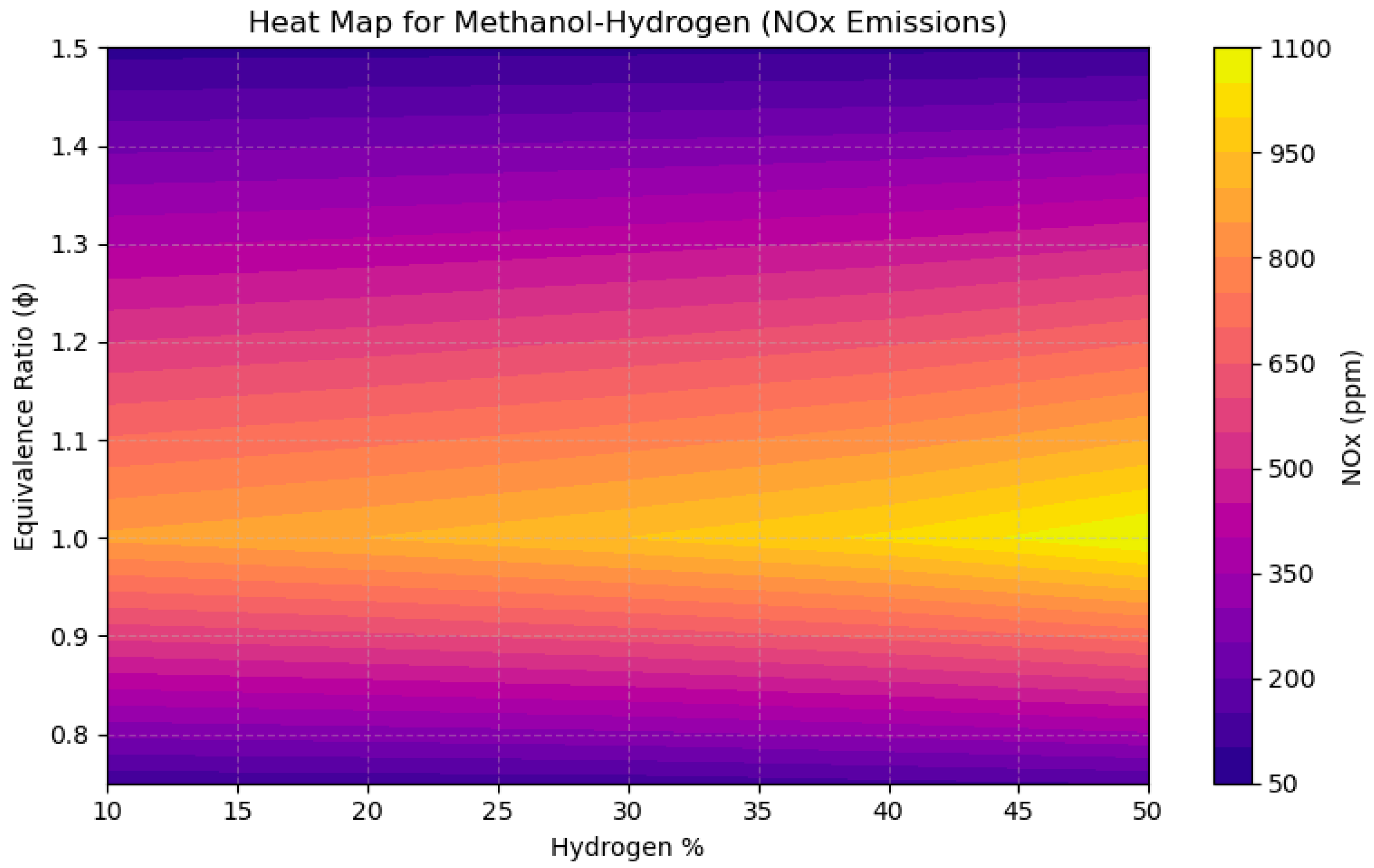
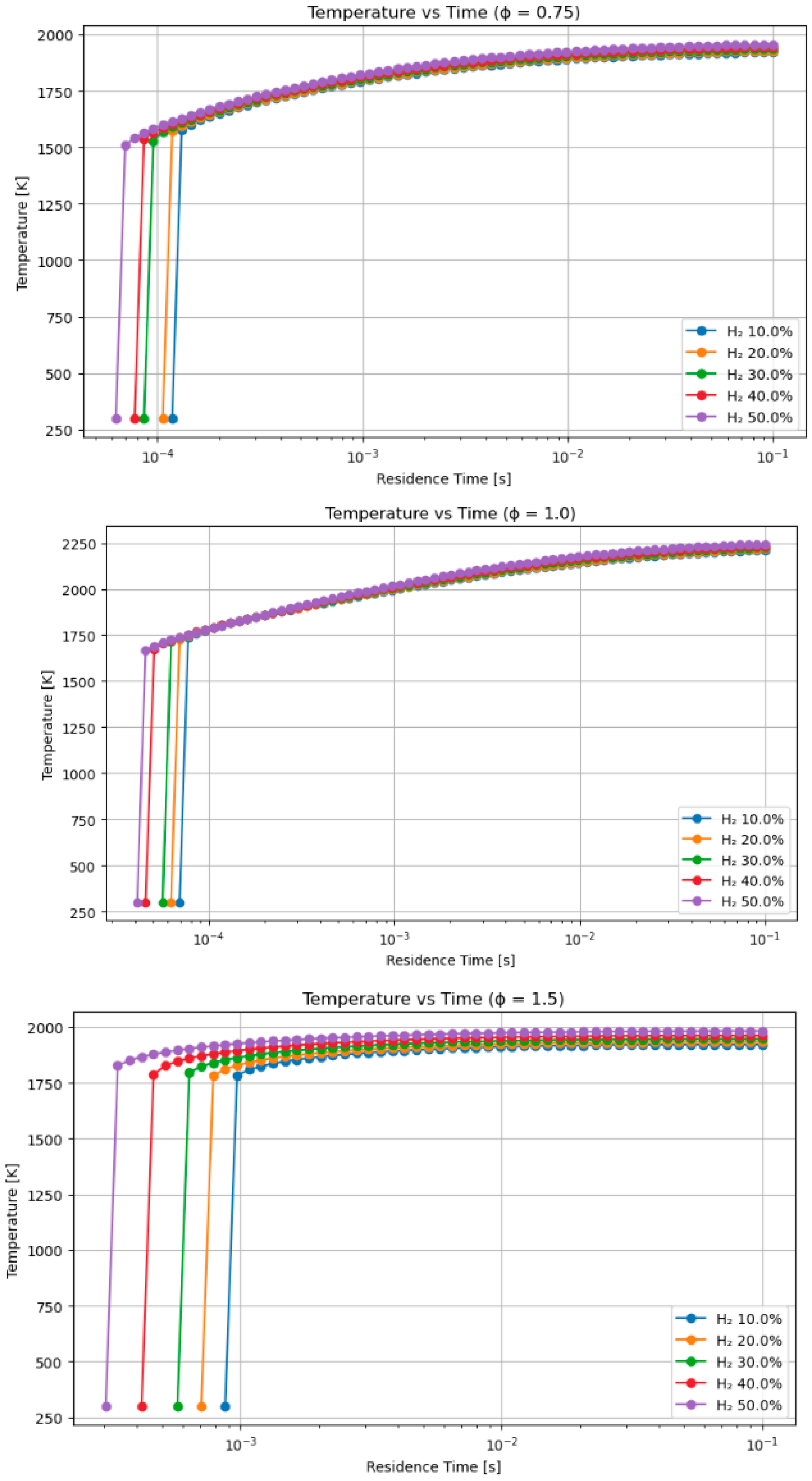
4.2. A Sensitivity Analysis of the System Involving a Blend of Hand Methanol, Focusing on Fuel Percentages and Equivalence Ratios
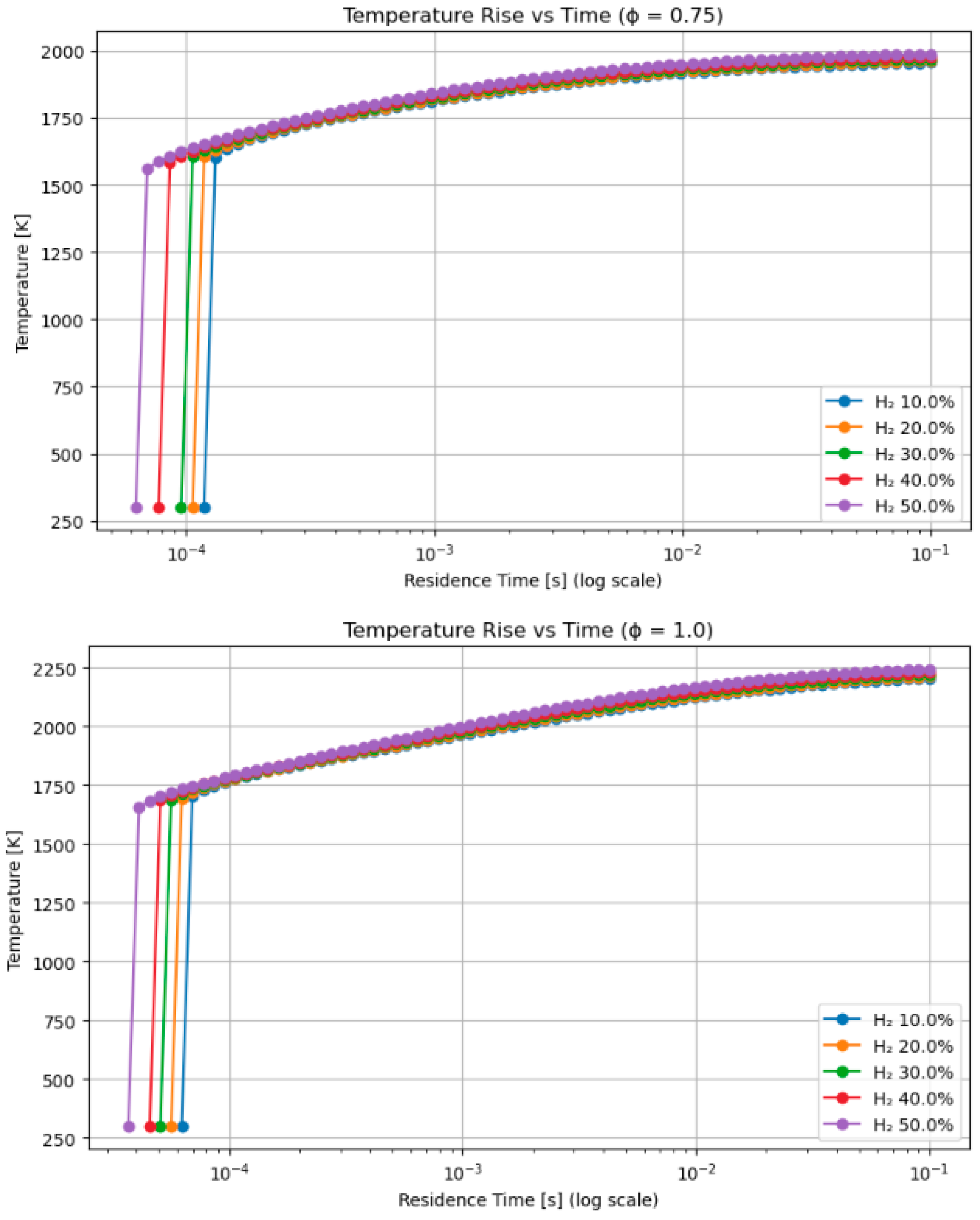
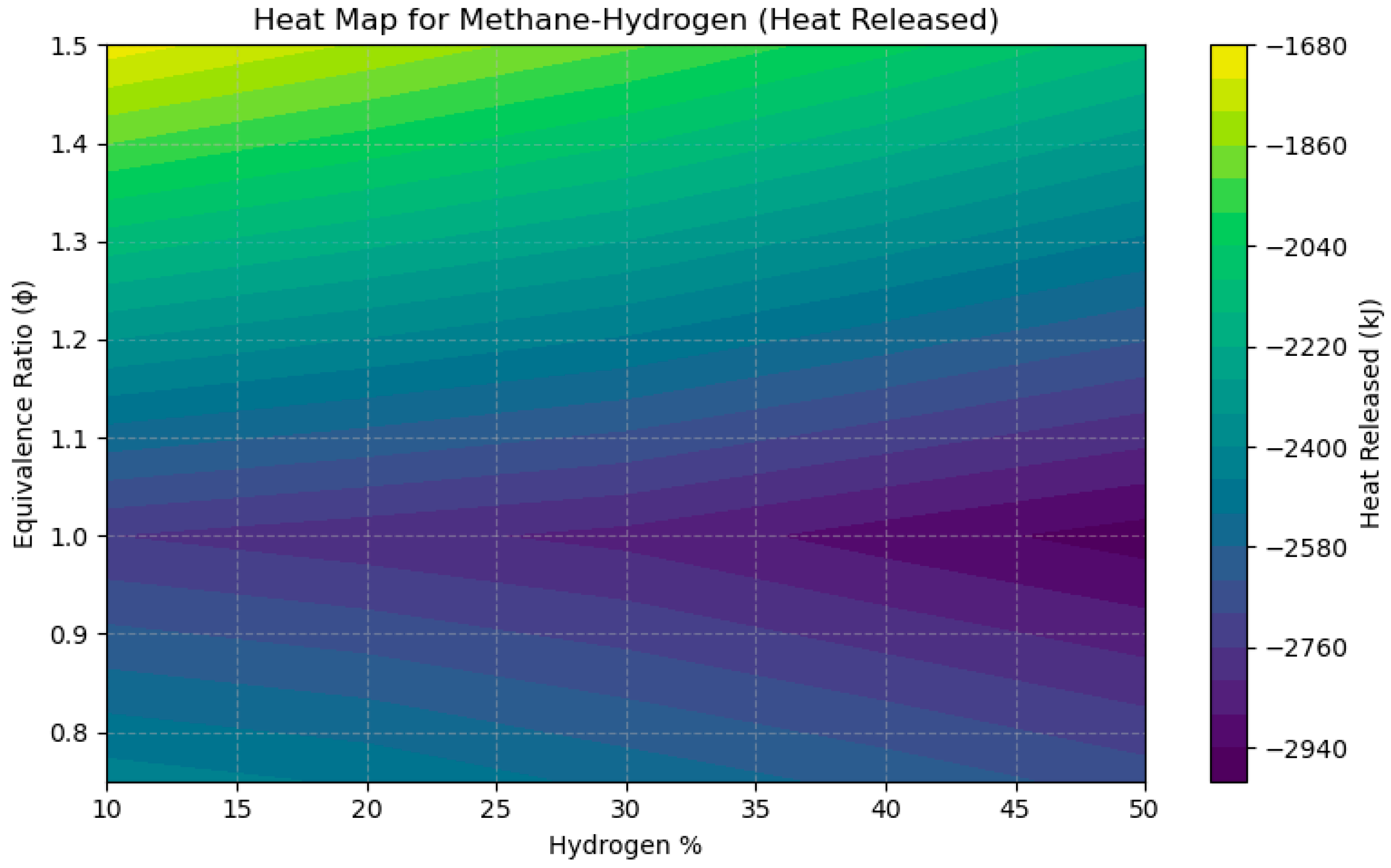
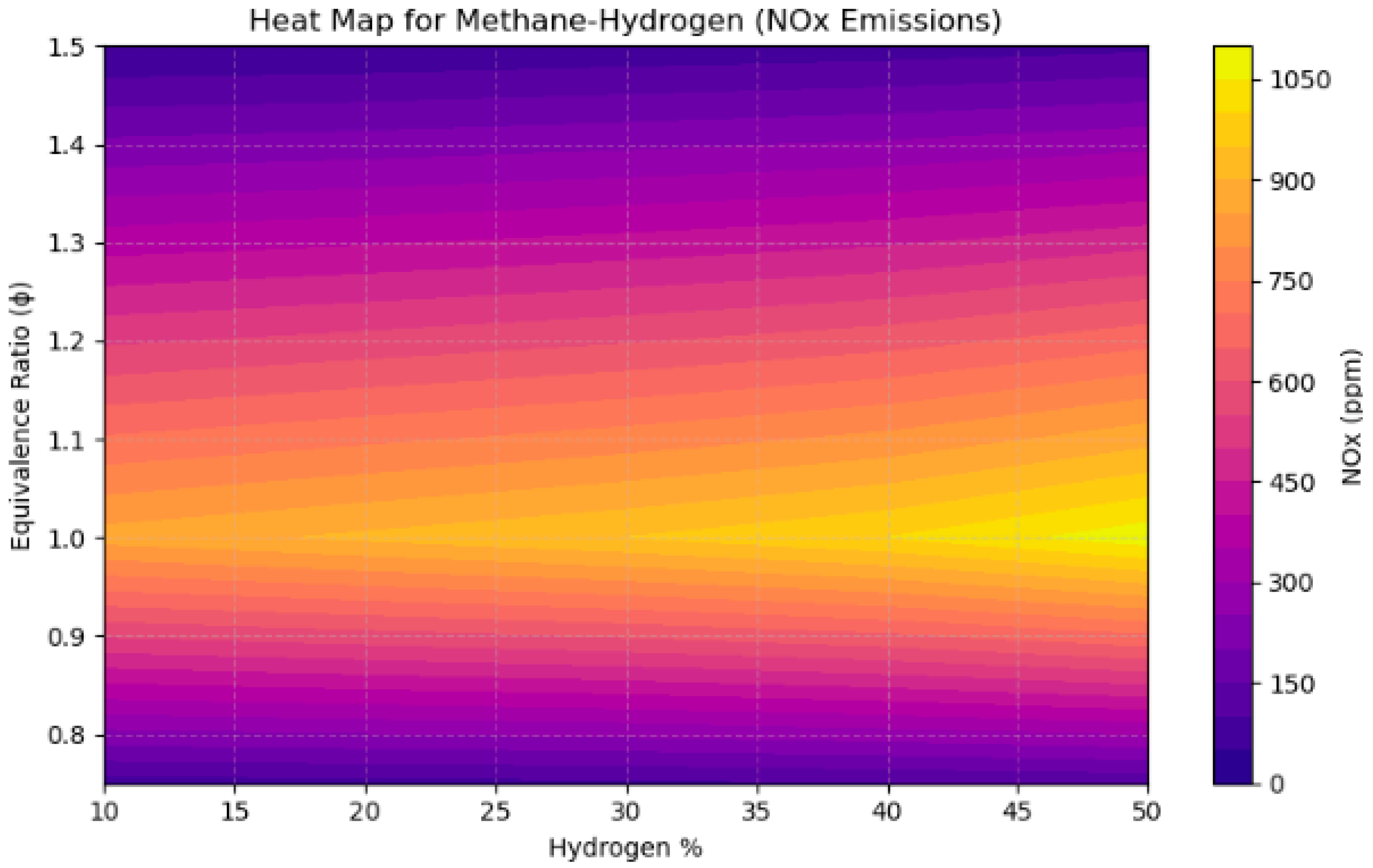
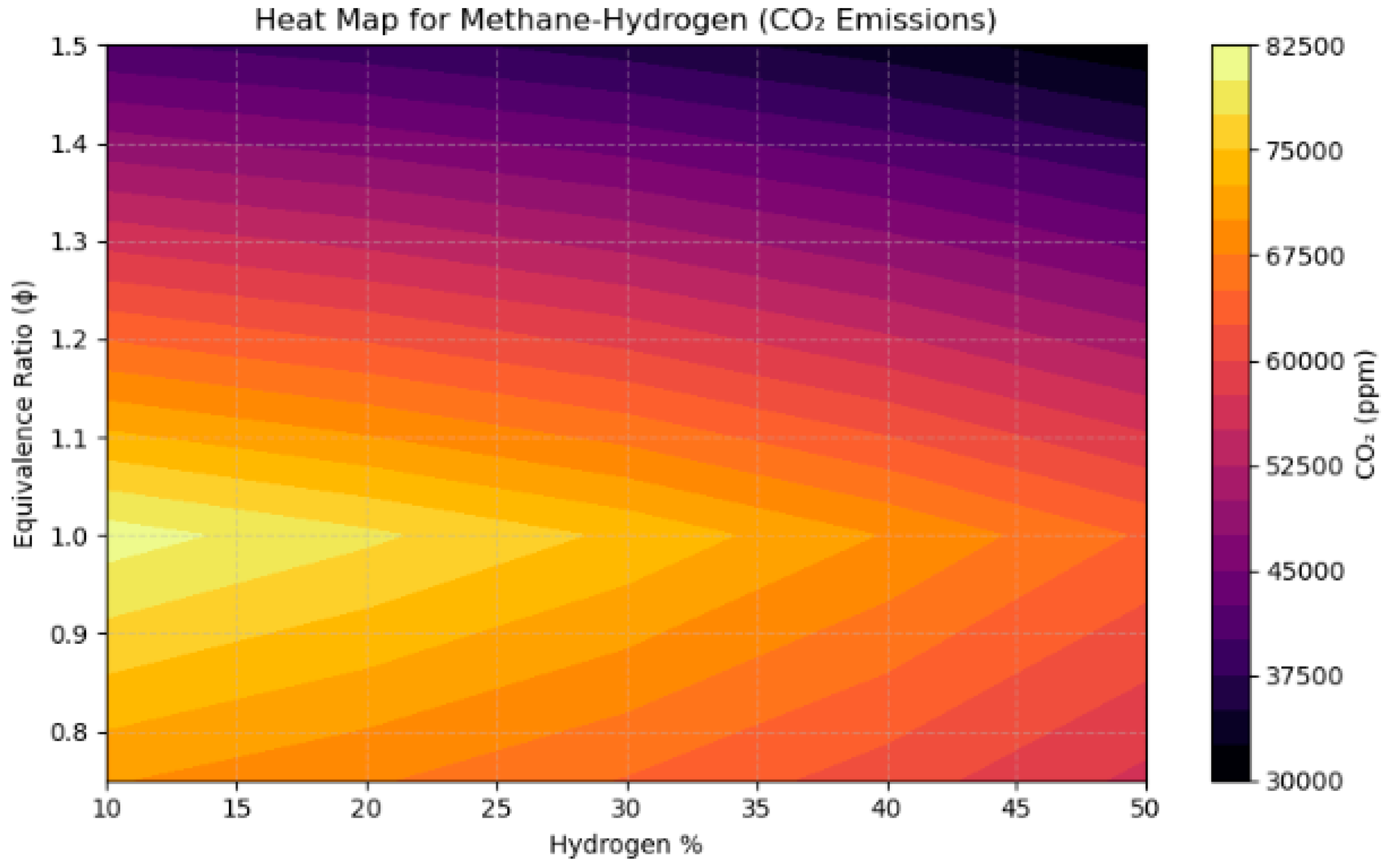
4.3. A Sensitivity Analysis of the System Involving a Blend of Hydrogen and Methane, Focusing on Fuel Percentages and Equivalence Ratios
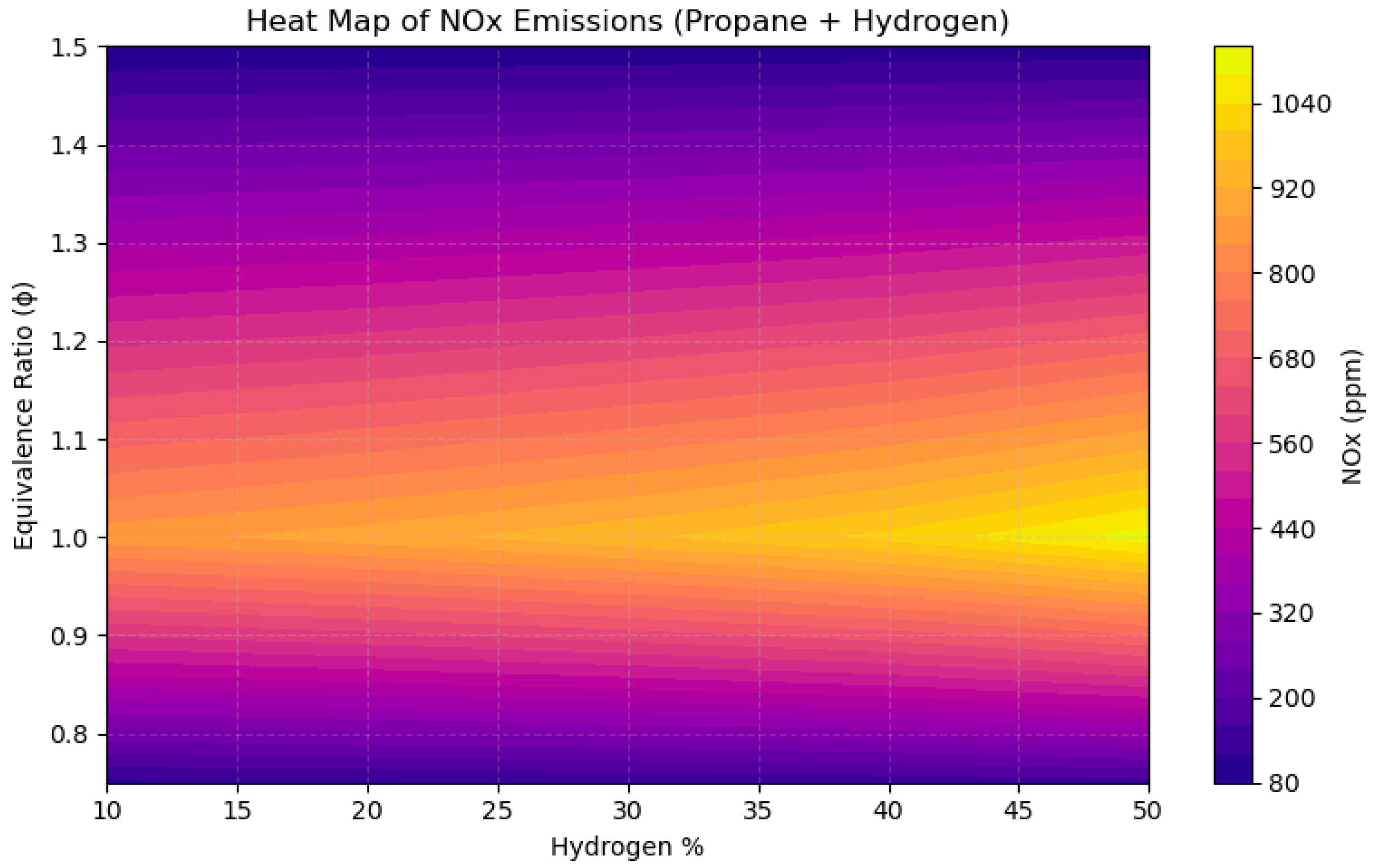
4.4. A Sensitivity Analysis of the System Involving a Blend of Hydrogen and Propane, Focusing on Fuel Percentages and Equivalence Ratios
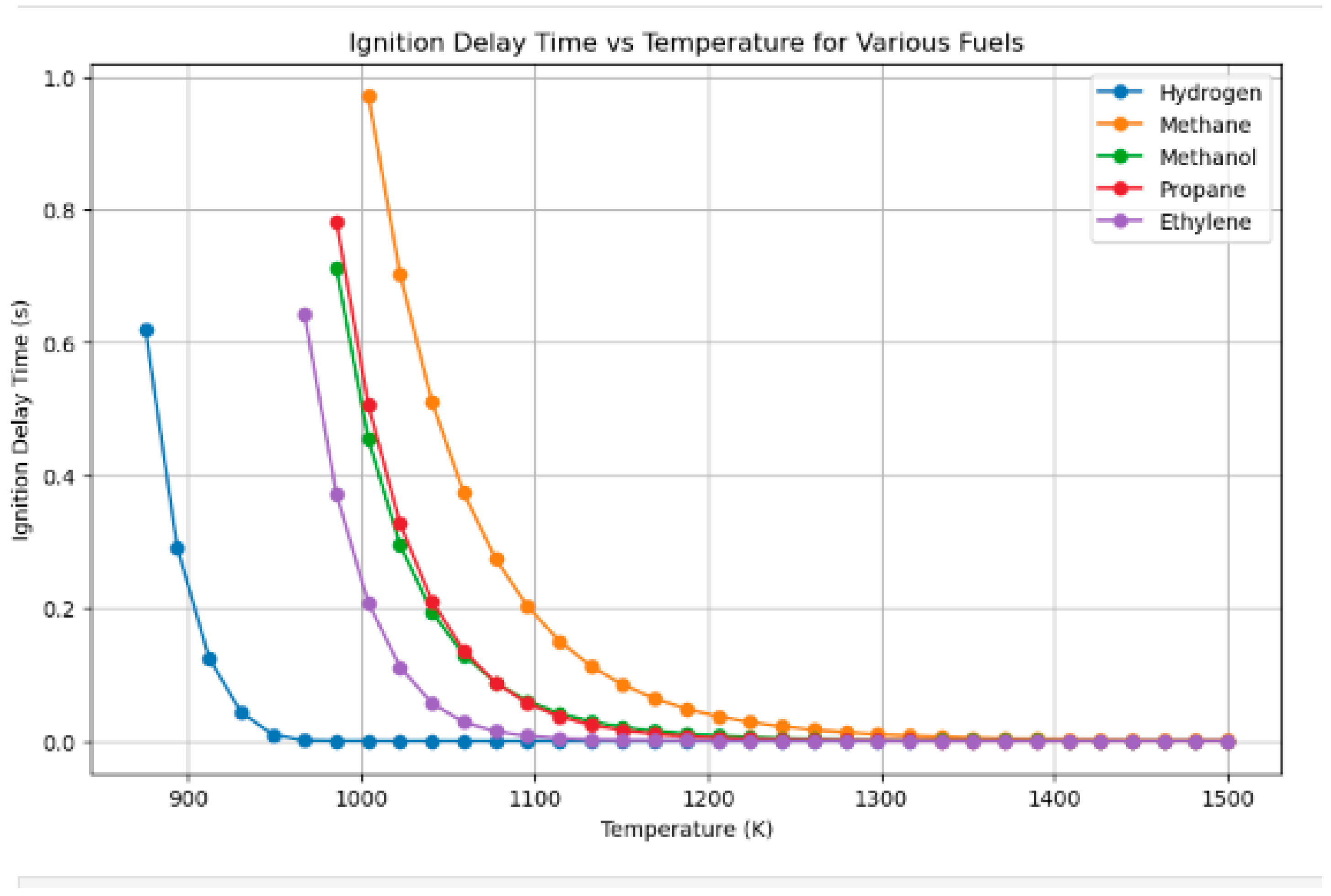
4.5. Ignition Delay for Different Fuels Compared to Hydrogen
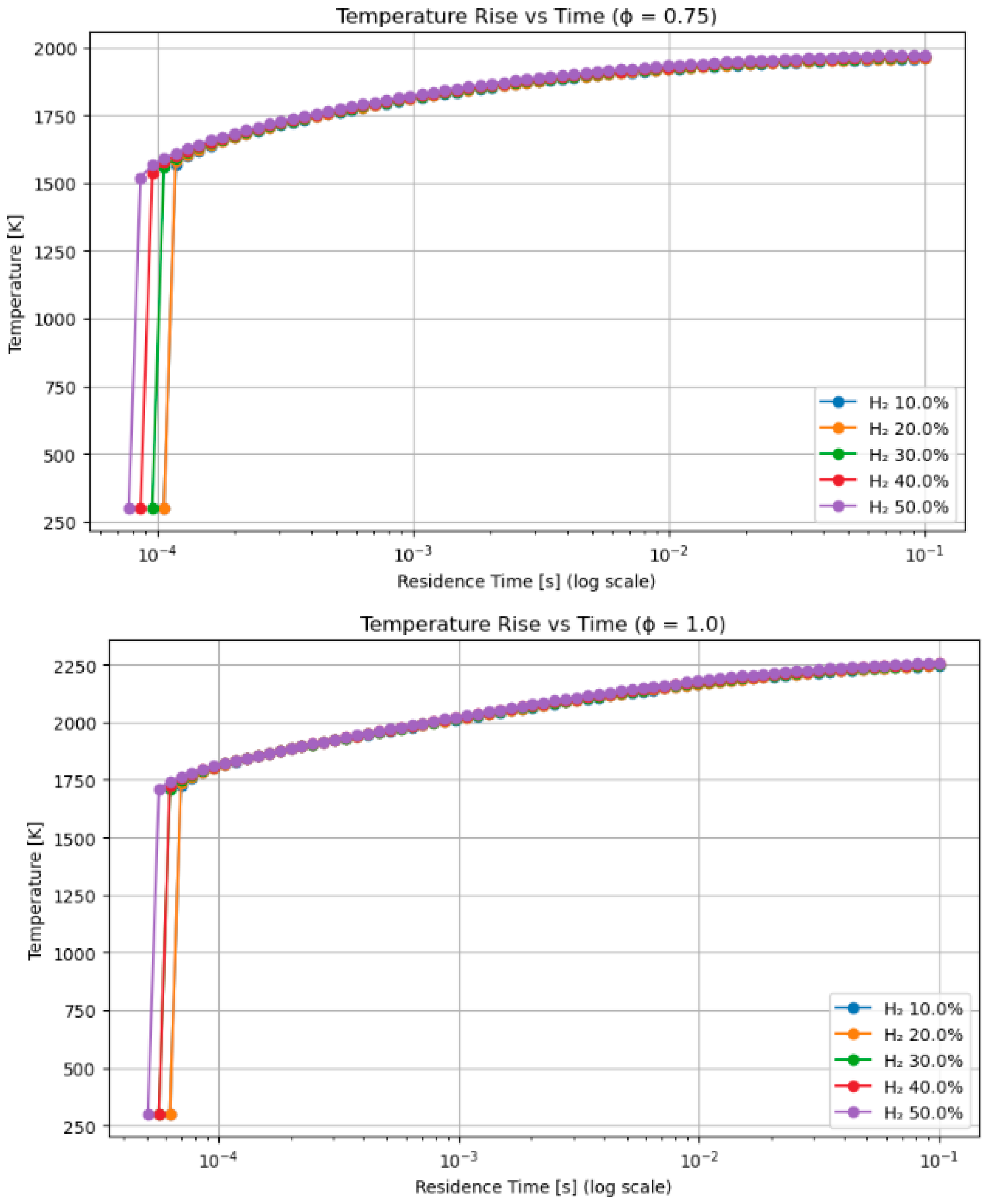
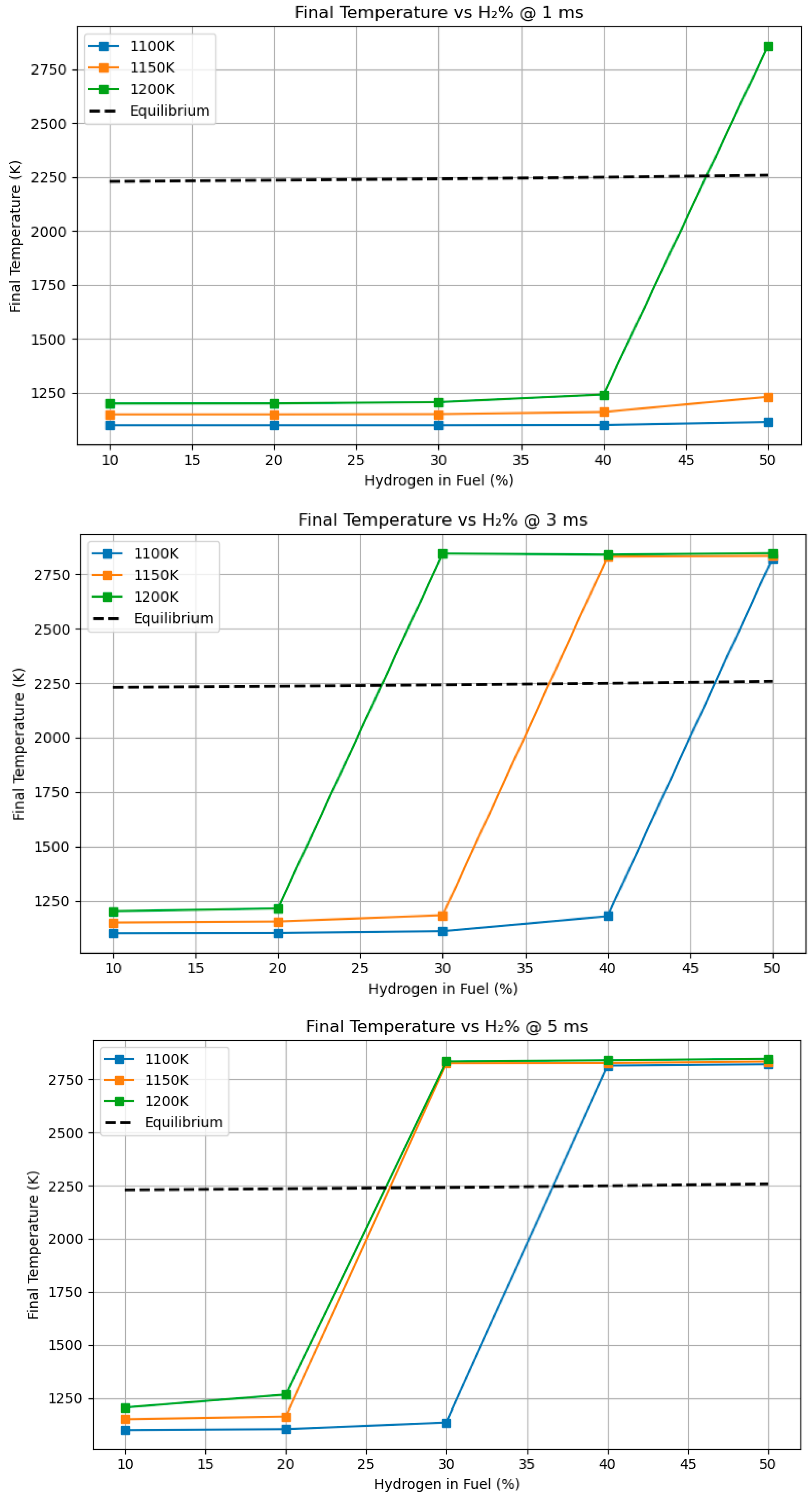
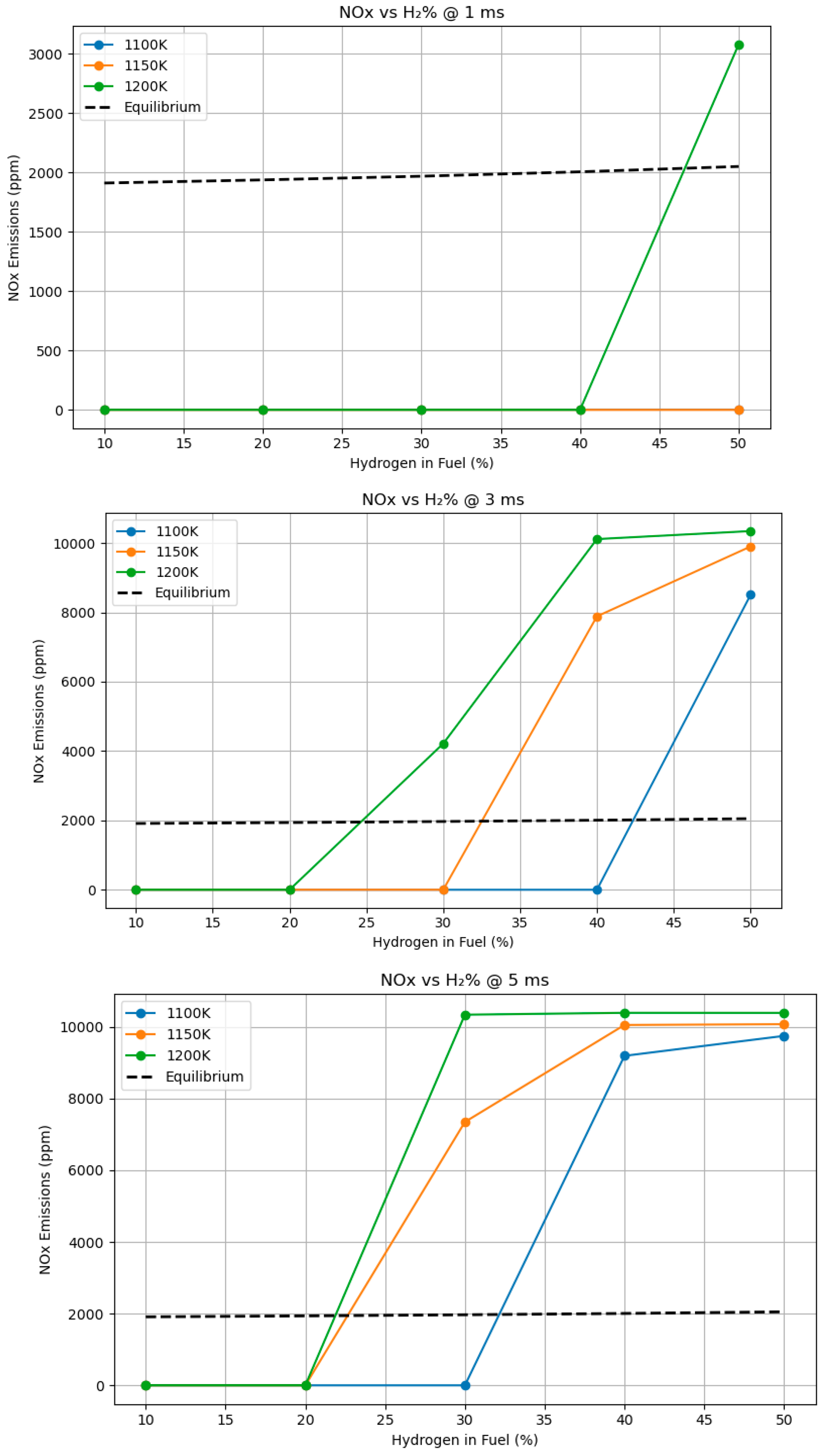
4.6. Influence of Ignition Technique and Residence Duration on Ultimate Flame Temperature and NOx in Hydrogen-Enriched Methane Blends
5. Validation and Practical Implications for Engine Design
Literature Validation
6. Conclusions
- This study illustrates, via Cantera-based PSR simulations and thermodynamic validation, the crucial significance of hydrogen enrichment in improving combustion performance and informing fuel-flexible engine strategies. The analysis of the combustion of hydrogen, methane, methanol, and propane at different equivalence ratios (ϕ = 0.75, 1.0, 1.5) and hydrogen blending levels (10–50% by mole) reveals numerous significant findings as follows:
- Hydrogen consistently elevates peak flame temperature, achieving up to 2350 K for H2-rich mixes, in contrast to 2100–2200 K for pure methane or methanol under stoichiometric conditions.
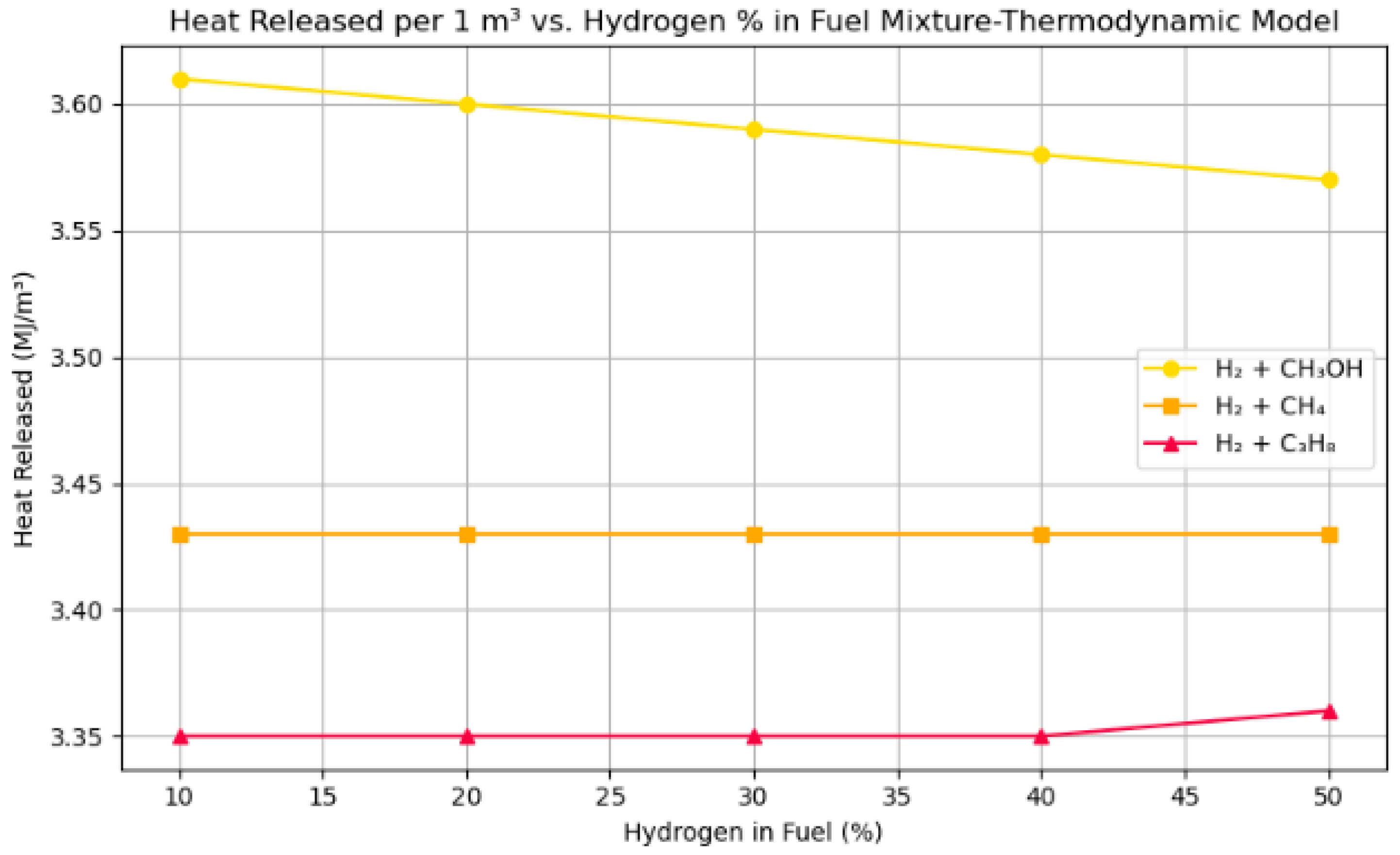
- The heat emission per unit volume reaches its maximum at ϕ = 1.0 for all fuels. A 30% H2–70% CH3OH mixture demonstrates a thermodynamic heat release of 3.59 MJ/m3, whereas Cantera forecasts 2.82–3.00 MJ, validating the consistency in both magnitude and trend.
- Hydrogen–methanol and hydrogen–methane mixtures exhibited ignition onset up to 30% faster at short residence durations (≤1 ms), highlighting its appropriateness for pre-chamber and rapid ignition combustion systems.
- NOx emissions reached their zenith near stoichiometric circumstances (ϕ = 1.0), with concentrations increasing by over 200 ppm when hydrogen content surpassed 40% in CH4 and CH3OH mixtures, attributable to heightened flame temperatures.
- CO2 emissions diminished linearly with the incorporation of hydrogen, decreasing by more than 40% as the hydrogen proportion escalated from 10% to 50% in CH4-based fuels, thus illustrating hydrogen’s capacity for decarbonization.
- The thermodynamic model, grounded in stoichiometric combustion analysis and higher heating values, precisely aligns with Cantera’s predictions for heat release. Thermodynamic estimations of 3.61 MJ/m3 for H2–CH3OH and 3.35 MJ/m3 for H2–C3H8 closely correspond with Cantera values of 2.82–3.02 MJ, hence confirming Cantera’s trustworthiness in equilibrium combustion modeling. Discrepancies of 10–15% arise from Cantera’s incorporation of real-gas effects, product dissociation, and enthalpy variations—elements not accounted for in idealized HHV-based computations.
- These findings affirm Cantera as a precise and physically coherent instrument for simulating hydrogen-enriched combustion systems, emphasizing the substantial impact of fuel mixture, equivalency ratio, and residence time on thermal performance and emissions. This research guides the design of hydrogen-integrated spark-ignition and pre-chamber engines by determining the ideal fuel blends and operating conditions that achieve great thermal efficiency while minimizing carbon and NOx emissions.
- This study integrates validated kinetic modeling with thermodynamic analysis to facilitate the advancement of cleaner, high-efficiency internal combustion technologies, providing practical guidance for fuel formulation, combustion chamber design, and emission control in relation to hydrogen co-firing and alternative fuel approaches.
Novelty and Engine Application
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krebs, S.; Biet, C. Predictive model of a premixed, lean hydrogen combustion for internal combustion engines. Transp. Eng. 2021, 5, 100086. [Google Scholar] [CrossRef]
- College of the Desert. Hydrogen Fuel Cell Engines and Related Technologies; Dempsey, J., Ed.; Revision 0; Energy Technology Training Center, College of the Desert: Palm Desert, CA, USA, 2001. [Google Scholar]
- Dhar, P.S. Compression ratio influence on combustion and emissions characteristic of hydrogen-diesel dual-fuel CI engine: Numerical study. Fuel 2018, 222, 852–858. [Google Scholar] [CrossRef]
- Efstathios, A.T. Hydrogen for Future Thermal Engines; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Homan, H.; Reynolds, R.; Deboer, P.; McLean, W. Hydrogen-fueled diesel engine without timed ignition. Int. J. Hydrogen Energy 1979, 4, 315–325. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, Y.R.; Byun, C.H.; Lee, J.T. Feasibility of compression ignition for hydrogen-fueled engine with neat hydrogen-air pre-mixture by using high compression. Int. J. Hydrogen Energy 2013, 38, 255–264. [Google Scholar] [CrossRef]
- Sandalcı, T.; Karagöz, Y. Experimental investigation of the combustion characteristics, emissions, and performance of hydrogen port fuel injection in a diesel engine. Int. J. Hydrogen Energy 2014, 39, 18480–18489. [Google Scholar] [CrossRef]
- Tsujimura, Y.S. The Combustion Improvements of Hydrogen/Diesel Dual Fuel Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2015. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Q.; Wang, Y.; Gu, M.; Huang, X. Reactive Molecular Dynamics Study of Pollutant Formation Mechanism in Hydrogen/Ammonia/Methanol Ternary Carbon-Neutral Fuel Blend Combustion. Molecules 2023, 28, 8140. [Google Scholar] [CrossRef]
- Dimitriou, P.; Tsujimura, T. A review of hydrogen as a compression ignition engine fuel. Int. J. Hydrogen Energy 2017, 42, 24470–24486. [Google Scholar] [CrossRef]
- Dimitriou, P.; Kumar, M.; Tsujimura, T.; Suzuki, Y. Combustion and emission characteristics of a hydrogen-diesel dual-fuel engine. Int. J. Hydrogen Energy 2018, 43, 13605–13617. [Google Scholar] [CrossRef]
- Luo, Q.-H.; Hu, J.-B.; Sun, B.-G.; Liu, F.-S.; Wang, X.; Li, C.; Bao, L.-Z. Effect of equivalence ratios on the power, combustion stability and NOx controlling strategy for the turbocharged hydrogen engine at low engine speeds. Int. J. Hydrogen Energy 2019, 44, 17095–17102. [Google Scholar] [CrossRef]
- Dam, Q.T. Modeling and simulation of an internal combustion engine using hydrogen: A MATLAB implementation approach. Eng. Perspect. 2024, 3, 108–118. [Google Scholar] [CrossRef]
- Verhelst, Y.W. Comparative analysis and optimization of hydrogen combustion mechanism for laminar burning velocity calculation in combustion engine modelling. Int. J. Hydrogen Energy 2023, 48, 112–125. [Google Scholar] [CrossRef]
- Wallnerb, S.V. Hydrogen-Fueled Internal Combustion Engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar]
- Molina, S.; Novella, R.; Gomez-Soriano, J.; Olcina-Girona, M. Experimental activities on a hydrogen-fueled spark-ignition engine for light-duty applications. Appl. Sci. 2023, 13, 12055. [Google Scholar] [CrossRef]
- Shivaprasad, K.V.; Raviteja, S.; Chitragar, P.; Kumar, G.N. Experimental Investigation of the Effect of Hydrogen Addition on Combustion Performance and Emissions Characteristics of a Spark Ignition High-Speed Gasoline Engine. Procedia Technol. 2014, 14, 141–148. [Google Scholar] [CrossRef]
- Huang, Q.L. Experimental investigations of the hydrogen injectors on the combustion characteristics and performance of a hydrogen internal combustion engine. Sustainability 2024, 16, 1940. [Google Scholar] [CrossRef]
- Bakar, R.A.; Widudo; Kadirgama, K.; Ramasamy, D.; Yusaf, T.; Kamarulzaman, M.K.; Sivaraos; Aslfattahi, N.; Samylingam, L.; Alwayzy, S.H. Experimental analysis on the performance, combustion/emission characteristics of a DI diesel engine using hydrogen in dual fuel mode. Int. J. Hydrogen Energy 2022, 52, 843–860. [Google Scholar] [CrossRef]
- Keromnes, W.K.-J. An experimental and detailed chemical kinetics model for hydrogen-methane mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef]
- Stoke, W.H. Hydrogen-Cum-Oil Gas as an Auxiliary Fuel for Airship Compression Ignition Engines; Report No. E 3219; British Royal Aircraft Establishment: Farnborough, UK, 1930. [Google Scholar]
- Chintala, V.; Subramanian, K. Hydrogen energy share improvement along with NOx (oxides of nitrogen) emission reduction in a hydrogen dual-fuel compression ignition engine using water injection. Energy Convers. Manag. 2014, 83, 249–259. [Google Scholar] [CrossRef]
- Tsujimura, T.; Suzuki, Y. The utilization of hydrogen in a hydrogen/diesel dual-fuel engine. Int. J. Hydrogen Energy 2017, 42, 24470–24486. [Google Scholar] [CrossRef]
- Tsujimura, M.D. A fully renewable and efficient backup power system with a hydrogen-biodiesel-fueled IC engine. Energy Procedia 2019, 157, 1305–1319. [Google Scholar] [CrossRef]
- David, G.; Goodwin, H.K. Cantera: An object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. Version 3.0.0. 2023. Available online: https://www.cantera.org (accessed on 1 October 2024).
- Shahpouri, S.; Gordon, D.; Hayduk, C.; Rezaei, R.; Koch, C.R.; Shahbakhti, M. Hybrid emission and combustion modeling of hydrogen-fueled engines. Int. J. Hydrogen Energy 2023, 48, 24037–24053. [Google Scholar] [CrossRef]
- Rosati, M.F.; Aleiferis, P.G. Hydrogen SI and HCCI combustion in a direct-injection optical engine. SAE Int. J. Engines 2009, 2, 1710–1736. [Google Scholar] [CrossRef]
- Ikegami, K.M. A study on hydrogen-fuelled diesel combustion (1st report). Prepr. Jpn. Soc. Mech. Eng. 1975, 750, 239–242. [Google Scholar]
- Chaichan, M.T.; Al-Zubaidi, D.S.M. A practical study of using hydrogen in dual-fuel compression ignition engine. Int. Publ. Adv. Sci. J. (IPASJ) 2015, 2, 1–10. [Google Scholar]
- Saravanan, N.; Nagarajan, G. Experimental investigation on a DI dual fuel engine with hydrogen injection. Int. J. Energy Res. 2009, 33, 295–308. [Google Scholar] [CrossRef]
- Smirnov, N.; Nikitin, V. Modeling and simulation of hydrogen combustion in engines. Int. J. Hydrogen Energy 2014, 39, 1122–1136. [Google Scholar] [CrossRef]
- Yakovenko, I.; Kiverin, A. Numerical Modeling of Hydrogen Combustion: Approaches and Benchmarks. Fire 2023, 6, 239. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Jia, B.; Zhang, Z.; Roskilly, A. Numerical Investigation on NOx Emission of a Hydrogen-Fuelled Dual-Cylinder Free-Piston Engine. Appl. Sci. 2023, 13, 1410. [Google Scholar] [CrossRef]



| Step | Description | Calculation/Value |
|---|---|---|
| 1 | Define fuel mixture (mole basis) | |
| 2 | Write combustion reactions | |
| 3 | Compute stoichiometric required | |
| 4 | Add nitrogen from air | |
| 5 | Total mixture before combustion | mol |
| 6 | Calculate mole density at | |
| 7 | Compute the fuel mole fraction | |
| 8 | Fuel moles in | of fuel |
| 9 | Heat of combustion (HHV) | |
| 10 | Total heat released from 6.05 mol of fuel | |
| Final | Heat released per of stoichiometric mixture |
| Fuel Blend | ||
|---|---|---|
| H2 + CH3OH | 3.61 to 3.57 | 2.82 to 3.00 |
| H2 + CH4 | 3.43 | 2.58 to 2.94 |
| H2 + C3H8 | 3.35 | 2.70 to 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbass, A. Influence of Fuel Types and Equivalence Ratios on NOx Emissions in Combustion: A Comparative Analysis of Methane, Methanol, Propane, and Hydrogen Blends. Clean Technol. 2025, 7, 42. https://doi.org/10.3390/cleantechnol7020042
Abbass A. Influence of Fuel Types and Equivalence Ratios on NOx Emissions in Combustion: A Comparative Analysis of Methane, Methanol, Propane, and Hydrogen Blends. Clean Technologies. 2025; 7(2):42. https://doi.org/10.3390/cleantechnol7020042
Chicago/Turabian StyleAbbass, Amr. 2025. "Influence of Fuel Types and Equivalence Ratios on NOx Emissions in Combustion: A Comparative Analysis of Methane, Methanol, Propane, and Hydrogen Blends" Clean Technologies 7, no. 2: 42. https://doi.org/10.3390/cleantechnol7020042
APA StyleAbbass, A. (2025). Influence of Fuel Types and Equivalence Ratios on NOx Emissions in Combustion: A Comparative Analysis of Methane, Methanol, Propane, and Hydrogen Blends. Clean Technologies, 7(2), 42. https://doi.org/10.3390/cleantechnol7020042






