Physicochemical Composition and Features of Skeleton Fractions Obtained from Fish Hydrolysate Production: Exploring Valuable Ca/P Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Materials
2.2. Chemical Analysis
2.3. Structural and Physicochemical Characterization
2.4. Statistical Analyses
3. Results and Discussion
3.1. Production Yields and Proximal Composition of Skeleton Material
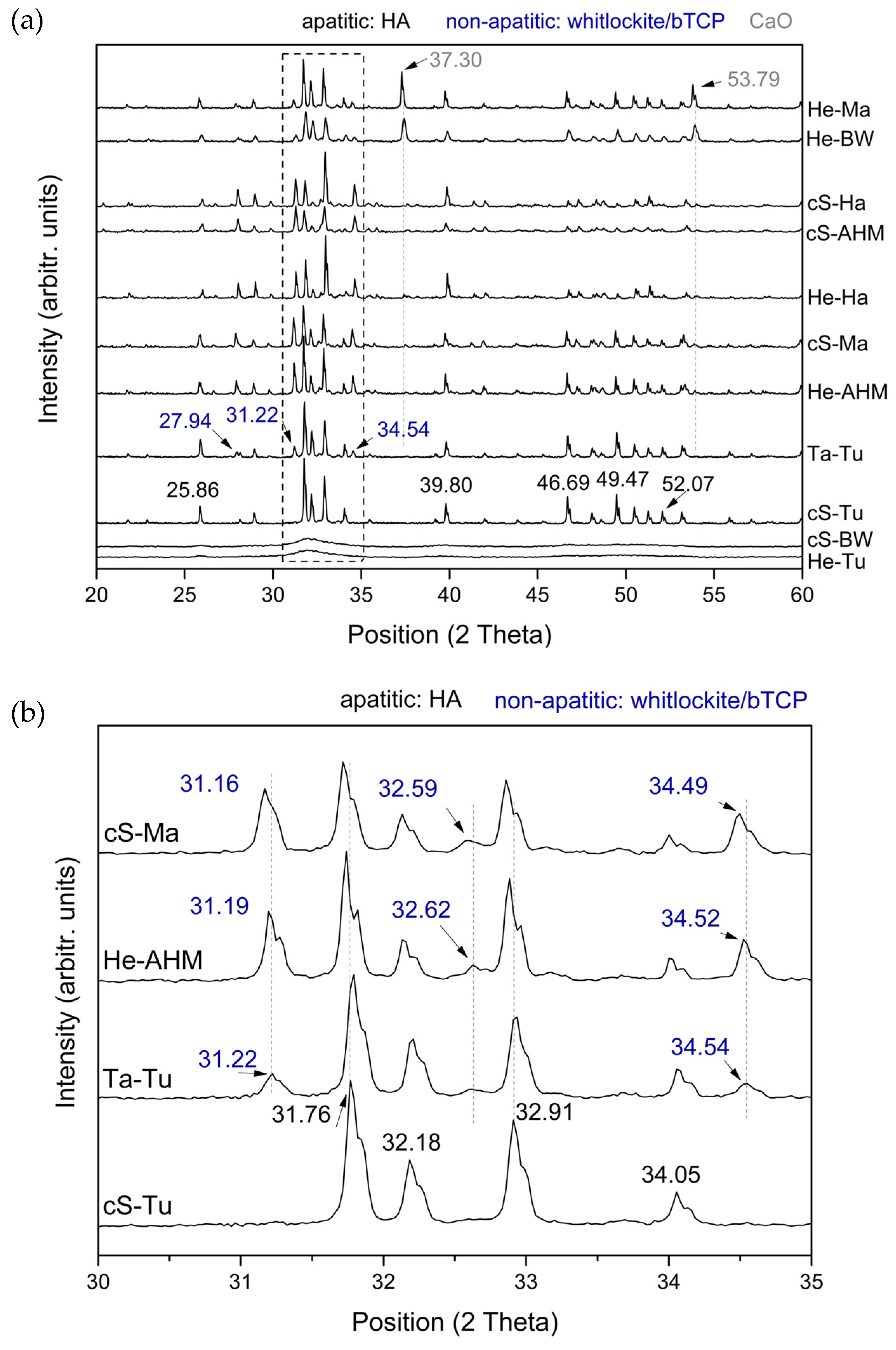
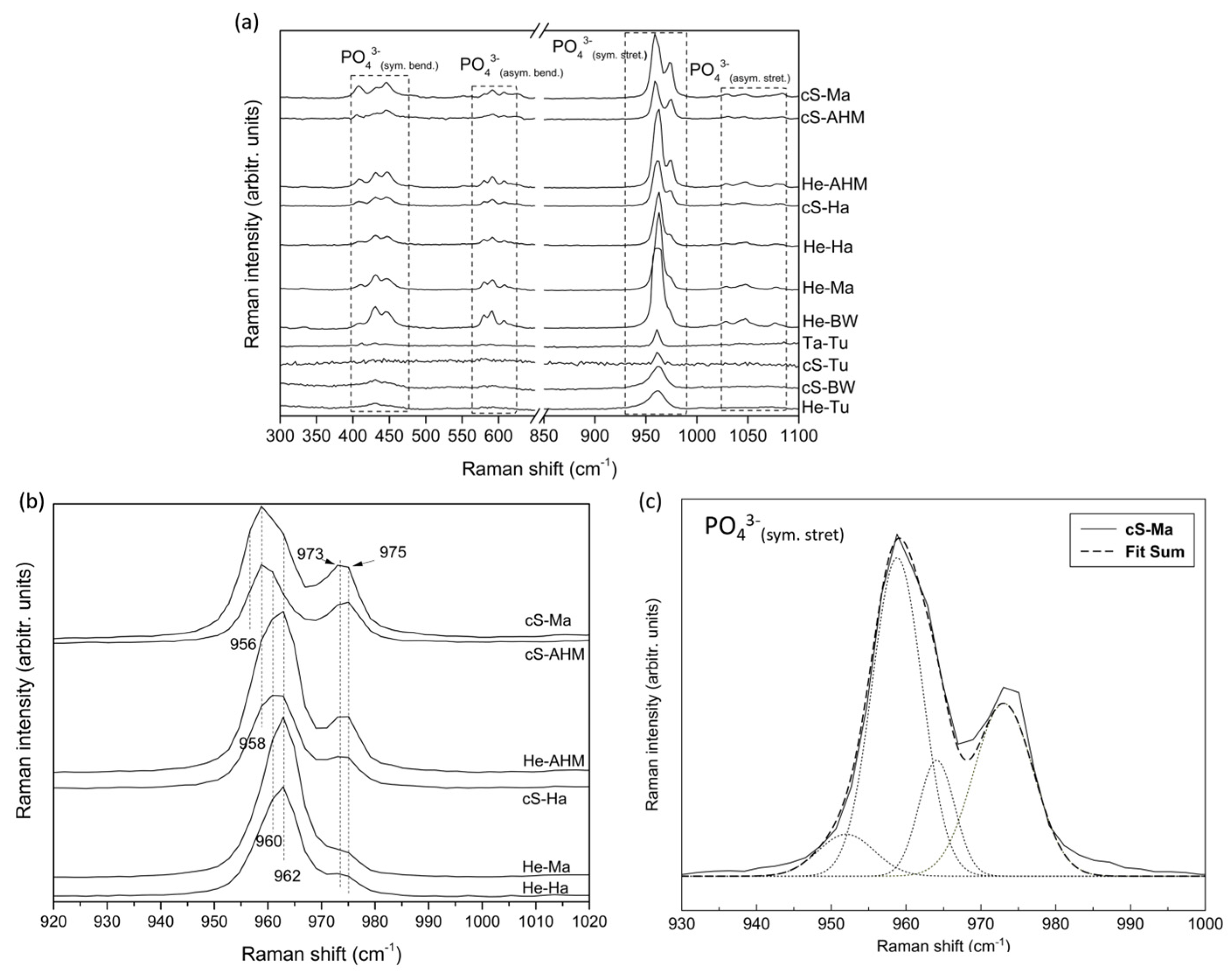
3.2. Structural and Physicochemical Characterization of Purified Calcium Phosphates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Italy, Rome, 2022. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Darghiasi, S.F.; Farazin, A.; Ghazali, H.S. Design of Bone Scaffolds with Calcium Phosphate and Its Derivatives by 3D Printing: A Review. J. Mech. Behav. Biomed. Mater. 2024, 151, 106391. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; De Carlos, A.; León, B. Biological Hydroxyapatite Obtained from Fish Bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Pérez-Davila, S.; Rodríguez-Valencia, C.; González, P.; Serra, J. The Improved Biological Response of Shark Tooth Bioapatites in a Comparative in Vitro Study with Synthetic and Bovine Bone Grafts. Biomed. Mater. 2016, 11, 035011. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Álvarez-Olcina, I.; Malvido-Fresnillo, P.; Vázquez, J.A.; Boutinguiza, M.; Comesaña, R.; Pou, J. Biogenic Calcium Phosphate from Fish Discards and By-products. Appl. Sci. 2021, 11, 3387. [Google Scholar] [CrossRef]
- Fijoł, N.; Abdelhamid, H.N.; Pillai, B.; Hall, S.A.; Thomas, N.; Mathew, A.P. 3D-Printed Monolithic Biofilters Based on a Polylactic Acid (PLA)-Hydroxyapatite (HAp) Composite for Heavy Metal Removal from an Aqueous Medium. RSC Adv. 2021, 11, 32408–32418. [Google Scholar] [CrossRef]
- Liu, W.K.; Liaw, B.S.; Chang, H.K.; Wang, Y.F.; Chen, P.Y. From Waste to Health: Synthesis of Hydroxyapatite Scaffolds from Fish Scales for Lead Ion Removal. JOM 2017, 69, 713–718. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Dong, Y.; Zhang, H.; Yun, R.; Liu, Z. Removal of Fluoride from Geothermal Water by Waste-Synthesized Al(OH)3-HAP@ZMS Composite Adsorbent: Sorption Capability and Mechanism. Water Air Soil Pollut. 2023, 234, 411. [Google Scholar] [CrossRef]
- Lizoul, B.; Kzaiber, F.; Chemaa, A.; Benharef, E.; El Hajri, J.; Zahidi, E. Use of Calcium Phosphates to Remove Nickel, Copper and Cobalt Ions from Aqueous Solutions. Int. J. Chemtech. Res. 2019, 12, 93–102. [Google Scholar] [CrossRef]
- Ozyhar, T.; Tschannen, C.; Thoemen, H.; Zoppe, J.O. Evaluating the Use of Calcium Hydrogen Phosphate Dihydrate as a Mineral-Based Fire Retardant for Application in Melamine-Urea-Formaldehyde (MUF)-Bonded Wood-Based Composite Materials. Fire Mater. 2022, 46, 595–604. [Google Scholar] [CrossRef]
- Sane, A.R.; Pham Minh, D.; Semlal, N.; Boulif, R.; Toussaint, C.; Germeau, A.; Nzihou, A. Clay/Phosphate-Based Ceramic Materials for Thermal Energy Storage—Part I: Effect of Synthetic Phosphate Content on Microstructure, Thermo-Physical and Thermo-Mechanical Properties. Open Ceram. 2023, 14, 100346. [Google Scholar] [CrossRef]
- Carella, F.; Seck, M.; Esposti, L.D.; Diadiou, H.; Maienza, A.; Baronti, S.; Vignaroli, P.; Vaccari, F.P.; Iafisco, M.; Adamiano, A. Thermal Conversion of Fish Bones into Fertilizers and Biostimulants for Plant Growth—A Low Tech Valorization Process for the Development of Circular Economy in Least Developed Countries. J. Environ. Chem. Eng. 2021, 9, 104815. [Google Scholar] [CrossRef]
- Wibisono, Y.; Ummah, S.R.; Hermanto, M.B.; Djoyowasito, G.; Noviyanto, A. Slow-Release Hydroxyapatite Fertilizer from Crab Shells Waste for Sustainable Crop Production. Results Eng. 2024, 21, 101781. [Google Scholar] [CrossRef]
- Dhar, M.; Jasrotia, R.; Langer, S.R. Manufacturing Organic Fertilizers and Manures. In Fish Waste to Valuable Products. Sustainable Materials and Technology; Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A., Eds.; Springer: Singapore, 2024. [Google Scholar]
- Vázquez, J.A.; Pedreira, A.; Durán, S.; Cabanelas, D.; Souto-Montero, P.; Martínez, P.; Mulet, M.; Pérez-Martín, R.I.; Valcarcel, J. Biorefinery for Tuna Head Wastes: Production of Protein Hydrolysates, High-Quality Oils, Minerals and Bacterial Peptones. J. Clean. Prod. 2022, 357, 131909. [Google Scholar] [CrossRef]
- AOAC. Methods of Analysis, 15th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1997. [Google Scholar]
- Havilah, E.J.; Wallis, D.M.; Morris, R.; Woolnough, J. A Micro-Colorimetric Method for Determination of Amonia in Kjeldajñ Dogests with a Manueal Spectrophtometer. Lab. Pract. 1977, 26, 545–547. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of Amino Acids on Sulfonated Polystyrene Resins an Improved System. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar]
- Valcarcel, J.; Sanz, N.; Vázquez, J.A. Optimization of the Enzymatic Protein Hydrolysis of By-Products from Seabream (Sparus aurata) and Seabass (Dicentrarchus labrax), Chemical and Functional Characterization. Foods 2020, 9, 1503. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of Aquaculture By-Products of Salmonids to Produce Enzymatic Hydrolysates: Process Optimization, Chemical Characterization and Evaluation of Bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef]
- Szpak, P. Fish Bone Chemistry and Ultrastructure: Implications for Taphonomy and Stable Isotope Analysis. J. Archaeol. Sci. 2011, 38, 3358–3372. [Google Scholar] [CrossRef]
- Wijayanti, I.; Benjakul, S.; Sookchoo, P. Effect of High Pressure Heating on Physical and Chemical Characteristics of Asian Sea Bass (Lates Calcarifer) Backbone. J. Food. Sci. Technol. 2021, 58, 3120–3129. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Structural Variations in Natural F, OH, and Cl Apatites. Am. Mineral. 1989, 74, 870–876. [Google Scholar]
- Hughes, J.M.; Jolliff, B.L.; Rakovan, J. The Crystal Chemistry of Whitlockite and Merrillite and the Dehydrogenation of Whitlockite to Merrillite. Am. Mineral. 2008, 93, 1300–1305. [Google Scholar] [CrossRef]
- Aguiar, H.; Chiussi, S.; López-Álvarez, M.; González, P.; Serra, J. Structural Characterization of Bioceramics and Mineralized Tissues Based on Raman and XRD Techniques. Ceram. Int. 2018, 44, 495–504. [Google Scholar] [CrossRef]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.C.; Silvestros, S.; Sagnou, M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and in Vitro Studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef]
- Posset, U.; Löcklin, E.; Thull, R.; Kiefer, W. Vibrational Spectroscopic Study of Tetracalcium Phosphate in Pure Polycrystalline Form and as a Constituent of a Self-Setting Bone Cement. J. Biomed. Mater. Res. 1998, 40, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Penel, G.; Delfosse, C.; Descamps, M.; Leroy, G. Composition of Bone and Apatitic Biomaterials as Revealed by Intravital Raman Microspectroscopy. Bone 2005, 36, 893–901. [Google Scholar] [CrossRef]
- Corno, M.; Busco, C.; Civalleri, B.; Ugliengo, P. Periodic Ab Initio Study of Structural and Vibrational Features of Hexagonal Hydroxyapatite Ca10(PO4)6(OH) 2. Phys. Chem. Chem. Phys. 2006, 8, 2464–2472. [Google Scholar] [CrossRef]
- De Aza, P.N.; Santos, C.; Pazo, A.; De Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 1. Raman Spectrum of β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- Kim, D.H.; Hwang, K.H.; Lee, J.D.; Park, H.C.; Yoon, S.Y. Long and Short Range Order Structural Analysis of In-Situ Formed Biphasic Calcium Phosphates. Biomater. Res. 2015, 19, 14. [Google Scholar] [CrossRef]
- Dalmônico, G.M.L.; Franczak, P.F.; Levandowski, N.; Camargo, N.H.A.; Dallabrida, A.L.; Da Costa, B.D.; Gil, O.G.; Cambra-Moo, O.; Rodríguez, M.A.; Canillas, M. An: In Vivo Study on Bone Formation Behavior of Microporous Granular Calcium Phosphate. Biomater. Sci. 2017, 5, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Truite, C.V.R.; Noronha, J.N.G.; Prado, G.C.; Santos, L.N.; Palácios, R.S.; Do Nascimento, A.; Volnistem, E.A.; da Silva Crozatti, T.T.; Francisco, C.P.; Sato, F.; et al. Bioperformance Studies of Biphasic Calcium Phosphate Scaffolds Extracted from Fish Bones Impregnated with Free Curcumin and Complexed with Β-Cyclodextrin in Bone Regeneration. Biomolecules 2022, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Dorcioman, G.; Grumezescu, V. A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards. Nanomaterials 2021, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.; Silva, M.F.; Pullar, R.C.; Braga Da Cruz, I.; Jorge, R.; Pintado, M.M.E.; Castro, P.M.L. Extraction and Characterisation of Apatite- and Tricalcium Phosphate-Based Materials from Cod Fish Bones. Mater. Sci. Eng. C 2013, 33, 103–110. [Google Scholar] [CrossRef]
- Belouafa, S. Biphasic Calcium Phosphate Derived from a Sardine By-Product. Curr. Trends. Biomed. Eng. Biosci. 2017, 6, 58–60. [Google Scholar] [CrossRef]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P.C. Fish Processing Industry Residues: A Review of Valuable Products Extraction and Characterization Methods. Waste Biomass Valorization 2020, 11, 3223–3246. [Google Scholar]


| Substrate | >0.5 mm | 0.5–0.25 mm | 0.25–0.1 mm | <0.1 mm |
|---|---|---|---|---|
| He-Ma | 16.5 | 45.5 | 31.5 | 6.5 |
| He-AHM | 12.5 | 30.5 | 45.5 | 11.5 |
| He-Ha | 37 | 27.5 | 30 | 15 |
| cS-Ma | 12.8 | 36.2 | 40.4 | 10.6 |
| cS-AHM | 30.5 | 39 | 23.5 | 7 |
| cS-Ha | 26.3 | 30 | 33.1 | 10.6 |
| Substrate | Ys (%) | Yds (%) |
|---|---|---|
| He-AHM | 17.3 ± 1.8 a,1 | 7.3 ± 0.9 a,1 |
| cS-AHM | 23.0 ± 3.1 b,2 | 9.7 ± 1.0 c,2 |
| He-BW | 19.0 ± 3.1 a,b,1 | 8.7 ± 1.4 a,c,1 |
| cS-BW | 19.1 ± 4.6 a,b,1 | 7.1 ± 1.0 a,1 |
| He-Ma | 15.2 ± 1.3 a,d,1 | 4.4 ± 0.6 b,1 |
| cS-Ma | 16.2 ± 1.9 a,1 | 5.4 ± 0.6 b,d,1 |
| He-Tu | 16.3 ± 0.7 a,1 | 8.4 ± 1.0 a,c,1 |
| cS-Tu | 16.1 ± 3.3 a,1 | 9.5 ± 1.4 a,c,1 |
| Ta-Tu | 12.9 ± 2.9 a,d,1 | 6.6 ± 0.9 a,d,2 |
| He-Ha | 13.0 ± 1.2 d,1 | 4.6 ± 0.2 b,1 |
| cS-Ha | 14.8 ± 1.3 a,d,1 | 4.8 ± 0.6 b,1 |
| Skeletons | Mo (%) | Ash (%) | OM (%) | Lip (%) | Pr-tN (%) |
|---|---|---|---|---|---|
| He-AHM | 2.6 ± 0.2 a,1 | 73.2 ± 0.9 a,1 | 24.2 ± 0.8 a,1 | 9.3 ± 0.4 a,d,1 | 14.9 ± 0.9 a,1 |
| cS-AHM | 4.3 ± 0.2 b,1 | 65.2 ± 0.6 b,2 | 30.5 ± 0.8 b,2 | 8.8 ± 0.7 a,1 | 21.7 ± 1.2 b,c,1 |
| He-BW | 3.3 ± 0.7 c,1 | 72.6 ± 0.5 a,1 | 24.1 ± 0.8 a,1 | 4.3 ± 0.4 b,1 | 19.8 ± 1.0 b,1 |
| cS-BW | 3.7 ± 0.6 b,c,1 | 70.2 ± 0.7 c,2 | 26.1 ± 0.4 c,2 | 2.6 ± 0.6 c,2 | 23.5 ± 0.8 c,2 |
| He-Ma | 3.3 ± 0.7 c,1 | 63.2 ± 1.2 d,1 | 33.5 ± 0.7 d,e,1 | 10.4 ± 0.6 d,1 | 23.1 ± 0.9 c,1 |
| cS-Ma | 4.8 ± 0.9 b,c,2 | 62.4 ± 0.7 d,1 | 32.8 ± 0.4 d,1 | 9.5 ± 0.5 a,d,1 | 23.3 ± 0.7 c,1 |
| He-Tu | 2.3 ± 0.5 a,1 | 64.5 ± 0.6 b,d,1 | 33.2 ± 0.8 d,e,1 | 6.1 ± 0.4 e,1 | 27.1 ± 1.2 d,1 |
| cS-Tu | 3.4 ± 0.3 c,2 | 62.2 ± 0.7 d,2 | 34.4 ± 0.5 e,1 | 7.5 ± 0.6 f,2 | 26.9 ± 0.9 d,1 |
| Ta-Tu | 3.0 ± 0.2 a,b,2 | 65.2 ± 0.9 b,1 | 31.8 ± 0.7 b,d,2 | 1.2 ± 0.3 g,3 | 30.6 ± 1.3 e,2 |
| He-Ha | 2.9 ± 0.2 a,b,1 | 67.3 ± 0.2 e,1 | 29.8 ± 0.3 b,1 | 1.6 ± 0.2 g,1 | 28.0 ± 0.6 d,1 |
| cS-Ha | 3.7 ± 0.5 b,c,2 | 67.0 ± 0.2 e,1 | 29.3 ± 0.7 b,1 | 1.6 ± 0.1 g,1 | 27.5 ± 0.4 d,1 |
| Skeletons | TEAA/TAA (%) | Gly (%) | Pro (%) | OHPro (%) |
|---|---|---|---|---|
| He-AHM | 32.7 ± 0.7 a,b,1 | 15.5 ± 0.7 a,b,1 | 8.7 ± 0.4 a,1 | 6.3 ± 0.4 a,b,1 |
| cS-AHM | 32.9 ± 1.1 a,b,1 | 15.9 ± 1.2 a,b,c,1 | 7.9 ± 0.3 b,2 | 6.0 ± 0.1 a,1 |
| He-BW | 32.6 ± 0.1 a,1 | 15.0 ± 0.3 a,1 | 8.4 ± 0.9 a,b,1 | 6.0 ± 0.1 a,1 |
| cS-BW | 32.0 ± 1.1 a,b,1 | 16.5 ± 0.4 b,2 | 8.5 ± 0.0 a,1 | 6.4 ± 0.2 a,b,2 |
| He-Ma | 32.7 ± 0.4 a,1 | 16.0 ± 1.1 a,b,1 | 7.9 ± 0.5 a,b,1 | 6.7 ± 0.3 b,1 |
| cS-Ma | 31.8 ± 0.9 a,b,1 | 18.4 ± 1.4 c,d,1 | 8.3 ± 0.1 a,1 | 6.8 ± 0.2 b,1 |
| He-Tu | 31.3 ± 0.7 b,1 | 18.6 ± 0.6 c,1 | 8.8 ± 0.4 a,1 | 7.0 ± 0.3 b,d,1 |
| cS-Tu | 32.4 ± 0.6 a,b,1 | 17.7 ± 0.5 c,d,1 | 9.6 ± 0.2 c,d,2 | 7.0 ± 0.2 b,d,1 |
| Ta-Tu | 28.5 ± 0.1 c,2 | 20.6 ± 0.7 e,2 | 9.5 ± 0.2 c,2 | 8.0 ± 0.1 c,2 |
| He-Ha | 31.4 ± 0.3 b,1 | 17.6 ± 0.1 d,1 | 9.1 ± 0.3 a,c,1 | 7.4 ± 0.2 d,1 |
| cS-Ha | 30.4 ± 0.2 d,2 | 19.0 ± 0.3 c,2 | 10.0 ± 0.2 d,2 | 7.5 ± 0.2 d,1 |
| Calcium Phosphates | Raman Intensity at 591 cm−1 (cps) | Raman Intensity at 962 cm−1 (cps) | Raman Intensity at 975 cm−1 (cps) | 975:591 Ratio | 962:975 Ratio |
|---|---|---|---|---|---|
| cS-Ma | 7100.98 | 40,481.22 | 27,744.58 | 3.9 | 1.5 |
| cS-AHM | 4238.03 | 18,927.44 | 15,811.22 | 3.7 | 1.2 |
| He-AHM | 10,243.78 | 62,593.50 | 22,773.47 | 2.2 | 2.8 |
| cS-Ha | 6381.06 | 36,131.13 | 12,901.20 | 2.0 | 2.8 |
| He-Ma | 8849.57 | 61,284.76 | 10,128.05 | 1.1 | 6.1 |
| He-Ha | 8212.10 | 43,140.67 | 9362.64 | 1.1 | 4.6 |
| Calcium Phosphates | Ca ± 0.86 (wt.%) | P ± 0.15 (wt.%) | Mg ± 0.03 (wt.%) |
|---|---|---|---|
| cS-Ma | 36.89 | 20.17 | 1.05 |
| Ta-Tu | 37.86 | 19.83 | 1.19 |
| cS-Tu | 38.15 | 19.08 | 0.80 |
| cS-BW | 33.34 | 16.28 | 0.86 |
| He-AHM | 25.06 | 12.21 | 0.27 |
| cS-AHM | 23.75 | 11.57 | 0.33 |
| cS-Ha | 23.87 | 11.25 | 0.26 |
| He-Tu | 33.07 | 14.27 | 0.49 |
| He-Ha | 26.08 | 10.29 | 0.22 |
| He-BW | 44.68 | 13.63 | 0.59 |
| He-Ma | 44.74 | 13.21 | 0.75 |
| Calcium Phosphate Structure | Skeletons |
|---|---|
| Hydroxyapatite and Whitlockite/β-TCP | He-AHM |
| cS-AHM | |
| cS-Ma | |
| Ta-Tu | |
| He-Ha | |
| cS-Ha | |
| Hydroxyapatite and Whitlockite/β-TCP + CaO crystals | He-BW |
| He-Ma | |
| Amorphous calcium phosphate | cS-BW |
| He-Tu | |
| Hydroxyapatite | cS-Tu |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Álvarez, M.; Pérez-Davila, S.; Vázquez, J.A.; Valcarcel, J.; Serra, J.; González, P. Physicochemical Composition and Features of Skeleton Fractions Obtained from Fish Hydrolysate Production: Exploring Valuable Ca/P Sources. Clean Technol. 2025, 7, 32. https://doi.org/10.3390/cleantechnol7020032
López-Álvarez M, Pérez-Davila S, Vázquez JA, Valcarcel J, Serra J, González P. Physicochemical Composition and Features of Skeleton Fractions Obtained from Fish Hydrolysate Production: Exploring Valuable Ca/P Sources. Clean Technologies. 2025; 7(2):32. https://doi.org/10.3390/cleantechnol7020032
Chicago/Turabian StyleLópez-Álvarez, Miriam, Sara Pérez-Davila, José Antonio Vázquez, Jesús Valcarcel, Julia Serra, and Pío González. 2025. "Physicochemical Composition and Features of Skeleton Fractions Obtained from Fish Hydrolysate Production: Exploring Valuable Ca/P Sources" Clean Technologies 7, no. 2: 32. https://doi.org/10.3390/cleantechnol7020032
APA StyleLópez-Álvarez, M., Pérez-Davila, S., Vázquez, J. A., Valcarcel, J., Serra, J., & González, P. (2025). Physicochemical Composition and Features of Skeleton Fractions Obtained from Fish Hydrolysate Production: Exploring Valuable Ca/P Sources. Clean Technologies, 7(2), 32. https://doi.org/10.3390/cleantechnol7020032








