Abstract
The expansion of fisheries and aquaculture in recent decades has led to a substantial increase in fish by-products. This study investigates the extraction and characterization of calcium phosphates from the by-products of representative species in these industries, aiming to identify potential sources for biotechnological and pharmaceutical applications. Clean bones obtained by enzyme hydrolysis from the heads, central skeletons, and/or tails of Atlantic horse mackerel, blue whiting, hake, mackerel, and farmed turbot were subjected to calcination to obtain calcium phosphates. The clean bone content in terms of nitrogen, lipids, organic matter, total protein, and amino acids was evaluated together with the chemical bonds, structures, and elemental composition of calcium phosphates. Results indicated a significantly higher yield of wet bone recovery (23%, p < 0.05) for the central skeleton of Atlantic horse mackerel and the highest mineral fraction for the heads of Atlantic horse mackerel (73.2%), followed by that of blue whiting (72.6%). Hake and turbot presented the lowest mineral fractions and, therefore, the highest protein content (27–31%, p < 0.05), with significant levels of collagen-related amino acids (p < 0.05). X-ray diffraction (XRD) and Fourier-transform Raman spectroscopy (FT-Raman) confirmed the biphasic calcium phosphate composition for most samples based on hydroxyapatite with contributions of whitlockite/β-tricalcium phosphate. The highest contribution to the non-apatite phase was made by the central skeletons of both mackerel and Atlantic horse mackerel.
1. Introduction
Based on the data from the Food and Agricultural Organization [1], total fisheries and aquaculture production have undergone a significant increase in recent decades. This growth has been driven by advances in fishing technologies and rapid developments in aquaculture, culminating in a global fish production of 178 million tonnes in the year 2020. Consequently, the amount of fish waste has also experienced a dramatic increase across the world, with large amounts of landed fish lost or their by-products discarded between landing and human food processing and consumption (heads, viscera, fins, bones, and scales), resulting in economic and environmental concerns [1,2]. In this aspect, the European Commission of Common Fisheries Policy (CFP) emphasizes the need to reduce or prohibit discards to comply with the landing obligation, intensifying the urgency to address fish waste management [2].
Traditionally, fish waste was often used for fishmeal and oil production with low profitability, or it is simply discarded [2]. Exploring new avenues from these waste products presents a potential opportunity to not only enhance their value but also reduce economic losses and minimize environmental impact [1]. In this context, research efforts have been intensified to build a circular bioeconomy that promotes sustainable practices in marine resource consumption and production through the reuse and recycling of this waste [2]. Fish by-products and discards are increasingly receiving attention in the fields of biotechnological and pharmaceutical applications as they provide a considerable and sustainable source of high-value biocompounds, such as collagen, gelatin, glycosaminoglycans, hyaluronic acids, calcium phosphates, etc., with significant potential in different fields [1,2].
The use of synthetic calcium phosphates mainly hydroxyapatite (HA) and/or β-tricalcium phosphate (β-TCP) in biomedical applications is well known and widely proven for bone regeneration purposes, providing biocompatible and osteoconductive properties [3]. Additionally, calcium phosphates of biological origin have garnered significant interest as bone substitutes, being the most used bovine sources due to their enhanced composition with relevant elements for bone metabolism, providing an increased biological activity, compared to synthetic alternatives [4]. Fish-derived calcium phosphates, such as those from the discarded teeth of commercial shark species, have been already successfully tested in vitro and in vivo for these purposes [5]. Moreover, the previous characterization of calcium phosphates obtained from tuna spine [4] and from the mixture of the different discarded parts of red scorpionfish, mackerel, and farmed salmon [6] indicated the biomedical potential of these fish-derived sources. These promising findings highlight the need for the in-depth evaluation of these fish-derived calcium phosphates from most commercial fish species, the ones which generate higher volumes of by-products and discards, differentiating the corresponding type and recovery yield of calcium phosphates for each body part of these discarded fish.
Other biotechnological applications of calcium phosphates are related to the removal of inorganic pollutants from aqueous solutions, such as heavy metal ions in wastewater treatments. Within the calcium phosphate mineral family, hydroxyapatite (HA, Ca10(PO4)6(OH)2) has been extensively proven to present a high removal capacity for divalent heavy metals from aqueous solutions, based on different immobilization processes such as ion exchange or dissolution–precipitation. Hydroxyapatite, obtained from fish scales and combined with polylactic acid to form 3D-printed filters, was proven to remove cadmium and lead from an aqueous medium [7]. With the same purpose, Liu and collaborators fabricated scaffolds from fish scales using a freeze-casting technique [8]. Moreover, the efficient fluoride removal from geothermal waste through shell-derived HA was also recently studied [9]. Calcium phosphates in the form of amorphous calcium phosphate (ACP, Ca9(PO4)6) or apatitictricalcium phosphate (Ca9(PO4)5(HPO4)OH), presenting a higher water solubility and a lower stability in a wide pH range, have also been proven to capture nickel, copper, and cobalt ions in solutions, with the concentrations of these metals being significantly reduced [10]. In this aspect, the high availability and low cost of fish-derived calcium phosphates are of exploratory interest for this application. Other potential industrial applications are related to the calcium phosphates’ capability to withstand high temperatures. In this regard, Ozyhar et al. [11] investigated the application of HA as a fire-retardant additive in wood-based materials. Following a similar approach, Sane and coauthors (2023) [12] proposed the use of HA for thermal energy storage purposes such as in concentrated solar power (CSP) plants or in units of heat recovery from industrial waste heat sources. All these applications require some thermal treatment to remove all the organic matter.
Finally, there are applications where the organic matter is not needed to be entirely removed, as in the utilization of calcium phosphate-based materials as fertilizers and biostimulants for plant growth. In this case, Carella et al. [13] proposed the partial and total calcinations of fish bones from fishery by-products where the partial calcined samples were composed of an organic part with bone collagen and fatty acids and an inorganic part of very poorly crystalline hydroxyapatite; the total calcined samples had a negligible content of organic matter and consisted of hydroxyapatite and β-TCP. All of the samples, partial and total, showed promising results in terms of seed germination, plant fertilization, and corn coleoptile biostimulation. Other studies [14,15] have also confirmed the benefits of enhancing soil nutrient content, controlling plant disease, eliminating unwanted plants, and inhibiting parasite growth.
Therefore, the aim of this study was to extend the range of fish-derived calcium phosphates available for these high-value applications. Five representative species from the fishing and aquaculture industries, which are highly commercialized and consumed and therefore are responsible for generating the highest volumes of by-products and waste, were selected, i.e., the main discard-producing species from the fishery industry, including (1) Atlantic horse mackerel (Scomber scombrus), (2) mackerel (Trachurus trachurus), and (3) blue whiting (Micromesistius poutassou), as well as the by-products from the industrial processing of (4) wild hake (Merluccius capensis/paradoxus) and (5) aquaculture turbot (Scophthalmus maximus). The amounts of organic matter, nitrogen, total proteins, lipids, and amino acids in clean bones are provided, along with the main chemical bonds (FT-Raman), structures (X-ray diffraction), and elemental composition (X-ray fluorescence) of calcium phosphates. The recovery yield of clean bones was calculated for each fish body part (heads, central skeletons, and tails) together with the relative quantification of the types of calcium phosphate.
2. Materials and Methods
2.1. Preparation of Raw Materials
The evaluated mineral fraction was obtained from the heads (He) and central skeletons (cS) of Atlantic horse mackerel (AHM, Scomber scombrus), blue whiting (BW, Micromesistius poutassou), hake (Ha, Merluccius capensis/paradoxus), and mackerel (Ma, Trachurus trachurus) and the heads, central skeletons, and tails (Ta) of farmed turbot (Tu, Scophthalmus maximus). AHM, BW, and Ma by-products, from individuals discarded by Galician fishing fleets (specimens with weights ranging from 100 to 300 g captured in the North Atlantic Ocean) were supplied by Opromar (Marín, Spain). Ha wastes were obtained from the on-board industrial filleting of hake by Nueva Pescanova S.L. (Vigo, Spain). The industrial by-products of Tu generated from filleting activities were provided by Stolt Sea Farm (Carnota, Spain). The fractions were separately obtained from the enzyme hydrolysis of the corresponding by-products (heads, central skeletons including the skin, and tails) of the mentioned species in a sustainable process aimed at the joint production of fish protein hydrolysates (FPHs), oils, and minerals under the biorefinery concept [16]. Briefly, each fish sample was homogenized by crushing and hydrolyzed by Novozym 37071 (Novozymes, Nordisk, Bagsvaerd, Denmark) under the following conditions: 0.1% (v/w) of enzyme concentration, 60 °C, pH 8.2, agitation of 250 rpm, solid/liquid ratio of (1:1), and 3 h of hydrolysis. After proteolysis, the bones without muscle remains were separated from the protein hydrolysates using filtration (250 µm). These solids were dried in an oven (80 °C/24 h), ground, and analyzed. Subsequently, they were calcined at 950 °C in a Carbolite CWF laboratory furnace (Carbolite Gero, Hope, Derbyshire, UK) at a heating rate of 5 °C/min. Once the calcination temperature was reached, calcium phosphates were isothermally kept inside the furnace for a period of 16 h, and then, they were cooled at a rate of 5 °C/min before being removed from the furnace. The calcination conditions used were optimized in previous studies [6,13] ensuring the removal of organic matter to obtain the purest mineral fraction as calcium phosphates from other bio-derived sources. The same calcination process was applied for all the discards and by-products in order to facilitate the scaling-up of this calcium phosphate production. In Figure 1 and Table 1, the examples of the material after the hydrolysis and calcination processes are shown, along with the particle sizes obtained from the different mineral fractions after sieving. On the other hand, the liquid hydrolysates together with their oils were subsequently processed (using the centrifugation, decantation, and thermal inactivation stages) for the separate production of oils and FPH. In this study, we mainly focus on the mineral fraction (bones).

Figure 1.
Mineral fraction obtained after hydrolysis and calcination of heads (He) and central skeletons (cS) of mackerel (Ma), Atlantic horse mackerel (AHM), and Hake (Ha).

Table 1.
Particle size range (in percentage, %) obtained from the mineral fraction after hydrolysis calcination and sieving.
2.2. Chemical Analysis
The proximal composition of the different dry skeletons was determined as follows: (1) the water (method 950.46), ash, and organic matter (method 900.2A) content using gravimetry [17]; (2) the total nitrogen concentration using spectrophotometric measurement, after Kjeldhal digestion and the subsequent ammonium reaction in an alkaline medium [18]; (3) the total protein content was calculated as total nitrogen × 6.25 and transformed to percentage versus dry weight; (4) the total lipid content utilizing Soxhlet extraction with diethyl ether and gravimetric quantification [19]; and (5) the amino acid profiles using the ninhydrin reaction, with an amino acid analyzer (Biochrom 30 series, Biochrom Ltd., Cambridge, UK), following the method of [20].
2.3. Structural and Physicochemical Characterization
The crystalline structure of the resulting calcium phosphates was analyzed by X-ray diffraction (XRD) using a X’Pert Pro Panalytical diffractometer (Malvern Panalytical, Malvern, UK). Monochromatic Cu-Kα radiation (λ = 1.5406 Å) was employed and covered a 2θ range of 4–100° (CACTI, UVigo, Vigo, Spain). Furthermore, to identify the main molecular vibrations and the relevant functional groups, Fourier-transform Raman spectroscopy (FT-Raman) was conducted using a B&W Tek i-Raman-785S device (Metrohm, Herisau, Switzerland), fitted with a maximum incident laser radiation of 340 mW and a BAC 100 Probe (785 nm). The measurements were carried out across the Raman shift range of 200–3200 cm−1, achieving an overall resolution of 4 cm−1. The Raman spectra obtained were corrected with a deconvoluted in the band area of 930–1000 cm−1 and a parabolic baseline using the MagicPlot Pro software. Finally, the elemental analysis of calcium phosphates was performed using X-ray fluorescence (XRF, Siemens SRS3000, CACTI, UVigo) with a certified reference material SARM 32- phosphate rock (Phalaborwa) prepared by MINTEK, Republic of South Africa, with certified values for the following constituents: P2O5 (39.96%), CaO (54.44%), F (2.49%), CO2 (1.61%), MgO (0.50%), SrO (0.52%), and Fe2O3 (0.14%).
2.4. Statistical Analyses
The one-way ANOVA test followed by means of Tukey test was applied to know the existence of significant differences between samples using Statistica 8.0 (StatSoft, Tulsa, OK, USA). Statistical significance was stablished at p < 0.05.
3. Results and Discussion
3.1. Production Yields and Proximal Composition of Skeleton Material
The yields of clean skeletons retrieved after the enzyme hydrolysis of fish substrates are summarized in Table 2, expressed as the percentage of wet skeletons (Ys, % w/w) and the percentage of dry skeletons processed at 80 °C/24 h (Yds, % w/w). Based on these values, a higher amount of wet skeleton (Ys) was determined for the central skeleton (including the skin) of Atlantic horse mackerel, cS-AHM, with a mean value of 23% w/w, and being statistically significant (p < 0.05), followed by the blue whiting samples, cS-BW and He-BW, with values of around 19% w/w. The lowest percentages in wet skeleton corresponded to the head and central skeleton of hake (He-Ha, cS-Ha) and the tails of turbot (Ta-Tu), with mean percentages around 13–15% w/w. Using a procedure similar to that reported in a previous study, but by employing Alcalase as the commercial protease, the mean percentage of wet skeletons from seabream and seabass was around 19–20% w/w and 11–14% w/w, respectively [21]. However, the mean percentage of wet skeletons from hydrolyzed salmonid heads and frames was between 9 and 12%, with lower values than those of our study [22]. Such low values can only be due to the lower presence of non-digested substrates (the bone fraction) in the wastes, since the degree of hydrolysis and protein concentration of the hydrolysates obtained from all fish species were comparable. When the yield of recovery of these materials after drying was determined (Table 1 as Yds, % w/w), the obtained data revealed similar values as the wet case (Ys): cS-AHM as the highest and He-Ma and He-Ha as the lowest sources of dry bones. The greater loss of mass in the mackerel, blue whiting, and hake samples is notable in comparison, for example, with turbot, indicating the lower amount of wet organic matter present in the waste collected after filtering the hydrolysates of this species. In general, a higher amount of dry material was obtained from the central skeletons in compared to the heads (and tails, Ta-Tu), although these differences were not statistically significant (p > 0.05). This preponderance of the central skeletons over the heads, in terms of mass, seems to be innate to the anatomical structure of fish since it has also been observed for species such as salmon, seabream, and seabass [21,22]. Unfortunately, a greater comparison cannot be made using results from other authors because this type of information (on the mass balances of bones) is unexplored in the literature.

Table 2.
Yields of skeleton material.
In Table 3, the proximal composition of the multiple bones is compiled. Moisture, although with slight and significant differences in some samples, was always lower than 5%. The ash content was higher than 62% for all samples, with the heads of Atlantic horse mackerel and blue whiting (He-AHM, He-BW) contributing the most to the mineral fraction. The organic matter in fish skeletons was around 29–34%, except in BW (24–26%) and He-AHM (24%). The presence of fat covering the skeletons was close to 10%, with the low levels of unhydrolyzed organic material making a significant contribution, in dry fish skeletons separated from AHM and Ma protein hydrolysates. The lipid content in Ha, BW, and Ta-Tu fractions was low and lesser than 4.3%. The protein concentration (27–31% w/w) was the highest (p < 0.05) in hake and turbot skeletons. In general, the protein levels found in our study were lower than those reported for deep-sea redfish and sardine bones [23]. Nevertheless, all samples evaluated in this study could have an interesting chemical composition for their potential use as fertilizers. In a previous study [13], the bones of sardine, presenting similar OM content when processed in the range of 300–900 °C, demonstrated an improved capacity for plant biostimulation.

Table 3.
Proximal composition of various skeletons, determined on a dry weight basis.
The presence of collagen in dry skeletons was assessed based on the content of the most relevant amino acids (glycine, proline, and hydroxyproline) that make up such a protein (Table 4). The highest percentage of these amino acids was observed in the tails of turbot (38.1% of Gly+Pro+OHPro) and the central skeletons of hake (36.5%). This value was significantly lower (p < 0.05) than 31%, which is the value of the bones of AHM. The percentages of essential amino acids were very close for most samples (around 32–33%), except in samples with a higher content of collagen-related amino acids (Ta-Tu and cS-Ha), in which the ratio was lower than 30.5%. Different skeleton-autoclaving procedures were performed to extract collagen from bones [24], but the yields of collagen/gelatin recovery were lower than 0.1% w of collagen/w of dry bone. These outputs restrict the potential use of bones for this bioproduction in a sustainable and profitable way.

Table 4.
Amino acids composition for each dry fish skeletons.
3.2. Structural and Physicochemical Characterization of Purified Calcium Phosphates
The crystalline structure and main functional groups of the corresponding calcium phosphates obtained after the controlled calcination of the different skeletons were evaluated using XRD. Figure 2a shows the diffraction patterns obtained for all the calcium phosphates in the general range. First, the presence of intense and sharp peaks attributed to the calcium phosphate structure can be observed with the more intense peaks obtained for the expected positions in the range of 30–35°, amplified for representative samples in Figure 2b, where calcium phosphates diffract more intensely. Moreover, differences are clearly observed when the patterns obtained for samples are compared, i.e., He-Tu and cS-BW showed low intense and wide bands in the area of interest that correspond to amorphous calcium phosphates, while the remaining samples showed intense sharp bands that correspond to crystalline calcium phosphates. Based on the attributions, the typical reflections located at 25.86, 31.76, 32.18, 32.91, 34.05, 39.80, 46.69, 49.47, and 52.07° correspond to the crystal planes (0,0,2), (1,2,1), (1,1,2), (3,0,0), (2,0,2) (3,1,0), (2,2,2), (1,2,3), and (4,0,2) of the apatite phase, i.e., hydroxyapatite (HA), Ca5(PO4)3(OH) [25]. Moreover, most of the diffraction patterns revealed other peaks at positions 27.94, 31.22, and 34.54° that can be attributed to the crystal planes (2,1,4), (0,2,10), and (2,2,0) of the non-apatite phase, i.e., whitlockite/β-tricalcium phosphate (TCP), Ca3(PO4)2 [26,27]. Finally, additional highly intense diffraction peaks at positions 37.30 and 53.79°, attributed to CaO crystals, can be observed in some samples.
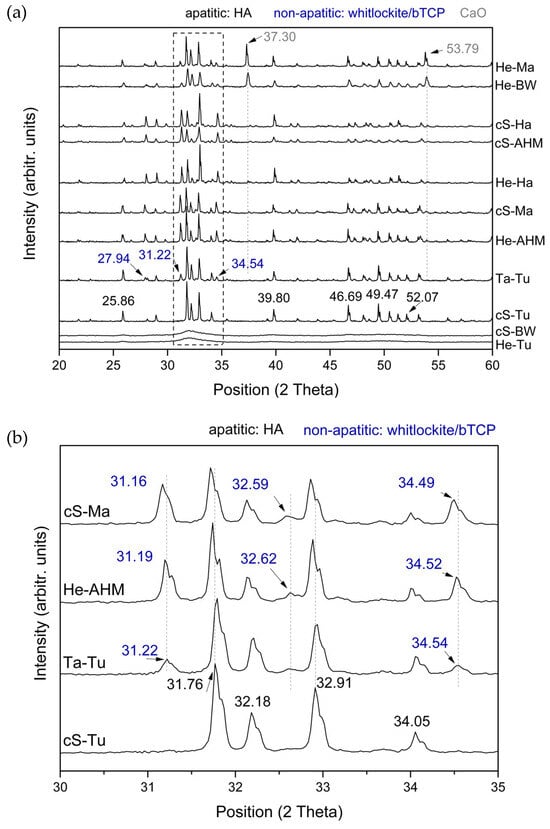
Figure 2.
X-ray diffraction patterns obtained for all the calcium phosphates in the general range (a) and in detail in the region where the most relevant peaks diffract (30–35°) for several representative samples (b).
The diffraction patterns in the region 30–35°, presented in Figure 2b, for cS-Ma, He-AHM, Ta-Tu, and cS-Tu, confirmed the major composition of HA crystals for the central skeleton of turbot, cS-Tu, and the additional contribution of the non-apatite crystal planes for cS-Ma, He-AHM, and Ta-Tu. According to the literature [26,27], the crystalline structure for a pure (100%) non-apatite phase such as β-TCP Ca3(PO4)2 or whitlockite Ca9Mg (PO3OH)(PO4) presents typical reflections located at 31.0°, 32.4°, and 34.4°, corresponding to the (0,2,10), (1,2,8), and (2,2,0) diffraction planes. The presence of both apatite and non-apatite contributions for the diffraction patterns of He-Ma, cS-Ma, He-BW, He-Ha, cS-Ha, cS-AHM, He-AHM, and Ta-Tu confirmed their biphasic composition. These results comply with those of previous studies [28], where the biphasic calcium phosphate particles with 41% wt. HA and 59% wt. of β-TCP, calcined in the same temperature range, presented diffraction peaks at 25.86° and 31.82° attributed to the crystal planes (0,0,2) and (1,2,1) of HA and at 27.78° and 34.45° attributed to the crystal planes (2,1,4) and (2,2,0) of β-TCP. Finally, the calcium phosphates obtained from He-Ma and He-BW presented additional high-intensity diffraction peaks at positions 37.30 and 53.79° attributed to CaO crystals. The diffraction peak at 53.79° was also slightly detected for other samples such as cS-Ha and He-Ha.
To complement these results, the main functional groups of these purified calcium phosphates were evaluated using FT-Raman to obtain the fingerprint region (300–1100 cm−1), where significant inorganic molecular vibrations are observed (Figure 3a). Thus, the Raman spectra exhibit the main vibration modes associated with PO43− groups, with the bands in the range of 400–485 cm−1 and 570–620 cm−1 attributed to the symmetric bending (ν2) and asymmetric bending (ν4) of PO43− group, respectively, the intense band at 930–990 cm−1 attributed to the symmetric stretching (ν1) of PO43− group, and the less intense peaks centered at 1029, 1047, and 1077 cm−1 attributed to the asymmetric stretching (ν3) of PO43− group [27,29,30,31,32,33]. However, differences were detected between the obtained Raman spectra when they were assessed in detail, in accordance with the XRD results; for instance, in the 900–1000 cm−1 region associated with the main vibrations of PO43− groups, i.e., symmetric stretching (ν1), the spectra of cS-Ma, cS-AHM, He-AHM, and cS-Ha clearly present a double band with peaks at 958–962 cm−1 and 975 cm−1, while the spectra of He-Tu and cS-BW show a single wide band at around 960 cm−1. Therefore, the fact that all the Raman spectra show the main vibration mode related with the symmetric stretching (ν1) of PO43− groups of calcium phosphates with an intense band in the range 930–990 cm−1, together with the presence of most bands of symmetric bending (ν2), asymmetric bending (ν4), and asymmetric stretching (ν3), confirms the calcium phosphate composition of all samples; however, the mentioned differences found pertaining to the double or single band in the characteristic region of 900–1000 cm−1 support the XRD results, indicating the presence of different phases of calcium phosphates.
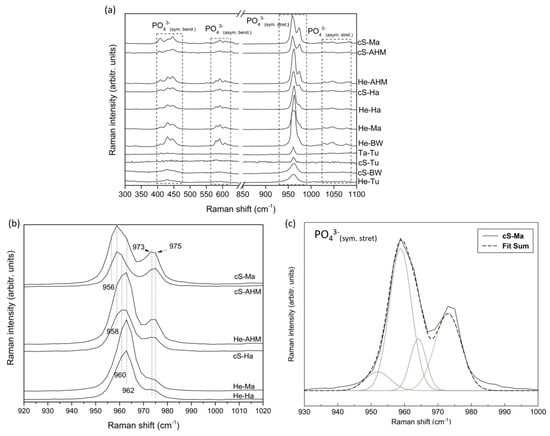
Figure 3.
FT-Raman spectra obtained for all the calcium phosphates in the fingerprint region (300–1100 cm−1) (a) in detail in the region 920–1020 cm−1 for several representative samples (b) and deconvolution of the FT-Raman double band with peaks at 958 and 973 cm−1 obtained for the central skeleton of mackerel, cS-Ma (c).
The Raman spectra in the region of 920–1020 cm−1 is presented in detail in Figure 3b for several representative samples, such as cS-Ma, cS-AHM, He-AHM, cS-Ha, He-Ma, and He-Ha, indicating a double band at 958 cm−1 and 973–975 cm−1 for cS-Ma and cS-AHM, at 960–962 cm−1 and 973–975 cm−1 for He-AHM and cS-Ha, and at 962 cm−1 with a weak band or shoulder at 975 cm−1 for He-Ma and He-Ha. It is proven in the literature that one vibrational band located at 962 cm−1 is attributed to the symmetric stretching (ν1) of PO43− groups of HA crystals [27,31,32]. It is also demonstrated that a wide band compose of two independent vibrations situated at 948 cm−1 and 970 cm−1 are both assigned to the symmetric stretching (ν1) of PO43− groups of pure non-apatite whitlockite/β-TCP structures [27,32,33,34]. Moreover, bands situated at 956 cm−1 and in the range 958–968 are attributed to HA and whitlockite/β-TCP structures, respectively [27]. The presence (Figure 3b) of a band at 973–975 cm−1 confirmed the presence of whitlockite/β-TCP structures and a band at 958–962 cm−1 confirmed the presence of HA structures in the samples evaluated in this study. Moreover, the fact that the peak at 962 cm−1 in He-Ha and He-Ma shifts gradually to lower numbers, up to 958 cm−1 in cS-Ma and cS-AHM, with an increase, at the same time, in the relative Raman intensity of the band 973–975 cm−1 for the latter, would indicate a gradual increase in the non-apatite contribution of cS-Ma and cS-AHM.
To confirm the presence of the peak at a position around 948 cm−1 is due to the non-apatite phase, the deconvolution of the double FT-Raman band is presented for the cS-Ma in Figure 3c. Accordingly, four bands situated around 951, 958, 964, and 973 cm−1 contribute to these double bands, where the bands situated at 951 and 973 cm−1 correspond to the non-apatite βTCP contribution. The presence of the band at 951 explains the shift in the main peak from higher to lower positions, from 962 to 958 cm−1, in the samples with the highest contribution.
Finally, a quantitative analysis using FT-Raman was carried out to confirm the tendency observed in the spectra for the higher and lower contributions of the non-apatite βTCP/whitlockite phase for the different calcium phosphates that were evaluated. The relative ratios established with the Raman intensities at 962 cm−1 in relation to 975 cm−1 and at 975 cm−1 in relation to 591 cm−1 are shown in Table 5 for cS-Ma, cS-AHM, He-AHM, cS-Ha, He-Ma, and He-Ha. The obtained ratios would confirm a higher relative contribution of β-TCP/whitlockite for cS-Ma and cS-AHM, a lower contribution for He-Ma and He-Ha, and an intermediate contribution for He-AHM and cS-Ha.

Table 5.
Raman intensities and corresponding ratios.
A comparison of the FT-Raman spectra for the calcium phosphates obtained from the same anatomic region, i.e., the head, for all sources is shown in Figure 4. These representation helps to infer that the obtained calcium phosphate structure does not depend on the anatomic region; therefore, a wide range of compositions from HA to increasing gradual contributions of non-apatite βTCP can be easily obtained from, for instance, the waste obtained from the heads of commercial fish species.
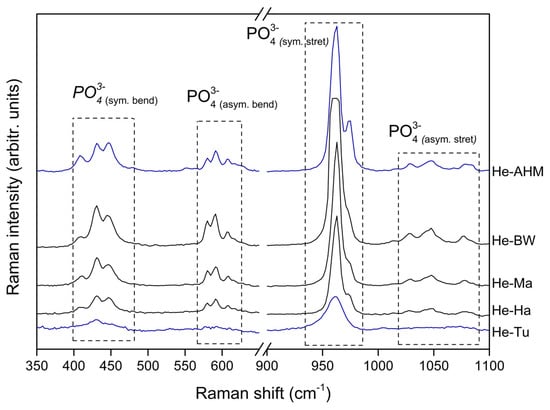
Figure 4.
FT-Raman spectra for the calcium phosphate obtained from the head of all fish sources evaluated.
The elemental composition of calcium phosphates was also evaluated using XRF and is presented in Table 6 as certified wt.% per element. All samples have Ca and P as major elements, followed by Mg. The presence of other elements such as Na, K, S, Cl, and Si was also identified, though in significantly lower amounts and not precisely measured (uncertified values). The Ca/P ratios ranged from 1.83, for cS-Ma, to 3.28 and 3.39, for He-BW and He-Ma. It is known that, for biphasic CaP or pure hydroxyapatite, the CaP ratio should be in the range of 1.5–1.67 [35]. The highest values obtained for He-BW and He-Ma are associated with the additional presence of CaO in the structure, as already indicated in Figure 1. The remaining samples present Ca/P ratios higher than expected, and this could be explained by the presence of CaO, although it makes a lower contribution, as observed for cS-Ha, cS-AHM, He-Ha, cS-Ma, and He-AHM in Figure 2a. All samples contain Mg, even the samples without a crystal structure (cS-BW and He-Tu) and the ones in which pure hydroxyapatite is detected (cS-Tu). This indicates that Mg is present in all samples; however, it is not always incorporated in their crystal structure as the non-apatite phase (whitlockite).

Table 6.
Elemental composition of alcium phosphates (wt. %) by X-ray fluorescence.
This study evaluates a wide range of fish-derived calcium phosphates obtained from five representative species of the fishing and aquaculture industries that are highly commercialized and consumed, thus generating high volumes of wastes that ensure the availability of raw materials. The results indicated that a variety of calcium phosphates can be obtained even within the same species for different wasted parts (heads, central skeletons, tails), as can be seen in the summary provided in Table 7. It is observed that, based on the source of the discard and the species, calcium phosphate is present in different crystalline structures: monophasic (apatite or non-apatite) structure and biphasic (mixture of both) or amorphous calcium phosphate, and as in the case of farmed turbot, where biphasic HA–whitlockite/β-TCP was obtained from the tails, pure hydroxyapatite from the central skeletons, and amorphous calcium phosphate from the heads. As reported by Duta and collaborators in their review [36], various biphasic calcium phosphate materials have been produced from by-products of other fish, such as bones from red scorpionfish (Scorpaena scrofa) and salmon (Salmo salar) where HA was the predominant component of the resulting powders, with minor amounts of β-tricalcium phosphate detected [6]. Similarly, a molar ratio of 75:25 (HA: β-TCP) was found in codfish bones [37]. Conversely, calcium phosphates obtained from sardine scales exhibited a similar composition but with a higher β-TCP content (54.2% of HA and 45.8% of β-TCP (wt%)) [38]. Ideia et al. reported similar results, finding that black scabbard fish bones consisted of homogeneous mixtures of hydroxyapatite and β-tricalcium phosphate (60% of HA and 40.1 of β-TCP) after calcination at 1000 °C, whereas the skin from grey triggerfish (Balistes capriscus) contained HA as the main phase, together with small amounts of other mineral phases, such as halite and rhenanite, which are known to enhance osteogenesis when used as bone substitute [39]. Other studies have also extracted pure HA from other species and parts, such as tilapia fish scales or the jaws of Prionace glauca shark for lead ion removal and bone regeneration purposes [5,8]. Furthermore, the yields of clean bone recovery in this study were also acquired from each discarded part of the fish body to estimate the potential profitability of calcium phosphate sources for each of these parts. By considering the percentage of wet clean bones that were obtained (% (w/w) or g/100 g of waste) using direct extraction with the hydrolysis and filtration process, this percentage was over 10% for all samples, with the central skeleton of Atlantic horse mackerel (cS-AHM) being the discard that provided the highest fraction of clean bones (p < 0.05), around 23 g per each 100 g of waste substrate (Table 1), followed by the discards from blue whiting (cS-BW, He-BW). The calcium phosphates obtained from these discards present different compositions, being biphasic HA–whitlockite/β-TCP for cS-AHM, with a high contribution of the non-apatite phase (Raman ratios, Table 4), amorphous calcium phosphate for cS-BW, and biphasic composition with the contribution of CaO crystals for He-BW. On the other hand, the lowest yield recovery percentages for wet clean bones corresponded to the heads and central skeletons of hake (He-Ha, cS-Ha) and the tails of turbot (Ta-Tu), with mean yield recovery percentages of around 13–15% w/w. These species presented the highest protein content (27–31%, p < 0.05) recovery, with significant levels of collagen-related amino acids (p < 0.05). The calcium phosphates obtained for three species corresponded to biphasic HA–whitlockite/β-TCP, with cS-Ha being one of the highest contributors of the non-apatite phase (Table 5). In general terms, this study demonstrates the feasibility of easily obtaining a wide range of compositions based on calcium phosphates by harnessing underutilized resources, to develop interesting solutions for high-value-added applications such as bone tissue regeneration, by combining the different phases of calcium phosphate to achieve the optimal balance in stability/resorbability, biotechnological applications (especially for HA source) in filters, fire-retardant additives, and thermal storage purposes; for all calcium phosphate phases that can be used as fertilizers and biostimulants, an interesting point is that these application do not require the total calcination of samples.

Table 7.
Type of calcium phosphate presented in the different skeletons.
4. Conclusions
The extraction and characterization analyses of the calcium phosphates obtained from the heads, central skeletons, and tails of Atlantic horse mackerel, mackerel, blue whiting, wild hake, and aquaculture turbot proved their use as sources of amorphous calcium phosphate, pure hydroxyapatite, and biphasic hydroxyapatite–whitlockite/β-TCP, contributing differently to the non-apatite phase according to the by-product or discard used. The evaluation of the production yield of recovery inferred that the central skeleton of Atlantic horse mackerel (23.0 ± 3.1%, with a significantly higher p-value, p < 0.05) and both the central skeleton and head of blue whiting were the samples with the highest mineral fraction. These two species provide biphasic HA–whitlockite/β-TCP along with CaO crystals in some cases and amorphous calcium phosphate. Farmed turbot provides the widest range of calcium phosphates: hydroxyapatite from the central skeleton, biphasic HA–whitlockite/β-TCP from the tail, and amorphous calcium phosphate from the head. According to the proposed Raman ratios, the biphasic calcium phosphates with the highest non-apatite contributions were obtained from the central skeletons of mackerel and Atlantic horse mackerel, and those with the lowest (purer hydroxyapatite) non-apatite contributions were obtained from the heads of mackerel and hake. Ongoing studies are investigating the valorization of these calcium phosphates to explore their potential applications in various fields, such as bone tissue regeneration. Thus, technoeconomic and environmental analyses are needed to evaluate the effectiveness and scalability of large-scale implementation of these calcium phosphates.
Author Contributions
Conceptualization, J.A.V.; methodology, J.A.V. and P.G.; validation, M.L.-Á., P.G. and J.A.V.; formal analysis, M.L.-Á., S.P.-D., J.V. and J.A.V.; investigation, M.L.-Á. and J.A.V.; resources, M.L.-Á., P.G., J.S., J.V., S.P.-D. and J.A.V.; writing—original draft preparation, M.L.-Á., S.P.-D. and J.A.V.; writing—review and editing, M.L.-Á., P.G., J.S., S.P.-D. and J.A.V.; visualization, M.L.-Á., P.G., J.S., S.P.-D. and J.A.V.; supervision, P.G., J.S. and J.A.V.; project administration, P.G., J.S. and J.A.V.; funding acquisition, P.G., J.S. and J.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
Authors are grateful to EU Interreg Program (IBEROS+) (0072_IBEROS_MAIS_1_E, Interreg-POCTEP 2021–2027) and GRC support program (ED431C 2021/49) and (GPC_IN607B 2024/010) from Xunta de Galicia, for financial support. Researchers from IIM-CSIC also want to thank the project LIFE-REFISH (Project 101074323, LIFE21-ENV-ES-LIFE REFISH) for financial support.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The technical staff from CACTI (UVigo) is gratefully acknowledged. We acknowledge the support of the CSIC Open Access Publication Support Initiative, through its Unit of Information Resources for Research (URICI), in the form of the publication fee.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Italy, Rome, 2022. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Darghiasi, S.F.; Farazin, A.; Ghazali, H.S. Design of Bone Scaffolds with Calcium Phosphate and Its Derivatives by 3D Printing: A Review. J. Mech. Behav. Biomed. Mater. 2024, 151, 106391. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; De Carlos, A.; León, B. Biological Hydroxyapatite Obtained from Fish Bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Pérez-Davila, S.; Rodríguez-Valencia, C.; González, P.; Serra, J. The Improved Biological Response of Shark Tooth Bioapatites in a Comparative in Vitro Study with Synthetic and Bovine Bone Grafts. Biomed. Mater. 2016, 11, 035011. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Álvarez-Olcina, I.; Malvido-Fresnillo, P.; Vázquez, J.A.; Boutinguiza, M.; Comesaña, R.; Pou, J. Biogenic Calcium Phosphate from Fish Discards and By-products. Appl. Sci. 2021, 11, 3387. [Google Scholar] [CrossRef]
- Fijoł, N.; Abdelhamid, H.N.; Pillai, B.; Hall, S.A.; Thomas, N.; Mathew, A.P. 3D-Printed Monolithic Biofilters Based on a Polylactic Acid (PLA)-Hydroxyapatite (HAp) Composite for Heavy Metal Removal from an Aqueous Medium. RSC Adv. 2021, 11, 32408–32418. [Google Scholar] [CrossRef]
- Liu, W.K.; Liaw, B.S.; Chang, H.K.; Wang, Y.F.; Chen, P.Y. From Waste to Health: Synthesis of Hydroxyapatite Scaffolds from Fish Scales for Lead Ion Removal. JOM 2017, 69, 713–718. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Dong, Y.; Zhang, H.; Yun, R.; Liu, Z. Removal of Fluoride from Geothermal Water by Waste-Synthesized Al(OH)3-HAP@ZMS Composite Adsorbent: Sorption Capability and Mechanism. Water Air Soil Pollut. 2023, 234, 411. [Google Scholar] [CrossRef]
- Lizoul, B.; Kzaiber, F.; Chemaa, A.; Benharef, E.; El Hajri, J.; Zahidi, E. Use of Calcium Phosphates to Remove Nickel, Copper and Cobalt Ions from Aqueous Solutions. Int. J. Chemtech. Res. 2019, 12, 93–102. [Google Scholar] [CrossRef]
- Ozyhar, T.; Tschannen, C.; Thoemen, H.; Zoppe, J.O. Evaluating the Use of Calcium Hydrogen Phosphate Dihydrate as a Mineral-Based Fire Retardant for Application in Melamine-Urea-Formaldehyde (MUF)-Bonded Wood-Based Composite Materials. Fire Mater. 2022, 46, 595–604. [Google Scholar] [CrossRef]
- Sane, A.R.; Pham Minh, D.; Semlal, N.; Boulif, R.; Toussaint, C.; Germeau, A.; Nzihou, A. Clay/Phosphate-Based Ceramic Materials for Thermal Energy Storage—Part I: Effect of Synthetic Phosphate Content on Microstructure, Thermo-Physical and Thermo-Mechanical Properties. Open Ceram. 2023, 14, 100346. [Google Scholar] [CrossRef]
- Carella, F.; Seck, M.; Esposti, L.D.; Diadiou, H.; Maienza, A.; Baronti, S.; Vignaroli, P.; Vaccari, F.P.; Iafisco, M.; Adamiano, A. Thermal Conversion of Fish Bones into Fertilizers and Biostimulants for Plant Growth—A Low Tech Valorization Process for the Development of Circular Economy in Least Developed Countries. J. Environ. Chem. Eng. 2021, 9, 104815. [Google Scholar] [CrossRef]
- Wibisono, Y.; Ummah, S.R.; Hermanto, M.B.; Djoyowasito, G.; Noviyanto, A. Slow-Release Hydroxyapatite Fertilizer from Crab Shells Waste for Sustainable Crop Production. Results Eng. 2024, 21, 101781. [Google Scholar] [CrossRef]
- Dhar, M.; Jasrotia, R.; Langer, S.R. Manufacturing Organic Fertilizers and Manures. In Fish Waste to Valuable Products. Sustainable Materials and Technology; Maqsood, S., Naseer, M.N., Benjakul, S., Zaidi, A.A., Eds.; Springer: Singapore, 2024. [Google Scholar]
- Vázquez, J.A.; Pedreira, A.; Durán, S.; Cabanelas, D.; Souto-Montero, P.; Martínez, P.; Mulet, M.; Pérez-Martín, R.I.; Valcarcel, J. Biorefinery for Tuna Head Wastes: Production of Protein Hydrolysates, High-Quality Oils, Minerals and Bacterial Peptones. J. Clean. Prod. 2022, 357, 131909. [Google Scholar] [CrossRef]
- AOAC. Methods of Analysis, 15th ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1997. [Google Scholar]
- Havilah, E.J.; Wallis, D.M.; Morris, R.; Woolnough, J. A Micro-Colorimetric Method for Determination of Amonia in Kjeldajñ Dogests with a Manueal Spectrophtometer. Lab. Pract. 1977, 26, 545–547. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of Amino Acids on Sulfonated Polystyrene Resins an Improved System. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar]
- Valcarcel, J.; Sanz, N.; Vázquez, J.A. Optimization of the Enzymatic Protein Hydrolysis of By-Products from Seabream (Sparus aurata) and Seabass (Dicentrarchus labrax), Chemical and Functional Characterization. Foods 2020, 9, 1503. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of Aquaculture By-Products of Salmonids to Produce Enzymatic Hydrolysates: Process Optimization, Chemical Characterization and Evaluation of Bioactives. Mar. Drugs 2019, 17, 676. [Google Scholar] [CrossRef]
- Szpak, P. Fish Bone Chemistry and Ultrastructure: Implications for Taphonomy and Stable Isotope Analysis. J. Archaeol. Sci. 2011, 38, 3358–3372. [Google Scholar] [CrossRef]
- Wijayanti, I.; Benjakul, S.; Sookchoo, P. Effect of High Pressure Heating on Physical and Chemical Characteristics of Asian Sea Bass (Lates Calcarifer) Backbone. J. Food. Sci. Technol. 2021, 58, 3120–3129. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Structural Variations in Natural F, OH, and Cl Apatites. Am. Mineral. 1989, 74, 870–876. [Google Scholar]
- Hughes, J.M.; Jolliff, B.L.; Rakovan, J. The Crystal Chemistry of Whitlockite and Merrillite and the Dehydrogenation of Whitlockite to Merrillite. Am. Mineral. 2008, 93, 1300–1305. [Google Scholar] [CrossRef]
- Aguiar, H.; Chiussi, S.; López-Álvarez, M.; González, P.; Serra, J. Structural Characterization of Bioceramics and Mineralized Tissues Based on Raman and XRD Techniques. Ceram. Int. 2018, 44, 495–504. [Google Scholar] [CrossRef]
- Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.C.; Silvestros, S.; Sagnou, M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and in Vitro Studies. Materials 2018, 11, 595. [Google Scholar] [CrossRef]
- Posset, U.; Löcklin, E.; Thull, R.; Kiefer, W. Vibrational Spectroscopic Study of Tetracalcium Phosphate in Pure Polycrystalline Form and as a Constituent of a Self-Setting Bone Cement. J. Biomed. Mater. Res. 1998, 40, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Penel, G.; Delfosse, C.; Descamps, M.; Leroy, G. Composition of Bone and Apatitic Biomaterials as Revealed by Intravital Raman Microspectroscopy. Bone 2005, 36, 893–901. [Google Scholar] [CrossRef]
- Corno, M.; Busco, C.; Civalleri, B.; Ugliengo, P. Periodic Ab Initio Study of Structural and Vibrational Features of Hexagonal Hydroxyapatite Ca10(PO4)6(OH) 2. Phys. Chem. Chem. Phys. 2006, 8, 2464–2472. [Google Scholar] [CrossRef]
- De Aza, P.N.; Santos, C.; Pazo, A.; De Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 1. Raman Spectrum of β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- Kim, D.H.; Hwang, K.H.; Lee, J.D.; Park, H.C.; Yoon, S.Y. Long and Short Range Order Structural Analysis of In-Situ Formed Biphasic Calcium Phosphates. Biomater. Res. 2015, 19, 14. [Google Scholar] [CrossRef]
- Dalmônico, G.M.L.; Franczak, P.F.; Levandowski, N.; Camargo, N.H.A.; Dallabrida, A.L.; Da Costa, B.D.; Gil, O.G.; Cambra-Moo, O.; Rodríguez, M.A.; Canillas, M. An: In Vivo Study on Bone Formation Behavior of Microporous Granular Calcium Phosphate. Biomater. Sci. 2017, 5, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Truite, C.V.R.; Noronha, J.N.G.; Prado, G.C.; Santos, L.N.; Palácios, R.S.; Do Nascimento, A.; Volnistem, E.A.; da Silva Crozatti, T.T.; Francisco, C.P.; Sato, F.; et al. Bioperformance Studies of Biphasic Calcium Phosphate Scaffolds Extracted from Fish Bones Impregnated with Free Curcumin and Complexed with Β-Cyclodextrin in Bone Regeneration. Biomolecules 2022, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Dorcioman, G.; Grumezescu, V. A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards. Nanomaterials 2021, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.; Silva, M.F.; Pullar, R.C.; Braga Da Cruz, I.; Jorge, R.; Pintado, M.M.E.; Castro, P.M.L. Extraction and Characterisation of Apatite- and Tricalcium Phosphate-Based Materials from Cod Fish Bones. Mater. Sci. Eng. C 2013, 33, 103–110. [Google Scholar] [CrossRef]
- Belouafa, S. Biphasic Calcium Phosphate Derived from a Sardine By-Product. Curr. Trends. Biomed. Eng. Biosci. 2017, 6, 58–60. [Google Scholar] [CrossRef]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P.C. Fish Processing Industry Residues: A Review of Valuable Products Extraction and Characterization Methods. Waste Biomass Valorization 2020, 11, 3223–3246. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).