Unravelling Diatoms’ Potential for the Bioremediation of Oil Hydrocarbons in Marine Environments
Abstract
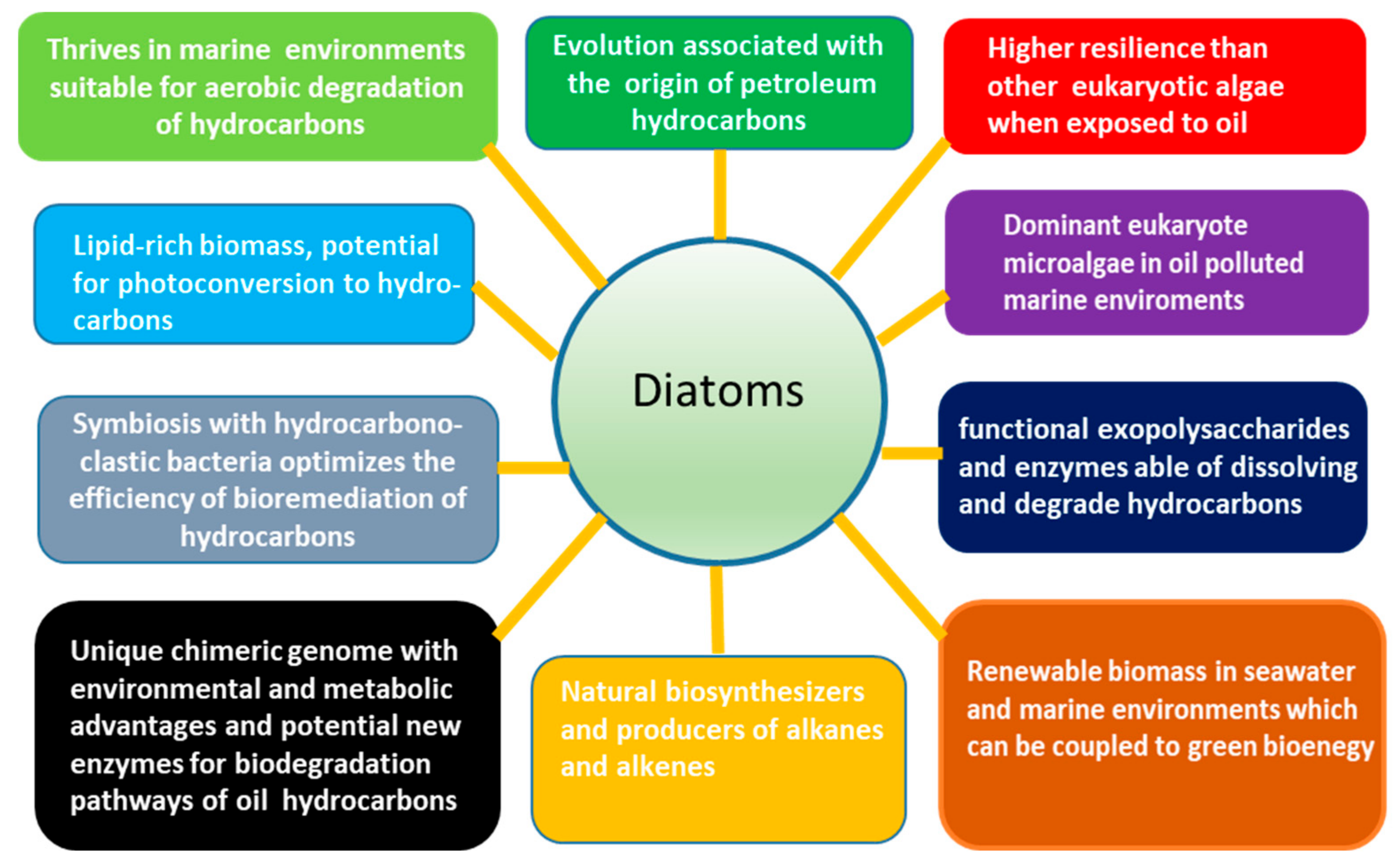
1. Introduction
2. Diatom Microalgae: Attributes and Potential for the Biodegradation and Bioremediation of Petroleum Hydrocarbons
3. Diatom Exopolysaccharides—Role as Biosurfactants and Biodegraders of Oil
4. Diatoms—Origin and Producers of Isoprenoids, Alkanes, and Alkenes
5. Marine Diatoms—Sustainable and Renewable Remediation of Polycyclic Aromatic Hydrocarbons (PAHs)
6. Diatom–Bacterial Synergy in the Removal of Petroleum Hydrocarbons
7. Environmental Conditions Affecting Diatoms’ Petroleum Hydrocarbon Biodegradation and Bioremediation Activities
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGenity, T.J.; Folwell, B.D.; McKew, B.A.; Sanni, G.O. Marine crude-oil biodegradation: A central role for interspecies interaction. Aquat. Biosyst. 2012, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Setta, S.; Liang, Y.; Brown, C.M.; Xuc, C.; Sweet, J.; Passowd, U.; Finkel, Z.B.; Irwin, A.J.; Santschi, P.H.; Quigg, A. Response of natural phytoplankton communities exposed to crude oil and chemical dispersants during a mesocosm experiment. Aquat. Toxicol. 2019, 206, 43–53. [Google Scholar] [CrossRef]
- Kerr, R.A.; Service, R.F. What can replace cheap oil and when. Science 2005, 309, 1. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial degradation of hydrocarbons: Basic principles for bioremediation: A review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef]
- Kanwal, M.; Ullah, H.; Gulzar, A.; Sadiq, T.; Ullah, M.; Sarfraz, M.; Gul, Z.; Aslam, M.W.; Khan, N.N.; Batool, T.; et al. Biodegradation of petroleum hydrocarbons and the factors effecting rate of biodegradation. Am. J. Biomed. Sci. Res. 2022, 16, 6. [Google Scholar] [CrossRef]
- Casau, M.; Dias, M.F.; Matias, J.C.O.; Nunes, L.J.R. Residual Biomass: A comprehensive review on the importance, uses and potential in a circular bioeconomy Approach. Resources 2022, 11, 35. [Google Scholar] [CrossRef]
- Stonik, V.; Stonik, I. Low-molecular-weight metabolites from diatoms: Structures, biological roles and biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, D.G.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; pp. 3–123. [Google Scholar]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical Controls and Feedbacks on Ocean Primary Production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Mohan, R.R. The contribution of diatoms to worldwide crude deposits. In The Science of Algal Fuels, Cellular Origin, Life in Extreme Habitats and Astrobiology; Gordon, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 357–382. [Google Scholar] [CrossRef]
- Mann, D.G.; Droop, S.J.M. Biodiversity, biogeography and conservation of diatoms. Hydrobiologia 1996, 336, 19–32. [Google Scholar] [CrossRef]
- Vinayak, V.; Manoylov, K.M.; Gateau, H.; Blanckaert, V.; Hérault, J.; Pencréac’h, G.; Marchand, J.; Gordon, R.; Schoefs, B. Diatom Milking: A Review and New Approaches. Mar. Drugs 2015, 13, 2629–2665. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Mahapatra, D.M.; Karthick, B.; Gordon, R. Milking Diatoms for Sustainable Energy: Biochemical Engineering versus Gasoline-Secreting Diatom Solar Panels. Ind. Eng. Chem. Res. 2009, 48, 8769–8788. [Google Scholar] [CrossRef]
- Atlas, R.M.; Hazen, T.C. Oil biodegradation and bioremediation: A tale of the two worst spills in U.S. Environ. Sci. Technol. 2011, 45, 6709–6715. [Google Scholar] [CrossRef]
- Domde, P.; Kapley, A.; Purohit, H.J. Impact of Bioaugmentation with a consortium of bacteria on the remediation of wastewater-containing hydrocarbons. Environ. Sci. Pollut. Res. Int. 2007, 14, 7–11. [Google Scholar]
- Stal, L.J.; de Brouwer, J.F.C. Biofilm formation by benthic diatoms and their influence on the stabilization of intertidal mudflats. Berichte–Forschungszentrum Terramare 2003, 12, 109–111. [Google Scholar]
- Cerniglia, C.E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 1992, 3, 351–368. [Google Scholar] [CrossRef]
- Genzer, J.L.; Kamalanathan, M.; Bretherton, L.; Hillhouse, J.; Xu, C.; Santschi, P.H.; Quigg, A. Diatom aggregation when exposed to crude oil and chemical dispersant: Potential impacts of ocean acidification. PLoS ONE 2020, 15, e0235473. [Google Scholar] [CrossRef]
- Gutierrez, T.; Rhodes, G.; Mishamandani, S.; William, D.B.; Whitman, B.; Nichols, P.D.; Semple, K.T.; Aitkena, M.D. Polycyclic aromatic hydrocarbon degradation of phytoplankton associated Arenibacter spp. and description of Arenibacter algicola sp. nov., an aromatic hydrocarbon-degrading bacterium. Appl. Environ. Microbiol. 2014, 80, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Chekroun, K.B.; Sánchez, E.; Baghour, M. The role of algae in bioremediation of organic pollutants. Int. Res. J. Public Environ. Health 2014, 1, 19–32. [Google Scholar]
- Thavasi, R.; Banat, I.M. Biosurfactants and Bioemulsifiers from Marine Sources. Chapter 5. In Biosurfactants Research Trends and Applications; Mulligan, C.N., Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 125–146. [Google Scholar]
- Zehnle, H.; Laso-Pérez, R.; Lipp, J.; Riedel, D.; Merino, D.B.; Teske, A.; Wegener, G. Candidatus Alkanophaga archaea from Guaymas Basin hydrothermal vent sediment oxidize petroleum alkanes. Nat. Microbiol. 2023, 8, 1199–1212. [Google Scholar] [CrossRef]
- Duan, W.; Du, S.; Meng, F.; Peng, X.; Peng, L.; Lin, Y.; Wang, G.; Wu, J. The pathway by which the marine diatom Thalassiosira sp. OUC2 biodegrades p-xilene, combined with a mechanistic analysis at the proteomic level. Ecotoxicol. Environ. Saf. 2020, 198, 110687. [Google Scholar] [CrossRef]
- Tremblay, J.; Yergeau, E.; Fortin, N.; Cobanli, S.; Elias, M.; King, T.L.; Lee, K.; Greer, C.W. Chemical dispersants enhance the activity of oil and gas condensate degrading marine bacteria. ISME J. 2018, 11, 2793–2808. [Google Scholar] [CrossRef]
- Prince, R.C. Bioremediation of marine oil spills. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin, Germany, 2010; pp. 2618–2626. [Google Scholar]
- Liebe, B.; Fock, H.P. Growth and adaptation of the green alga Chlamydomonas reinhardii diesel exhaust particle extracts. J. Gen. Microbiol. 1992, 138, 973–978. [Google Scholar] [CrossRef]
- Todd, S.J.; Cain, R.B.; Schmid, S. Biotransformation of naphthalene and diaryl ethers by green microalgae. Biodegradation 2002, 13, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, A.X.; Movafeghi, A.; Mohammadi-Nassab, A.D.; Abedi, E.; Bahrami, A. Potential of the Green Alga Chlorella Vulgaris for Biodegradation of Crude Oil Hydrocarbons. Mar. Pollut. Bull. 2017, 123, 286–290. [Google Scholar] [CrossRef]
- Warshawsky, D.; Cody, T.; Radike, M.; Reilman, R.; Schumann, B.; LaDow, K.; Schneider, J. Biotransformation of benzo[a]pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light. Chem.-Biol. Interact. 1995, 97, 131–148. [Google Scholar] [CrossRef]
- Walker, J.D.; Colwell, R.R.; Petrakis, L. Degradation of petroleum by an alga, Prototheca zopfii. Appl. Microbiol. 1975, 30, 79–81. [Google Scholar] [CrossRef]
- Mueller, J.G.; Cerniglia, C.E.; Pritchard, P.H. Bioremediation of environments contaminated by polycyclic aromatic hydrocarbons. In Bioremediation: Principles and Applications; Crawford, R.L., Crawford, D.L., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 125–194. [Google Scholar]
- Cerniglia, C.E.; Gibson, D.T.; Van Baalen, C. Naphthalene metabolism by diatoms isolated from the Kachemak Bay region of Alaska. J. Gen. Microbiol. 1982, 128, 987–990. [Google Scholar] [CrossRef]
- Antic, M.; Jovancicevic, B.; Vivic, M.M.; Schwarzbauer, J. Petroleum pollutant degradation by surface water microorganisms. Environ. Sci. Pollut. Res. 2006, 13, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, C.E.; Gibson, D.T. Fungal oxidation of benzo (a)pyrene and (+)-trans-7,8-dihydroxy-7,8-dihydrobenzo(a) pyrene: Evidence for the formation of a benzo(a)pyrene 7,8-diol-9,10-epoxide. J. Biol. Chem. 1980, 255, 5159–5163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luan, T.G.; Lu, N.N.; Lan, C.Y. Toxicity of Fluoranthene and Its Biodegradation by Cyclotella caspia Alga. J. Integr. Plant Biol. 2006, 48, 169–180. [Google Scholar] [CrossRef]
- Hong, Y.W.; Yuan, D.X.; Lin, Q.M.; Yang, T.L. Accumulation and biodegradation of phenanthrene and fluoranthene by the algae enriched from a mangrove aquatic ecosystem. Mar. Pollut. Bull. 2008, 56, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Headley, J.V.; Du, J.L.; Peru, K.M.; Gurprasad, N.; McMartin, D.W. Evaluation of algal phytodegradation of petroleum naphthenic acids. J. Environ. Sci. Health Part A 2008, 43, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Mandal, T.K.; Patra, S. Biodegradation of phenol by a novel diatom BD1IITG-kinetics and biochemical studies. Int. J. Environ. Sci. Technol. 2016, 13, 529–542. [Google Scholar]
- Lovell, C.R.; Eriksen, N.T.; Lewitus, A.J.; Chen, W.P. Resistance of the marine diatom Thalassiosira sp. To toxicity of phenolic compounds. Mar. Ecol. Prog. Ser. 2002, 229, 11–18. [Google Scholar] [CrossRef]
- Kahla, O.; Ben Garali, S.M.; Karray, F.; Ben Abdallah, M.; Kallel, N.; Mhiri, N.; Zaghden, H.; Barhoumi, B.; Pringault, O.; Quéméneur, M.; et al. Efficiency of benthic diatom-associated bacteria in the removal of benzo(a)pyrene and fluoranthene. Sci. Total Environ. 2021, 751, 141399. [Google Scholar] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Golyshin, P.N. Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 2007, 18, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kostka, J.E.; Teske, A.P.; Joye, S.B.; Head, I.M. The metabolic pathways and environmental controls of hydrocarbon biodegradation in marine ecosystems. Front. Microbiol. 2014, 5, 471. [Google Scholar] [CrossRef]
- Kumar, L.; Bharadvaja, N. Enzymatic bioremediation: A smart tool to fight environmental pollutants. In Smart Bioremediation Technologies; Bhatt, P., Ed.; Academic Press: London, UK, 2019; pp. 98–118. [Google Scholar] [CrossRef]
- Kuttiyathil, M.S.; Mohamed, M.M.; Al-Zuhair, S. Using microalgae for remediation of crude petroleum oil-water emulsion. Biotechnol. Prog. 2020, 37, e3098. [Google Scholar] [CrossRef]
- Osuna-Cruz, C.M.; Bilcke, G.; Vancaester, E.; De Decker, S.; Bones, A.M.; Winge, P.; Poulsen, N.; Bulankova, P.; Verhelst, B.; Audoor, S.; et al. The Seminavis robusta genome provides insights into the evolutionary adaptations of benthic diatoms. Nat. Commun. 2020, 11, 3320. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Liu, Y.; Xie, Z.; Cao, J.; Zhang, H.; Liu, J.; Bao, T.; Sun, C.; Liu, B.; et al. The phylogeny and metabolic potentials of an n-alkane-degrading Venatorbacter bacterium isolated from deep-sea sediment of the Mariana Trench. Front. Microbiol. 2023, 14, 1108651. [Google Scholar] [CrossRef]
- Baghour, M. Algal degradation of organic pollutants. In Handbook of Ecomaterials; Martinez, L.M.T., Kharissova, L.O., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2019; pp. 565–586. [Google Scholar]
- Paniagua Michel, J.J.; Fathepure, B.Z. Microbial Consortia and Biodegradation of Petroleum Hydrocarbons in Marine Environments. In Microbial Action on Hydrocarbons; Kumar, V., Kumar, P., Prasad, R., Eds.; Springer: Singapore, 2018; pp. 1–20. [Google Scholar]
- Blanco-Vieites, M.; Suárez-Montes, D.; Delgado, F.; Álvarez-Gil, M.; Hernández Battez, A.; Rodríguez, E. Removal of heavy metals and hydrocarbons by microalgae from wastewater in the steel industry. Algal Res. 2022, 64, 102700. [Google Scholar] [CrossRef]
- Brown, A.E.; Finnerty, G.L.; Camargo-Valero, M.A.; Ross, A.B. Valorisation of macroalgae via the integration of hydrothermal carbonization and anaerobic digestion. Bioresour. Technol. 2020, 312, 123539. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Bio/Technol. 2019, 18, 335–388. [Google Scholar]
- Sen, B.; Mehmet, A.; Feray, S.; Mehmet, K.; Ozgur, C. Relationship of algae to water pollution and waste water treatment. Water Treat. 2013, 14, 335–354. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223, 1–12. [Google Scholar] [CrossRef]
- Olajire, A.A.; Essien, J. Aerobic Degradation of Petroleum Components by Microbial Consortia. J. Pet. Environ. Biotechnol. 2014, 5, 1000195. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Coulon, F.; McKew, B.A.; Osborn, A.M.; McGenity, T.J.; Timmis, K.N. Effects of temperature and biostimulation on oil-degrading microbial communities in temperate estuarine waters. Environ. Microbiol. 2007, 9, 177–186. [Google Scholar] [CrossRef]
- Cui, Z.; Lai, Q.; Dong, C.; Shao, Z. Biodiversity of polycyclic aromatic hydrocarbon-degrading bacteria from deep sea sediments of the Middle Atlantic Ring. Environ. Microbiol. 2008, 10, 2138–2149. [Google Scholar] [CrossRef]
- Pereira, E.; Napp, A.P.; Allebrandt, S.; Barbosa, R.; Reuwsaat, J.; Lopes, W.; Kmetzsch, L.; Staats, C.C.; Schrank, A.; Dallegrave, A.; et al. Biodegradation of aliphatic and polycyclic aromatic hydrocarbons in seawater by autochthonous microorganisms. Int. Biodeterior. Biodegrad. 2019, 145, 104789. [Google Scholar] [CrossRef]
- Maeda, Y.; Nojima, D.; Yoshino, T.; Tanaka, T. Structure and properties of oil bodies in diatoms. Phil. Trans. R. Soc. B 2017, 372, 20160408. [Google Scholar] [CrossRef]
- Hildebrand, M.; Davis, A.K.; Smith, S.R.; Traller, J.C.; Abbriano, R. The place of diatoms in the biofuels industry. Biofuels 2012, 3, 221–240. [Google Scholar] [CrossRef]
- Silva, E.J.; Correa, P.F.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Recovery of contaminated marine environments by biosurfactant-enhanced bioremediation. Colloids Surf. B Interfaces 2018, 172, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What Is in Store for EPS Microalgae in the Next Decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef]
- Antoniou, E.; Fodelianakis, S.; Korkakaki, E.; Kalogerakis, N. Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source. Front. Microbiol. 2015, 6, 274. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Youssef, N.H.; McInerney, M.J.; Sabatini, D.A. Rhamnolipid biosurfactant mixtures for environmental remediation. Water Res. 2008, 42, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Sarkar, S.; Govil, T.; González-Faune, P.; Cabrera-Barajas, G.; Bandopadhyay, R.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: Biotechnologies and Wastewater Remediation. Front. Microbiol. 2021, 12, 721365. [Google Scholar] [CrossRef]
- Manga, E.B.; Celik, P.A.; Cabuk, A.; Banat, I.M. Biosurfactants: Opportunities for the development of a sustainable future. Curr. Opin. Colloid Interface Sci. 2021, 56, 101514. [Google Scholar] [CrossRef]
- Karamchandani, B.M.; Pawar, A.A.; Pawar, S.S.; Syed, S.; Mone, N.S.; Dalvi, S.G.; Rahman, P.K.S.M.; Banat, I.M.; Satpute, S.K. Biosurfactants’ multifarious functional potential for sustainable agricultural practices. Front. Bioeng. Biotechnol. 2022, 10, 1047279. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, D.; Sukhbir-Singh, G.M.; Karamchandani, B.M.; Aseri, G.K.; Banat, I.M.; Satpute, S.K. Biosurfactants: Forthcomings and Regulatory Affairs in Food-Based Industries. Molecules 2023, 28, 2823. [Google Scholar] [CrossRef]
- Shniukova, E.I.; Zolotareva, E.K. Diatom Exopolysaccharides: A Review. Int. J. Algae 2015, 17, 50–67. [Google Scholar] [CrossRef]
- Liang, Y.; Bretherton, L.; Brown, C.M.; Passow, U.; Quigg, A.S.; Irwin, A.J.; Finkel, Z.V. Transcriptome-wide responses of aggregates of the diatom Odontella aurita to oil. Mar. Ecol. Prog. Ser. 2021, 671, 67–79. [Google Scholar] [CrossRef]
- Hoagland, K.D.; Rosowsky, J.R.; Gretz, M.R.; Roemer, S.C. Diatom extracellular polymeric substances: Function, fine structure, chemistry, and physiology. J. Phycol. 1993, 29, 537–566. [Google Scholar] [CrossRef]
- Wu, Y.; Campbell, D.A.; Irwin, A.J.; Suggett, D.J.; Finkel, Z.V. Ocean acidification enhances the growth rate of larger diatoms. Limnol. Oceanogr. 2014, 59, 1027–1034. [Google Scholar] [CrossRef]
- Haug, A.; Myklestad, S. Polysaccharides of Marine Diatoms with Special Reference to Chaetoceros Species. Mar. Biol. 1976, 34, 217–222. [Google Scholar] [CrossRef]
- Thornton, D.C. Dissolved organic matter (DOM) release by phytoplankton in the contemporary and future ocean. Eur. J. Phycol. 2014, 49, 20–46. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides. In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.C., Eds.; Springer: Berlin, Germany, 1999; pp. 73–92. [Google Scholar]
- Steele, D.J.; Franklin, D.J.; Underwood, G.J.C. Protection of cells from salinity stress by extracellular polymeric substances in diatom biofilms. Biofouling 2014, 30, 987–998. [Google Scholar] [CrossRef]
- Staats, N.; de Winder, B.; Stal, L.; Mur, L.R. Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur. J. Phycol. 1999, 34, 61–169. [Google Scholar] [CrossRef]
- Quigg, A.; Passow, U.; Chin, W.-C.; Xu, C.; Doyle, S.; Bretherton, L.; Kamalanathan, M.; Williams, A.K.; Sylvan, J.B.; Finkel, Z.V.; et al. The role of microbial exopolymers in determining the fate of oil and chemical dispersants in the ocean. Limnol. Oceanogr. Lett. 2016, 1, 3–26. [Google Scholar] [CrossRef]
- Quigg, A.; Passow, U.; Daly, K.L.; Burd, A.; Hollander, D.J.; Schwing, P.T.; Lee, K. Marine Oil Snow Sedimentation and Flocculent Accumulation (MOSSFA) events: Learning from the past to predict the future. In Deep Oil Spills–Facts, Fate and Effects; Murawski, S.A., Ainsworth, C., Gilbert, S., Hollander, D., Paris, C.B., Schluter, M., Wetzel, D., Eds.; Springer: Cham, Switzerland, 2020; pp. 199–224. [Google Scholar]
- Passou, U.; Carlson, C.A. The biological pump in a high CO2 world. Mar. Ecol. Prog. Ser. 2012, 470, 249–271. [Google Scholar] [CrossRef]
- Basu, S.; Mackey, K.R.M. Phytoplankton as key mediators of the biological carbon pump: Their responses to a changing climate. Sustainability 2018, 10, 869. [Google Scholar] [CrossRef]
- Daly, K.L.; Passow, U.; Chanton, J.; Hollander, D. Assessing the impacts of oil-associated marine snow formation and sedimentation during and after the Deepwater Horizon oil spill. Anthropocene 2016, 13, 18–33. [Google Scholar] [CrossRef]
- Gregson, B.H.; McKew, B.A.; Holland, R.D.; Nedwed, T.J.; Prince, R.C.; McGenity, T.J. Marine oil snow, a microbial perspective. Front. Mar. Sci. 2020, 8, 619484. [Google Scholar] [CrossRef]
- Passow, U.; Stout, S.A. Character and sedimentation of “lingering” Macondo oil to the deep-sea after the Deepwater Horizon oil spill. Mar. Chem. 2020, 218, 103733. [Google Scholar] [CrossRef]
- Yang, R.; Wei, D.; Pohnert, G. Nitrogen utilization analysis reveals the synergetic effect of arginine and urea in promoting fucoxanthin biosynthesis in the mixotrophic marine diatom Phaeodactylum tricornutum. Front. Mar. Sci. 2022, 9, 947726. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Meng-Hsuen, C.; Bacosa, H.; Schwehr, K.; Shih-Ming, T.; Doyle, S.; Yard, A.; Mapes, S.; Vasequez, C.; Bretherton, L.; et al. Role of Polysaccharides in diatom Thalassiosira pseudonana and its associated bacteria in hydrocarbon presence. Plant Physiol. 2019, 180, 1898–1911. [Google Scholar] [CrossRef]
- Waghmode, S.; Suryavanshi, M.; Dama, L.; Kansara, S.; Ghattargi, V.; Das, P.; Banpurkar, A.; Satpute, S.K. Genomic insights of halophilic Planococcus maritimus SAMP MCC 3013 and detail investigation of its biosurfactant production. Front. Microbiol. 2019, 10, 235. [Google Scholar] [CrossRef]
- Araújo, W.J.; Oliveira, J.S.; Araújo, S.C.S.; Minnicelli, C.F.; Silva-Portela, R.C.B.; Da Fonseca, M.M.B.; Freitas, J.F.; Silva-Barbalho, K.K.; Napp, A.P.; Pereira, J.E.S.; et al. Microbial Culture in Minimal Medium with Oil Favors Enrichment of Biosurfactant Producing Genes. Front. Bioeng. Biotechnol. 2020, 11, 962. [Google Scholar] [CrossRef]
- Cane, R.F. Coorongite and the genesis of oil shale. Geochim. Cosmochim. Acta 1969, 33, 257–265. [Google Scholar] [CrossRef]
- Rowland, S.J.; Robson, J.N. The widespread occurrence of highly branched acyclic C20, C25 and C30 hydrocarbons in recent sediments and biota—A review. Mar. Environ. Res. 1990, 30, 191–216. [Google Scholar] [CrossRef]
- Damste, J.; Baas, M.; Geenevasen, J.A.; Kenig, F. Structural identification of sedimentary C21 and C22 highly branched isoprenoid alkanes. Org. Geochem. 2005, 36, 511–517. [Google Scholar] [CrossRef]
- Sakari, M.; Zakaria, M.P.; Lajis, N.H.; Mohamed, C.; Bahry, P.S.; Anita, S.; Chandru, K. Characterization, distribution, sources and origins of aliphatic hydrocarbons from surface sediment of Prai Strait, Penang, Malaysia: A widespread anthropogenic input. Environ. Asia 2008, 2, 1–14. [Google Scholar]
- Speight, J.G.; Arjoon, K.K. Bioremediation of Petroleum and Petroleum Products; John Wiley & Sons. Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Salem, MA, USA, 2018. [Google Scholar]
- Sorigué, D.; Légeret, B.; Cuiné, B.; Morales, P.; Mirabella, B.; Guédeney, M.; Li-Beisson, Y.; Jetter, R.; Peltier, G.; Beisson, F. Microalgae Synthesize Hydrocarbons from Long-Chain Fatty Acids via a Light-Dependent Pathway. Plant Physiol. 2016, 171, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.E.; Finkel, Z.V.; Grzebyk, D.; Knoll, A.H.; Falkowski, P.G. Evolutionary trajectories and biogeochemical impacts of marine eukaryotic phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 523–556. [Google Scholar] [CrossRef]
- Holba, A.G.; Tegelaar, E.W.; Huizinga, B.J.; Moldowan, J.M.; Singletary, M.S.; McCaffrey, M.A.; Dzou, L.I.P. 24-norcholestanes as age-sensitive molecular fossils. Geology 1998, 26, 783–786. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Dunstan, G.A. Sterol biomarkers for microalgae from the green algal class Prasinophyceae. Org. Geochem. 1994, 21, 1211–1218. [Google Scholar] [CrossRef]
- Levitan, O.; Dinamarca, J.; Hochman, G.; Falkowski, P.G. Diatoms: A fossil fuel of the future. Trends Biotechnol. 2014, 32, 117–124. [Google Scholar] [CrossRef]
- Grossi, V.; Beker, B.; Geenevasen, J.A.J.; Schouten, S.; Raphel, D.; Fontaine, M.F.; Damste, J.S.S. C25 highly branched isoprenoid alkenes from the marine benthic diatom Pleurosigma strigosum. Phytochemistry 2004, 65, 3049–3055. [Google Scholar] [CrossRef]
- Lee, R.F.; Nevenzel, J.C.; Paffenhofer, G.A.; Benson, A.A.; Patton, S.; Kavanagh, T.E. A unique hexaene hydrocarbon from a diatom (Skeletonema costatum). Biochim. Biophys. Acta. 1970, 202, 386. [Google Scholar] [CrossRef]
- Lee, R.F.; Loeblich III, A.R. Distribution of 21:6 hydrocarbon and its relationship to 22:6 fatty acid in algae. Phytochemistry 1971, 10, 593–602. [Google Scholar] [CrossRef]
- Shishlyannikov, S.; Alyona, A.; Nikonova, A.A.; Klimenkov, I.V.; Gorshkov, A.G. Accumulation of petroleum hydrocarbons in intracellular lipid bodies of the freshwater diatom Synedra acus subsp. radians. Environ. Sci. Pollut. Res. 2016, 24, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Simon, D.P.; Diaz-Garza, A.M.; Fantino, E.; Messaabi, A.; Meddeb-Mouelhi, F.; Germain, H.; Desgagné-Penix, I. Diatoms Biotechnology: Various Industrial Applications for a Greener Tomorrow. Front. Mar. Sci. 2021, 8, 636613. [Google Scholar] [CrossRef]
- Brocks, J.J.; Summons, R.E. Sedimentary Hydrocarbons, Biomarkers for Early Life. Tratise Geochem. 2023, 8, 63–115. [Google Scholar] [CrossRef]
- Leterme, S.C. The Oil Production Capacity of Diatoms. Ann. Aquac. Res. 2015, 2, 1007. [Google Scholar]
- Novak, M.; Gostinčar, C.; Gunde-Cimerman, N. Microorganisms populating the water-related indoor biome. Appl. Microbiol. Biotechnol. 2020, 104, 6443–6462. [Google Scholar] [CrossRef]
- Leyland, B.; Boussiba, S.; Khozin-Goldberg, I. A review of diatom lipid droplets. Biology 2020, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Medlin, K.L.; Williams, D.M.; Sims, P.A. The evolution of the diatoms (Bacillariophyta). I. Origin of the group and assessment of the monophyly of its major divisions. Eur. J. Phycol. 1993, 28, 261–275. [Google Scholar] [CrossRef]
- Harada, N.; Hirose, Y.; Chihong, S.; Kurita, H.; Sato, M.; Onodera, J.; Kazuyoshi, M.; Ito, F. A novel characteristic of a phytoplankton as a potential source of straight-chain alkanes. Sci. Rep. 2021, 11, 14190. [Google Scholar] [CrossRef]
- Bamforth, S.M.; Singleton, I. Bioremediation of polycyclic aromatic hydrocarbons. J. Chem. Technol. Biotechnol. 2005, 80, 723–736. [Google Scholar] [CrossRef]
- Mersch-Sundermann, V.; Mochayedi, S.; Kevekordes, S. Genotoxicity of polycyclic aromatic hydrocarbons in Escherichia coli PQ37. Mutat. Res. 1992, 278, 1–9. [Google Scholar] [CrossRef]
- Yamada, I.; Takada, H.; Toyoda, K.; Yoshida, A.; Shibata, A.; Nomura, H.; Wada, M.; Nishimura, M.; Okamoto, K.; Ohwada, K. Study on the fate of petroleum-derived polycyclic aromatic hydrocarbons (PAHs) and the effect of chemical dispersant using an enclosed ecosystem, mesocosm. Mar. Pollut. Bull. 2003, 47, 105–113. [Google Scholar] [CrossRef]
- Ben Othman, H.; Pick, F.R.; Hlailia, A.S. Leboulanger CH. Effects of polycyclic aromatic hydrocarbons on marine and freshwater microalgae—A review. J. Hazard. Mater. 2023, 441, 29869. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001, 56, 650–663. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Gibson, D.T.; Van Baalen, C. Algal oxidation of aromatic hydrocarbons: Formation of l-naphthol from naphthalene by Agmenellum quadruplicatum, strain PR-6. Biochem. Biophys. Res. Commun. 1979, 88, 50–58. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Muñoz, R.; Rolvering, C.; Guieysse, B.; Mattiasson, B. Aerobic phenanthrene biodegradation in a two-phase partitioning bioreac-tor. Water Sci. Technol. 2005, 52, 265–271. [Google Scholar] [CrossRef]
- Al-Turki, A.I. Microbial polycyclic aromatic hydrocarbons degradation in soil. Res. J. Environ. Toxicol. 2009, 3, 1–8. [Google Scholar] [CrossRef]
- Aslam, S.N.; Strauss, J.; Thomas, D.N.; Mock, T.; Underwood, G.C. Identifying metabolic pathways for production of extracellular polymeric substances by the diatom Fragilariopsis cylindrus inhabiting sea ice. ISME J. 2018, 12, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.G.; Lewin, J.; Johnson, P.G. Marine polymers. IV Diatom polysaccharides. Bot. Mar. 1972, 15, 102–108. [Google Scholar] [CrossRef]
- Netzer, R.; Henry, I.A.; Ribicic, D.; Wibberg, D.; Brönner, U.; Brakstada, U.G. Petroleum hydrocarbon and microbial community structure successions in marine oil-related aggregates associated with diatoms relevant for Arctic conditions. Marine Pollution Bulletin 2018, 135, 759–768. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Heitkamp, M.A. Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment; Varanasi, U., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 41–68. [Google Scholar]
- Cerniglia, C.E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv. Appl. Microbiol. 1984, 30, 31–71. [Google Scholar] [PubMed]
- Wang, X.C.; Zhao, H.M. Uptake and Biodegradation of Polycyclic Aromatic Hydrocarbons by Marine Seaweed. J. Coast. Res. 2007, 50, 1056–1061. [Google Scholar]
- Carvalho, R.N.; Burchardt, A.D.; Sena, F.; Mariani, G.; Mueller, A.; Bopp, S.K.; Umlauf, G.; Lettieri, T. Gene biomarkers in diatom Thalassiosira pseudonana exposed to polycyclic aromatic hydrocarbons from contaminated marine surface sediments. Aquat. Toxicol. 2011, 101, 244–253. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R.; Nunes-Nesi, A. The central carbon and energy metabolism of marine diatoms. Metabolites 2013, 3, 325–346. [Google Scholar] [CrossRef]
- Gutierrez, T.; Banat, I.M. Isolation of glycoprotein bioemulsifiers produced by marine bacteria. In Hydrocarbon and Lipid Microbiology Protocols; McGenity, T., Timmis, K., Nogales, B., Eds.; Springer protocols handbooks; Springer: Berlin, Germany, 2014. [Google Scholar] [CrossRef]
- Ozhan, K.; Parson, M.L.; Bargu, S. How were phytoplankton affected by the deepwater horizon oil spill? Bioscience 2014, 64, 829–836. [Google Scholar] [CrossRef]
- Ozhan, K.; Bargu, S. Distinct responses of Gulf of Mexico Phytoplankton communities to crude oil and the dispersant corexit EC9500A under nutrient regimes. Ecotoxicology 2014, 23, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Coulon, F.; Chronopoulou, P.M.; Païssé, A.F.; Goñi-Urriza, M.; Peperzak, L.; Acuña-Alvarez, L.; McKew, B.A.; Brussaard, C.P.D.; Underwood, G.J.C.; Timmis, K.N.; et al. Central role of dynamic tidal biofilms dominated by aerobic hydrocarbonoclastic bacteria and diatoms in the biodegradation of hydrocarbons in coastal mudflats. Appl. Environ. Microbiol. 2012, 78, 3638–3648. [Google Scholar] [CrossRef]
- Gutierrez, T.; Green, D.H.; Nichols, P.D.; Whitman, W.B.; Semple, K.T.; Aitken, M.D. Algiphilus aromaticivorans gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium isolated from a culture of the marine dinoflagellate Lingulodinium polyedrum, and proposal of Algiphilaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 2743–2749. [Google Scholar] [CrossRef]
- Gutierrez, T.; Green, D.H.; Whitman, W.B.; Nichols, P.D.; Semple, K.T.; Aitken, M.D. Polycyclovorans algicola gen. nov. sp. nov., an aromatic hydrocarbon-degrading marine bacterium found associated with laboratory cultures of marine phytoplankton. Appl. Environ. Microbiol. 2013, 79, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mishamandani, S.; Gutierrez, T.; Berry, D.; Aitken, M.D. Response of the bacterial community associated with a cosmopolitan marine diatom to crude oil shows a preference for the biodegradation of aromatic hydrocarbons. Environ. Microbiol. 2016, 18, 1817–1833. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo[a]pyrene. Int. Biodeterior. Biodegrad. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Kelley, I.; Freeman, J.P.; Evans, F.E.; Cerniglia, E.E. Identification of a carboxylic acid metabolite from the catabolism of fluoranthene by a Mycobacterium sp. Appl. Environ. Microbiol. 1991, 57, 636–641. [Google Scholar] [CrossRef]
- Albaiges, J.; Frei, R.W.; Merian, E. Chemistry and Analysis of Hydrocarbons in the Environment; Science Publishers: New York, NY, USA, 1983. [Google Scholar]
- Wilson, S.C.; Jones, K.C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): A review. Environ. Pollut. 1993, 81, 229–249. [Google Scholar] [CrossRef]
- Cohen, Y. Bioremediation of oil by marine microbial mats. Int. Microbiol. 2002, 5, 189–193. [Google Scholar] [CrossRef]
- Meulenberg, R.; Rijnaarts, H.H.M.; Doddema, H.J.; Field, J.A. Partially oxidized polycyclic aromatic hydrocarbons show an increased bioavailability and biodegradability. FEMS Microbiol. Lett. 1997, 15, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.B.; Singh, H.; Biju, V.G.; Krishnamurthy, N.B. Classification, source, and effect of environmental pollutants and their biodegradation. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Doghri, I.; Lavaud, J.; Dufour, A.; Bazire, A.; Lanneluc, I.; Sablé, S. Cell-bound exopolysaccharides from an axenic culture of the intertidal mudflat Navicula phyllepta diatom affect biofilm formation by benthic bacteria. J. Appl. Phycol. 2017, 29, 165–177. [Google Scholar] [CrossRef]
- Sondes Melliti, B.G.; Sahraoui, I.; Ben Othman, H.; Abdessalem, K.; de la Iglesia, P.; Diogène, J.; Lafabrie, C.; Andree, K.B.; Fernández-Tejedor, M.; Mejri, K.; et al. Capacity of the potentially toxic diatoms Pseudo-nitzschia mannii and Pseudo-nitzschia hasleana to tolerate polycyclic aromatic hydrocarbons. Ecotoxicol. Environ. Saf. 2021, 214, 112082. [Google Scholar] [CrossRef] [PubMed]
- Swannell, R.F.; Lee, K.; McDonagh, M. Field evaluations of marine spill bioremediation. Microbiol. Rev. 1996, 60, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Bacosa, H.P.; Ancla, S.M.B.; Arcadio, C.G.L.A.; Dalogdog, J.R.A.; Ellos, D.M.C.; Hayag, H.D.A.; Jarabe, J.G.P.; Karim, A.J.T.; Navarro, C.K.P.; Palma, M.P.I.; et al. From Surface Water to the Deep Sea: A Review on Factors Affecting the Biodegradation of Spilled Oil in Marine Environment. J. Mar. Sci. Eng. 2022, 10, 426. [Google Scholar] [CrossRef]


| Genera | Substrata | References |
|---|---|---|
| Phylum Chlorophyta | ||
| Chlamydomonas reinhardii | Phenanthrene | [26] |
| Chlorella vulgaris | Naphthalene | [27] |
| Nonadecane | [28] | |
| Ankistrodesmus capricornutum | Benzo(a)pyrene | [29] |
| Prototheca zopfii | n-alkanes and PAHs | [30] |
| Dunaliella salina | Naphthalene | [31] |
| Scenedesmus acutus | Benzo(a)pyrene | [29] |
| Phylum Heterokontophyta | ||
| Class Bacillariophyta | ||
| Navicula sp. | Naphthalene | [32] |
| Achnanthes minutissima | Alkanes | [33] |
| Cyclotella caspia | Fluoranthene | [34] |
| Cylindrotheca sp. | Naphthalene | [35] |
| Synedra sp., Amphora sp. | Naphthalene | [32] |
| Skeletonema costatum | Fluoranthene | [36] |
| Nitzschia sp. | Phenanthrene | |
| Nitzschia sp., Navicula sp. | Naphthenic acid | [37] |
| Diatom BD1IITG | Phenol | [38] |
| Thalassiosira sp. | Phenol | [39] |
| Nitzchia sp. and Bacteria | Benzo(a)pyrene | [40] |
| Genera Marivita, Erythrobacter, | Fluoranthene | |
| Alcaligenes | ||
| Cylindrotheca sp., Amphora sp. | Naphthalene | [35] |
| Navicula sp., Nitzschia sp., | Naphthalene | [32] |
| Diatoms | Remarks | References |
|---|---|---|
| Navicula sp., Nitzschia sp. Synedra sp. | Oxidation with 6–9 mg/L of naphthalene at 6 and 12 °C | [35] |
| Cylindrotheca sp., Amphora sp. | Oxidation of 78 µM naphthalene at 30 °C | [32] |
| Nitzschia sp. | Absorption and metabolic degradation of phenanthrene (53%) | [36] |
| Skeletonema costatum | Absorption and metabolic degradation of phenanthrene (16%) | |
| Navicula sp. 1 and 2, Nitzschia sp. | Naphthenic acids cis- and trans isomers, uptake and degradation at 5.5 mg/L | [37] |
| Thalassiosira sp. | Growth and degradation in 0.25 mM phenol and 1 mM benzoate | [39] |
| Achnanthes minutissima | n-Alkanes and isoprenoids biodegradation | [33] |
| Diatom BD1IITG | Phenol degradation in the range 50–100 mg/L in refinery waste-water. Highest degradation rate and production of biosurfactant with emulsifying activity at 100 mg/L | [38] |
| Cyclotella caspia | Biodegradation of fluoranthene (86%) | [34] |
| Nitzchia sp. and Bacteria Genera Marivita, Erythrobacter Alcaligenes | Biodegradation of benzo(a)pyrene (79%) and fluoranthene (82%) by co-metabolic synergy | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paniagua-Michel, J.; Banat, I.M. Unravelling Diatoms’ Potential for the Bioremediation of Oil Hydrocarbons in Marine Environments. Clean Technol. 2024, 6, 93-115. https://doi.org/10.3390/cleantechnol6010007
Paniagua-Michel J, Banat IM. Unravelling Diatoms’ Potential for the Bioremediation of Oil Hydrocarbons in Marine Environments. Clean Technologies. 2024; 6(1):93-115. https://doi.org/10.3390/cleantechnol6010007
Chicago/Turabian StylePaniagua-Michel, J., and Ibrahim M. Banat. 2024. "Unravelling Diatoms’ Potential for the Bioremediation of Oil Hydrocarbons in Marine Environments" Clean Technologies 6, no. 1: 93-115. https://doi.org/10.3390/cleantechnol6010007
APA StylePaniagua-Michel, J., & Banat, I. M. (2024). Unravelling Diatoms’ Potential for the Bioremediation of Oil Hydrocarbons in Marine Environments. Clean Technologies, 6(1), 93-115. https://doi.org/10.3390/cleantechnol6010007







