Wastewater Treatment Utilizing Industrial Waste Fly Ash as a Low-Cost Adsorbent for Heavy Metal Removal: Literature Review
Abstract
1. Introduction
2. Causes of Heavy Metal Pollution
3. Concept of Heavy Metals
4. Levels of Heavy Metal Pollution
5. Health Effects of Heavy Metals
6. Methods for Heavy Metal Removal
6.1. Biosorption Approach
6.2. Industrial Waste Adsorbents
6.3. Fly Ash Material
6.4. Use of FA for Heavy Metal Removal
6.4.1. Raw FA for Heavy Metal Removal
6.4.2. Treated FA for Heavy Metal Removal
7. Adsorption-Related Factors Analysis
7.1. Operational Parameters
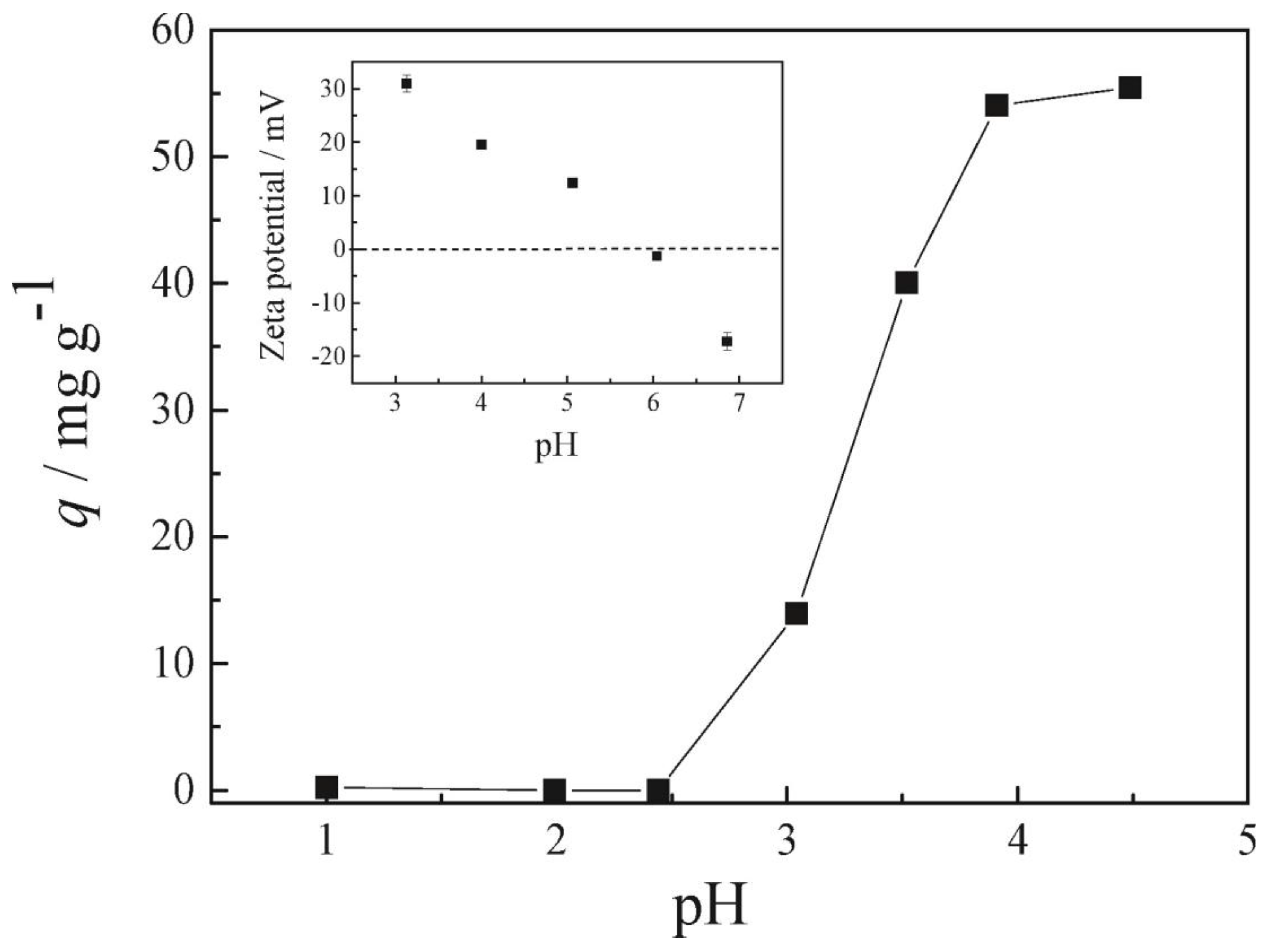
7.1.1. pH Effect
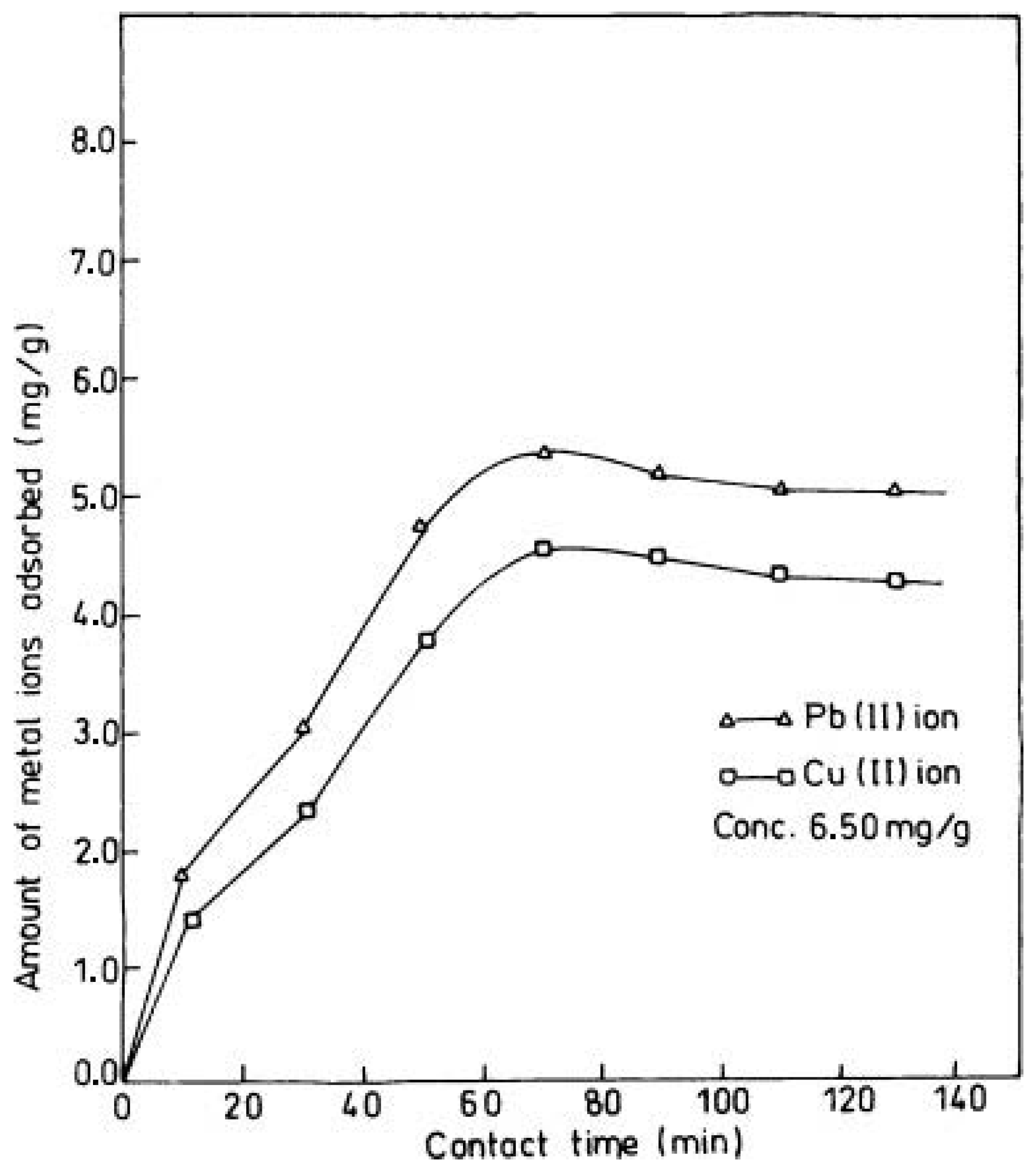
7.1.2. Effect of Ion Concentration
7.1.3. Adsorption Temperature
7.2. Effect of FA Constituents
7.3. Effect of Materials Addition
7.4. Adsorption Isotherms
7.5. Adsorption Kinetics
7.6. Adsorption Capacity
7.7. Removal Mechanism
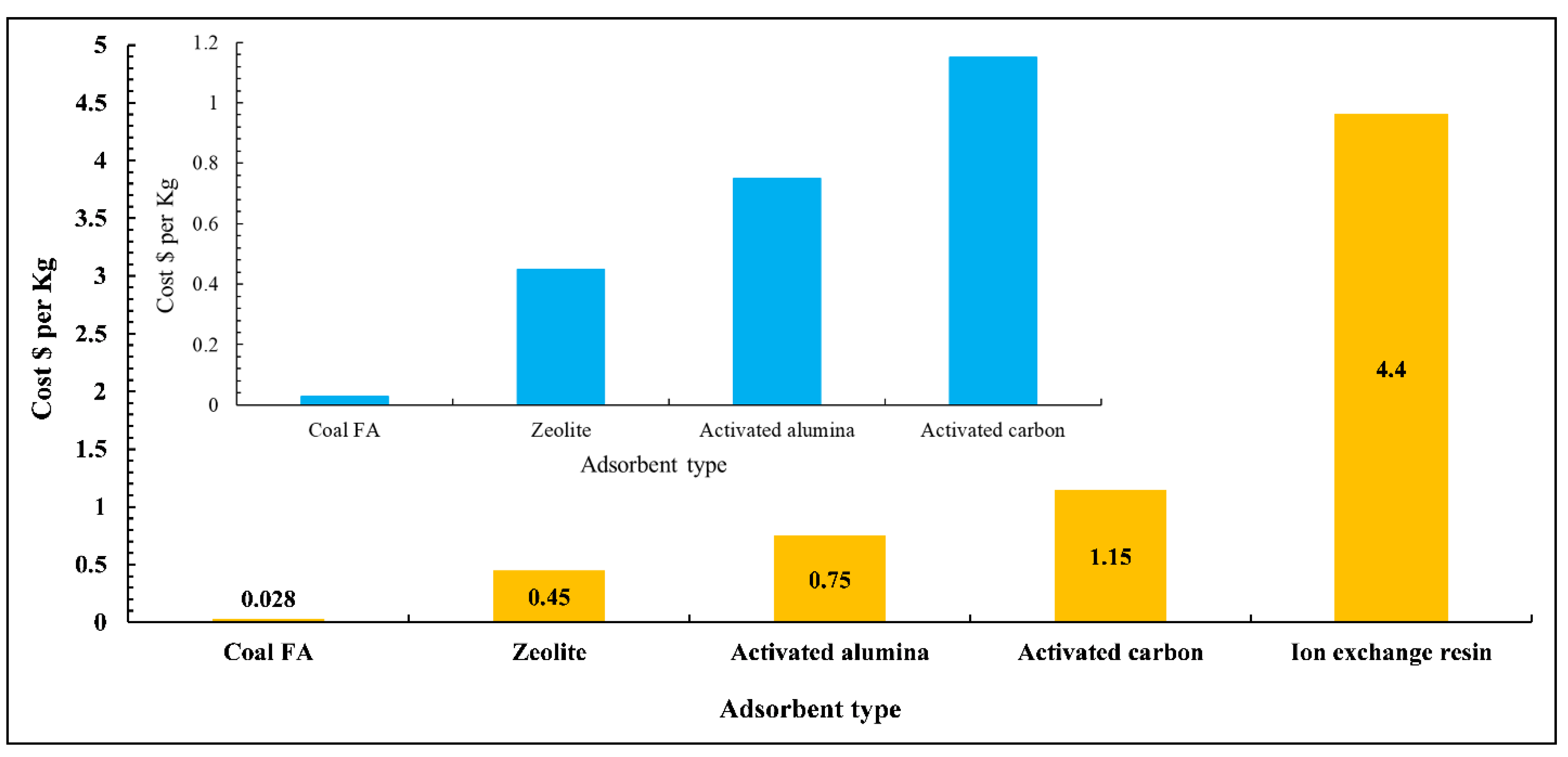
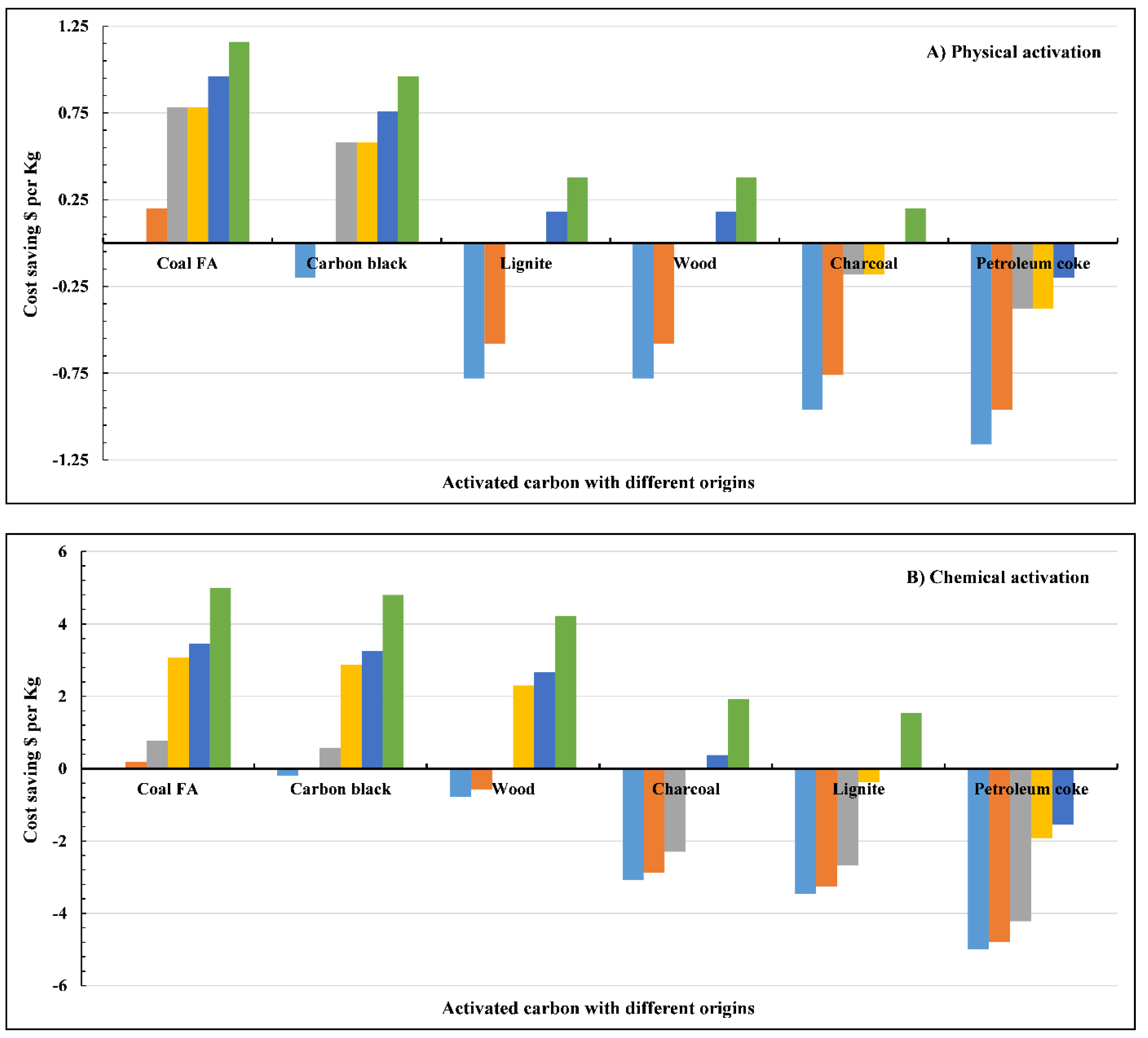
8. Cost Analysis
9. FA Regeneration and Reusability
10. Future Perspectives
11. Conclusions
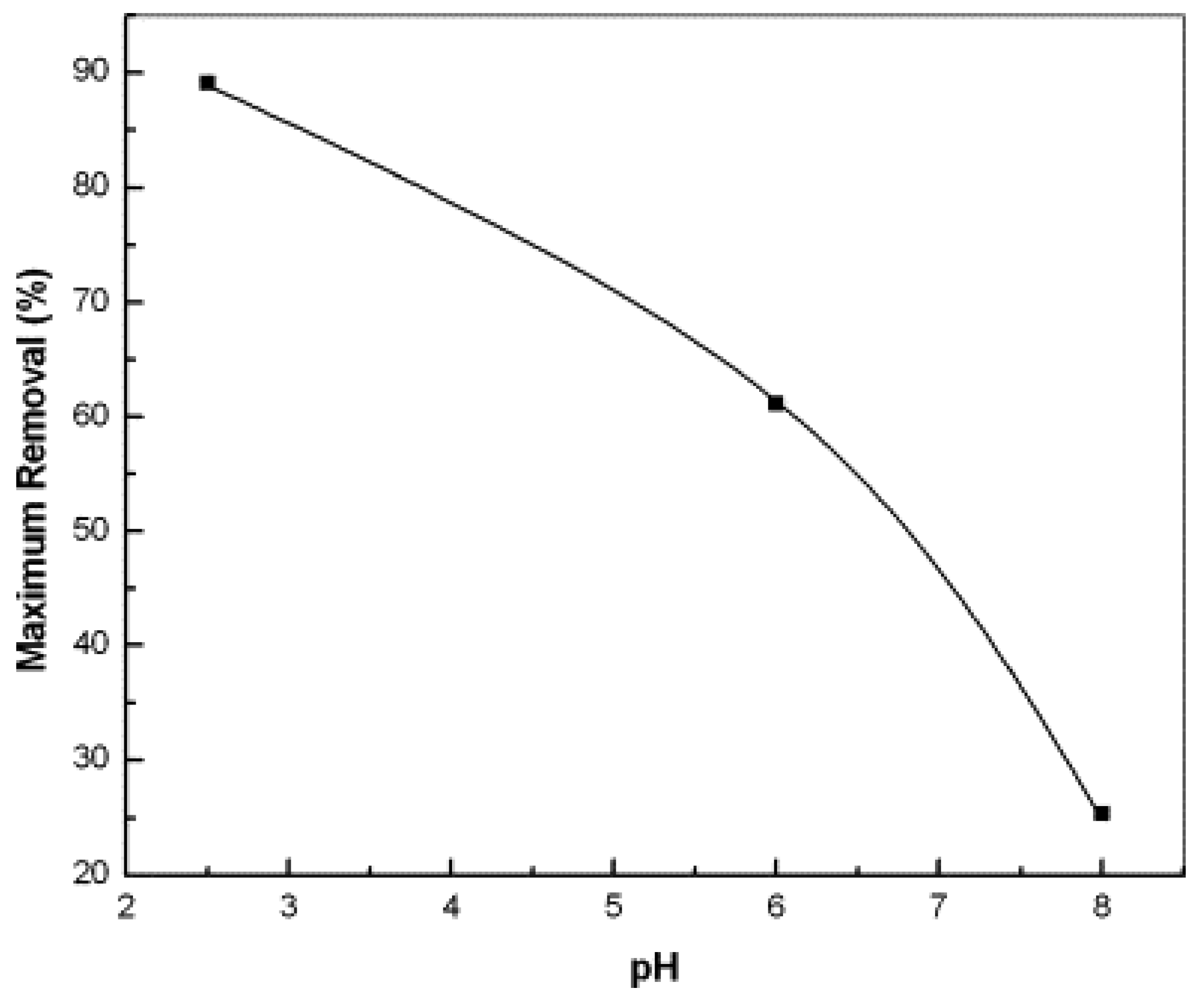
- Using FA as an adsorbent, the adsorption process is affected by many factors. Among them, pH significantly impacts the heavy metals removal performance. In general, the removal of heavy metals is improved at lower pH values, especially within the pH range of 2–5.
- Using FA, it was found that the removal rate increases when there are lower concentrations of heavy metals and vice versa. However, the removal rate is influenced by both adsorbate and heavy metal concentration.
- The findings revealed that adsorption temperature positively affects the removal process. However, a negative impact was also registered.
- It is concluded that the carbon content within FA relates to the surface area, resulting in a significant role during heavy metal removal. It was also found that the FA capability is enhanced when higher CaO percentages are within its content.
- It was found that the addition of nanomaterials can lead to enhanced FA’s ability to remove heavy metals. Examples of such materials include CaO, CaCO3, Ag, and Fe3O4 nanoparticles.
- It was observed that most heavy metal removal follows the Langmuir isotherm. The Langmuir fit suitability indicates that the adsorbed heavy metals tend to form a monolayer on the FA surface.
- Regarding kinetics removal, it was found that the pseudo-second-order kinetics well describes the removal of heavy metals. However, some metals had a removal behavior consistent with the pseudo-first-order kinetics equation.
- It is indicated that the adsorption process using FA as an adsorbent has two different steps: diffusion within the boundary layer, which is influenced by the external mass transfer impact, and intraparticle diffusion within the adsorbent pores.
- The cost analysis found that using treated FA with physical and chemical treatments is highly cost-effective and can achieve significant savings compared to commercial adsorbents.
- Despite being used in several applications, the ability of FA to undergo a transformation into a zeolite material via specific treatment holds promise for new application areas. Nevertheless, more investigation is necessary to validate this methodology.
- In conclusion, FA is known for its cost-effective origin as a waste material, making it a potentially advantageous resource for water treatment applications and diverse utilizations owing to its significant chemical and mineralogical composition.
Funding
Acknowledgments
Conflicts of Interest
References
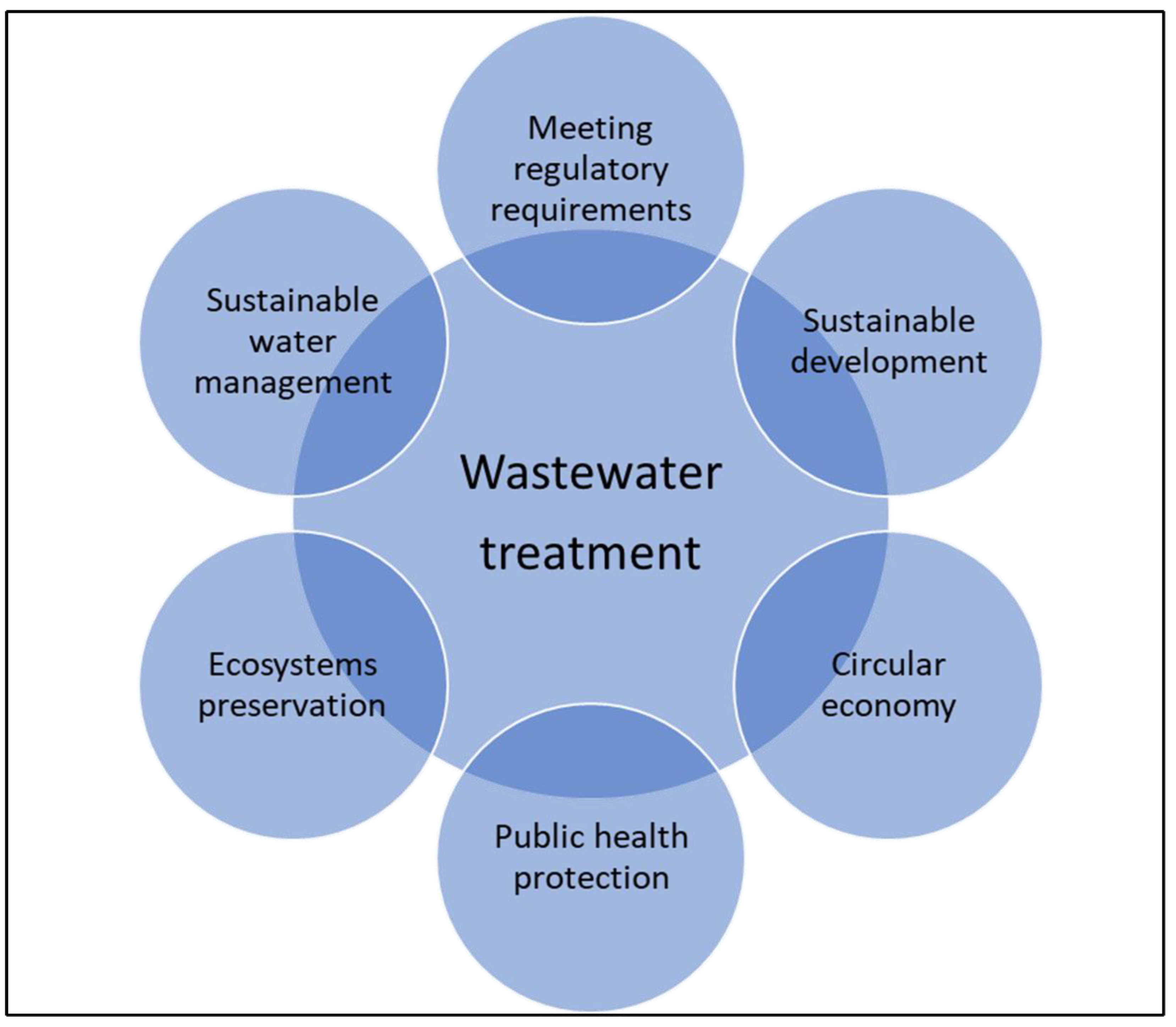
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A.G. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Naik, K.S.; Stenstrom, M.K. Evidence of the influence of wastewater treatment on improved public health. Water Sci. Technol. 2012, 66, 644–652. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Bartram, J.; Clasen, T.; Colford, J.M., Jr.; Cumming, O.; Curtis, V.; Bonjour, S.; Dangour, A.D.; De France, J.; Fewtrell, L.; et al. Burden of disease from inadequate water, sanitation and hygiene in low-and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health 2014, 19, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Mainardis, M.; Cecconet, D.; Moretti, A.; Callegari, A.; Goi, D.; Freguia, S.; Capodaglio, A.G. Wastewater fertigation in agriculture: Issues and opportunities for improved water management and circular economy. Environ. Pollut. 2022, 296, 118755. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and phosphorus recovery from wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef]
- Mara, D. Waste stabilization ponds: Past, present and future. Desalination Water Treat. 2009, 4, 85–88. [Google Scholar] [CrossRef]
- Pasqualino, J.C.; Meneses, M.; Castells, F. Life cycle assessment of urban wastewater reclamation and reuse alternatives. J. Ind. Ecol. 2011, 15, 49–63. [Google Scholar] [CrossRef]
- Pintilie, L.; Torres, C.M.; Teodosiu, C.; Castells, F. Urban wastewater reclamation for industrial reuse: An LCA case study. J. Clean. Prod. 2016, 139, 1–14. [Google Scholar] [CrossRef]
- Anderson, J. The environmental benefits of water recycling and reuse. Water Sci. Technol. Water Supply 2003, 3, 1–10. [Google Scholar] [CrossRef]
- Voulvoulis, N.; Arpon, K.D.; Giakoumis, T. The EU Water Framework Directive: From great expectations to problems with implementation. Sci. Total Environ. 2017, 575, 358–366. [Google Scholar] [CrossRef]
- Preisner, M.; Neverova-Dziopak, E.; Kowalewski, Z. An analytical review of different approaches to wastewater discharge standards with particular emphasis on nutrients. Environ. Manag. 2020, 66, 694–708. [Google Scholar] [CrossRef] [PubMed]
- von Sperling, M.; de Lemos Chernicharo, C.A. Urban wastewater treatment technologies and the implementation of discharge standards in developing countries. Urban Water 2002, 4, 105–114. [Google Scholar] [CrossRef]
- Lourenço, N.; Nunes, L.M. Life-cycle assessment of decentralized solutions for wastewater treatment in small communities. Water Sci. Technol. 2021, 84, 1954–1968. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, J.; Savenije, H.H. A simple approach to assess water scarcity integrating water quantity and quality. Ecol. Indic. 2013, 34, 441–449. [Google Scholar] [CrossRef]
- Cho, I.; Heo, E.; Park, J. Water resource R&D efficiency in Korea-toward sustainable integrated water resources management. Water Policy 2021, 23, 581–598. [Google Scholar]
- Pallavi, S.; Yashas, S.R.; Anilkumar, K.M.; Shahmoradi, B.; Shivaraju, H.P. Comprehensive understanding of urban water supply management: Towards sustainable water-socio-economic-health-environment nexus. Water Resour. Manag. 2021, 35, 315–336. [Google Scholar] [CrossRef]
- Padilla-Rivera, A.; Güereca, L.P. A proposal metric for sustainability evaluations of wastewater treatment systems (SEWATS). Ecol. Indic. 2019, 103, 22–33. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Korhonen, J.; Nuur, C.; Feldmann, A.; Birkie, S.E. Circular economy as an essentially contested concept. J. Clean. Prod. 2018, 175, 544–552. [Google Scholar] [CrossRef]
- Winans, K.; Kendall, A.; Deng, H. The history and current applications of the circular economy concept. Renew. Sustain. Energy Rev. 2017, 68, 825–833. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Oulego, P.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Towards the implementation of circular economy in the wastewater sector: Challenges and opportunities. Water 2020, 12, 1431. [Google Scholar] [CrossRef]
- Smol, M.; Adam, C.; Preisner, M. Circular economy model framework in the European water and wastewater sector. J. Mater. Cycles Waste Manag. 2020, 22, 682–697. [Google Scholar] [CrossRef]
- Preisner, M.; Smol, M.; Horttanainen, M.; Deviatkin, I.; Havukainen, J.; Klavins, M.; Ozola-Davidane, R.; Kruopienė, J.; Szatkowska, B.; Appels, L.; et al. Indicators for resource recovery monitoring within the circular economy model implementation in the wastewater sector. J. Environ. Manag. 2022, 304, 114261. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Aderhold, D.; Edyvean, R.G.J. Comparison between biosorbents for the removal of metal ions from aqueous solutions. Water Res. 1998, 32, 216–224. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.A.B.; Zainol, M.R.R.M.A.; Murshed, M.F.; Faris, M.A.; Bayuaji, R. Review on adsorption of heavy metal in wastewater by using geopolymer. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 97, p. 01023. [Google Scholar]
- Roccaro, P.; Sgroi, M.; Vagliasindi, F.G. Removal of xenobiotic compounds from wastewater for environment protection: Treatment processes and costs. Chem. Eng. Trans. 2013, 32, 505–510. [Google Scholar]
- Wang, L.K.; Hung, Y.T.; Shammas, N.K. (Eds.) Physicochemical Treatment Processes; Humana Press: Totowa, NJ, USA, 2005; Volume 3, pp. 3141–3197. [Google Scholar]
- Bodalo-Santoyo, A.; Gómez-Carrasco, J.L.; Gomez-Gomez, E.; Maximo-Martin, F.; Hidalgo-Montesinos, A.M. Application of reverse osmosis to reduce pollutants present in industrial wastewater. Desalination 2003, 155, 101–108. [Google Scholar] [CrossRef]
- Walsh, F.C.; Reade, G.W. Electrochemical techniques for the treatment of dilute metal-ion solutions. In Studies in Environmental Science; 1994; Volume 59, pp. 3–44. [Google Scholar]
- Ersahin, M.E.; Ozgun, H.; Dereli, R.K.; Ozturk, I.; Roest, K.; van Lier, J.B. A review on dynamic membrane filtration: Materials, applications and future perspectives. Bioresour. Technol. 2012, 122, 196–206. [Google Scholar] [CrossRef]
- Zhang, P.; Hahn, H.H.; Hoffmann, E. Different behavior of iron (III) and aluminum (III) salts to coagulate silica particle suspension. Acta Hydrochim. Hydrobiol. 2003, 31, 145–151. [Google Scholar] [CrossRef]
- Rykowska, I.; Wasiak, W.; Byra, J. Extraction of copper ions using silica gel with chemically modified surface. Chem. Pap. 2008, 62, 255–259. [Google Scholar] [CrossRef]
- Vishwakarma, V. Recovery and recycle of wastewater contaminated with heavy metals using adsorbents incorporated from waste resources and nanomaterials-A review. Chemosphere 2021, 273, 129677. [Google Scholar]
- Gupta, V.K.; Carrott, P.J.M.; Carrott, M.M.L.R. Low-cost adsorbents: Growing approach to wastewater treatment-a review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Singh, T.S.; Pant, K.K. Equilibrium, kinetics and thermodynamic studies for adsorption of As (III) on activated alumina. Sep. Purif. Technol. 2004, 36, 139–147. [Google Scholar] [CrossRef]
- Repo, E.; Kurniawan, T.A.; Warchol, J.K.; Sillanpää, M.E. Removal of Co (II) and Ni (II) ions from contaminated water using silica gel functionalized with EDTA and/or DTPA as chelating agents. J. Hazard. Mater. 2009, 171, 1071–1080. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Cd (II) and Pb (II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009, 333, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of acid modification on adsorption of hexavalent chromium (Cr (VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalination Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Repo, E.; Malinen, L.; Koivula, R.; Harjula, R.; Sillanpää, M. Capture of Co (II) from its aqueous EDTA-chelate by DTPA-modified silica gel and chitosan. J. Hazard. Mater. 2011, 187, 122–132. [Google Scholar] [CrossRef]
- Iakovleva, E.; Sillanpää, M. The use of low-cost adsorbents for wastewater purification in mining industries. Environ. Sci. Pollut. Res. 2013, 20, 7878–7899. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment-a review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Dhir, B. Potential of biological materials for removing heavy metals from wastewater. Environ. Sci. Pollut. Res. 2014, 21, 1614–1627. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Turan, N.G.; Mesci, B. Use of pistachio shells as an adsorbent for the removal of Zinc (II) ion. Clean-Soil Air Water 2011, 39, 475–481. [Google Scholar] [CrossRef]
- Pesavento, M.; Profumo, A.; Alberti, G.; Conti, F. Adsorption of lead (II) and copper (II) on activated carbon by complexation with surface functional groups. Anal. Chim. Acta 2003, 480, 171–180. [Google Scholar] [CrossRef]
- Qadeer, R.; Akhtar, S. Kinetics study of lead ion adsorption on active carbon. Turk. J. Chem. 2005, 29, 95–100. [Google Scholar]
- Uzun, I.; Güzel, F. Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk. J. Chem. 2000, 24, 291–298. [Google Scholar]
- Kołodyńska, D.; Krukowska, J.A.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Minocha, A.K. Conventional and non-conventional adsorbents for removal of pollutants from water-A review. Indian J. Chem. Technol. 2006, 13, 203–217. [Google Scholar]
- Shaheen, S.M.; Derbalah, A.S.; Moghanm, F.S. Removal of heavy metals from aqueous solution by zeolite in competitive sorption system. Int. J. Environ. Sci. Dev. 2012, 3, 362–367. [Google Scholar] [CrossRef]
- Bose, P.; Bose, M.A.; Kumar, S. Critical evaluation of treatment strategies involving adsorption and chelation for wastewater containing copper, zinc and cyanide. Adv. Environ. Res. 2002, 7, 179–195. [Google Scholar] [CrossRef]
- Choi, H.J.; Yu, S.W.; Kim, K.H. Efficient use of Mg-modified zeolite in the treatment of aqueous solution contaminated with heavy metal toxic ions. J. Taiwan Inst. Chem. Eng. 2016, 63, 482–489. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalination 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Singh, S.P.; Ma, L.Q.; Harris, W.G. Heavy metal interactions with phosphatic clay: Sorption and desorption behavior. J. Environ. Qual. 2001, 30, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, N.; Martin, D.F. Extraction of selected heavy metals using modified clays. J. Environ. Sci. Health 2005, 40, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Aşçı, Y.; Nurbaş, M.; Açıkel, Y.S. Sorption of Cd (II) onto kaolin as a soil component and desorption of Cd (II) from kaolin using rhamnolipid biosurfactant. J. Hazard. Mater. 2007, 139, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Hussain, S.T.; Ali, S.A.K. Removal of heavy metal by ion exchange using bentonite clay. J. Ecol. Eng. 2021, 22, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.G.; Gupta, S.S. Kaolinite and montmorillonite as adsorbents for Fe (III), Co (II) and Ni (II) in aqueous medium. Appl. Clay Sci. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Şölener, M.; Tunali, S.; Özcan, A.S.; Özcan, A.; Gedikbey, T. Adsorption characteristics of lead (II) ions onto the clay/poly (methoxyethyl) acrylamide (PMEA) composite from aqueous solutions. Desalination 2008, 223, 308–322. [Google Scholar] [CrossRef]
- Vengris, T.; Binkien, R.; Sveikauskait, A. Nickel, copper and zinc removal from waste water by a modified clay sorbent. Appl. Clay Sci. 2001, 18, 183–190. [Google Scholar] [CrossRef]
- de Almeida Neto, A.F.; Vieira, M.G.A.; da Silva, M.G.C. Adsorption and desorption processes for copper removal from water using different eluents and calcined clay as adsorbent. J. Water Process Eng. 2014, 3, 90–97. [Google Scholar] [CrossRef]
- Bertagnolli, C.; Kleinübing, S.J.; Da Silva, M.G.C. Preparation and characterization of a Brazilian bentonite clay for removal of copper in porous beds. Appl. Clay Sci. 2011, 53, 73–79. [Google Scholar] [CrossRef]
- Oubagaranadin, J.U.K.; Murthy, Z.V.; Mallapur, V.P. Removal of Cu (II) and Zn (II) from industrial wastewater by acid-activated montmorillonite-illite type of clay. Comptes Rendus Chim. 2010, 13, 1359–1363. [Google Scholar] [CrossRef]
- Selim, K.H.A.; Rostom, M.; Youssef, M.A.; Abdel-Khalek, N.A.; Abdel-Khalek, M.A.; Hassan, E.S.R. Surface modified bentonite mineral as a sorbent for Pb2+ and Zn2+ ions removal from aqueous solutions. Physicochem. Probl. Miner. Process. 2020, 56, 145–157. [Google Scholar]
- Babu, B.V.; Ramakrishnan, V. An Approach for Ranking of Adsorbents Based on Method of Preparation and Isotherm Fitting. In Proceedings of the International Symposium, 56th Annual Session of IIChE (CHEMCON-2003), Bhubaneswar, India, 19–22 December 2003. [Google Scholar]
- Wang, S.; Wu, H. Environmental-benign utilisation of fly ash as low-cost adsorbents. J. Hazard. Mater. 2006, 136, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Chen, J. Utilization of coal fly ash for the production of glass-ceramics with unique performances: A brief review. J. Mater. Sci. Technol. 2014, 30, 1208–1212. [Google Scholar] [CrossRef]
- Wang, Q.; Song, X.; Liu, Y. China’s coal consumption in a globalizing world: Insights from Multi-Regional Input-Output and structural decomposition analysis. Sci. Total Environ. 2020, 711, 134790. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, G.; Gu, X.; Gao, Y.; Zhao, P. High value-added applications of coal fly ash in the form of porous materials: A review. Ceram. Int. 2021, 47, 22302–22315. [Google Scholar] [CrossRef]
- Alterary, S.S.; Marei, N.H. Fly ash properties, characterization, and applications: A review. J. King Saud Univ.-Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Basu, M.; Pande, M.; Bhadoria, P.B.S.; Mahapatra, S.C. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Shaikh, S.M.S.; Kumar, M.S. Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: A review. Chemosphere 2018, 213, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ukwattage, N.L.; Ranjith, P.G.; Bouazza, M. The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 2013, 109, 400–408. [Google Scholar] [CrossRef]
- Meer, I.; Nazir, R. Removal techniques for heavy metals from fly ash. J. Mater. Cycles Waste Manag. 2018, 20, 703–722. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. J. Mater. Res. Technol. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Heavy metals removal/stabilization from municipal solid waste incineration fly ash: A review and recent trends. J. Mater. Cycles Waste Manag. 2022, 24, 1693–1717. [Google Scholar] [CrossRef]
- Vogelsang, C.; Umar, M. Municipal solid waste fly ash-derived zeolites as adsorbents for the recovery of nutrients and heavy metals-A Review. Water 2023, 15, 3817. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H. Water pollution during China’s industrial transition. Environ. Dev. 2013, 8, 57–73. [Google Scholar] [CrossRef]
- Nabi, S.A.; Shahadat, M.; Bushra, R.; Shalla, A.H. Heavy-metals separation from industrial effluent, natural water as well as from synthetic mixture using synthesized novel composite adsorbent. Chem. Eng. J. 2011, 175, 8–16. [Google Scholar] [CrossRef]
- du Plessis, A. Persistent degradation: Global water quality challenges and required actions. One Earth 2022, 5, 129–131. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Olsson, G. Application of membrane bioreactor technology in treating high strength industrial wastewater: A performance review. Desalination 2012, 305, 1–11. [Google Scholar] [CrossRef]
- Shahadat, M.; Isamil, S. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: A review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Gunatilake, S.K. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. Stud. 2015, 1, 14. [Google Scholar]
- Chowdhury, S.; Mazumder, M.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Removal of heavy metals from wastewater using carbon nanotubes. Sep. Purif. Rev. 2014, 43, 311–338. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, Q.; Nadeem, R.; Iqbal, M.; Saeed, R.; Ansari, T.M. Organic acids pretreatment effect on Rosa bourbonia phyto-biomass for removal of Pb (II) and Cu (II) from aqueous media. Bioresour. Technol. 2013, 132, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Zhang, C.; Rong, H.; Zeng, G. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6509–6518. [Google Scholar] [CrossRef]
- Kılıc, M.; Kırbıyık, C.; Çepelioğullar, Ö.; Pütün, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Ojedokun, A.T.; Bello, O.S. Sequestering heavy metals from wastewater using cow dung. Water Resour. Ind. 2016, 13, 7–13. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Kadhom, M.A.; Alminshid, A.H. Removal of heavy metals from wastewater using agricultural byproducts. J. Water Supply: Res. Technol.-Aqua 2020, 69, 99–112. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2022, 287, 131959. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 1–9. [Google Scholar] [CrossRef]
- Okeimen, F.E.; Onyenkpa, V.U. Binding of cadmium, copper, lead and nickel ions with melon (Citrullus vulgaris) seed husk. Biol. Waste 2000, 29, 11–16. [Google Scholar]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Appenroth, K.J. Definition of “heavy metals” and their role in biological systems. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–29. [Google Scholar]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, W.; Dang, Z.; Hu, Q.; Wu, F.; Feng, C.; Giesy, J.P. Refocusing on nonpriority toxic metals in the aquatic environment in China. Environ. Sci. Technol. 2017, 51, 3117–3118. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Steinmaus, C.M. Health effects of arsenic and chromium in drinking water: Recent human findings. Annu. Rev. Public Health 2009, 30, 107–122. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Parimala, V.; Meng, X. Detoxification of hexavalent chromium from coastal water using lignocellulosic waste. Water SA 2004, 30, 541–545. [Google Scholar] [CrossRef][Green Version]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy metal pollution: Source, impact, and remedies. In Biomanagement of Metal-Contaminated Soils; 2011; pp. 1–28. [Google Scholar]
- Tran, T.K.; Leu, H.J.; Chiu, K.F.; Lin, C.Y. Electrochemical treatment of heavy metal-containing wastewater with the removal of COD and heavy metal ions. J. Chin. Chem. Soc. 2017, 64, 493–502. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Baysal, A.; Ozbek, N.; Akman, S. Determination of trace metals in waste water and their removal processes. Waste Water-Treat. Technol. Recent Anal. Dev. 2013, 1, 145–171. [Google Scholar]
- Kharayat, Y. Distillery wastewater: Bioremediation approaches. J. Integr. Environ. Sci. 2012, 9, 69–91. [Google Scholar] [CrossRef]
- Afolabi, T.J.; Alade, A.O.; Jimoh, M.O.; Fashola, I.O. Heavy metal ions adsorption from dairy industrial wastewater using activated carbon from milk bush kernel shell. Desalination Water Treat. 2016, 57, 14565–14577. [Google Scholar] [CrossRef]
- Lokhande, R.S.; Singare, P.U.; Pimple, D.S. Toxicity study of heavy metals pollutants in waste water effluent samples collected from Taloja industrial estate of Mumbai, India. Resour. Environ. 2011, 1, 13–19. [Google Scholar]
- Alluri, H.K.; Ronda, S.R.; Settalluri, V.S.; Bondili, J.S.; Suryanarayana, V.; Venkateshwar, P. Biosorption: An eco-friendly alternative for heavy metal removal. Afr. J. Biotechnol. 2007, 6, 2924–2931. [Google Scholar]
- Akpoveta, O.V.; Osakwe, S.A. Determination of heavy metal contents in refined petroleum products. IOSR J. Appl. Chem. 2014, 7, 1–2. [Google Scholar]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S. Negative environmental impacts of antibiotic-contaminated effluents from pharmaceutical industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Verma, V.K.; Gupta, R.K.; Rai, J.P.N. Biosorption of Pb and Zn from pulp and paper industry effluent by water hyacinth (Eichhornia crassipes). J. Sci. Ind. Res. 2005, 64, 778–781. [Google Scholar]
- Kowalska, I.; Kabsch-Korbutowicz, M.; Majewska-Nowak, K.; Pietraszek, M. Removal of detergents from industrial wastewater in ultrafiltration process. Environ. Prot. Eng. 2005, 31, 207. [Google Scholar]
- Samuel, F.A.; Mohan, V.; Rebecca, L.J. Physicochemical and heavy metal analysis of sugar mill effluent. J. Chem. Pharm. Res. 2014, 6, 585–587. [Google Scholar]
- Chowdhury, M.; Mostafa, M.G.; Biswas, T.K.; Mandal, A.; Saha, A.K. Characterization of the effluents from leather processing industries. Environ. Process. 2015, 2, 173–187. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Igwe, J.; Abia, A.A. A bioseparation process for removing heavy metals from waste water using biosorbents. Afr. J. Biotechnol. 2006, 5, 1167–1179. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, I.Y.; Tolani, S.; Ofosu, K.; Mohamed, O.A.; Wanekaya, A.K. Polymeric nanofibers for the removal of Cr (III) from tannery waste water. J. Environ. Manag. 2013, 129, 410–413. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhu, J.; Zhang, Y.; Liu, T. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Akpor, O.B.; Ohiobor, G.O.; Olaolu, D.T. Heavy metal pollutants in wastewater effluents: Sources, effects and remediation. Adv. Biosci. Bioeng. 2014, 2, 37–43. [Google Scholar] [CrossRef]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Lee, J.C.; Son, Y.O.; Pratheeshkumar, P.; Shi, X. Oxidative stress and metal carcinogenesis. Free. Radic. Biol. Med. 2012, 53, 742–757. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Elzwayie, A.; Afan, H.A.; Allawi, M.F.; El-Shafie, A. Heavy metal monitoring, analysis and prediction in lakes and rivers: State of the art. Environ. Sci. Pollut. Res. 2017, 24, 12104–12117. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 1–24. [Google Scholar] [CrossRef]
- Jadaa, W.; Mohammed, H. Heavy Metals--Definition, Natural and Anthropogenic Sources of Releasing into Ecosystems, Toxicity, and Removal Methods--An Overview Study. J. Ecol. Eng. 2023, 24, 249–271. [Google Scholar] [CrossRef]
- EPA, National Primary Drinking Water Regulations: List of Contaminants and Their Maximum Contami nant Levels (MCLs). 2009. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 12 July 2023).
- Guidelines for Canadian Drinking Water Quality. 2022. Available online: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/guidelines-canadian-drinking-water-quality-summary-table.html (accessed on 10 July 2023).
- Guidelines for Drinking Water Quality (4th ed.). 2011. Available online: https://www.who.int/publications/i/item/9789241548151 (accessed on 13 July 2023).
- Rezakazemi, M.; Dashti, A.; Harami, H.R.; Hajilari, N. Inamuddin Fouling-resistant membranes for water reuse. Environ. Chem. Lett. 2018, 16, 715–763. [Google Scholar] [CrossRef]
- Villaescusa, I.; Bollinger, J.C. Arsenic in drinking water: Sources, occurrence and health effects (a review). Rev. Environ. Sci. Bio/Technol. 2008, 7, 307–323. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Volesky, B. Advances in biosorption of metals: Selection of biomass types. FEMS Microbiol. Rev. 1994, 14, 291–302. [Google Scholar] [CrossRef]
- Puranik, P.R.; Paknikar, K.M. Biosorption of lead and zinc from solutions using Streptoverticillium cinnamoneum waste biomass. J. Biotechnol. 1997, 55, 113–124. [Google Scholar] [CrossRef]
- Cotruvo, J.A. 2017 WHO guidelines for drinking water quality: First addendum to the fourth edition. J. -Am. Water Work. Assoc. 2017, 109, 44–51. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Naqash, N.; Prakash, S.; Kapoor, D.; Singh, R. Interaction of freshwater microplastics with biota and heavy metals: A review. Environ. Chem. Lett. 2020, 18, 1813–1824. [Google Scholar] [CrossRef]
- Hong, Y.J.; Liao, W.; Yan, Z.F.; Bai, Y.C.; Feng, C.L.; Xu, Z.X.; Xu, D.Y. Progress in the research of the toxicity effect mechanisms of heavy metals on freshwater organisms and their water quality criteria in China. J. Chem. 2020, 2020, 9010348. [Google Scholar] [CrossRef]
- Drake, P.L.; Hazelwood, K.J. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [PubMed]
- Uluisik, I.; Karakaya, H.C.; Koc, A. The importance of boron in biological systems. J. Trace Elem. Med. Biol. 2018, 45, 156–162. [Google Scholar] [CrossRef]
- Yasuda, M.; Miwa, A.; Kitagawa, M. Morphometric studies of renal lesions in itai-itai disease: Chronic cadmium nephropathy. Nephron 1995, 69, 14–19. [Google Scholar] [CrossRef]
- Barceloux, D.G.; Barceloux, D. Cobalt. J. Toxicol. Clin. Toxicol. 1999, 37, 201–216. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Dunlop, P.; Müller, N.L.; Wilson, J.; Flint, J.; Churg, A. Hard metal lung disease: High resolution CT and histologic correlation of the initial findings and demonstration of interval improvement. J. Thorac. Imaging 2005, 20, 301–304. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Zhu, X.; Wang, X. Removal of chromium from aqueous solution by using oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2009, 162, 1542–1550. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, M.; Zhen, H.; Chen, L.; Shi, P.; Huang, Z. Genotoxicity of tri-and hexavalent chromium compounds in vivo and their modes of action on DNA damage in vitro. PLoS ONE 2014, 9, e103194. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Singh, K.P.; Singh, V.K. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J. Hazard. Mater. 2006, 135, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Strausak, D.; Mercer, J.F.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res. Bull. 2001, 55, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Vieira, R.H.S.F. Sargassum seaweed as biosorbent for heavy metals. Water Res. 2000, 34, 4270–4278. [Google Scholar] [CrossRef]
- Chan, Y.H.; Chen, J.; Liu, Q.; Wark, S.E.; Son, D.H.; Batteas, J.D. Ultrasensitive copper (II) detection using plasmon-enhanced and photo-brightened luminescence of CdSe quantum dots. Anal. Chem. 2010, 82, 3671–3678. [Google Scholar] [CrossRef]
- Young, H.A.; Geier, D.A.; Geier, M.R. Thimerosal exposure in infants and neurodevelopmental disorders: An assessment of computerized medical records in the Vaccine Safety Datalink. J. Neurol. Sci. 2008, 271, 110–118. [Google Scholar] [CrossRef]
- Bonzongo, J.C.J.; Lyons, W.B.; Heim, K.J.; Chen, Y.U.; Warwick, J.J.; Miller, G.C.; Lechler, P.J. Mercury pathways in the carson river-lahontan reservoir system, nevada, usa. Environ. Toxicol. Chem. Int. J. 1996, 15, 677–683. [Google Scholar]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health Part B 2017, 20, 55–80. [Google Scholar] [CrossRef]
- Gupta, R.C. (Ed.) Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Yang, S.; Li, J.; Shao, D.; Hu, J.; Wang, X. Adsorption of Ni (II) on oxidized multi-walled carbon nanotubes: Effect of contact time, pH, foreign ions and PAA. J. Hazard. Mater. 2009, 166, 109–116. [Google Scholar] [CrossRef]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L. Primary concept of nickel toxicity-an overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- Grimsrud, T.K.; Berge, S.R.; Haldorsen, T.; Andersen, A. Exposure to different forms of nickel and risk of lung cancer. Am. J. Epidemiol. 2002, 156, 1123–1132. [Google Scholar] [CrossRef]
- Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Tripathi, A.; Ranjan, M.R. Heavy metal removal from wastewater using low cost adsorbents. J. Bioremediation Biodegrad. 2015, 6, 315. [Google Scholar] [CrossRef]
- Pedersen, A.J. Characterization and electrodialytic treatment of wood combustion fly ash for the removal of cadmium. Biomass Bioenergy 2003, 25, 447–458. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Mishra, V.; Balomajumder, C.; Agarwal, V.K. Kinetics, mechanistic and thermodynamics of Zn (II) ion sorption: A modeling approach. Clean-Soil Air Water 2012, 40, 718–727. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Čolić, M.; Ristić, M.Đ.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes. Chem. Eng. J. 2010, 157, 238–248. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions-A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Perić, J.; Trgo, M.; Medvidović, N.V. Removal of zinc, copper and lead by natural zeolite-a comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of Palladium (II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant. Gels 2022, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Du, X.; Liu, H.; Zhang, Q.; Ma, L. Facile one-step fabrication of polymer microspheres with high magnetism and armored inorganic particles by Pickering emulsion polymerization. Colloids Surf. A: Physicochem. Eng. Asp. 2012, 414, 289–295. [Google Scholar] [CrossRef]
- Mehta, D.; Mazumdar, S.; Singh, S.K. Magnetic adsorbents for the treatment of water/wastewater-a review. J. Water Process Eng. 2015, 7, 244–265. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, M.; Zhao, D. Synthesis and properties of magnetic Cu (II) ion imprinted composite adsorbent for selective removal of copper. Desalination 2008, 228, 135–149. [Google Scholar] [CrossRef]
- Mishra, V.; Balomajumder, C.; Agarwal, V.K. Zn (II) ion biosorption onto surface of eucalyptus leaf biomass: Isotherm, kinetic, and mechanistic modeling. Clean-Soil Air Water 2010, 38, 1062–1073. [Google Scholar] [CrossRef]
- Jiménez-Cedillo, M.J.; Olguín, M.T.; Fall, C.; Colin-Cruz, A. As (III) and As (V) sorption on iron-modified non-pyrolyzed and pyrolyzed biomass from Petroselinum crispum (parsley). J. Environ. Manag. 2013, 117, 242–252. [Google Scholar] [CrossRef]
- Khosravi, J.; Alamdari, A. Copper removal from oil-field brine by coprecipitation. J. Hazard. Mater. 2009, 166, 695–700. [Google Scholar] [CrossRef]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Lee, J.U.; Moon, S.H.; Kim, K.W. Competitive adsorption characteristics of Co2+, Ni2+, and Cr3+ by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 2004, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Ananpattarachai, J.; Pongpom, S. Sol–gel preparation and properties study of TiO2 thin film for photocatalytic reduction of chromium (VI) in photocatalysis process. Sci. Technol. Adv. Mater. 2005, 6, 352. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; San Ly, Q. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Deliyanni, E.A.; Peleka, E.N.; Matis, K.A. Removal of zinc ion from water by sorption onto iron-based nanoadsorbent. J. Hazard. Mater. 2007, 141, 176–184. [Google Scholar] [CrossRef]
- Brown, P.A.; Gill, S.A.; Allen, S.J. Metal removal from wastewater using peat. Water Res. 2000, 34, 3907–3916. [Google Scholar] [CrossRef]
- Shah, B.A.; Shah, A.V.; Singh, R.R. Sorption isotherms and kinetics of chromium uptake from wastewater using natural sorbent material. Int. J. Environ. Sci. Technol. 2009, 6, 77–90. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Yahya, M.A.; Al-Shannag, M. On the performance of bioadsorption processes for heavy metal ions removal by low-cost agricultural and natural by-products bioadsorbent: A review. Desalination Water Treat. 2017, 85, 339–357. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Renge, V.C.; Khedkar, S.V.; Pande, S.V. Removal of heavy metals from wastewater using low cost adsorbents: A review. Sci. Rev. Chem. Commun. 2012, 2, 580–584. [Google Scholar]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, S. Removal of heavy metals from waste water by using various adsorbents-a review. Indian J. Sci. Technol. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Lee, Y.R.; Soe, J.T.; Zhang, S.; Ahn, J.W.; Park, M.B.; Ahn, W.S. Synthesis of nanoporous materials via recycling coal fly ash and other solid wastes: A mini review. Chem. Eng. J. 2017, 317, 821–843. [Google Scholar] [CrossRef]
- Sommerville, R.; Blissett, R.; Rowson, N.; Blackburn, S. Producing a synthetic zeolite from improved fly ash residue. Int. J. Miner. Process. 2013, 124, 20–25. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.T.; Alastuey, A.; Hernández, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Yao, Z.T.; Xia, M.S.; Sarker, P.K.; Chen, T.Z. A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 2014, 120, 74–85. [Google Scholar] [CrossRef]
- Singh, N.B.; Agarwal, A.; De, A.; Singh, P. Coal fly ash: An emerging material for water remediation. Int. J. Coal Sci. Technol. 2022, 9, 44. [Google Scholar] [CrossRef]
- Tiwari, M.; Sahu, S.K.; Bhangare, R.C.; Ajmal, P.Y.; Pandit, G.G. Elemental characterization of coal, fly ash, and bottom ash using an energy dispersive X-ray fluorescence technique. Appl. Radiat. Isot. 2014, 90, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-H.T.; Nguyen, H.T.; Ahmed, S.F.; Rajamohan, N.; Yusuf, M.; Sharma, A. Emerging waste-to-wealth applications of fly ash for environmental remediation: A review. Environ. Res. 2023, 227, 115800. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A. The impacts of combustion emissions on air quality and climate-From coal to biofuels and beyond. Atmos. Environ. 2009, 43, 23–36. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Gadore, V.; Ahmaruzzaman, M. Tailored fly ash materials: A recent progress of their properties and applications for remediation of organic and inorganic contaminants from water. J. Water Process Eng. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Volli, V.; Shu, C.M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- Panday, K.K.; Prasad, G.; Singh, V.N. Copper (II) removal from aqueous solutions by fly ash. Water Res. 1985, 19, 869–873. [Google Scholar] [CrossRef]
- Lin, C.J.; Chang, J.E. Effect of fly ash characteristics on the removal of Cu (II) from aqueous solution. Chemosphere 2001, 44, 1185–1192. [Google Scholar] [CrossRef]
- Grover, M.; Narayanaswamy, M.S. Removal of hexavalent chromium by adsorption on flyash. J. Environ. Eng. Div. 1982, 63, 36–39. [Google Scholar]
- Dasmahapatra, G.P.; Pal, T.K.; Bhadra, A.K.; Bhattacharya, B. Studies on separation characteristics of hexavalent chromium from aqueous solution by fly ash. Sep. Sci. Technol. 1996, 31, 2001–2009. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Upadhyay, S.N.; Weng, C.H. Studies on an economically viable remediation of chromium rich waters and wastewaters by PTPS fly ash. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 222–228. [Google Scholar] [CrossRef]
- Sen, A.K.; De, A.K. Adsorption of mercury (II) by coal fly ash. Water Res. 1987, 21, 885–888. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Adsorption of mercury from wastewater by fly ash. Adsorpt. Sci. Technol. 1992, 9, 130–147. [Google Scholar] [CrossRef]
- Diamadopoulos, E.; Ioannidis, S.; Sakellaropoulos, G.P. As (V) removal from aqueous solutions by fly ash. Water Res. 1993, 27, 1773–1777. [Google Scholar] [CrossRef]
- Yadava, K.P.; Tyagi, B.S.; Panday, K.K.; Singh, V.N. Fly ash for the treatment of Cd (II) rich effluents. Environ. Technol. 1987, 8, 225–234. [Google Scholar] [CrossRef]
- Weng, C.H.; Huang, C.P. Adsorption characteristics of Zn (II) from dilute aqueous solution by fly ash. Colloids Surf. A Physicochem. Eng. Asp. 2004, 247, 137–143. [Google Scholar] [CrossRef]
- Visa, M.; Isac, L.; Duta, A. Fly ash adsorbents for multi-cation wastewater treatment. Appl. Surf. Sci. 2012, 258, 6345–6352. [Google Scholar] [CrossRef]
- Sočo, E.; Kalembkiewicz, J. Adsorption of nickel (II) and copper (II) ions from aqueous solution by coal fly ash. J. Environ. Chem. Eng. 2013, 1, 581–588. [Google Scholar] [CrossRef]
- Papandreou, A.D.; Stournaras, C.J.; Panias, D.; Paspaliaris, I. Adsorption of Pb (II), Zn (II) and Cr (III) on coal fly ash porous pellets. Miner. Eng. 2011, 24, 1495–1501. [Google Scholar] [CrossRef]
- Bayat, B. Comparative study of adsorption properties of Turkish fly ashes: I. The case of nickel (II), copper (II) and zinc (II). J. Hazard. Mater. 2002, 95, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Gandhimathi, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Alinnor, I.J. Adsorption of heavy metal ions from aqueous solution by fly ash. Fuel 2007, 86, 853–857. [Google Scholar] [CrossRef]
- Bayat, B. Comparative study of adsorption properties of Turkish fly ashes: II. The case of chromium (VI) and cadmium (II). J. Hazard. Mater. 2002, 95, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Bayat, B. Combined removal of zinc (II) and cadmium (II) from aqueous solutions by adsorption onto high-calcium Turkish fly ash. Water Air Soil Pollut. 2002, 136, 69–92. [Google Scholar] [CrossRef]
- Ayala, J.; Blanco, F.; Garcìa, P.; Rodriguez, P.; Sancho, J. Asturian fly ash as a heavy metals removal material. Fuel 1998, 77, 1147–1154. [Google Scholar] [CrossRef]
- Gupta, G.; Torres, N. Use of fly ash in reducing toxicity of and heavy metals in wastewater effluent. J. Hazard. Mater. 1998, 57, 243–248. [Google Scholar] [CrossRef]
- Apak, R.; Tütem, E.; Hügül, M.; Hizal, J. Heavy metal cation retention by unconventional sorbents (red muds and fly ashes). Water Res. 1998, 32, 430–440. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mohan, D.; Sharma, S. Removal of lead from wastewater using bagasse fly ash—A sugar industry waste material. Sep. Sci. Technol. 1998, 33, 1331–1343. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Utilisation of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep. Purif. Technol. 2000, 18, 131–140. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, S. Removal of zinc from aqueous solutions using bagasse fly ash—A low cost adsorbent. Ind. Eng. Chem. Res. 2003, 42, 6619–6624. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, C.K.; Ali, I.; Sharma, M.; Saini, V.K. Removal of cadmium and nickel from wastewater using bagasse fly ash—A sugar industry waste. Water Res. 2003, 37, 4038–4044. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Removal of lead and chromium from wastewater using bagasse fly ash—A sugar industry waste. J. Colloid Interface Sci. 2004, 271, 321–328. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Naiya, T.K.; Mandal, S.N.; Das, S.K. Adsorption, kinetics and equilibrium studies on removal of Cr (VI) from aqueous solutions using different low-cost adsorbents. Chem. Eng. J. 2008, 137, 529–541. [Google Scholar] [CrossRef]
- Rao, M.; Parwate, A.V.; Bhole, A.G. Removal of Cr6+ and Ni2+ from aqueous solution using bagasse and fly ash. Waste Manag. 2002, 22, 821–830. [Google Scholar] [CrossRef]
- Cetin, S.; Pehlivan, E. The use of fly ash as a low cost, environmentally friendly alternative to activated carbon for the removal of heavy metals from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 83–87. [Google Scholar] [CrossRef]
- Rao, M.; Parwate, A.V.; Kadu, P.A.; Bhole, A.G. Performance of low-cost adsorbents for the removal of copper and lead. J. Water Supply: Res. Technol.-AQUA 2003, 52, 49–58. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Jayaram, R.V.; Joshi, M.V. Removal of nickel (II) and zinc (II) from wastewater using fly ash and impregnated fly ash. Sep. Sci. Technol. 2003, 38, 1015–1032. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Joshi, M.V.; Jayaram, R.V. Removal of Cr (VI) and Hg (II) from aqueous solutions using fly ash and impregnated fly ash. Sep. Sci. Technol. 2005, 39, 1611–1629. [Google Scholar] [CrossRef]
- Ricou, P.; Lecuyer, I.; Le Cloirec, P. Removal of Cu2+, Zn2+ and Pb2+ by adsorption onto fly ash and fly ash/lime mixing. Water Sci. Technol. 1999, 39, 239–247. [Google Scholar] [CrossRef]
- Ricou-Hoeffer, P.; Lecuyer, I.; Le Cloirec, P. Experimental design methodology applied to adsorption of metallic ions onto fly ash. Water Res. 2001, 35, 965–976. [Google Scholar] [CrossRef]
- Weng, C.H.; Huang, C.P. Treatment of metal industrial wastewater by fly ash and cement fixation. J. Environ. Eng. 1994, 120, 1470–1487. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.S.; Xiu, F.R. Arsenic (V) removal from aqueous system using adsorbent developed from a high iron-containing fly ash. Sci. Total Environ. 2009, 407, 5780–5786. [Google Scholar] [CrossRef] [PubMed]
- Visa, M.; Duta, A. TiO2/fly ash novel substrate for simultaneous removal of heavy metals and surfactants. Chem. Eng. J. 2013, 223, 860–868. [Google Scholar] [CrossRef]
- Shyam, R.; Puri, J.K.; Kaur, H.; Amutha, R.; Kapila, A. Single and binary adsorption of heavy metals on fly ash samples from aqueous solution. J. Mol. Liq. 2013, 178, 31–36. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Liao, N.; Kim, J.H.; Kim, H.J.; Park, C.H.; Kim, C.S. In-situ deposition of silver-iron oxide nanoparticles on the surface of fly ash for water purification. J. Colloid Interface Sci. 2015, 453, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Adamczuk, A.; Kołodyńska, D. Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem. Eng. J. 2015, 274, 200–212. [Google Scholar] [CrossRef]
- Qiu, W.; Zheng, Y. Arsenate removal from water by an alumina-modified zeolite recovered from fly ash. J. Hazard. Mater. 2007, 148, 721–726. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Sorption of Cu2+, Cd2+, and Pb2+ using modified zeolite from coal fly ash. Chem. Eng. J. 2008, 144, 245–258. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- Qiu, W.; Zheng, Y. Removal of lead, copper, nickel, cobalt, and zinc from water by a cancrinite-type zeolite synthesized from fly ash. Chem. Eng. J. 2009, 145, 483–488. [Google Scholar] [CrossRef]
- He, X.; Yao, B.; Xia, Y.; Huang, H.; Gan, Y.; Zhang, W. Coal fly ash derived zeolite for highly efficient removal of Ni2+ in waste water. Powder Technol. 2020, 367, 40–46. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sun, X.; Wang, L.; Sun, X. Evaluation of zeolites synthesized from fly ash as potential adsorbents for wastewater containing heavy metals. J. Environ. Sci. 2009, 21, 127–136. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338–347. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of novel zeolites produced from fly ash by hydrothermal treatment in alkaline solution and its evaluation as an adsorbent for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 103687. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z.; Hu, Y. Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ. Sci. Pollut. Res. 2016, 23, 2778–2788. [Google Scholar] [CrossRef] [PubMed]
- Somerset, V.; Petrik, L.; Iwuoha, E. Alkaline hydrothermal conversion of fly ash precipitates into zeolites 3: The removal of mercury and lead ions from wastewater. J. Environ. Manag. 2008, 87, 125–131. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Tzanoudaki, M.; Haralambous, K.J.; Loizidou, M. Regeneration of natural zeolite polluted by lead and zinc in wastewater treatment systems. J. Hazard. Mater. 2011, 189, 773–786. [Google Scholar] [CrossRef]
- Shoumkova, A.; Stoyanova, V. Zeolites formation by hydrothermal alkali activation of coal fly ash from thermal power station “Maritsa 3”, Bulgaria. Fuel 2013, 103, 533–541. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd (II), Co (II), Cu (II), Pb (II), and Zn (II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, F.S.; Zhu, J.; Liu, Z. Effective utilization of waste ash from MSW and coal co-combustion power plant-Zeolite synthesis. J. Hazard. Mater. 2008, 153, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Panek, R.; Medykowska, M.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Jędruchniewicz, K.; Franus, M. Simultaneous removal of Pb2+ and Zn2+ heavy metals using fly ash Na-X zeolite and its carbon Na-X (C) composite. Materials 2021, 14, 2832. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.K.; Nagae, M.; Matsuda, M.; Miyake, M. Zeolite formation from coal fly ash and heavy metal ion removal characteristics of thus-obtained Zeolite X in multi-metal systems. J. Environ. Manag. 2009, 90, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Murukutti, M.K.; Jena, H. Synthesis of nano-crystalline zeolite-A and zeolite-X from Indian coal fly ash, its characterization and performance evaluation for the removal of Cs+ and Sr2+ from simulated nuclear waste. J. Hazard. Mater. 2022, 423, 127085. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Wang, L.; Li, X.; Luo, Q.; Na, P. Zeolite P synthesis based on fly ash and its removal of Cu (II) and Ni (II) ions. Chin. J. Chem. Eng. 2019, 27, 341–348. [Google Scholar] [CrossRef]
- Bu, N.; Liu, X.; Song, S.; Liu, J.; Yang, Q.; Li, R.; Zhang, J. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution. Adv. Powder Technol. 2020, 31, 2699–2710. [Google Scholar] [CrossRef]
- Rio, S.; Delebarre, A.; Hequet, V.; Cloirec, P.L.; Blondin, J. Metallic ion removal from aqueous solutions by fly ashes: Multicomponent studies. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2002, 77, 382–388. [Google Scholar] [CrossRef]
- Rio, S.; Delebarre, A. Removal of mercury in aqueous solution by fluidized bed plant fly ash. Fuel 2003, 82, 153–159. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhu, Z.H. Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. J. Hazard. Mater. 2007, 139, 254–259. [Google Scholar] [CrossRef]
- Pattanayak, J.; Mondal, K.; Mathew, S.; Lalvani, S.B. A parametric evaluation of the removal of As (V) and As (III) by carbon-based adsorbents. Carbon 2000, 38, 589–596. [Google Scholar] [CrossRef]
- Papandreou, A.; Stournaras, C.J.; Panias, D. Copper and cadmium adsorption on pellets made from fired coal fly ash. J. Hazard. Mater. 2007, 148, 538–547. [Google Scholar] [CrossRef]
- Hsu, T.C.; Yu, C.C.; Yeh, C.M. Adsorption of Cu2+ from water using raw and modified coal fly ashes. Fuel 2008, 87, 1355–1359. [Google Scholar] [CrossRef]
- Mimura, H.; Yokota, K.; Akiba, K.; Onodera, Y. Alkali hydrothermal synthesis of zeolites from coal fly ash and their uptake properties of cesium ion. J. Nucl. Sci. Technol. 2001, 38, 766–772. [Google Scholar] [CrossRef][Green Version]
- Shawabkeh, R.; Al-Harahsheh, A.; Hami, M.; Khlaifat, A. Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater. Fuel 2004, 83, 981–985. [Google Scholar] [CrossRef]
- Wu, X.W.; Ma, H.W.; Zhang, L.T.; Wang, F.J. Adsorption properties and mechanism of mesoporous adsorbents prepared with fly ash for removal of Cu (II) in aqueous solution. Appl. Surf. Sci. 2012, 261, 902–907. [Google Scholar] [CrossRef]
- Visa, M.; Chelaru, A.M. Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl. Surf. Sci. 2014, 303, 14–22. [Google Scholar] [CrossRef]
- Chandra Srivastava, V.; Deo Mall, I.; Mani Mishra, I. Modelling individual and competitive adsorption of cadmium (II) and zinc (II) metal ions from aqueous solution onto bagasse fly ash. Sep. Sci. Technol. 2006, 41, 2685–2710. [Google Scholar] [CrossRef]
- Kelleher, B.P.; O’Callaghan, M.N.; Leahy, M.J.; O’Dwyer, T.F.; Leahy, J.J. The use of fly ash from the combustion of poultry litter for the adsorption of chromium (III) from aqueous solution. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2002, 77, 1212–1218. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mohan, D.; Sharma, S.; Park, K.T. Removal of chromium (VI) from electroplating industry wastewater using bagasse fly ash—A sugar industry waste material. Environmentalist 1998, 19, 129–136. [Google Scholar] [CrossRef]
- Panday, K.K.; Prasad, G.; Singh, V.N. Removal of Cr (V1) from aqueous solutions by adsorption on fly ash-wollastonite. J. Chem. Technol. Biotechnol. Chem. Technol. 1984, 34, 367–374. [Google Scholar] [CrossRef]
- Pehlivan, E.; Cetin, S.; Yanık, B.H. Equilibrium studies for the sorption of zinc and copper from aqueous solutions using sugar beet pulp and fly ash. J. Hazard. Mater. 2006, 135, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Terdkiatburana, T.; Tadé, M.O. Single and co-adsorption of heavy metals and humic acid on fly ash. Sep. Purif. Technol. 2008, 58, 353–358. [Google Scholar] [CrossRef]
- Qiu, Q.; Jiang, X.; Lv, G.; Chen, Z.; Lu, S.; Ni, M.; Deng, X. Adsorption of heavy metal ions using zeolite materials of municipal solid waste incineration fly ash modified by microwave-assisted hydrothermal treatment. Powder Technol. 2018, 335, 156–163. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Application of Xanthan gum/n-acetyl cysteine modified mica bionanocomposite as an adsorbent for the removal of toxic heavy metals. Groundw. Sustain. Dev. 2018, 7, 101–108. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Tripathy, S.; Panigrahi, M.K.; Equeenuddin, S.M. Evaluation of the use of an alkali modified fly ash as a potential adsorbent for the removal of metals from acid mine drainage. Appl. Water Sci. 2013, 3, 567–576. [Google Scholar] [CrossRef]
- Oliveira, J.A.; Cunha, F.A.; Ruotolo, L.A. Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. J. Clean. Prod. 2019, 229, 956–963. [Google Scholar] [CrossRef]
- Chaiyasith, S.; Chaiyasith, P.; Septhum, C. Removal of cadmium and nickel from aqueous solution by adsorption onto treated fly ash from Thailand. Sci. Technol. Asia 2006, 13–20. [Google Scholar]
- Wang, J.; Ban, H.; Teng, X.; Wang, H.; Ladwig, K. Impacts of pH and ammonia on the leaching of Cu (II) and Cd (II) from coal fly ash. Chemosphere 2006, 64, 1892–1898. [Google Scholar] [CrossRef]
- Huang, C.P.; Smith, E.H. Removal of Cd (II) from plating waste water by an activated carbon process. In Chemistry in Water Reuse; Cooper, W.J., Ed.; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1981; Volume 2, pp. 355–398. [Google Scholar]
- Ali, A.A.H.; El-Bishtawi, R. Removal of lead and nickel ions using zeolite tuff. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1997, 69, 27–34. [Google Scholar] [CrossRef]
- Weng, C.H.; Wang, J.H.; Huang, C.P. Adsorption of Cr (VI) onto TiO2 from dilute aqueous solutions. Water Sci. Technol. 1997, 35, 55–62. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Nabarawy, T.; Samra, S.E. Sorption properties of che.mically-activated carbons: 1. Sorption of cadmium (II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2004, 235, 153–163. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Siloko, I.U. Effect of hexavalent chromium on the environment and removal techniques: A review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhang, Z.; Wang, H.; Zhou, S.; Zhou, W. A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution. Fuel 2016, 185, 181–189. [Google Scholar] [CrossRef]
- Uğurlu, M.; Karaoğlu, M.H. Adsorption of ammonium from an aqueous solution by fly ash and sepiolite: Isotherm, kinetic and thermodynamic analysis. Microporous Mesoporous Mater. 2011, 139, 173–178. [Google Scholar] [CrossRef]
- Asl, S.H.; Ahmadi, M.; Ghiasvand, M.; Tardast, A.; Katal, R. Artificial neural network (ANN) approach for modeling of Cr (VI) adsorption from aqueous solution by zeolite prepared from raw fly ash (ZFA). J. Ind. Eng. Chem. 2013, 19, 1044–1055. [Google Scholar] [CrossRef]
- Asl, S.M.H.; Masomi, M.; Hosseini, M.; Javadian, H.; Ruiz, M.; Sastre, A.M. Synthesis of hydrous iron oxide/aluminum hydroxide composite loaded on coal fly ash as an effective mesoporous and low-cost sorbent for Cr (VI) sorption: Fuzzy logic modeling. Process Saf. Environ. Prot. 2017, 107, 153–167. [Google Scholar]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Stavropoulos, G.G.; Zabaniotou, A.A. Minimizing activated carbons production cost. Fuel Process. Technol. 2009, 90, 952–957. [Google Scholar] [CrossRef]
- Kumar, M.; Goswami, L.; Singh, A.K.; Sikandar, M. Valorization of coal fired-fly ash for potential heavy metal removal from the single and multi-contaminated system. Heliyon 2019, 5, e02562. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, J.; Khare, R.; Cameotra, S.S.; Ali, A. Batch sorption dynamics, kinetics and equilibrium studies of Cr (VI), Ni (II) and Cu (II) from aqueous phase using agricultural residues. Appl. Water Sci. 2013, 3, 207–218. [Google Scholar] [CrossRef]
- Bunluesin, S.; Kruatrachue, M.; Pokethitiyook, P.; Upatham, S.; Lanza, G.R. Batch and continuous packed column studies of cadmium biosorption by Hydrilla verticillata biomass. J. Biosci. Bioeng. 2007, 103, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Tauanov, Z.; Shah, D.; Itskos, G.; Inglezakis, V. Optimized production of coal fly ash derived synthetic zeolites for mercury removal from wastewater. In IOP Conference Series: Materials Science and Engineering; 2017; Volume 230, p. 012044. [Google Scholar]
- Attari, M.; Bukhari, S.S.; Kazemian, H.; Rohani, S. A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J. Environ. Chem. Eng. 2017, 5, 391–399. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Ram, L.C.; Srivastava, N.K.; Jha, S.K.; Sinha, A.K.; Masto, R.E.; Selvi, V.A. Management of lignite fly ash for improving soil fertility and crop productivity. Environ. Manag. 2007, 40, 438–452. [Google Scholar] [CrossRef]
- Masto, R.E.; Sengupta, T.; George, J.; Ram, L.C.; Sunar, K.K.; Selvi, V.A.; Sinha, A.K. The impact of fly ash amendment on soil carbon. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 554–562. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. An appraisal of the potential use of fly ash for reclaiming coal mine spoil. J. Environ. Manag. 2010, 91, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements-a review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Characterization of sintered coal fly ashes. Fuel 2008, 87, 1334–1340. [Google Scholar] [CrossRef]
- Kniess, C.T.; De Lima, J.C.; Prates, P.B.; Kuhnen, N.C.; Riella, H.G. Dilithium dialuminium trisilicate phase obtained using coal bottom ash. J. Non-Cryst. Solids 2007, 353, 4819–4822. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Ye, Y. Dilithium dialuminium trisilicate crystalline phase prepared from coal fly ash. J. Mater. Eng. Perform. 2012, 21, 877–881. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Mericboyu, A. Comparison of the properties of glass, glass-ceramic and ceramic materials produced from coal fly ash. J. Hazard. Mater. 2008, 153, 418–425. [Google Scholar] [CrossRef] [PubMed]




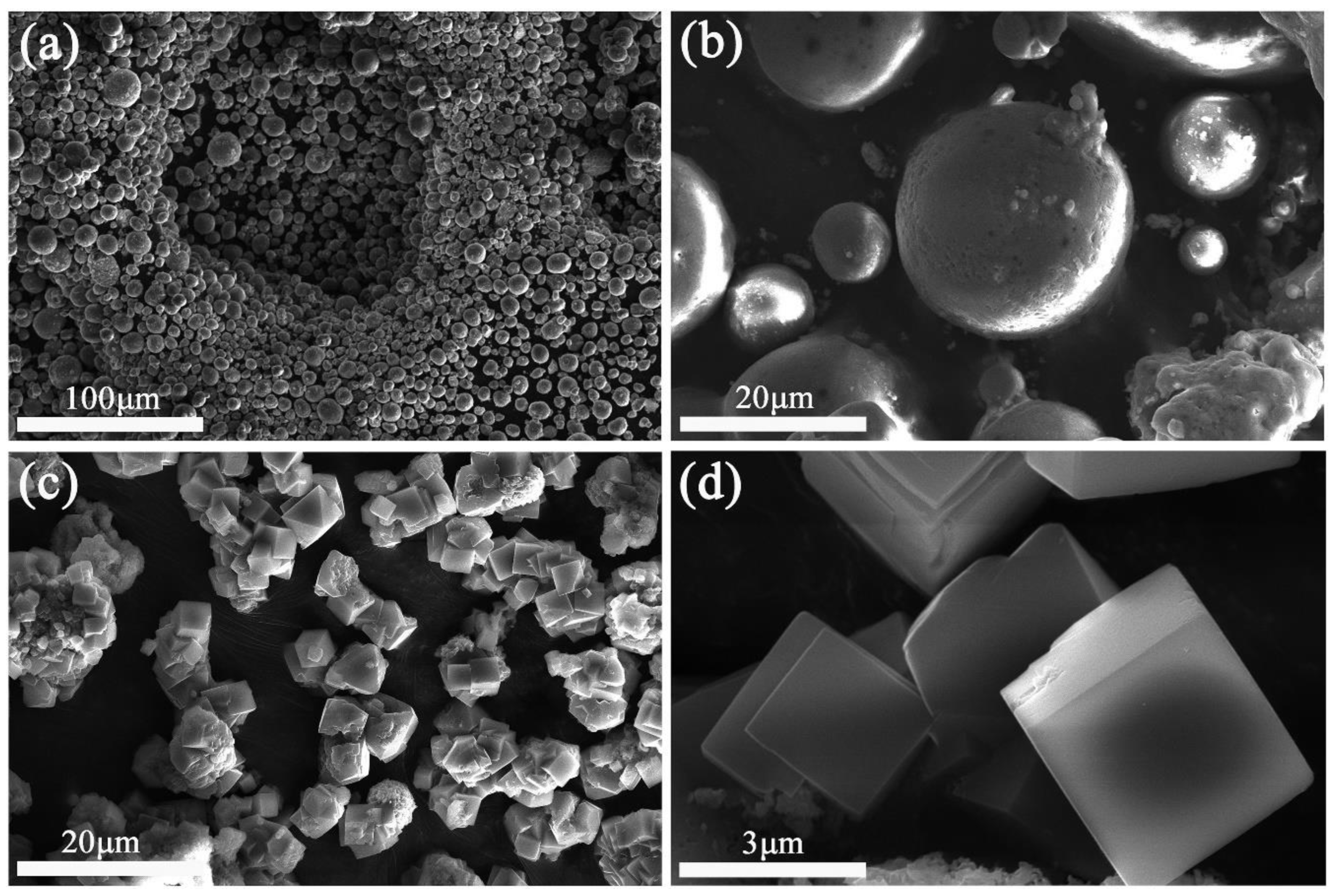


| N | Industry Sector Type/Process | Heavy Metals That Released from Different Sources | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | As | Cd | Co | Cr | Cu | Fe | Hg | Mn | Ni | Pb | Sb | Se | Zn | |||
| 1 | Aircraft manufacturing | × | × | × | × | × | × | × | [124] | |||||||
| 2 | Blast furnace | × | × | × | [125] | |||||||||||
| 3 | Chemicals production | × | × | × | × | × | × | × | × | × | [126] | |||||
| 4 | Coal burning | × | × | [125] | ||||||||||||
| 5 | Distillery | × | × | × | × | × | [127] | |||||||||
| 6 | Dairy industry | × | × | × | × | × | [128] | |||||||||
| 7 | Dyes manufacturing | × | × | × | × | × | × | × | [129] | |||||||
| 8 | Electrolysis processes | × | [125] | |||||||||||||
| 9 | Electroplating process | × | × | [130] | ||||||||||||
| 10 | Engineering industry | × | × | × | × | × | × | × | [129] | |||||||
| 11 | Fertilizers industry | × | × | × | × | × | × | × | × | × | [124] | |||||
| 12 | Fine chemicals industry | × | × | × | × | × | × | × | [129] | |||||||
| 13 | Food additives industry | × | × | [125] | ||||||||||||
| 14 | Food industry | × | × | × | × | × | [128] | |||||||||
| 15 | High-tension lines manufacturing | × | × | [125] | ||||||||||||
| 16 | Household waste | × | × | × | × | × | [125] | |||||||||
| 17 | Metal smelting | × | × | × | × | × | [125] | |||||||||
| 18 | Oil refinery | × | × | × | × | × | × | × | × | [124] | ||||||
| 19 | Organic chemistry | × | × | × | × | × | × | × | [124] | |||||||
| 20 | Paper mill | × | × | × | × | × | × | × | × | × | [124,129] | |||||
| 21 | Pesticides industry | × | × | × | × | × | [125,130] | |||||||||
| 22 | Petroleum combustion | × | [125] | |||||||||||||
| 23 | Petroleum industry | × | × | × | × | × | [131] | |||||||||
| 24 | Pharmaceuticals industry | × | × | × | × | × | × | × | [132] | |||||||
| 25 | Plastic manufacturing | × | × | × | × | × | [133] | |||||||||
| 26 | Pulp and paper industry | × | × | × | × | × | [134] | |||||||||
| 27 | Steel manufacturing | × | × | × | × | × | × | × | × | × | [124] | |||||
| 28 | Soap and detergents | × | × | × | × | × | × | × | [135] | |||||||
| 29 | Sugar industry | × | × | × | × | × | × | [136] | ||||||||
| 30 | Tanning industry | × | × | × | × | × | × | [137] | ||||||||
| 31 | Textile and dyeing | × | × | × | × | × | × | × | [138] | |||||||
| 32 | Wastewater sludge | × | × | × | × | × | [125] | |||||||||
| N | Metal | MCL Values 1 | |||
|---|---|---|---|---|---|
| US 2 | Canada 3 | UK 4 | WHO 5 | ||
| 1 | Al | 0.2 | 0.1 | 0.2 | - |
| 2 | Ag | - | - | - | - |
| 3 | As | 0.01 | 0.01 | 0.01 | 0.01 |
| 4 | B | - | 5.0 | 1 | 2.4 |
| 5 | Ba | 2.0 | 2.0 | - | 0.7 |
| 6 | Be | 0.004 | - | - | - |
| 7 | Cd | 0.005 | 0.007 | 0.005 | 0.003 |
| 8 | Cr | 0.1 | 0.05 | 0.05 | 0.05 |
| 9 | Cu | 0.25 * | 2.0 | 2.0 | 2.0 |
| 10 | Fe | - | - | 0.2 | - |
| 11 | Hg | 0.00003 | 0.001 | 0.001 | 0.006 |
| 12 | Mn | - | 0.12 | 0.05 | - |
| 13 | Ni | 0.2 * | - | 0.02 | 0.07 |
| 14 | Pb | 0.006 * | 0.005 | 0.01 | 0.01 |
| 15 | Sb | 0.006 | 0.006 | - | 0.02 |
| 16 | Se | 0.05 | 0.05 | 0.01 | 0.04 |
| 17 | U | 0.03 | 0.02 | - | 0.03 |
| 18 | Zn | 0.8 * | - | - | - |
| N | Metal | Health Hazards Resulting From Exposure To Heavy Metals | Ref. |
|---|---|---|---|
| 1 | Ag | Lowered blood pressure, diarrhea, gastric irritation, and reduced breathing; occurrence of fatty degeneration in the kidneys and liver along with modifications in blood cell composition. | [166] |
| 2 | As | It specifically affects the outer layer of the skin, resulting in damage and potentially leading to the onset of skin cancer in its later stages; diverse complications involving the circulatory system, including arterial issues and the presence of diabetes; cancerous conditions involving the skin, lungs, and kidneys, as well as other internal malignancies; the potential for increased infant mortality and lower birth weight in newborns; neurological issues; developmental challenges, neurobehavioral disorders, blood-related conditions, and genotoxic effects. | [94,99,104] |
| 3 | B | Headaches, lowered body temperature, fatigue, kidney problems, skin inflammation, hair loss, loss of appetite, and digestive disorders. | [167] |
| 4 | Ba | Increased blood pressure levels | [153] |
| 5 | Be | Digestive disorders | [153] |
| 6 | Cd | Various complications affect the kidneys, resulting in damage, severe bone pain, liver disorders, hypertension, and a substantial risk of cancer development. | [99,101,106,168] |
| 7 | Co | The primary organs affected are the respiratory system and skin, with the possibility of developing hypersensitivity lung disease leading to irreversible fibrosis as well as dermatitis caused by a reaction of inflammation. | [169,170,171] |
| 8 | Cr | Symptoms of nausea and significant diarrhea, obstruction of the lungs, and impairment of liver and kidney functions; a substance with nephrotoxic properties with a high likelihood of causing cancer; and it has an association with disorders of the skin, nervous system, and digestive system, as well as the development of malignancies in different organs like the lungs and thyroid. | [172,173,174] |
| 9 | Cu | Short-term effects may include hypertension, sleeplessness, rapid respiration, seizures, and muscular cramps; a tendency to accumulate in different areas, including the skin and brain, giving rise to significant toxic implications that can ultimately result in long-term harm, particularly to the kidneys and liver; occurrence of Wilson’s disease and Menkes syndrome. | [94,106,175,176,177] |
| 10 | Hg | In the immediate term, it primarily targets the neurological system, causing significant damage to the central neural system and exhibiting nephrotoxic effects; over the long term, it can have severe implications on multiple organs, particularly brain and kidneys, as well as various bodily systems like immune and respiratory; and it is linked to neurodevelopmental challenges, encompassing conditions such as tic disorders and delayed speech. | [94,178,179,180] |
| 11 | Mn | The central neurological system is the primary organ affected by the Mn toxic effects. Chronic exposure to Mn leads to alterations in neurological and neurobehavioral functions. Neurobehavioral signs encompass changes in mood, impaired motor skills, slower response time, limb numbness, and impaired memory. | [181] |
| 12 | Ni | A range of respiratory conditions like asthma and Chronic lung disease are associated with it; it manifests in various symptoms, such as dry cough, nasal congestion, bluish skin, chest tightness, rapid breathing, breathlessness, and dizziness; and it is associated with various detrimental health effects, including skin allergies, pulmonary illnesses like fibrosis, neural damage, kidney disorders, and pulmonary system malignancies. | [94,182,183,184] |
| 13 | Pb | Infants are vulnerable to damage in their central neural system, while children may exhibit conduct problems and encounter learning challenges, including difficulties with concentration and acquiring new skills; it is connected to various health implications: blood diseases like anemia, hypertension, disorders, neural system damage, kidney illnesses, and cognitive impairment. | [94,122,162,185,186] |
| 14 | Sb | Lowering of blood sugar content and markedly increased levels of cholesterol. | [104] |
| 15 | Se | Various health effects like artery problems, loss of both nails and hair and hands and legs numbness. | [104,153] |
| 16 | Zn | It is linked to a range of health hazards, such as fatigue, increased thirst, feelings of depression, increased nervousness, stomach sickness, skin inflammation, muscular cramps, and vomiting. | [94,99,106] |
| N | Technique Used | Key Benefits | Key Drawbacks | Ref. |
|---|---|---|---|---|
| 1 | Adsorption |
|
| [99,112,193] |
| 2 | Adsorption using magnetic materials |
|
| [194,195,196,197]. |
| 3 | Biosorption |
|
| [97,198,199] |
| 4 | Chemical precipitation |
|
| [94,105,200]. |
| 5 | Electrochemical treatment |
|
| [99,104,201] |
| 6 | Flocculation and coagulation |
|
| [94,112,201]. |
| 7 | Flotation method |
|
| [112,189,201] |
| 8 | Ion-exchange method |
|
| [104,105,202] |
| 9 | Membrane filtration |
|
| [94,201,203]. |
| 10 | Photocatalysis |
|
| [99,201,204]. |
| 11 | Reverse osmosis |
|
| [189,201] |
| Chemical Composition of FA | (w/w %) | ||||||
|---|---|---|---|---|---|---|---|
| N | Major Constituents | No1 a | No2 b | No3 c | No4 d | No5 e | No6 f |
| 1 | Silica (SiO2) | 53.32 | 51.0 | 47.42 | 15.14 | 53.50 | 36.06 |
| 2 | Alumina (Al2O3) | 22.05 | 20.0 | 19.16 | 7.54 | 15.71 | 15.38 |
| 3 | Iron(III) oxide (Fe2O3) | 8.97 | 12.5 | 10.89 | 3.30 | 8.81 | 8.28 |
| 4 | Calcium oxide (CaO) | 5.24 | 4.0 | 12.52 | 23.66 | 0.29 | 34.96 |
| 5 | Magnesium oxide (MgO) | 2.44 | 2.0 | 1.21 | 4.50 | 2.94 | 2.26 |
| 6 | Potassium oxide (K2O) | 2.66 | 0.8 | 2.42 | 0.28 | 1.19 | 0.12 |
| 7 | Sulfur trioxide (SO3) | - | - | 2.82 | 13.22 | 1.11 | - |
| 8 | Titanium dioxide (TiO2) | 1.07 | - | 1.11 | 1.03 | 0.12 | 0.93 |
| 9 | Sodium oxide (Na2O) | 0.63 | 0.7 | 0.52 | 0.57 | 0.77 | - |
| N | Trace elements | (mg/Kg) | |||||
| 10 | Arsenic (As) | 100.0 | - | 12.0 | - | - | - |
| 11 | Cadmium (Cd) | - | 4.0 | 0.2 | 8.0 | - | 1.0 |
| 12 | Chromium (Cr) | 100.0 | 71.0 | 327.3 | 298.0 | 454.5 | 70.0 |
| 13 | Copper (Cu) | 60.0 | 73.0 | 1.5 | 40.0 | 98.8 | 80.0 |
| 14 | Lead (Pb) | 35.0 | 141.0 | 7.6 | 80.0 | 79.0 | - |
| 15 | Manganese (Mn) | 800.0 | 956.0 | 378.2 | 219.0 | 790.4 | 10.0 |
| 16 | Nickel (Ni) | 55.0 | 73.0 | 297.4 | 119.0 | 1976 | - |
| 17 | Zinc (Zn) | 160.0 | 98.0 | 118.8 | 80.0 | 112.6 | 5000 |
| Physical properties | |||||||
| 18 | Density (g cm−3) | - | 0.62 | - | 1.05 | 0.88 | 2.51 |
| 19 | Loss on ignition | 1.58 | 7.50 | 2.42 | 2.31 | 3.78 | 4.49 |
| 20 | Surface area (m2 g−1) | - | - | 10.20 | 0.34 | 0.12 | 0.41 |
| Metal Ion | FA Raw/Treated | pH | Capacity | Isotherm Model | Removal Kinetics | Ref. |
|---|---|---|---|---|---|---|
| As(III) | FA-derived char-carbon | - | 89.24 | No model applied | No model applied | [291] |
| As(V) | FA | 4.0 | 27.78 | Langmuir model Freundlich model | [236] | |
| As(V) | FA-coated chitosan | 6.0 | 19.1 | Freundlich model | Pseudo-second-order model Intra particle diffusion model | [268] |
| As(V) | FA-derived cancrinite zeolite | 6.0 | 5.1 | No model applied | No model applied | [269] |
| As(V) | FA-derived cancrinite zeolite/modified alumina | 6.0 | 34.5 | [269] | ||
| As(V) | FA-derived char-carbon | - | 34.46 | [291] | ||
| As(V) | FA-high iron oxide | - | 19.46 | Langmuir model | [264] | |
| Cd(II) | Bagasse FA | - | 1.24 | Langmuir model Freundlich model | [253] | |
| Cd(II) | Bagasse FA | 6.0 | 6.19 | Langmuir model Freundlich model Redlich–Peterson | [298] | |
| Cd(II) | FA | - | 0.09 | Freundlich model | Pseudo-second-order model | [243] |
| Cd(II) | FA | 6.0 | 0.83 | Langmuir model | No model applied | [246] |
| Cd(II) | FA | 7.2 | 198.2 | Langmuir model | [249] | |
| Cd(II) | FA | - | 0.05 | No model applied | [263] | |
| Cd(II) | FA-Afsin-Elbistan | 7.0 | 0.29 | Langmuir model | [245] | |
| Cd(II) | FA-derived zeolite | 5.0 | 52.12 | Langmuir model | Pseudo-second-order model | [277] |
| Cd(II) | FA-derived zeolite X | - | 870 a | Langmuir model D-R model | Pseudo-second-order model Vermeulen model External mass transfer model Weber–Morris model | [270] |
| Cd(II) | FA-pellets | - | 18.98 | Langmuir model | Pseudo-second-order model | [292] |
| Cd(II) | FA-Seyitomer | 7.0 | 0.22 | Langmuir model | No model applied | [245] |
| Cd(II) | FA-treated HCl | 6.6 | 180.4 | Langmuir model | [249] | |
| Cd(II) | FA-TiO2 | - | 86.2 b | Langmuir model Freundlich model | Pseudo-second-order model Intra particle diffusion model | [265] |
| Cd(II) | FA-treated NaOH | 5.6 | 30.21 | Langmuir model | Pseudo-second-order model Intra particle diffusion model | [239] |
| Cd(II) | FA-washed water | 6.7 | 195.2 | Langmuir model | No model applied | [249] |
| Cd(II) | Oil shale FA-derived zeolite | 7.0 | 95.6 | Sips model | [295] | |
| Co(II) | FA-derived zeolite 4A | 3.0 | 13.72 | Langmuir model | Pseudo-second-order model | [271] |
| Co(II) | FA-derived cancrinite zeolite | - | 1242 a | Langmuir model | Pseudo-first-order model | [272] |
| Cr(III) | Bagasse FA | 5.0 | 4.35 | Langmuir model Freundlich model | No model applied | [254] |
| Cr(III) | FA | - | 52.6 | Langmuir model | Pseudo-second-order model | [299] |
| Cr(III,VI) | FA-coated chitosan | 4.0 | 36.22 | Freundlich model | Pseudo-second-order model Intra particle diffusion model | [268] |
| Cr(III) | FA-derived zeolite 4A | 3.0 | 41.61 | Langmuir model | Pseudo-second-order model | [271] |
| Cr(III) | FA-pellets | 7.0 | 22.94 | Langmuir model | Pseudo-second-order model | [241] |
| Cr(VI) | Bagasse FA | 1.0 | 5000.0 a | Langmuir model Freundlich model | No model applied | [300] |
| Cr(VI) | FA | - | 23.86 | Langmuir model Freundlich model | Pseudo-second-order model Intra particle diffusion model | [255] |
| Cr(VI) | FA | - | 1.38 | Langmuir model | Pseudo-first-order model | [260] |
| Cr(VI) | FA/CaCO3(10:1)-H3PO4 | 5.0 | - | Temkin model | No model applied | [266] |
| Cr(VI) | FA-impregnated Al | - | 1.82 | Langmuir model | Pseudo-first-order model | [260] |
| Cr(VI) | FA-impregnated Fe | - | 1.67 | Langmuir model | Pseudo-first-order model | [260] |
| Cr(VI) | FA-wollastonite | - | 2.92 | Langmuir model | No model applied | [301] |
| Cs+ | FA-derived zeolite | 9.5 | 3340a | Langmuir model | [294] | |
| Cu(II) | Bagasse FA | - | 2.26 | Langmuir model Freundlich model | [251] | |
| Cu(II) | FA | 6.5 | 1.39 | Langmuir model | [229] | |
| Cu(II) | FA | 3.0 | - | Freundlich model | [240] | |
| Cu(II) | FA | - | 0.05 | Freundlich model | Pseudo-second-order model | [243] |
| Cu(II) | FA | 6.0 | 207.3 | Langmuir model | No model applied | [249] |
| Cu(II) | FA | 6.2 | 0.1 | No model applied | [290] | |
| Cu(II) | FA | 5.0 | 178.5 | Langmuir model Freundlich model DKR model | Pseudo-second-order model | [293] |
| Cu(II) | FA | 5.0 | 7.0 | Langmuir model | No model applied | [302] |
| Cu(II) | FA | 5.0 | 7.0 | No model applied | Pseudo-first-order model | [303] |
| Cu(II) | FA-Afsin-Elbistan | 6.0 | 1.35 | Langmuir model | No model applied | [242] |
| Cu(II) | FA-coated chitosan | 4.0 | 28.65 | Freundlich model | Pseudo-second-order model Intra particle diffusion model | [268] |
| Cu(II) | FA-derived cancrinite zeolite | - | 2081 a | Langmuir model | Pseudo-first-order model | [272] |
| Cu(II) | FA-derived geopolymer | 6.2 | 90.0 | No model applied | No model applied | [290] |
| Cu(II) | FA-derived mesoporous material | 4.5 | 221 | Freundlich model D-R model | [296] | |
| Cu(II) | FA-derived zeolite | 5.0 | 56.06 | Langmuir model | Pseudo-second-order model | [277] |
| Cu(II) | FA-derived zeolite A | 3.0 | 82.74 | Langmuir model Freundlich model | No model applied | [274] |
| Cu(II) | FA-derived zeolite 4A | 3.0 | 50.45 | Langmuir model | Pseudo-second-order model | [271] |
| Cu(II) | FA-derived zeolite P | - | 105.8 | Langmuir model | Second-order exchange second-order saturation model. | [286] |
| Cu(II) | FA-derived zeolite X | - | 1430 a | Langmuir model D-R model | Pseudo-second-order model Vermeulen model External mass transfer model Weber–Morris model | [270] |
| Cu(II) | FA-pellets | - | 20.92 | Langmuir model | Pseudo-second-order model | [292] |
| Cu(II) | FA-Seyitomer | 6.0 | 1.25 | Langmuir model | No model applied | [242] |
| Cu(II) | FA-TiO2 | - | 21.0 b | Langmuir model Freundlich model | Pseudo-second-order model Intra particle diffusion model | [265] |
| Cu(II) | FA-treated 550 °C | 6.2 | 99.0 | Langmuir model | Pseudo-second-order model | [290] |
| Cu(II) | FA-treated 600 °C | 5.0 | 126.4 | Langmuir model Freundlich model DKR model | Pseudo-second-order model | [293] |
| Cu(II) | FA-treated HCl | 5.7 | 198.5 | Langmuir model | No model applied | [249] |
| Cu(II) | FA-treated NaOH | 5.0 | 76.7 | Langmuir model Freundlich model DKR model | Pseudo-second-order model | [293] |
| Cu(II) | FA-washed water | 5.8 | 205.8 | Langmuir model | No model applied | [249] |
| Hg(II) | FA | - | 11.0 | Langmuir model | Pseudo-first-order model | [260] |
| Hg(II) | FA | 5.0 | 0.73 | Langmuir model Freundlich model | No model applied | [235] |
| Hg(II) | FA-derived zeolite-24 | - | 25.5 | Langmuir model | [276] | |
| Hg(II) | FA-impregnated Al | - | 12.5 | Langmuir model | Pseudo-first-order model | [260] |
| Hg(II) | FA-impregnated Fe | - | 13.4 | Langmuir model | Pseudo-first-order model | [260] |
| Mn(II) | FA | - | - | Freundlich model | Pseudo-first-order model | [243] |
| Mn(II) | FA-derived zeolite | 5.0 | 30.89 | Langmuir model | Pseudo-second-order model | [277] |
| Ni(II) | Bagasse FA | - | 1.12 | Langmuir model Freundlich model | No model applied | [253] |
| Ni(II) | FA | 8.0 | 4.5 | Freundlich model 2nd Langmuir | [240] | |
| Ni(II) | FA | - | 0.03 | Langmuir model | Pseudo-first-order model | [256] |
| Ni(II) | FA | 4.0 | 0.16 | Langmuir model Freundlich model | No model applied | [257] |
| Ni(II) | FA | 6.0 | 14.0 | Langmuir model | Pseudo-first-order model | [259] |
| Ni(II) | FA-Afsin-Elbistan | 8.0 | 0.99 | Langmuir model | No model applied | [242] |
| Ni(II) | FA/CaCO3(10:1)-H3PO4 | 5.0 | - | Temkin model | [266] | |
| Ni(II) | FA/CaCO3(10:1)-H3PO4 | 5.0 | 0.31–7.1 b | Freundlich model | [266] | |
| Ni(II) | FA/CaCO3(15:1)-H3PO4 | 5.0 | 0.4–6.6 b | Freundlich model | [266] | |
| Ni(II) | FA-derived cancrinite zeolite | - | 1532 a | Langmuir model | Pseudo-first-order model | [272] |
| Ni(II) | FA-derived zeolite | 5.0 | 34.40 | Langmuir model | Pseudo-second-order model | [277] |
| Ni(II) | FA-derived zeolite A | - | 57.74 | Langmuir model | Pseudo-second-order model | [273] |
| Ni(II) | FA-derived zeolite 4A | 3.0 | 8.96 | Langmuir model | Pseudo-first-order model | [271] |
| Ni(II) | FA-derived zeolite P | - | 50.29 | Langmuir model | First-order empirical model | [286] |
| Ni(II) | FA-impregnated Fe | 6.0 | 14.93 | Langmuir model | Pseudo-first-order model | [259] |
| Ni(II) | FA-impregnated Al | 6.0 | 15.75 | Langmuir model | Pseudo-first-order model | [259] |
| Ni(II) | FA-Seyitomer | 8.0 | 1.16 | Langmuir model | No model applied | [242] |
| Pb(II) | Bagasse FA | 6.0 | 2.50 | Langmuir model Freundlich model | [254] | |
| Pb(II) | FA | - | 0.08 | Freundlich model | Pseudo-second-order model | [243] |
| Pb(II) | FA | - | 416.6 | Langmuir model | No model applied | [267] |
| Pb(II) | FA | 5.0 | 18.0 | - | Pseudo-first-order model | [303] |
| Pb(II) | FA/Ag-Fe3O4 | - | 526.5 | Langmuir model | Pseudo-second-order model Intra particle diffusion model | [267] |
| Pb(II) | FA/CaCO3(10:1)-H3PO4 | 5.0 | - | Freundlich model | No model applied | [266] |
| Pb(II) | FA/CaCO3(10:1)-H3PO4 | 5.0 | 1.4–9.1 b | Temkin model | [266] | |
| Pb(II) | FA/CaCO3(15:1)-H3PO4 | 5.0 | 1.3–8.9 b | Freundlich model | [266] | |
| Pb(II) | FA-derived cancrinite zeolite | - | 2130 a | Langmuir model | Pseudo-first-order model | [272] |
| Pb(II) | FA-derived zeolite | 5.0 | 65.75 | Langmuir model | Pseudo-second-order model | [277] |
| Pb(II) | FA-derived zeolite-24 | - | 38.0 | Langmuir model | No model applied | [276] |
| Pb(II) | FA-derived Na-X zeolite | 5.0 | 676.59 | Langmuir model | Pseudo-second-order model | [283] |
| Pb(II) | FA-derived Na-X zeolite | 5.0 | 693.29 | Langmuir model | Pseudo-second-order model | [283] |
| Pb(II) | FA-derived zeolite X | - | 2030 a | Langmuir model D-R model | Pseudo-second-order model Vermeulen model External mass transfer model Weber–Morris model | [270] |
| Pb(II) | FA-pellets | 7.0 | 45.25 | Langmuir model | Pseudo-second-order model | [241] |
| Pb(II) | FA-treated NaOH | 5.6 | 2500.0 | Freundlich model | Pseudo-second-order model | [239] |
| Pb(II) | Oil shale FA-derived zeolite | 7.0 | 70.58 | Redlich–Peterson | No model applied | [295] |
| Zn(II) | Bagasse FA | - | 2.34 | Langmuir model Freundlich model | [251] | |
| Zn(II) | Bagasse FA | 4.0 | 202.0 a | Langmuir model Freundlich model | [252] | |
| Zn(II) | Bagasse FA | 6.0 | 7.03 | Langmuir model Freundlich model Redlich–Peterson | [298] | |
| Zn(II) | FA | 7.0 | 6.01 a | Langmuir model | [238] | |
| Zn(II) | FA | - | 0.03 | Freundlich model | Pseudo-first-order model | [243] |
| Zn(II) | FA | 7.0 | 2.78 | Langmuir model | No model applied | [246] |
| Zn(II) | FA | 4.0 | 0.17 | Langmuir model Freundlich model | [257] | |
| Zn(II) | FA | 6.5 | 6.49 | Langmuir model | Pseudo-first-order model | [259] |
| Zn(II) | FA | - | 0.27 | - | No model applied | [263] |
| Zn(II) | FA | 4.0 | 7.84 | Freundlich model | [302] | |
| Zn(II) | FA-Afsin-Elbistan | 7.0 | 1.16 | Langmuir model | [242] | |
| Zn(II) | FA-coated chitosan | 2.0 | 55.52 | Freundlich model | Pseudo-second-order model Intra particle diffusion model | [268] |
| Zn(II) | FA-derived cancrinite zeolite | - | 1154 a | Langmuir model | Pseudo-first-order model | [272] |
| Zn(II) | FA-derived zeolite | - | 91.72 | Langmuir model | No model applied | [282] |
| Zn(II) | FA-derived zeolite A | 3.0 | 47.34 | Langmuir model Freundlich model | [274] | |
| Zn(II) | FA-derived zeolite 4A | 3.0 | 30.80 | Langmuir model | Pseudo-second-order model | [271] |
| Zn(II) | FA-derived Na-X(C) zeolite | 5.0 | 321.91 | Langmuir model | Pseudo-second-order model | [283] |
| Zn(II) | FA-derived Na-X(C) zeolite | 5.0 | 640.94 | Langmuir model | Pseudo-second-order model | [283] |
| Zn(II) | FA-impregnated Al | 6.5 | 7.0 | Langmuir model | Pseudo-first-order model | [259] |
| Zn(II) | FA-impregnated Fe | 6.5 | 7.50 | Langmuir model | Pseudo-first-order model | [259] |
| Zn(II) | FA/MSW derived zeolite | - | 121.97 | Langmuir model | No model applied | [282] |
| Zn(II) | FA-pellets | 8.0 | 17.7 | Langmuir model | Pseudo-second-order model | [241] |
| Zn(II) | FA-Seyitomer | 7.0 | 1.30 | Langmuir model | No model applied | [242] |
| Zn(II) | FA-treated NaOH | 5.6 | 18.87 | Langmuir model | Pseudo-second-order model Intra particle diffusion model | [239] |
| N | Isotherm Model Name | Model Formula |
|---|---|---|
| 1 | Dubinin–Kaganer–Radushkevich isotherm model | |
| 2 | Freundlich isotherm model | |
| 3 | Henry isotherm model | |
| 4 | Langmuir isotherm model | |
| 5 | Redlich-Peterson isotherm model | |
| 6 | Sips isotherm model | |
| 7 | Tempkin isotherm model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadaa, W. Wastewater Treatment Utilizing Industrial Waste Fly Ash as a Low-Cost Adsorbent for Heavy Metal Removal: Literature Review. Clean Technol. 2024, 6, 221-279. https://doi.org/10.3390/cleantechnol6010013
Jadaa W. Wastewater Treatment Utilizing Industrial Waste Fly Ash as a Low-Cost Adsorbent for Heavy Metal Removal: Literature Review. Clean Technologies. 2024; 6(1):221-279. https://doi.org/10.3390/cleantechnol6010013
Chicago/Turabian StyleJadaa, Waleed. 2024. "Wastewater Treatment Utilizing Industrial Waste Fly Ash as a Low-Cost Adsorbent for Heavy Metal Removal: Literature Review" Clean Technologies 6, no. 1: 221-279. https://doi.org/10.3390/cleantechnol6010013
APA StyleJadaa, W. (2024). Wastewater Treatment Utilizing Industrial Waste Fly Ash as a Low-Cost Adsorbent for Heavy Metal Removal: Literature Review. Clean Technologies, 6(1), 221-279. https://doi.org/10.3390/cleantechnol6010013