A Green Approach to Valorizing Abundant Aquatic Weeds for Nutrient-Rich Edible Paper Sheets Production in Bangladesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples and Preparation of Edible Paper Sheets
2.2. Analysis of Proximate Composition
2.3. Determination of Amino Acid Composition
2.4. Determination of Mineral and Heavy Metals Contents
2.5. Gas Chromatography Mass Spectrometry (GC-MS) Analysis
2.6. Statistical Analyses of Experimental Data
3. Results and Discussion
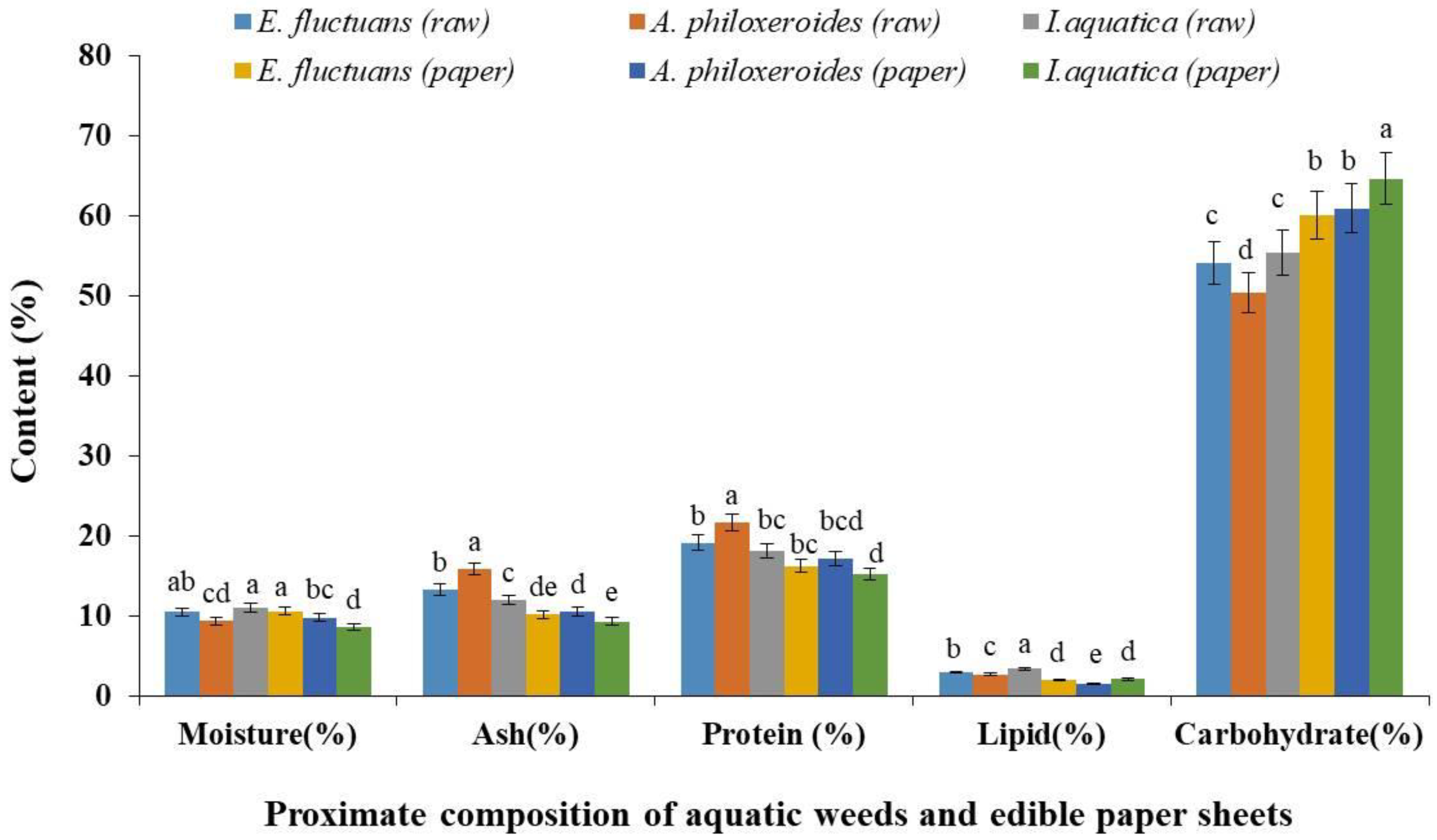
3.1. Proximate Composition Analysis
3.1.1. Moisture Content of Edible Aquatic Weed Paper Sheets
3.1.2. Ash Content of Edible Aquatic Weed Paper Sheets
3.1.3. Crude Protein Content of Edible Aquatic Weed Paper Sheets
3.1.4. Lipid Content of Edible Aquatic Weed Paper Sheets
3.1.5. Carbohydrate Content of Edible Aquatic Weed Paper Sheets
3.2. Minerals and Heavy Metal Content of Edible Aquatic Weed Paper Sheets
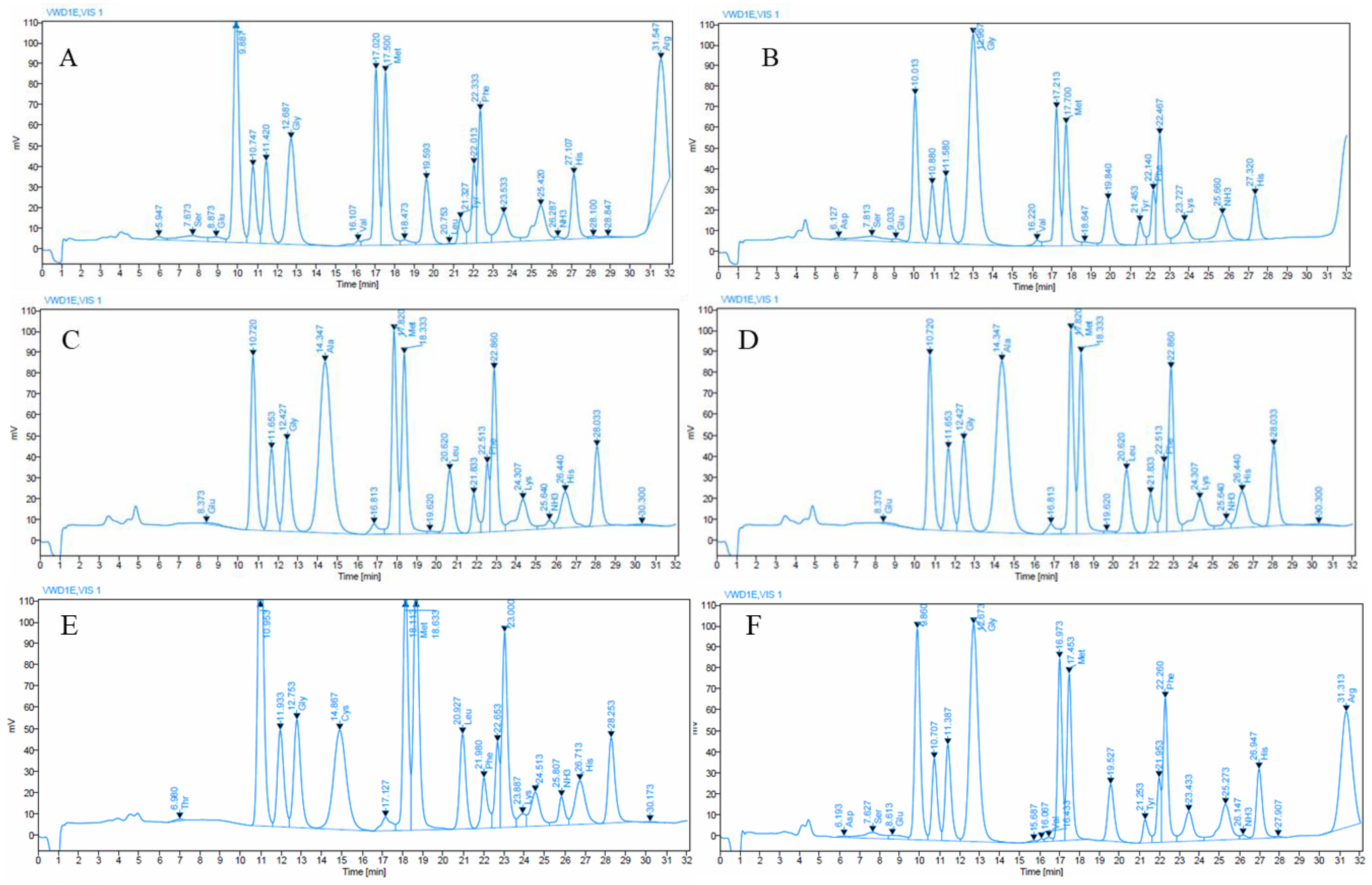
3.3. Amino Acid Composition in Aquatic Weed Paper Sheets
3.4. Major Bioactive Compounds in Aquatic Paper Sheets Determined via GC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, T.R. Management of haors, baors, and beels in Bangladesh. Lessons Lake Basin Manag. 2009, 1, 50352–50357. [Google Scholar]
- Roberts, C.K.; Barnard, R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol. 2005, 98, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Andriani, R.; Wulansari, A.; Dewi, E.K.; Husen, A.H. Physical characteristics of artificial nori made from Ptilophora pinnatifida and Moringa oleifera leaves. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 584, p. 012033. [Google Scholar]
- Rahman, A.H.M.M.; Gulshana, M.I.A. Taxonomy and medicinal uses on amaranthaceae family of Rajshahi, Bangladesh. Appl. Ecol. Environ. Sci. 2014, 2, 54–59. [Google Scholar]
- Hundiwale, J.C.; Patil, A.V.; Kulkarni, M.V.; Patil, D.A.; Mali, R.G. A current update on phytopharmacology of the genus Alternanthera. J. Pharm. Res. 2012, 5, 1924–1929. [Google Scholar]
- Sarma, U.; Borah, V.V.; Saikia, K.K.; Hazarika, N.K. Enhydra fluctuans: A review on its pharmacological importance as a medicinal plant and prevalence and use in North-East India. Int. J. Pharmcy Pharm. Sci. 2014, 6, 48–50. [Google Scholar]
- Saha, S.; Paul, S. A review on phytochemical constituents and pharmacological properties of EnhydrafluctuansLour. J. Pharmacog. Phytochem. 2019, 8, 887–893. [Google Scholar]
- Bhakta, J.; Majumdar, P.; Munekage, Y. Antimicrobial efficacies of methanol extract of Asteracantha longifolia, Ipomoea aquatica and Enhydra fluctuans against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Micrococcus luteus. Int. J. Alter. Med. 2009, 7, 125–135. [Google Scholar]
- Ullah, M.O.; Haque, M.; Urmi, K.F.; Zulfiker, A.H.M.; Anita, E.S.; Begum, M.; Hamid, K. Anti–bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac. J. Trop. Biomed. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Kuri, S.; Billah, M.M.; Rana, S.M.; Naim, Z.; Islam, M.M.; Hasanuzzaman, M.; Banik, R. Phytochemical and in vitro biological investigations of methanolic extracts of Enhydra fluctuans Lour. Asian Pac. J. Trop. Biomed. 2014, 4, 299–305. [Google Scholar] [CrossRef]
- Odulate, D.O.; Idowu, A.A.; Fabusoro, A.A.; Odebiyi, C.O. Growth performance of juvenile Clarias gariepinus (Burchell, 1822) fed Ipomoea aquatica based diets. J. Fish. Aquat. Sci. 2014, 9, 468. [Google Scholar] [CrossRef][Green Version]
- Prasad, K.N.; Shivamurthy, G.R.; Aradhya, S.M. Ipomoea aquatica, an underutilized green leafy vegetable: A review. Int. J. Botany. 2008, 4, 123–129. [Google Scholar]
- Gad, M.H.; Demeyer, K.; Vander Heyden, Y.; Manelings, D. Cytotoxic, antioxidant, and antidiabetic activities versus UPLC-ESI-QTOF-MS chemical-profile analysis of Ipomoea aquatica fractions. Planta Medica 2021, 87, 1089–1100. [Google Scholar]
- Padmavathy, A.; Rasny, M.R.M.; Reyadh, R.; Khan, J. Evaluation of antibacterial activity of different extracts of Ipomoea aquatica leaves against Escherichia coli and Salmonella typhi. J. Manag. Sci. 2017, 15, 51–67. [Google Scholar]
- Bhaigybati, T.; Sanasam, S.; Gurumayum, J.; Bag, G.C.; Singh, L.R.; Devi, P.G. Phytochemical profiling, antioxidant activity, antimicrobial activity and GC-MS analysis of Ipomoea aquatica Forsk collected from EMA market, Manipur. J. Pharmacogn. Phytochem. 2020, 9, 2335–2342. [Google Scholar]
- UNICEF. The State of Food Security and Nutrition in the World 2021; FAO: Rome, Italy, 2022. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Ullah, H.; Gul, B.; Khan, H.; Zeb, U. Effect of salt stress on proximate composition of duckweed (Lemna minor L.). Heliyon 2021, 7, e07399. [Google Scholar] [CrossRef]
- Islam, M.A.; Mohibbullah, M.; Suraiya, S.; Sarower-E-Mahfuj, M.; Ahmed, S.; Haq, M. Nutritional characterization of freshwater mud eel (Monopterus cuchia) muscle cooked by different thermal processes. Food Sci. Nutr. 2020, 8, 6247–6258. [Google Scholar] [CrossRef]
- Kumaravel, S.; Alagusundaram, K. Determination of mineral content in Indian spices by ICP-OES. Orient. J. Chem. 2014, 30, 631–636. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sifat, S.A.D.; Hossain, M.A.; Salleh, S. Comparative assessment of bioactive compounds, antioxidant capacity and nutritional quality of red sea weeds and water spinch. Reg. Stud. Mar. Sci. 2021, 46, 101878. [Google Scholar]
- Hasan, M.M.; Nahar, S.; Arafat, S.T.; Debnath, S.; Parvez, M.S.; Rahman, S.M.; Ahsan, M.N. Proximate composition of edible aquatic vegetables: A preliminary assessment of four species from Bangladesh. Khulna Univ. Stud. 2016, 13, 49–53. [Google Scholar] [CrossRef]
- Pulipati, S.; Devi, B.S.; Devi, G.R.; Bhanuja, M. Pharmacognostic studies of Alternanthera philoxeroides (mart.) griseb. J. Pharmacogn. Phytochem. 2015, 4, 202–204. [Google Scholar]
- Harper, J.M. Ash content determination in foods: A review of current techniques and applications. Food Chem. 2016, 197, 1280–1287. [Google Scholar]
- Umar, K.J.; Hassan, L.G.; Dangoggo, S.M.; Ladan, M.J. Nutritional composition of water spinach (Ipomoea aquatica Forsk.) leaves. J. Appl. Sci. 2007, 7, 803–809. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, B.K.; Bhattacharjee, S.; Seal, T. Nutritional composition, mineral content, antioxidant activity and quantitative estimation of water soluble vitamins and phenolics by RP-HPLC in some lesser used wild edible plants. Heliyon 2019, 5, e01431. [Google Scholar] [CrossRef]
- Suraiya, S.; Ahmmed, M.K.; Haq, M. Immunity boosting roles of biofunctional compounds available in aquafoods: A review. Heliyon 2022, 8, e09547. [Google Scholar] [CrossRef] [PubMed]
- Satter, M.M.A.; Khan, M.M.R.L.; Jabin, S.A.; Abedin, N.; Islam, M.F.; Shaha, B. Nutritional quality and safety aspects of wild vegetables consume in Bangladesh. Asian Pac. J. Trop. Biomed. 2016, 6, 125–131. [Google Scholar] [CrossRef]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Determination of the tolerable upper intake level of leucine in acute dietary studies in young men. Am. J. Clin. Nutr. 2012, 96, 759–767. [Google Scholar] [CrossRef]
- Levine, M.E.; Wu, H. Protein methylation and demethylation: New insights into enzymatic reactions and biological functions. BioEssays 2020, 42, 1900206. [Google Scholar]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prevent. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Dutta, P. Pharmacognostical evaluation and preliminary phytochemical analysis of Alternantheraphiloxeroides. Int. J. MediPharm Res. 2015, 1, 7–13. [Google Scholar]
- Igwenyi, I.O.; Offor, C.E.; Ajah, D.A.; Nwankwo, O.C.; Ukomah, J.I.; Aja, P.M. Chemical compositions of Ipomea aquatica (Green kangkong). Int. J. Pharma Bio Sci. 2011, 2, B593–B598. [Google Scholar]
- Sree, L.; Vijayalakshmi, K. Proximate composition, nutritional evaluation and mineral analysis in the leaves of an indigenous medicinal plant Alternanthera sessilis. Int. J. Health Sci. 2018, 8, 55–62. [Google Scholar]
- Othman, A.; Ismail, A.; Hassan, F.A.; Yusof, B.N.M.; Khatib, A. Comparative evaluation of nutritional compositions, antioxidant capacities, and phenolic compounds of red and green sessile joyweed (Alternanthera sessilis). J. Func. Food. 2016, 21, 263–271. [Google Scholar] [CrossRef]
- Leterme, P.; Buldgen, A.; Estrada, F.; Londoño, A.M. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chem. 2006, 95, 644–652. [Google Scholar] [CrossRef]
- Ndamitso, M.M.; Etsuyankpa, M.B.; Jacob, J.O.; Mathew, J.T.; Shaba, E.Y.; Olisedeme, K.C. The nutritional values and functional properties of wild Ipomoea aquatica (water spinach) found in the Fadama areas of Minna, Niger state, Nigeria. Acad. Res. Int. 2015, 6, 1–8. [Google Scholar]
- Suthari, S.; Kiran, B.R.; Prasad, M.N.V. Health risks of leafy vegetable Alternanthera philoxeroides (Alligator weed) rich in phytochemicals and minerals. Eurobiotech. J. 2017, 1, 293–302. [Google Scholar] [CrossRef]
- Dewanji, A. Amino acid composition of leaf proteins extracted from some aquatic weeds. J. Agr. Food Chem. 1993, 41, 1232–1236. [Google Scholar] [CrossRef]
- Akbar, M.; Amin, A.; Khalil, T.; Iqbal, M.S.; Nazir, A.; Taswar, A. Antibacterial activity of Alternanthera philoxeroides (Mart.) Griseb. against bacterial phytopathogens: Erwinia carotovora, Ralstonia solanacearum and Xanthomonas axonopodis. Allelopath. J. 2021, 53, 83–92. [Google Scholar] [CrossRef]
- Pamila, U.A.; Karpagam, S. Antimicrobial activity and Phytochemical content of an edible plant Alternanthera philoxeroides (Mart.) Griseb. In Proceedings of the National Seminar on Phytochemicals as Therapeutics; Allied Publishers: New Delhi, India, 2017; p. 23. [Google Scholar]
- Muselli, A.; Bighelli, A.; Minh Hoi, T.; Phuong Thao, N.T.; Huy Thai, T.; Casanova, J. Dihydroperillaldehydes from Enhydra fluctuans Lour. essential oil. Flavour Fragr. J. 2000, 15, 299–302. [Google Scholar] [CrossRef]

| Sample | Quantity (g) | Corn Flour (g) | Testing Salt (g) | Water (mL) |
|---|---|---|---|---|
| E. fluctuans | 400 | 4 | 2 | 200 |
| A. philoxeroides | 400 | 4 | 2 | 200 |
| I. aquatica | 400 | 4 | 2 | 200 |
| Minerals and Heavy Metals | Enhydra fluctuans (Raw) | Alternanthera philoxeroides (Raw) | Ipomoea aquatica (Raw) | Enhydra fluctuans (Paper) | Alternanthera philoxeroides (Paper) | Ipomoea aquatica (Paper) |
|---|---|---|---|---|---|---|
| Na | 29.1 ± 0.52 b | 27.7 ± 0.38 c | 28.9 ± 0.55 b | 30.4 ± 0.36 a | 29.3 ± 0.36 ab | 29.2 ± 0.24 b |
| Ca | 397.1 ± 2.49 c | 442.85 ± 4.53 b | 281.9 ± 4.09 d | 433.3 ± 4.94 b | 489.65 ± 5.55 a | 126.8 ± 2.17 e |
| Zn | 6.01 ± 0.27 b | 16.29 ± 0.81 a | 6.11 ± 0.32 b | 5.92 ± 1.21 c | 15.55 ± 0.92 a | 4.55 ± 1.13 d |
| Ni | 1.24 ± 0.12 b | 1.15 ± 0.37 b | 1.39 ± 0.26 a | 0.34 ± 0.08 d | 1.22 ± 0.32 b | 0.77 ± 0.17 c |
| Cu | 2.35 ± 0.25 c | 4.39 ± 0.33 a | 3.74 ± 0.16 ab | 3.57 ± 0.3 b | 4.13 ± 0.17 a | 2.16 ± 0.39 c |
| Pb | Nd | Nd | 0.14 ± 0.03 a | 0.04 ± 0.002 b | Nd | 0.18 ± 0.05 a |
| As | Nd | Nd | 0.02 ± 0.001 a | Nd | Nd | 0.03 ± 0.001 a |
| Cr | 0.53 ± 0.06 b | 0.45 ± 0.03 c | 0.78 ± 0.03 a | 0.57 ± 0.02 b | 0.58 ± 0.04 b | 0.69 ± 0.05 a |
| Amino Acid | Enhydra fluctuans (Raw) | Alternanthera philoxeroides (Raw) | Ipomoea aquatica (Raw) | Enhydra fluctuans (Paper) | Alternanthera philoxeroides (Paper) | Ipomoea aquatica (Paper) |
|---|---|---|---|---|---|---|
| Essential Amino Acid | ||||||
| Thr | Nd | Nd | 24.99 ± 0.67 a | Nd | Nd | 24.99 ± 0.75 a |
| Val | 77.22 ± 1.56 a | Nd | Nd | 77.22 ± 1.23 a | Nd | 27.49 ± 0.71 b |
| Met | 2377.29 ± 13.31 b | 2265.31 ± 7.45 c | Nd | 1741.06 ± 6.5 d | 2616.44 ± 9.62 a | Nd |
| Phe | 2139.22 ± 8.74 b | 948.75 ± 6.28 d | 1914.1 ± 5.87 c | 2735.9 ± 14.67 a | 925.65 ± 7.21 d | 2187.9 ± 13.45 b |
| Lys | Nd | 512.12 ± 4.61 c | 217.54 ± 2.87 d | 557.72 ± 3.98 b | 807.38 ± 3.87 a | Nd |
| His | 1160.95 ± 8.67 c | 753.56 ± 6.7 d | 1243.87 ± 13.48 b | 1972.67 ± 11.7 a | 748.57 ± 4.5 d | 1177.22 ± 8.9 c |
| Leu | 7.2 ± 0.12 d | 886.76 ± 4.52 c | 1388.6 ± 7.43 a | Nd | 1066.99 ± 5.23 b | Nd |
| Non-Essential Amino Acid | ||||||
| Asp | Nd | Nd | Nd | 439.93 ± 3.34 b | Nd | 27.95 ± 4.83 a |
| Ser | 226.28 ± 1.35 a | Nd | Nd | 221.55 ± 1.56 a | Nd | 198.45 ± 0.94 b |
| Glu | 175.2 ± 1.43 c | 645.45 ± 2.21 b | Nd | 73 ± 1.08 d | 1964.97 ± 10.29 a | 148.89 ± 1.32 c |
| Gly | 1275 ± 6.69 d | 1234.74 ± 5.84 d | 6012.12 ± 15.65 a | 2778.75 ± 7.45 c | 821.25 ± 5.53 e | 5609.25 ± 18.75 b |
| Ala | Nd | 8721.25 ± 14.76 a | Nd | Nd | 7195.54 ± 16.28 b | Nd |
| Cys | Nd | Nd | 2327.43 ± 7.59 | Nd | Nd | Nd |
| Tyr | 500.46 ± 4.5 b | Nd | Nd | 3420.82 ± 12.6 a | Nd | 427.16 ± 4.34 c |
| Pro | 2025.98 ± 9.68 | Nd | Nd | Nd | Nd | Nd |
| Arg | 4539.6 ± 15.37 a | Nd | Nd | Nd | Nd | 4250.82 ± 12.43 b |
| Total | 14,558.42 | 15,967.94 | 13,128.67 | 14,018.64 | 16,146.81 | 14,079.43 |
| SL. No | Retention Time | Identified Compounds | Molecular wt. | Molecular Formula | Peak Area (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| EfR | ApR | IaR | EfP | ApP | IaP | |||||
| 1 | 3.053 | N-Methyl-3,4-Methylenedioxyamphetamine | 193 | C11H15NO2 | - | - | 0.05 | - | - | - |
| 2 | 3.174 | N-Methyl-3,4-Methylenedioxyamphetamine | 194 | C11H15NO2 | - | - | - | - | - | 0.03 |
| 3 | 3.697 | Cyclohexyl Propionate | 156 | C9H16CO2 | - | 0.03 | - | - | - | 0.03 |
| 4 | 3.698 | Cyclohexylmethylsilane | 128 | C7H16Si | 0.08 | |||||
| 5 | 3.712 | 2-Ethylbutyl Propionate | 158 | C9H18O2 | 0.1 | |||||
| 6 | 3.818 | Butanoic Acid, 2-Methyl | 102 | C7H12O3 | - | - | - | - | - | 0.1 |
| 7 | 4.287 | Cyclobutanespiro-2′-Bicyclo[1.1.0]Butane-4′-Spirocyclobutane | 134 | C10H14 | - | - | - | - | - | 0.68 |
| 8 | 4.269 | 3-Cyclohexene-1-Acetaldehyde, Apha, 4-Dimethyl | 152 | C15H24O3 | 0.22 | |||||
| 9 | 4.247 | Benzene,1,3-Dimethyl | 106 | C8H10 | - | - | 0.70 | 0.30 | 0.91 | - |
| 8 | 4.289 | Pyrimidine, 4-Methyl- | 94 | C5H6N2 | - | - | 0.49 | - | - | - |
| 10 | 5.69 | Acrylic Acid, (5-Cyclopropylidenepentyl) Ester | 180 | C11H16O2 | - | - | - | 0.24 | - | - |
| 11 | 5.801 | Cyclohexane, 1-Methylene-4-(1-Methylethenyl)- | 136 | C10H16 | - | 0.07 | - | 0.21 | - | |
| 12 | 5.822 | Benzenesulfonamide, N-(4-Hydroxyphenyl)- | 249 | C12H11NO3S | - | - | 0.38 | - | - | - |
| 13 | 5.83 | Trifluoroacetic Acid, 4-Methylcyclohex-3-Enylmethyl Ester. | 222 | C10H13F3O2 | - | - | - | - | - | 1.74 |
| 14 | 5.843 | 1,2-Cyclooctadiene | 108 | C8H12 | - | - | - | 0.91 | 0.58 | 0.75 |
| 15 | 6.217 | 1-Hexene, 3-Methyl-6-Phenyl-4-(1-Phenylethoxy)- | 294 | C21H26O | - | - | 0.22 | 0.15 | - | - |
| 16 | 6.277 | 1-Hexene, 6-Phenyl-4-(1-Phenylethoxy) | 280 | C20H24O | - | - | - | 0.07 | - | - |
| 17 | 6.773 | Benzaldehyde,3-Chloro-5-Methoxy – | 290 | C16H15ClO3 | - | - | - | 0.29 | - | - |
| 18 | 6.907 | Limonene | 136 | C10H16 | - | - | 0.78 | 1.40 | 1.31 | |
| 19 | 7.69 | Azacyclohexan-3-One, 1-Tert-Butyl- | 155 | C9H17O | - | - | - | 0.52 | 1.41 | - |
| 20 | 7.712 | 3-Ethyl-2-Pentadecanone | 254 | C17H34O | - | - | 1.3 | - | - | - |
| 21 | 7.715 | Clofexamide | 284 | C14H21ClN2O2 | - | 0.30 | 3.25 | - | 1.41 | - |
| 22 | 7.72 | 3-Ethyl-2-Pentadecanone | 254 | C17H34O | - | - | 0.68 | - | 1.41 | 2.40 |
| 23 | 7.81 | Carbonic Acid, Decyl Phenyl Ester | 278 | C17H26O3 | - | - | - | - | 5.20 | - |
| 24 | 7.813 | Carbonic Acid | 320 | C20H32O3 | - | - | - | - | 4.19 | - |
| 25 | 9.214 | 2-Propanoic Acid, Ethyl Ester | 100 | C5H8O2 | - | - | - | - | - | 3.83 |
| 26 | 9.425 | 1H-Imidazol-2-Amine | 83 | C3H5N3 | - | 1.59 | - | - | - | - |
| 27 | 9.432 | (E)-4-Oxohex-2-Enal | 112 | C6H8O2 | - | 1.05 | - | 1.70 | - | - |
| 28 | 9.851 | 2-Ethylthiolane, S,S-Dioxide | 148 | C6H12O2S | - | - | - | - | 2.42 | |
| 29 | 9.725 | D-Methionine | 149 | C5H11NO2S | - | - | - | - | 3.22 | - |
| 30 | 9.85 | Benzeneacetaldehyde | 120 | C8H8O | 1.7 | 1.19 | 5.4 | 0.88 | 3.40 | 4.2 |
| 31 | 9.856 | Cyclohexene, (1-Methylpropyl)- | 140 | C10H20 | - | - | - | - | - | 3.24 |
| 32 | 10.476 | Sulfurous Acid, Di(Cyclohexylmethyl) Ester | 274 | C14H26O3S | - | - | - | - | 1.25 | - |
| 33 | 10.482 | Cycloheptanemethanol | 128 | C8H16O | - | - | - | - | 2.78 | - |
| 34 | 10.494 | Z-24-Tritriaconten-2-One | 474 | C33H64O | 0.15 | - | - | - | - | - |
| 35 | 12.063 | 2-Cyclohexen-1-Ol, 4-Amino-5,6-Dimethoxy- | 173 | C8H15NO3 | - | - | 0.57 | - | - | - |
| 36 | 12.117 | 1-Propanamine, N,N-Dimethyl-3-[[1-(Phenylmethyl)-1h-Indazol-3-Yl]Oxy]- | 309 | C19H23N3O | - | - | - | - | - | 0.52 |
| 37 | 12.123 | 1,3-Propanediamine, N,N,N′,N′-Tetramethyl | 130 | C25H54N2O2 | - | - | - | - | - | 1.72 |
| 38 | 12.404 | Succinic Acid, 2-Chloro-5-Methylphenyl Tetradecyl Ester | 438 | C25H39ClO4 | 1.34 | - | - | 0.86 | - | - |
| 39 | 12.431 | Acrylic Acid, Butyldimethylsilylmethyl Ester | 200 | C10H20O2Si | - | 0.9 | - | - | - | - |
| 40 | 12.432 | L-Proline, 2Tms Derivative | 259 | C11H25NO2Si2 | - | - | 1.45 | - | - | - |
| 41 | 12.857 | Succinic Acid, 2-Chloro-5-Methylphenyl Tridecyl Ester | 424 | C24H37ClO4 | 0.86 | - | - | - | - | - |
| 42 | 12.43 | Cyclopentanol, Tms Derivative | 158 | C8H18OSi | - | 0.47 | - | - | - | - |
| 43 | 12.443 | 2-Butenoic Acid, 3-Methoxy-4-Nitro-, Methyl Ester | 174 | C6H9NO5 | - | 0.47 | - | - | - | 0.42 |
| 44 | 12.445 | Dimethylisopropylsilyloxycyclobutane | 172 | C9H20OSi | - | - | 1.45 | - | - | - |
| 45 | 13.04 | Orcinyl Di-Tiglate | 288 | C17H20O4 | - | 0.90 | - | - | - | - |
| 46 | 13.109 | Glycine | 171 | C2H5NO2 | - | 0.95 | - | - | - | - |
| 47 | 13.091 | (E)-4-Oxohex-2-Enal | 112 | C6H8O2 | - | - | - | - | 4.43 | - |
| 48 | 13.124 | Phenyl Angelate, 2-Allyl | 216 | C9H16O2 | - | - | - | - | 0.91 | - |
| 49 | 14.382 | Benzaldehyde, 4-Methyl- | 120 | C13H14N4S | 1.93 | - | - | - | - | - |
| 50 | 14.388 | 2-Butenoic Acid | 86 | C4H6O2 | - | 0.23 | - | - | - | - |
| 51 | 14.398 | Benzaldehyde, 3-Benzyloxy-2-Fluoro- | 230 | C15H13FO3 | - | - | - | - | 1.35 | - |
| 52 | 14.436 | Methyl 4-O-Benzyl-.Alpha.-L-Rhamnopyranoside | 268 | C14H20O5 | - | - | - | - | - | 1.50 |
| 53 | 14.564 | 2-Dimethyl(Octyl)Silyloxybutane | 244 | C14H32OSi | - | - | 3.46 | - | - | - |
| 54 | 14.817 | Phenylalanine | 165 | C9H11NO2 | - | - | - | - | - | 1.75 |
| 55 | 15.027 | 1,3-Bis(Trimethylsilyloxy)Pentane | 248 | C11H22O2Si2 | - | - | 1.39 | - | - | - |
| 56 | 15.072 | 2-Methyl-3-Ethyl-3-Hydroxyglutaric Acid | 406 | C17H38O8Si3 | - | - | 0.72 | - | - | 0.36 |
| 57 | 15.983 | Cis-2,4-Dimethylthiane, S,S-Dioxide | 162 | C7H14O2S | - | - | - | - | - | 1.73 |
| 58 | 15.984 | Cyclobutanone, 2-(1,1-Dimethylethyl)- | 126 | C8H14O | - | - | - | - | 1.04 | |
| 59 | 16.289 | 2-Hydroxy-5-Methylbenzohydrazide | 166 | C8H10N2O2 | - | - | - | - | 0.62 | - |
| 60 | 16.441 | Propanoic Acid, Propyl Ester | 116 | C6H12O2 | - | - | 7.02 | - | - | - |
| 61 | 16.627 | Silane, Dimethoxydimethyl | 120 | C4H12OSi | - | - | 3.45 | - | - | 1.04 |
| 62 | 16.93 | Ethanone, 2-Ethoxy-1,2-Diphenyl | 240 | C16H16O2 | - | 0.46 | - | - | - | - |
| 63 | 16.931 | 4-Acetoxy-3-Methoxystyrene | 192 | C11H12O3 | 0.54 | |||||
| 64 | 16.933 | 4-Hydroxy-2-Methylacetophenone | 150 | C9H10O2 | - | - | - | - | 2.23 | - |
| 65 | 17.284 | 4-Quinolinol | 145 | C9H7NO | - | - | - | - | - | 1.87 |
| 66 | 17.628 | Indole | 117 | C8H7N | - | 0.54 | - | - | - | - |
| 67 | 17.697 | 7-Methyl-4-(1-Pyrrolidinyl)Pyrido[3,2-C]Pyridazine | 214 | C12H14N4 | - | 0.74 | - | - | 1.24 | - |
| 68 | 18.204 | Phenol, 2,6-Dimethoxy-, Acetate | 196 | C10H12O4 | 0.19 | - | - | - | - | - |
| 69 | 18.70 | Carveol | 152 | C10H16O | - | - | 0.80 | - | - | - |
| 70 | 18.861 | Phenanthrene, 3,6-Dimethoxy-9,10-Dimethyl- | 266 | C18H18O2 | 0.96 | - | - | - | - | - |
| 71 | 19.101 | P-Mentha-1,8-Dien-7-Yl Acetate | 194 | C12H18O2 | - | - | - | - | - | 0.14 |
| 72 | 20.209 | 1-Methylene-2b-Hydroxymethyl-3,3-Dimethyl-4b-(3-Methylbut-2-Enyl)-Cy | 222 | C15H26O | 0.13 | - | - | 0.38 | - | - |
| 73 | 20.532 | Piperidine, 2-(Tetrahydro-2-Furanyl)- | 155 | C9H17NO | - | 0.89 | - | - | - | - |
| 74 | 20.563 | 3-Carene | 136 | C10H16 | - | - | - | 0.55 | - | - |
| 75 | 20.606 | Pipradrol | 267 | C18H21NO | 1.26 | 0.88 | - | 1.46 | - | |
| 76 | 20.956 | 1-Bromo-3,7-Dimethyl-2,6-Octadiene | 217 | C10H17Br | - | - | - | - | - | 0.83 |
| 77 | 20.965 | 3,7,11-Trimethyl-3-Hydroxy-6,10-Dodecadien-1-Yl Acetate | 282 | C17H30O3 | - | - | - | 0.38 | - | - |
| 78 | 23.294 | 2(4h)-Benzofuranone, 5,6,7,7a-Tetrahydro-4,4,7a-Trimethyl-, (R)- | 180 | C11H16O2 | - | - | - | - | 1.67 | - |
| 79 | 24.104 | Quinoline, 2-Ethyl- | 157 | C11H11N | 0.11 | 0.19 | 0.41 | - | 0.27 | - |
| 80 | 24.851 | Dl-Alanyl-Dl-Alanine | 482 | C17H28N2O5 | - | 0.16 | - | - | - | - |
| 81 | 26.446 | 1,5-Diphenyl-2H-1,2,4Trizoline-3-Thione- | 253 | C14H11N3S | - | - | - | 0.35 | - | - |
| 82 | 26.869 | Phthalic Acid, Di(3,4- Dimethylphenyl)Ester | 374 | C24H22O4 | - | - | - | 0.29 | - | - |
| 83 | 26.784 | Carbonic Acid,2-Ethylhexyl Nonyl Ester | 300 | C18H36O3 | - | - | 0.56 | - | - | - |
| 84 | 26.791 | Carbonic Acid, Bis(2-Ethylhexyl)Ester | 286 | C18H34O6 | - | - | 1.8 | - | - | - |
| 85 | 27.837 | Imidazole, 2-Cyano- | 230 | C7H10N4O3S | - | - | 0.44 | - | - | - |
| 86 | 27.84 | Borazine,2,4-Dimethyl | 109 | C2H9B2O3 | - | - | 1.22 | - | - | - |
| 87 | 27.845 | 1-Amino-2-Methylpyridinium Iodine | 236 | C6H9IN2 | - | - | 0.67 | - | - | - |
| 88 | 27.96 | Methyl 11-Methyl-Dodecanoate | 228 | C14H28O2 | 1.12 | 4.46 | 6.31 | 1.14 | 2.54 | 2.51 |
| 89 | 27.971 | Tridecanoic Acid,12-Methyl-Methyl Ester | 242 | C15H30O2 | 0.42 | 0.86 | - | 0.89 | - | - |
| 90 | 27.988 | Heptacosanoic Acid, 25-Methyl-,Methyl Ester | 438 | C29H58O2 | 3.14 | 0.23 | - | 0.78 | 1.5 | - |
| 91 | 27.318 | Octadecanoic Acid, 11-Methyl-Methyl Ester | 312 | C20H40O2 | - | - | - | - | - | 1.21 |
| 92 | 27.997 | Undecanoic Acid,10-Methyl-,Methyl Ester | 214 | C13H26O2 | - | 0.29 | 8.05 | - | 0.05 | - |
| 93 | 27.996 | Methyl 8-Methyl-Nonanoate | 186 | C11H22O2 | - | - | - | - | - | 1.21 |
| 94 | 27.998 | Tetradecanoic Acid, 10,13-Dimethyl-,Methyl Ester | 270 | C17H34O2 | 1.6 | - | 0.25 | - | - | - |
| 95 | 29.083 | Dodecanoic Acid | 200 | C12H24O2 | - | 3.31 | - | - | 8.71 | 1.75 |
| 96 | 29.107 | 12-Bromododecanoic Acid | 278 | C12H23BrO2 | 7.93 | - | 1.25 | 7.93 | - | - |
| 97 | 29.102 | N-Hexadecanoic Acid | 256 | C16H32O2 | 1.3 | 5.46 | - | 2.25 | - | 16.07 |
| 98 | 29.569 | Triallylvinylsilane | 178 | C11H18Si | - | - | - | 4.23 | - | - |
| 99 | 29.585 | 6-Hydroxy-4,4,7A-Trimethyl-5,6,7,7A-TetrahydrobenZofuran-2(4H)-One | 196 | C11H16O3 | 8.71 | - | - | 1.89 | - | 3.21 |
| 100 | 29.587 | (R)-3-Methylene-6-(S)-1,2,2-Trimethylcyclopentyl | 204 | C15H24 | - | - | - | - | 8.60 | - |
| 101 | 29.588 | 1-Cyclopentene-1-Methanol, 2-Methyl-5-(1-Methylethyl)- | 154 | C10H18O | 1.17 | - | - | - | 3.61 | - |
| 102 | 29.589 | 2-N-Butyladamantane | 192 | C14H24 | - | - | - | - | - | 1.81 |
| 103 | 29.591 | 5-Methoxy-Cyclooctene | 140 | C9H16O | - | - | - | 1.56 | - | - |
| 104 | 30.304 | Adenine | 135 | C5H5N5 | - | - | 4.48 | - | - | - |
| 105 | 31.44 | PhytylTetradecanoate | 506 | C34H66O2 | 2.30 | - | - | - | 5.29 | - |
| 106 | 31.442 | PhytylPalmitat | 534 | C35H38O2 | 8.60 | - | - | - | - | - |
| 107 | 31.446 | Phytol | 296 | C20H40O | 14.02 | 1.39 | 2.89 | 3.20 | 14.02 | 2.55 |
| 108 | 31.747 | Chloroacetic Acid, Ddec-9-Ynyl Ester | 258 | C14H23ClO2 | - | - | - | 0.64 | - | - |
| 109 | 31.751 | Methyl 10-Trans,12-Cis-Octadecadienoate | 294 | C19H34O2 | 3.19 | 2.54 | 2.33 | - | - | - |
| 110 | 31.755 | 3,10-Dioxatricyclo[4.3.1.0(2,4)]Dec-7-Ene | 138 | C8H10O2 | - | - | - | 2.80 | - | - |
| 111 | 31.756 | Cyclohexene, 1-Pentyl- | 152 | C11H20 | - | - | - | - | - | 0.05 |
| 112 | 31.766 | Cis,cis-2,8-Dimethylspiro[5.5]Undecane | 180 | C13H24 | - | - | - | - | 3.19 | - |
| 113 | 31.954 | N-Propyl 9,12-Octadecadienoate | 322 | C21H38O2 | - | 1.41 | - | - | - | - |
| 114 | 32.16 | 11,14,17-Eicosatrienoic Acid, Methyl Ester | 320 | C21H36O2 | 1.59 | 12.33 | 1.91 | - | 3.81 | 1.66 |
| 115 | 32.163 | 1-Methyl-3-Ethyladamantane | 178 | C13H22 | - | - | - | 1.51 | - | - |
| 116 | 32.175 | Methyl 8,11,14-Heptadecatrienoate | 278 | C18H32O2 | - | - | - | - | 5.29 | - |
| 117 | 32.182 | 9,12,15-Octadecatrienoic Acid, Methyl Ester | 292 | C24H40O4 | - | 7.19 | 11.66 | - | - | - |
| 118 | 32.483 | Methyl 9,12-Heptadecadienoate | 280 | C18H32O2 | - | 1.84 | - | - | - | - |
| 119 | 32.810 | 2,4,7-Trioxabicyclo [4.4.0]Dec-9-Ene,8-Decyloxy-3-Phenyl | 374 | C23H34O4 | 5.29 | - | - | - | - | - |
| 120 | 32.859 | 1-Silacyclohexa-2,5-Diene | 96 | C5H8Si | - | 4.61 | - | - | - | - |
| 121 | 32.876 | Methyl 2-Hydroxy-Octadega Hydroxy-Octadeca-9,12,15-Trienoate | 308 | C19H32O3 | - | - | - | - | 4.11 | |
| 122 | 32.893 | 1,4-Dimethyladamantane | 164 | C12H20 | - | - | 7.89 | - | - | |
| 123 | 32.906 | ThymylTiglate | 232 | C16H20O3 | 3.81 | - | - | - | - | - |
| 124 | 33.26 | BicycloI [5.1.0]Octane, 8-Methylene- | 122 | C9H14 | - | - | 5.69 | - | - | - |
| 125 | 33.262 | BUTYL 9,12,15-OCTADECATRIENOATE | 334 | C22H38O2 | - | 12.3 | - | - | - | - |
| 126 | 33.265 | Methyl 10,13,16-Docosatrienoate | 348 | C23H40O2 | - | - | - | - | - | 13.42 |
| 127 | 35.962 | Kauran-16-Ol | 290 | C20H34O | 5.29 | - | - | 15.43 | - | - |
| 128 | 35.507 | Androst-5-En-4-Onet | 272 | C19H28O | - | - | - | 41.01 | - | - |
| 129 | 36.804 | 1-Cyclohexanone, 2-Methyl-2-(3-Methyl-2-Oxobutyl | 196 | C12H20O2 | - | - | - | - | - | 2.41 |
| 130 | 36.811 | (1,5,5,8-Tetramethyl Bicyclo [4.2.1]NON-9-YL)-Acetic Acid | 238 | C15H26O2 | - | - | - | - | - | 4.89 |
| 131 | 36.988 | Kauran-18-Al, 16-Hydroxy-, (4.Beta.)- | 304 | C20H32O2 | 2.45 | - | - | - | 4.83 | 7.54 |
| 132 | 37.545 | Carbamimidothioic Acid, | 313 | C16H15N3O2S | 9.57 | - | - | - | - | - |
| 133 | 37.299 | Tamoxifin | 371 | C26H29NO | - | 26.80 | - | - | - | - |
| 134 | 38.741 | 1,1′-Biphenyl, 6-[(2-Dimethylamino)Ethyl]-6′-[2-Phenylethyl]- | 419 | C27H36NO3 | - | 4.28 | - | - | - | - |
| 135 | 38.97 | Succinic Acid, 1, 1, 1-Trifluroprop-2-YL(2-Chlorocyclohexyl) Methyle | 344 | C14H20ClF3O4 | - | - | 11.20 | - | - | - |
| 136 | 38.972 | 1-Oxacyclododec-6-Ene-2,10-Dione, 7-Methyl | 210 | C12H18O3 | 1.3 | - | 2.40 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suraiya, S.; Bristy, S.A.; Ali, M.S.; Biswas, A.; Ali, M.R.; Haq, M. A Green Approach to Valorizing Abundant Aquatic Weeds for Nutrient-Rich Edible Paper Sheets Production in Bangladesh. Clean Technol. 2023, 5, 1269-1286. https://doi.org/10.3390/cleantechnol5040064
Suraiya S, Bristy SA, Ali MS, Biswas A, Ali MR, Haq M. A Green Approach to Valorizing Abundant Aquatic Weeds for Nutrient-Rich Edible Paper Sheets Production in Bangladesh. Clean Technologies. 2023; 5(4):1269-1286. https://doi.org/10.3390/cleantechnol5040064
Chicago/Turabian StyleSuraiya, Sharmin, Suraiya Afrin Bristy, Md. Sadek Ali, Anusree Biswas, Md. Rasal Ali, and Monjurul Haq. 2023. "A Green Approach to Valorizing Abundant Aquatic Weeds for Nutrient-Rich Edible Paper Sheets Production in Bangladesh" Clean Technologies 5, no. 4: 1269-1286. https://doi.org/10.3390/cleantechnol5040064
APA StyleSuraiya, S., Bristy, S. A., Ali, M. S., Biswas, A., Ali, M. R., & Haq, M. (2023). A Green Approach to Valorizing Abundant Aquatic Weeds for Nutrient-Rich Edible Paper Sheets Production in Bangladesh. Clean Technologies, 5(4), 1269-1286. https://doi.org/10.3390/cleantechnol5040064







