ZnO for Photoelectrochemical Hydrogen Generation
Abstract
:1. Introduction
2. Principles of PEC Hydrogen Production
- (1)
- Large amount of absorbed photons.
- (2)
- Reducing the level of electron-hole recombination.
- (3)
- Fast charge carriers transportation to the centers of RedOx reactions.
- (4)
- Reducing number of charge carriers trapping zones.
- (5)
- Increase in the surface area of the RedOx centers.
2.1. Requirements for Semiconductors for PEC Reactions
2.2. PEC Cell Photoanode Materials
- (1)
- choosing appropriate materials to serve as photocatalysts with favorable band levels and high absorption coefficients in their bands;
- (2)
- creating extremely crystalline layers of photocatalysts covering the entire electrode, with the correct concentrations of dominant carriers;
- (3)
- stablishing an ohmic contact (to minimize the Schottky barrier) between the semiconductor and any co-catalysts, if they are employed;
- (4)
- development of high-activity co-catalysts for surface electrocatalysis;
- (5)
- Efficient location and maximization of the concentration of reductive/oxidative co-catalysts on photocatalyst surfaces [35].
- (1)
- the conductivity level of the narrow-gap semiconductor being investigated should have a more negative potential than that of the wide-gap semiconductor;
- (2)
- the position of the conduction level in the wide-gap semiconductor must be more negative than the recovery potential;
- (3)
- electron injection should occur rapidly and efficiently;
- (1)
- transport of reactants in the liquid phase to the catalyst’s surface;
- (2)
- adsorption of reactants on the photocatalyst’s surface, activated by photon energy during this stage;
- (3)
- photocatalytic reactions taking place on the catalyst’s surface;
- (4)
- desorption of RedOx reaction products from the photocatalyst’s surface [41].
2.3. Photoactive ZnO
2.4. Modification of ZnO for the Water-Splitting Reaction
- (1)
- co-catalysts can effectively lower the activation energy or induce an overvoltage, thereby facilitating the release of hydrogen and oxygen on the semiconductor surface;
- (2)
- co-catalysts aid in the separation of electron-hole pairs at the interface between the co-catalyst and the semiconductor;
- (3)
- co-catalysts serve to mitigate the issue of photocorrosion in the semiconductor material [117].
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sathre, R.; Scown, C.D.; Morrow, W.R.; Stevens, J.C.; Sharp, I.D.; Ager, J.W.; Walczak, K.; Houle, F.A.; Greenblatt, J.B. Life-cycle net energy assessment of large-scale hydrogen production via photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 3264–3278. [Google Scholar] [CrossRef]
- Prevot, M.S.; Sivula, K. Photoelectrochemical Tandem Cells for Solar Water Splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Aruchamy, A. Photoelectrochemistry and Photovoltaics of Layered Semiconductors (Physics and Chemistry of Materials with Low-Dimensional Structures, 14); Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Townsend, T.K. Inorganic Metal Oxide Nanocrystal Photocatalysts for Solar Fuel Generation from Water; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- NASA. Semiconductor Photoelectrochemistry-NASA Technical Paper 2088 [Re-Imaged from 2008 Original for Greater Clarity]; NASA: Washington, DC, USA, 2016; Volume 97.
- Lee, S.L.; Chang, C.J. Recent Progress on Metal Sulfide Composite Nanomaterials for Photocatalytic Hydrogen Production. Catalysts 2019, 9, 457. [Google Scholar] [CrossRef]
- Liao, C.H.; Huang, C.W.; Wu, J.C.S. Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Fekete, M.; Riedel, W.; Patti, A.F.; Spiccia, L. Photoelectrochemical Water Oxidation by Screen Printed ZnO Nanoparticle Films: Effect of pH on Catalytic Activity and Stability. Nanoscale 2014, 6, 7585–7593. [Google Scholar] [CrossRef]
- Kedruk, Y.Y.; Baigarinova, G.A.; Gritsenko, L.V.; Cicero, G.; Abdullin, K.A. Facile Low-Cost Synthesis of Highly Photocatalitycally Active Zinc Oxide Powders. Front. Mater. 2022, 9, 869493. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. PhotocatalyticWater Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Wang, G.M.; Ling, Y.C.; Wang, H.Y.; Lu, X.H.; Li, Y. Chemically modified nanostructures for photoelectrochemical water splitting. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 35–51. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.Z. Hydrogen generation from photoelectrochemical water splitting based on nanomaterials. Laser Photonics Rev. 2010, 4, 517–528. [Google Scholar] [CrossRef]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Nano Today 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry. Science 1980, 207, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Currao, A. Photoelectrochemical water splitting. Chimia 2007, 61, 815–819. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Lin, Y.J.; Yuan, G.B.; Liu, R.; Zhou, S.; Sheehan, S.W.; Wang, D.W. Semiconductor nanostructure-based photoelectrochemical water splitting: A brief review. Chem. Phys. Lett. 2011, 507, 209–215. [Google Scholar] [CrossRef]
- Wang, X.D.; Estrade, S.; Lino, Y.J.; Yu, F.; Lopez-Conesa, L.; Zhou, H.; Gurramb, S.K.; Peiro, F.; Fan, Z.Y.; Shen, H.; et al. Enhanced Photoelectrochemical Behavior of H-TiO2 Nanorods Hydrogenated by Controlled and Local Rapid Thermal Annealing. Nanoscale Res. Lett. 2017, 12, 336. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.S.; Zhang, L.; Zhang, J.J.; Wilkinson, D.P. Nano-architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.X.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells (vol 110, pg 6446, 2010). Chem. Rev. 2011, 111, 5815. [Google Scholar] [CrossRef]
- Maeda, K. Z-Scheme Water Splitting Using Two Different Semiconductor Photocatalysts. Acs Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 surfaces-principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Bard, A.J.; Memming, R.; Miller, B. Terminology in semiconductor electrochemistry and photoelectrochemical energy-conversion-(recommendations 1991). Pure Appl. Chem. 1991, 63, 569–596. [Google Scholar] [CrossRef]
- Fujishima, A. Electrochemical Evidence for the Mechanism of the Primary Stage of Photosynthesis; Honda, K., Ed.; Bulletin of the chemical Society of Japan: Tokyo, Japan, 1971; Volume 44, pp. 1148–1150. [Google Scholar]
- Maeda, K.; Domen, K. New non-oxide photocatalysts designed for overall water splitting under visible light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Wolcott, A.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.P.; Zhang, J.Z. Photoelectrochemical Study of Nanostructured ZnO Thin Films for Hydrogen Generation from Water Splitting. Adv. Funct. Mater. 2009, 19, 1849–1856. [Google Scholar] [CrossRef]
- Joya, K.S.; Joya, Y.F.; Ocakoglu, K.; van de Krol, R. Water-Splitting Catalysis and Solar Fuel Devices: Artificial Leaves on the Move. Angew. Chem. Int. Ed. 2013, 52, 10426–10437. [Google Scholar] [CrossRef]
- Kudo, A. Photocatalyst materials for water splitting. Catal. Surv. Asia 2003, 7, 31–38. [Google Scholar] [CrossRef]
- Takanabe, K.; Domen, K. Preparation of Inorganic Photocatalytic Materials for Overall Water Splitting. ChemCatChem 2012, 4, 1485–1497. [Google Scholar] [CrossRef]
- Yerga, R.M.N.; Galvan, M.C.A.; del Valle, F.; de la Mano, J.A.V.; Fierro, J.L.G. Water Splitting on Semiconductor Catalysts under Visible-Light Irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.M.; Huang, Y.B.; Liu, J.; Liu, K.; Wang, Z.J.; Zhao, C.; Qu, S.C.; Wang, Z.G. Engineering the photoelectrochemical behaviors of ZnO for efficient solar water splitting. J. Semicond. 2020, 41, 091702. [Google Scholar] [CrossRef]
- Hieu, H.N.; Nghia, N.V.; Vuong, N.M.; Van Bui, H. Omnidirectional Au-embedded ZnO/CdS core/shell nanorods for enhanced photoelectrochemical water-splitting efficiency. Chem. Commun. 2020, 56, 3975–3978. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. Type-II Heterostructure of ZnO and Carbon Dots Demonstrates Enhanced Photoanodic Performance in Photoelectrochemical Water Splitting. Inorg. Chem. 2020, 59, 6988–6999. [Google Scholar] [CrossRef]
- Iwase, A.; Kudo, A. Photoelectrochemical water splitting using visible-light-responsive BiVO4 fine particles prepared in an aqueous acetic acid solution. J. Mater. Chem. 2010, 20, 7536–7542. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Aroutiounian, V.M.; Arakelyan, V.M.; Shahnazaryan, G.E. Metal oxide photoelectrodes for hydrogen generation using solar radiation-driven water splitting. Sol. Energy 2005, 78, 581–592. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Oxynitride materials for solar water splitting. MRS Bull. 2011, 36, 25–31. [Google Scholar] [CrossRef]
- Das, C.; Roy, P.; Yang, M.; Jha, H.; Schmuki, P. Nb doped TiO2 nanotubes for enhanced photoelectrochemical water-splitting. Nanoscale 2011, 3, 3094–3096. [Google Scholar] [CrossRef]
- Jitputti, J.; Pavasupree, S.; Suzuki, Y.; Yoshikawa, S. Synthesis and photocatalytic activity for water-splitting reaction of nanocrystalline mesoporous titania prepared by hydrothermal method. J. Solid State Chem. 2007, 180, 1743–1749. [Google Scholar] [CrossRef]
- Li, L.; Duan, L.L.; Xu, Y.H.; Gorlov, M.; Hagfeldt, A.; Sun, L.C. A photoelectrochemical device for visible light driven water splitting by a molecular ruthenium catalyst assembled on dye-sensitized nanostructured TiO2. Chem. Commun. 2010, 46, 7307–7309. [Google Scholar] [CrossRef] [PubMed]
- Paracchino, A.; Mathews, N.; Hisatomi, T.; Stefik, M.; Tilley, S.D.; Gratzel, M. Ultrathin films on copper(I) oxide water splitting photocathodes: A study on performance and stability. Energy Environ. Sci. 2012, 5, 8673–8681. [Google Scholar] [CrossRef]
- Galinska, A.; Walendziewski, J. Photocatalytic water splitting over Pt-TiO2 in the presence of sacrificial reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Shankar, K.; Basham, J.I.; Allam, N.K.; Varghese, O.K.; Mor, G.K.; Feng, X.J.; Paulose, M.; Seabold, J.A.; Choi, K.S.; Grimes, C.A. Recent Advances in the Use of TiO2 Nanotube and Nanowire Arrays for Oxidative Photoelectrochemistry. J. Phys. Chem. C 2009, 113, 6327–6359. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of Zinc-Oxide varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Nanto, H.; Minami, T.; Takata, S. Zinc-Oxide thin-film ammonia gas sensors with high-sensitivity and excellent selectivity. J. Appl. Phys. 1986, 60, 482–484. [Google Scholar] [CrossRef]
- Becheri, A.; Durr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Xie, Y.P.; He, Y.P.; Irwin, P.L.; Jin, T.; Shi, X.M. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Z.; Ren, L.L.; Cui, Z.H.; Chen, C.Q.; Pan, H.B.; Chen, J.Z. Ag/ZnO flower heterostructures as a visible-light driven photocatalyst via surface plasmon resonance. Appl. Catal. B-Environ. 2012, 126, 298–305. [Google Scholar] [CrossRef]
- Shanmugam, N.R.; Muthukumar, S.; Prasad, S. A review on ZnO-based electrical biosensors for cardiac biomarker detection. Future Sci. OA 2017, 3, FSO196. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Leung, K.T. Vertical growth of two-dimensional zinc oxide nanostructures on ITO-coated glass: Effects of deposition temperature and deposition time. J. Phys. Chem. C 2008, 112, 1357–1364. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Kang, Z.H. 3D Branched ZnO Nanowire Arrays Decorated with Plasmonic Au Nanoparticles for High-Performance Photoelectrochemical Water Splitting. Acs Appl. Mater. Interfaces 2014, 6, 4480–4489. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chang, S.J. Doped ZnO 1D Nanostructures: Synthesis, Properties, and Photodetector Application. Small 2014, 10, 4562–4585. [Google Scholar] [CrossRef]
- Bai, X.; Yi, L.; Liu, D.L.; Nie, E.Y.; Sun, C.L.; Feng, H.H.; Xu, J.J.; Jin, Y.; Jiao, Z.F.; Sun, X.S. Electrodeposition from ZnO nano-rods to nano-sheets with only zinc nitrate electrolyte and its photoluminescence. Appl. Surf. Sci. 2011, 257, 10317–10321. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Dai, C.H.; Liang, Q.M.; Yang, P.; Yang, H.H.; Yan, J.H. Constructing ZnO/ZnCr2O4@TiO2-NTA Nanocomposite for Photovoltaic Conversion and Photocatalytic Hydrogen Evolution. J. Electron. Mater. 2019, 48, 1724–1729. [Google Scholar] [CrossRef]
- Huang, M.C.; Wang, T.H.; Wu, B.J.; Lin, J.C.; Wu, C.C. Anodized ZnO nanostructures for photoelectrochemical water splitting. Appl. Surf. Sci. 2016, 360, 442–450. [Google Scholar] [CrossRef]
- Lin, Y.G.; Hsu, Y.K.; Chen, Y.C.; Chen, L.C.; Chen, S.Y.; Chen, K.H. Visible-light-driven photocatalytic carbon-doped porous ZnO nanoarchitectures for solar water-splitting. Nanoscale 2012, 4, 6515–6519. [Google Scholar] [CrossRef]
- Goux, A.; Pauporte, T.; Chivot, J.; Lincot, D. Temperature effects on ZnO electrodeposition. Electrochim. Acta 2005, 50, 2239–2248. [Google Scholar] [CrossRef]
- Xu, F.; Lu, Y.N.; Xie, Y.; Liu, Y.F. Controllable morphology evolution of electrodeposited ZnO nano/micro-scale structures in aqueous solution. Mater. Des. 2009, 30, 1704–1711. [Google Scholar] [CrossRef]
- Pauporte, T.; Lincot, D. Hydrogen peroxide oxygen precursor for zinc oxide electrodeposition II-Mechanistic aspects. J. Electroanal. Chem. 2001, 517, 54–62. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Yacoubi-Ouslim, A.; Wang, G.Y. Structure study of electrodeposited ZnO nanowires. Microelectron. J. 2005, 36, 625–628. [Google Scholar] [CrossRef]
- Chettah, H.; Abdi, D. Effect of the electrochemical technique on nanocrystalline ZnO electrodeposition, its structural, morphological and photoelectrochemical properties. Thin Solid Film. 2013, 537, 119–123. [Google Scholar] [CrossRef]
- Xu, L.F.; Guo, Y.; Liao, Q.; Zhang, J.P.; Xu, D.S. Morphological control of ZnO nanostructures by electrodeposition. J. Phys. Chem. B 2005, 109, 13519–13522. [Google Scholar] [CrossRef]
- Fahoume, M.; Maghfoul, O.; Aggour, M.; Hartiti, B.; Chraibi, F.; Ennaoui, A. Growth and characterization of ZnO thin films prepared by electrodeposition technique. Sol. Energy Mater. Sol. Cells 2006, 90, 1437–1444. [Google Scholar] [CrossRef]
- Lupan, O.; Guerin, V.M.; Tiginyanu, I.M.; Ursaki, V.V.; Chow, L.; Heinrich, H.; Pauporte, T. Well-aligned arrays of vertically oriented ZnO nanowires electrodeposited on ITO-coated glass and their integration in dye sensitized solar cells. J. Photochem. Photobiol. A-Chem. 2010, 211, 65–73. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yuan, Y.; Liang, L.H.; Cheng, Y.X.; Shi, G.Y.; Jin, L.T. Preparation and photoelectrocatalytic activity of ZnO nanorods embedded in highly ordered TiO2 nanotube arrays electrode for azo dye degradation. J. Hazard. Mater. 2008, 158, 517–522. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Chen, X.Y.; Leung, Y.H.; Ng, A.M.C. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, B.; Demchenko, D.O.; Zheng, H.; Hughes, S.M.; Merkle, M.G.; Dahmen, U.; Wang, L.W.; Alivisatos, A.P. Selective Facet Reactivity during Cation Exchange in Cadmium Sulfide Nanorods. J. Am. Chem. Soc. 2009, 131, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Synthesis of ultrathin CdS nanosheets as efficient visible-light-driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.Y.; Mao, C.B.; Gao, X.X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef]
- Mews, A.; Eychmuller, A.; Giersig, M.; Schooss, D.; Weller, H. Preparation, Characterization, and Photophysics of the Quantum-Dot Quantum-Well System CdS/HgS/CdS. J. Phys. Chem. 1994, 98, 934–941. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P. Alternate synthetic strategy for the preparation of CdS nanoparticles and its exploitation for water splitting. Int. J. Hydrogen Energy 2006, 31, 891–898. [Google Scholar] [CrossRef]
- Henglein, A. Photo-Degradation and Fluorescence of Colloidal-Cadmium Sulfide in Aqueous-Solution. Berichte Bunsenges. Für Phys. Chem. 1982, 86, 301–305. [Google Scholar] [CrossRef]
- Yin, Y.; Jin, Z.; Hou, F. Enhanced solar water-splitting efficiency using core/sheath heterostructure CdS/TiO2 nanotube arrays. Nanotechnology 2007, 18, 495608. [Google Scholar] [CrossRef]
- Li, C.L.; Yuan, J.A.; Han, B.Y.; Jiang, L.; Shangguan, W.F. TiO2 nanotubes incorporated with CdS for photocatalytic hydrogen production from splitting water under visible light irradiation. Int. J. Hydrogen Energy 2010, 35, 7073–7079. [Google Scholar] [CrossRef]
- Shangguan, W.F.; Yoshida, A. Photocatalytic hydrogen evolution from water on nanocomposites incorporating cadmium sulfide into the interlayer. J. Phys. Chem. B 2002, 106, 12227–12230. [Google Scholar] [CrossRef]
- Xu, J.; Cao, X.J. Characterization and mechanism of MoS2/CdS composite photocatalyst used for hydrogen production from water splitting under visible light. Chem. Eng. J. 2015, 260, 642–648. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, H.; Lou, S.; Ding, J.; Zhang, D.; Zhu, H.X.; Fan, T.X. Butterfly wing architecture assisted CdS/Au/TiO2 Z-scheme type photocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 8244–8253. [Google Scholar] [CrossRef]
- Matsumura, M.; Furukawa, S.; Saho, Y.; Tsubomura, H. Cadmium-Sulfide Photocatalyzed Hydrogen-Production from Aqueous-Solutions of Sulfite-Effect of Crystal-Structure and Preparation Method of the Catalyst. J. Phys. Chem. 1985, 89, 1327–1329. [Google Scholar] [CrossRef]
- Tak, Y.; Hong, S.J.; Lee, J.S.; Yong, K. Fabrication of ZnO/CdS core/shell nanowire arrays for efficient solar energy conversion. J. Mater. Chem. 2009, 19, 5945–5951. [Google Scholar] [CrossRef]
- Xing, C.J.; Zhang, Y.J.; Yan, W.; Guo, L.J. And structure-controlled solid solution of Cd1-xZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrogen Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Wang, X.W.; Liu, G.; Lu, G.Q.; Cheng, H.M. Stable photocatalytic hydrogen evolution from water over ZnO-CdS core-shell nanorods. Int. J. Hydrogen Energy 2010, 35, 8199–8205. [Google Scholar] [CrossRef]
- Lin, H.L.; Wei, L.; Wu, C.C.; Chen, Y.X.; Yan, S.S.; Mei, L.M.; Jiao, J. High-Performance Self-powered Photodetectors Based on ZnO/ZnS Core-Shell Nanorod Arrays. Nanoscale Res. Lett. 2016, 11, 420. [Google Scholar] [CrossRef]
- Hong, E.; Choi, T.; Kim, J.H. Application of content optimized ZnS-ZnO-CuS-CdS heterostructured photocatalyst for solar water splitting and organic dye decomposition. Korean J. Chem. Eng. 2015, 32, 424–428. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. ZnO@CdS heterostructures: An efficient photoanode for photoelectrochemical water splitting. New J. Chem. 2019, 43, 7001–7010. [Google Scholar] [CrossRef]
- Ma, D.D.; Shi, J.W.; Zou, Y.J.; Fan, Z.Y.; Ji, X.; Niu, C.M. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. Acs Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef]
- An, C.H.; Peng, S.N.; Sun, Y.G. Facile Synthesis of Sunlight-Driven AgCl:Ag Plasmonic Nanophotocatalyst. Adv. Mater. 2010, 22, 2570–2574. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.A.; Brongersma, M.L.; Kik, P.G.; Meltzer, S.; Requicha, A.A.G.; Atwater, H.A. Plasmonics-A route to nanoscale optical devices. Adv. Mater. 2001, 13, 1501–1505. [Google Scholar] [CrossRef]
- Brolo, A.G. Plasmonics for future biosensors. Nat. Photonics 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Bakranov, N.; Aldabergenov, M.; Ibrayev, N.; Abdullin, K.; Kudaibergenov, S. The Study of Photoelectrochemical Water Splitting by ZnO Nanostructures and ZnO/Ag Nanocomposites. In Proceedings of the 7th IEEE International Conference Nanomaterials-Application and Properties (NAP), Odessa, Ukraine, 10–15 September 2017. [Google Scholar]
- Zhong, C.; Shang, Z.C.; Zhao, C.X.; Luo, H.A.; Cao, Y.; Yan, D.J.; You, K.Y. Co-Catalyst Ti3C2TX MXene-Modified ZnO Nanorods Photoanode for Enhanced Photoelectrochemical Water Splitting. Top. Catal. 2023, 66, 12–21. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Chen, C.J.; Cheng, L.C.; Wu, P.C.; Cheng, B.H.; Ho, Y.Z.; Tseng, M.L.; Hsu, Y.Y.; Chan, T.S.; et al. Plasmon Inducing Effects for Enhanced Photoelectrochemical Water Splitting: X-ray Absorption Approach to Electronic Structures. ACS Nano 2012, 6, 7362–7372. [Google Scholar] [CrossRef]
- Moakhar, R.S.; Kushwaha, A.; Jalali, M.; Goh, G.K.L.; Dolati, A.; Ghorbani, M. Enhancement in solar driven water splitting by Au-Pd nanoparticle decoration of electrochemically grown ZnO nanorods. J. Appl. Electrochem. 2016, 46, 819–827. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, Y.; Zhao, J.L.; Wang, S.G.; Li, Y.D.; Dai, H.T.; Sun, X.W. Advanced three-component ZnO/Ag/CdS nanocomposite photoanode for photocatalytic water splitting. J. Power Sources 2014, 269, 466–472. [Google Scholar] [CrossRef]
- Zhang, C.L.; Shao, M.F.; Ning, F.Y.; Xu, S.M.; Li, Z.H.; Wei, M.; Evans, D.G.; Duan, X. Au nanoparticles sensitized ZnO nanorod@nanoplatelet core-shell arrays for enhanced photoelectrochemical water splitting. Nano Energy 2015, 12, 231–239. [Google Scholar] [CrossRef]
- Ma, C.H.; Liu, Z.F.; Tong, Z.F.; Han, C.C.; Cai, Q.J. Plasmonic Ag nanoparticles and p-type CuO-modified ZnO nanorods for efficient photoelectrochemical water splitting. Appl. Phys. A Mater. Sci. Process. 2019, 125, 451. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Feng, S.; Yang, W.S.; Zhu, Y.; Zhao, Y.Y.; Liu, Z.Y.; Yang, H.B.; Fu, W.Y. Au/CdS Core-Shell Sensitized Actinomorphic Flower-Like ZnO Nanorods for Enhanced Photocatalytic Water Splitting Performance. Nanomaterials 2021, 11, 233. [Google Scholar] [CrossRef]
- Sreedhar, A.; Reddy, I.N.; Ta, Q.T.H.; Namgung, G.; Noh, J.S. Plasmonic Ag nanowires sensitized ZnO flake-like structures as a potential photoanode material for enhanced visible light water splitting activity. J. Electroanal. Chem. 2019, 832, 426–435. [Google Scholar] [CrossRef]
- Qi, H.; Alexson, D.; Glembocki, O.; Prokes, S.M. Plasmonic coupling on dielectric nanowire core-metal sheath composites. Nanotechnology 2010, 21, 085705. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.J.; Yu, J.G.; Cheng, B.; Ong, H.C. Microwave-Hydrothermal Preparation and Visible-Light Photoactivity of Plasmonic Photocatalyst Ag-TiO2 Nanocomposite Hollow Spheres. Chem. Asian J. 2010, 5, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Alexson, D.; Glembocki, O.; Prokes, S.M. The effect of size and size distribution on the oxidation kinetics and plasmonics of nanoscale Ag particles. Nanotechnology 2010, 21, 215706. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.J.; Mokkapati, S.; Catchpole, K.R. Plasmonic light-trapping for Si solar cells using self-assembled, Ag nanoparticles. Prog. Photovolt. 2010, 18, 500–504. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Zheng, L.R.; Zhan, Y.Y.; Lin, X.Y.; Zheng, Q.; Wei, K.M. Ag/ZnO heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef]
- Lin, D.D.; Wu, H.; Zhang, R.; Pan, W. Enhanced Photocatalysis of Electrospun Ag-ZnO Heterostructured Nanofibers. Chem. Mater. 2009, 21, 3479–3484. [Google Scholar] [CrossRef]
- You, J.B.; Zhang, X.W.; Fan, Y.M.; Yin, Z.G.; Cai, P.F.; Chen, N.F. Effects of the morphology of ZnO/Ag interface on the surface-plasmon-enhanced emission of ZnO films. J. Phys. D Appl. Phys. 2008, 41, 205101. [Google Scholar] [CrossRef]
- Mahanti, M.; Basak, D. Highly enhanced UV emission due to surface plasmon resonance in Ag-ZnO nanorods. Chem. Phys. Lett. 2012, 542, 110–116. [Google Scholar] [CrossRef]
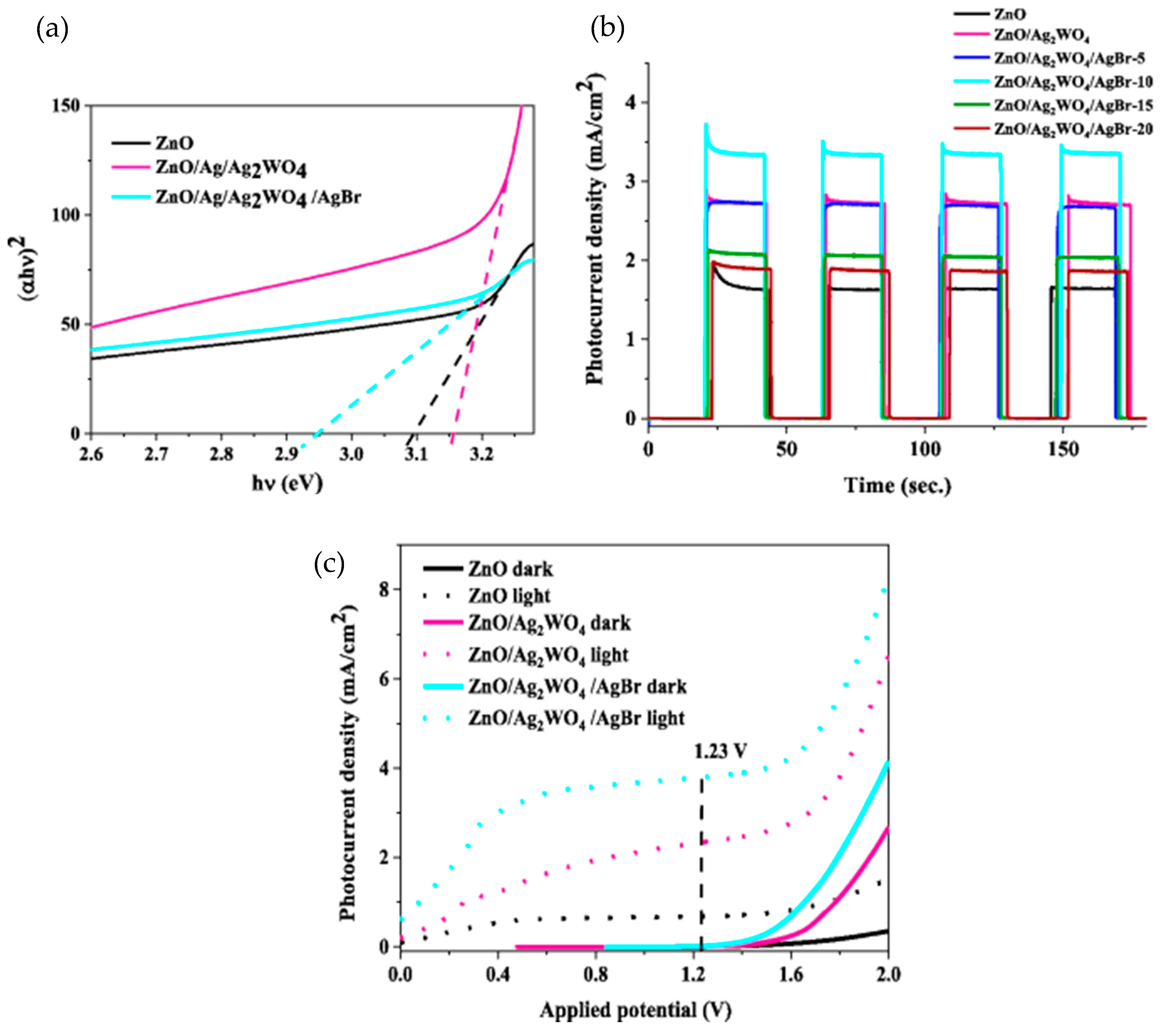
- Adam, R.E.; Pirhashemi, M.; Elhag, S.; Liu, X.J.; Habibi-Yangjeh, A.; Willander, M.; Nur, O. ZnO/Ag/Ag2WO4 photo-electrodes with plasmonic behavior for enhanced photoelectrochemical water oxidation. RSC Adv. 2019, 9, 8271–8279. [Google Scholar] [CrossRef]
- Mustafa, E.; Adam, R.E.; Rouf, P.; Willander, M.; Nur, O. Solar-Driven Photoelectrochemical Performance of Novel ZnO/Ag2WO4/AgBr Nanorods-Based Photoelectrodes. Nanoscale Res. Lett. 2021, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.R.; Zhang, J.; Yu, J.G.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Qiu, K.J.; Bai, H.Y.; Wang, F.G.; Ge, Y.L.; Cui, W.C.; Zheng, G.L.; Cui, J.G.; Fan, W.Q. Ni-MOF in-situ Decorating ZnO photoelectrode for photoelectrochemical water splitting. Funct. Mater. Lett. 2018, 11, 1850085. [Google Scholar] [CrossRef]
- Patil, S.S.; Johar, M.A.; Hassan, M.A.; Waseem, A.; Bagal, I.V.; Shinde, D.E.; Ryu, S.W. Synergic effect of ZnO nanostructures and cobalt phosphate co-catalyst on photoelectrochemical properties of GaN. Mater. Chem. Phys. 2021, 260, 124141. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.W.; Fan, K.; Liu, Z.Q.; Song, B.; Yu, J.G. MOF-Based Transparent Passivation Layer Modified ZnO Nanorod Arrays for Enhanced Photo-Electrochemical Water Splitting. Adv. Energy Mater. 2018, 8, 1800101. [Google Scholar] [CrossRef]
- Kim, M.W.; Joshi, B.; Samuel, E.; Seok, H.; Aldalbahi, A.; Almoigli, M.; Swihart, M.T.; Yoon, S.S. Electrosprayed MnO2 on ZnO nanorods with atomic layer deposited TiO2 layer for photoelectrocatalytic water splitting. Appl. Catal. B Environ. 2020, 271, 118928. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Y.H.; Li, H.; Liang, Z.Q.; Wu, H.H.; Zhang, J.C.; Zeng, H.J.; Geyer, S.M.; Jiang, L. A highly active three-dimensional Z-scheme ZnO/Au/g-C3N4 photocathode for efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 263, 118180. [Google Scholar] [CrossRef]
- Mao, J.Y.; Zhong, H.L.; Liu, X.P.; Qian, Q.R.; Luo, Y.J.; Xiao, L.R.; Xue, H. Electrospinning Preparation of GaN:ZnO Solid Solution Nanorods with Visible-Light-Driven Photocatalytic Activity toward H-2 Production. Appl. Sci. 2021, 11, 10854. [Google Scholar] [CrossRef]
- Pina-Perez, Y.; Aguilar-Martinez, O.; Acevedo-Pena, P.; Santolalla-Vargas, C.E.; Oros-Ruiz, S.; Galindo-Hernandez, F.; Gomez, R.; Tzompantzi, F. Novel ZnS-ZnO composite synthesized by the solvothermal method through the partial sulfidation of ZnO for H-2 production without sacrificial agent. Appl. Catal. B Environ. 2018, 230, 125–134. [Google Scholar] [CrossRef]
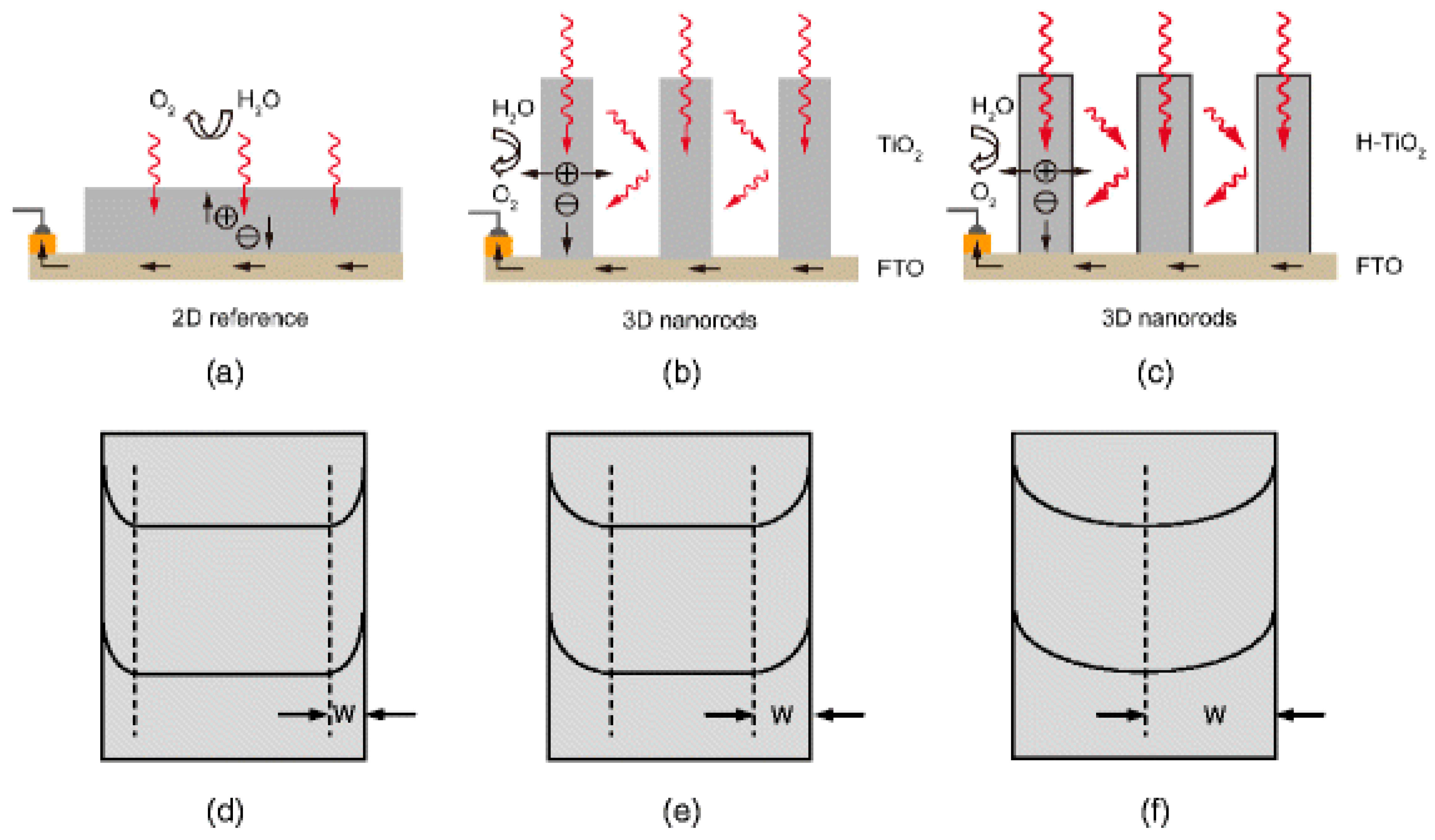






| (ZnO)Catalysts/Co-Catalysts | Morphology | Photocurrent Density (IPCE) without Co-Catalysts | Photocurrent Density (IPCE) with Co-Catalysts | Bias | Electrolyte | Hydrogen Evolution Reaction | Ref. |
|---|---|---|---|---|---|---|---|
| (ZnO)/Ti3C2TX | nanorods/flask | 0.83 mA cm−2 | 1.2 mA/cm2 | 1.23 VRHE | 1 M potassium borate (pH 9.3) | [99] | |
| (ZnO)/Ni-MOF | film | (6.4%) | (11.0%) | 0.5 V | Na2SO4 | [118] | |
| GaN/(ZnO)/CoPi |
|
| 1.23 VRHE | 0.5 M NaOH | [119] | ||
| Ni(OH)2/ZIF-8/(ZnO)/NF | nanorods/branches | 0.92 mA/cm2 | 1.95 mA/cm2 (40.05%) | 1.23 VRHE | 0.1 M KOH | [120] | |
| (ZnO)/MnO2 | nanorods | 0.49 mA/cm2 | 0.95 mA/cm2 | 1.2 VAg/AgCl | 0.5-M Na2SO3 | [121] | |
| (ZnO)/Au/g-C3N4/Pt | 3D urchin-like | 0.3 mA/cm2 | 0 VRHE | 0.2 Na2SO4 | 6.75 μmol/h·cm2 | [122] | |
| GaN:(ZnO)/Rh2−yCryO3 | nanorods | distilled water containing 10 vol% methyl alcohol | 53.44 μmoL·g−1·h−1 | [123] | |||
| ZnS-ZnO | composite | 0.03 M NaClO4 (methanol-water solution) | 247 µmol H2 h−1·g−1 | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakranova, D.; Nagel, D. ZnO for Photoelectrochemical Hydrogen Generation. Clean Technol. 2023, 5, 1248-1268. https://doi.org/10.3390/cleantechnol5040063
Bakranova D, Nagel D. ZnO for Photoelectrochemical Hydrogen Generation. Clean Technologies. 2023; 5(4):1248-1268. https://doi.org/10.3390/cleantechnol5040063
Chicago/Turabian StyleBakranova, Dina, and David Nagel. 2023. "ZnO for Photoelectrochemical Hydrogen Generation" Clean Technologies 5, no. 4: 1248-1268. https://doi.org/10.3390/cleantechnol5040063
APA StyleBakranova, D., & Nagel, D. (2023). ZnO for Photoelectrochemical Hydrogen Generation. Clean Technologies, 5(4), 1248-1268. https://doi.org/10.3390/cleantechnol5040063




