1. Introduction
The surface of carbon nanostructures is a privileged factor for gas detecting and adsorbing gas devices [
1,
2,
3,
4,
5,
6].
In addition, enough implanting of the compounds with transition metals might enhance their adsorbing ability and adjust their adsorbing selectivity as the excellent dopant applicants [
7,
8,
9,
10,
11,
12,
13,
14].
Jayaprakash and coworkers in 2016 have investigated the effect of deficiency on selectivity of nano pristine graphene [
15]. They accomplished the DFT method to depict the redox reactivity of pristine and defected graphene surfaces accompanying the rearranging of Stone–Wales and double vacancy deficiencies in their model, which have indicated changes in the bond length of carbon–carbon in the graphene nanosheet [
15].
In addition, the outreach of a ReaxFF reactive potential has been studied, which is able to explain the chemistry and dynamics of C-condensed phases using the density functional theory method for achieving the equation of state for graphite and the formation energies of defects in graphene [
16]. These calculations were applied to rearrange the parameters of ReaxFFCHO towards a new potential surface as ReaxFFC-2013 based on the DFT method for Stone–Wales transformations in carbon structures [
16].
Sensing and grabbing toxic and harmful gases like CO, CO
2, NO, N
2O, CH
4, SO
2 and H
2S can largely help maintain human health and the ecosystem [
17,
18,
19,
20].
There are different usages of carbon nanocompounds, such as the adsorbing of hydrogen, hazardous compounds, gas and designing the sensor instruments [
21,
22,
23,
24,
25,
26].
Recently, many materials including carbon structures have been designed and applied for the adsorptive removal of environmental pollutant gases [
27,
28,
29]. Thus, it is essential to make high-implement gas detectors for distinguishing these compounds [
30,
31].
Thus, this research wants to investigate the adsorption of hazardous gases of CO2 on the carbon nanographene which has been decorated by transition metals of iron, nickel, zinc, manganese, cobalt, and copper, respectively, using a DFT (density functional theory) approach to discover the adsorbing parameters of the various TM-embedded nanographene surfaces.
2. Materials and Methods
2.1. Adsorptive Removal of Toxic Gases
This article brings up the adsorbing of CO2 onto transition-metals-embedded carbon nanographene. This part defines the first process of bond formation arising during CO2 chemisorption and runs over the consequences gained for adsorbing of CO2 onto the (Fe, Ni, Zn) embedding of carbon nanographene. The resulted data from transition-metal-embedded graphene surface is measured for two toxic gases. Bonding of the CO2 gas molecules to a TM atom on the GR surface can be observed, as first launched by the giving of the lone pair on the C-atom to the unoccupied d orbitals of the TM atom. The donor potency of CO2 in this procedure is recognized as being much smaller, and the stability of the TM-C bond is confirmed to be captured by the back donation of electrons from occupied d orbitals on the metal into unoccupied antibonding π* orbitals on the CO2 gas molecules. It is assumed that the two steps, donation and back donation, intend to augment each other in a cooperative state.
2.2. Langmuir Adsorption Model and Charge Density Analysis
Langmuir adsorbing can be defined through a physical and chemical interaction on the area of the resembling solid state that adsorbs compounds without any interactions with each other, making a mono layer of particles on the solid-state surface.
The Langmuir adsorption equation is the following [
32]:
where
is the fractional occupancy of the adsorbing sites; the ratio of
, the volume of adsorbed gas onto the solid, to
, the volume of a monolayer gas particles coating the entire of the solid surface and totally filled by the adsorbate particle;
is the equilibrium constant and
is the adsorbate’s partial pressure. A continuing monolayer of adsorbate particles coating a resembling solid surface is the basic concept for this adsorbing system [
33,
34,
35,
36,
37].
Different studies have concentrated on the gas-adsorbing susceptibilities of C-nanosurfaces which denote a good accord with the Langmuir adsorbing template. The adsorption of toxic NO gas on the Mn-embedded, Co-embedded and Cu-embedded graphene nanosheets has been approved by the most appropriate Langmuir isotherm, which indicates the nature of chemisorption for the bond distance between
and
molecules and TM-embedded C-nanographene, the equilibrium electron diffusion of the adsorbed particles between the solid and gas phases, and a monolayer feature. The gas molecules of
and
are kept on TM-embedded C-nanographene with Langmuir chemisorption (
Scheme 1).
In fact, the mechanism of the gas-sensing phenomenon in the (Fe, Ni, Zn) embedding of C-nanographene would be due to charge transfer between the surface and CO
2 molecules adsorbed. The changes of charge density analysis in the adsorption process have illustrated that Fe-embedded, Ni-embedded and Zn-embedded C-nanographene show the Mulliken charge of −1.345, −2.087 and −1.416, respectively, before the adsorption of carbon dioxide, and −1.898, −1.763 and −3.221, respectively, after the adsorption of carbon dioxide (
Figure 1 and
Table 1).
The chemical shielding (CS) tensors in principal axes system evaluate the isotropic chemical shielding (σ
iso), anisotropic chemical shielding (σ
aniso) [
38]:
,
.
Therefore, the changes of charge density for the Langmuir adsorption of carbon dioxide on Fe-embedded, Ni-embedded, and Zn-embedded C-nanographene alternatively are │∆Q
Zn-doped│= −1.805 ˃˃│∆Q
Fe-doped│= −0.553 ˃˃˃│∆Q
Ni-doped│= +0.324 (
Figure 1 and
Table 1). The values of the changes of charge density have illustrated a more significant charge transfer for Zn-embedded C-nanographene.
2.3. ONIOM Model
Our own n-layered Integrated molecular Orbital and Molecular mechanics, or ONIOM, merges three theoretical levels that are combined for reducing the sequence of validity as the high, medium and low degrees of theory. In this model, a high-degree level has been performed using the density functional theory insight of the CAM-B3LYP functional, which merges the hybrid qualities of B3LYP and the correction of long-range term [
39] with a 6-31+G (d,p) basis set [
40] for some carbon atoms in nanographene and oxygen atoms in the adsorption zone, and an EPR-III basis set for nitrogen and LANL2DZ for some iron, nickel, zinc, manganese, cobalt and copper atoms through adsorbing CO
2 in the adsorption zone. A medium-degree level has been considered on the other carbon atoms of nanographene in the adsorption zone owing to semi-empirical methodologies. At last, a low-degree level has been depicted on the other iron, nickel, zinc, manganese, cobalt and copper atoms through adsorption of CO
2 with MM2 force fields of molecular mechanic methods
=
(
Scheme 2) [
41].
In other words, the three-degree model of ONIOM leads to exploring a ground order more precisely than the one-degree model, which might treat a medium-sized order exactly as a huge order with admissible validity [
42].
In this article, the structures have been computed using CAM-DFT method on the mechanisms of adsorption of CO
2 by the (Fe, Ni, Zn) embedding of C-nanographene through bonding between transition metals and gas molecules. It has been discovered that the surface binding zone preference of O-atoms of CO
2 in an adsorption zone are greatly influenced by the existence of neighboring atoms in the C-nanographene. The calculated pair distribution functions in the CO
2→Fe/Ni/Zn embedding of C- GR has depicted that the creation of complexes leads to shorter bond lengths of O→Fe (1.90 Å), O→Ni (1.88 Å) and O→Zn (1.98 Å), once balanced to the analogous increment. Furthermore, the graphene sheet has been optimized and the C–C bond length in GR has been calculated at about 1.42 Å, which has the appropriate accord with the experimental amount (
Scheme 2) [
43]. After the doping of TM on the graphene sheet, the bond length of Fe–C was 2.29 Å, O→Ni, 2.01 Å and Zn-C, 2.33 Å, which was larger than corresponding bond length of the TM–TM atom in the mass.
The transition-metal-embedded graphene sheet has been made by a hard system and Z-Matrix format of which a blank line has been positioned. The hard potential energy surface has been exposed at a CAM-B3LYP functional [
44,
45], and concerns LANL2DZ/6-31+ G (d,p) basis sets to appoint frontier molecular orbital, Mulliken charges, nuclear magnetic resonance properties, dipole moment, thermodynamic characteristics and other quantum attributes [
46]. In this research, CO
2 molecules have been adsorbed onto TM-embedded C-nanographene toward the formation of CO
2→Fe/Ni/Zn embedding of C- GR sheets using Gaussian 16 revision C.01 software [
47]. This software is applied for molecular designing, causing automated scientific sequences to simplify more fast and extensive quantum chemistry computations [
47].
3. Results
Based on the computational results, transition metals of manganese, iron, cobalt, nickel, copper and zinc embedded on the nanographene have been investigated as efficient surfaces for the adsorption toxic gas of carbon dioxide (CO2) causing air pollution. These experiments have been accomplished using spectroscopy analysis through some physico-chemical attributes.
3.1. NMR Spectra
The analysis of altering in magnetic properties of surfaces upon interaction with gases can be an appropriate route for detecting the gases [
48,
49,
50]. In fact, the application of magnetic attributes can be replaced with electrical parameters changes owing to the interaction between graphene nanosheet and CO
2 molecules.
Concerning nuclear magnetic resonance spectroscopy, parameters of isotropic (σ
iso) and anisotropy (σ
aniso) shielding tensors of NMR spectroscopy for certain atoms in the active site of CO
2 adsorption on the (Fe, Ni, Zn)-embedded carbon nanographene, through the creation of the binding between gas molecules and the solid surface, have been evaluated using Gaussian 16 revision C.01 software [
47] and represented in
Table 1.
The fluctuation of the magnetic properties of a GR nanosheet doped with transition metals persuades a frequency shielding in the magnetostatic surface wave oscillator that can be ascribable in the existence of various gases.
Considering
Table 1, the degeneracy of NMR graphs via chemical shielding (ppm) for CO
2 adsorption on the (Fe, Ni, Zn)-embedded carbon nanographene has been depicted. The data of NMR spectroscopy in
Table 1 shows approximately the identical chemical shielding behavior of isotropic and anisotropy parameters for CO
2 → Fe @C- GR and CO
2 → Ni @C- GR embedding on the surface of nanographene, respectively.
Therefore, following the changes of magnetic attributes of the graphene sheet doped with transition metals after CO2 adsorption, the TM-doped GR surface can be applied as an appropriately selective magnetic gas sensor CO2 detector.
3.2. Natural Bond Orbital (NBO) Analysis
Natural bond orbital (NBO) analysis has been employed to investigate the intermolecular and intra-molecular interactions [
51] occurring from chemical bonds in the discussed model.
Therefore, NBO analysis of the CO
2 adsorbed on the (Fe, Ni, Zn)-embedded carbon nanographene has illustrated the character of electronic conjugation between bonds in the gas molecules and TM-doped C- GR (
Table 2 and
Figure 2).
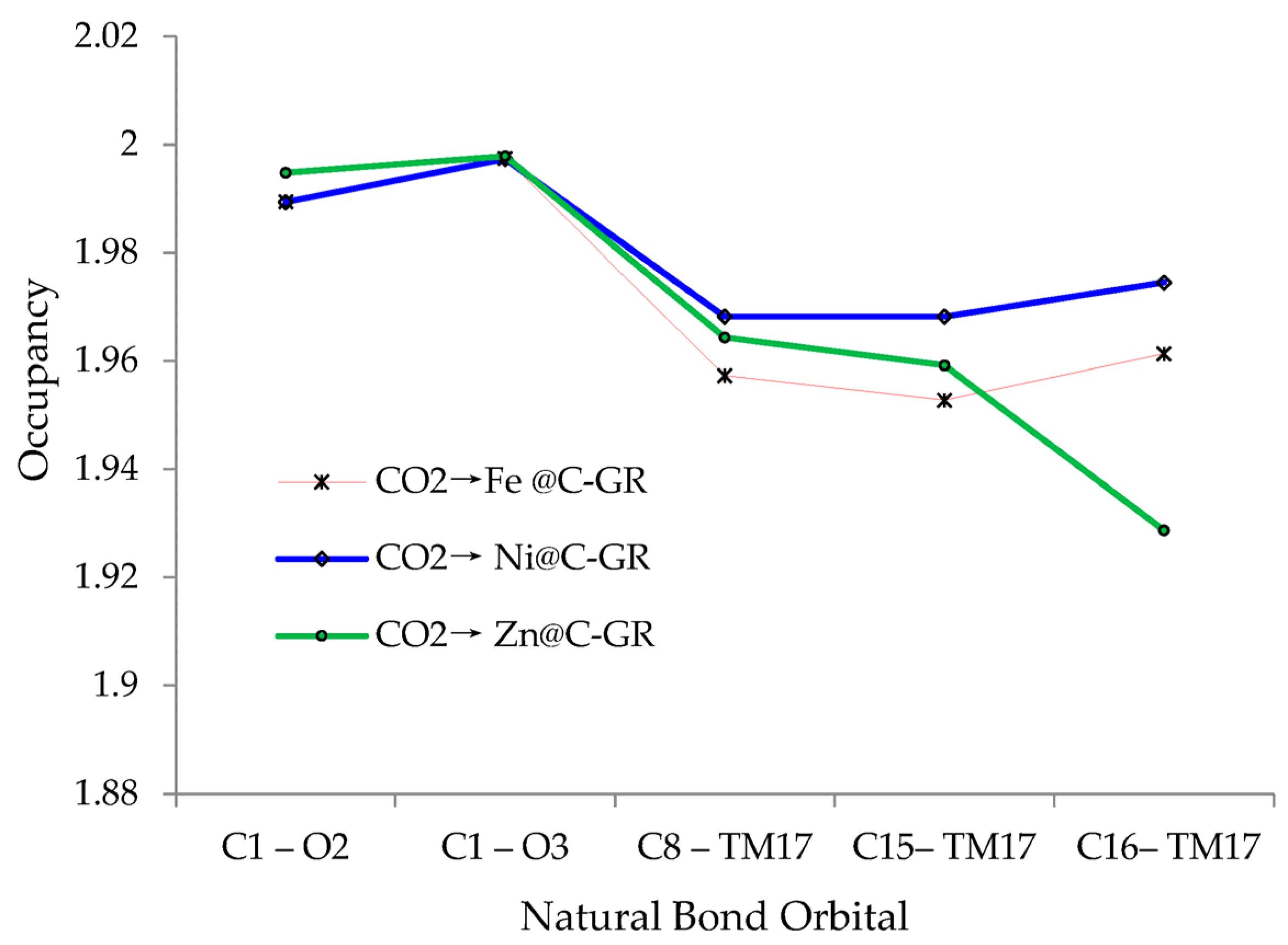
In
Figure 2, it has been observed the fluctuation of occupancy of natural bond orbitals for CO
2→Fe@C- GR, CO
2→Ni@C- GR, CO
2→Zn@C- GR surfaces through the Langmuir adsorption process by indicating the active oxygen atom in carbon dioxide becoming close to the nanographene.
3.3. Thermodynamic Properties and IR Spectroscopy Analysis
The capacity of carbon dioxide (CO
2) adsorption on carbon nanostructures decreases with temperature, which exhibits the exothermic nature of the adsorption process, while the thermodynamic parameters represent low isosteric heats of the adsorption process [
52].
Thermodynamic parameters have been estimated due to Gaussian 16 revision C.01 software using CAM-B3LYP/LANL2DZ, 6-31+G (d,p) through for the adsorption of toxic carbon dioxide (
on the surfaces of the (Fe, Ni, Zn) embedding of nanographene as the gas sensor which can be used as the selective detectors for environmental hazardous gases (
Table 3).
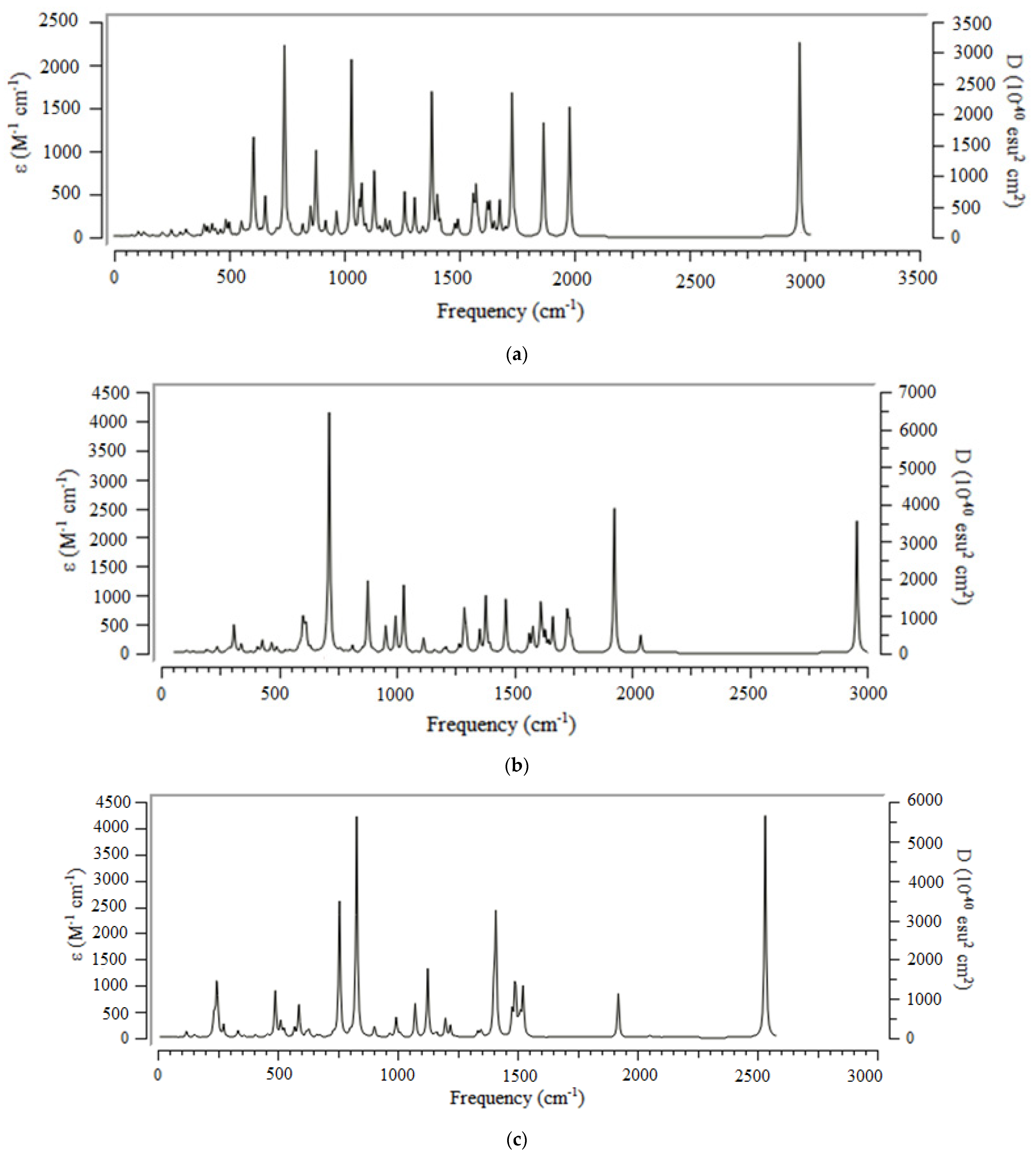
Furthermore, the infrared spectra for the adsorption of CO
2 by (Fe, Ni, Zn) embedded onto C-nanographene have been reported in
Figure 3a–c.
The graphs of
→Fe @C- GR,
→Ni @C- GR, and
→Zn @C- GR have shown the frequency range around 500 cm
−1–3000 cm
−1 with the strongest peaks in IR spectrum around 750 cm
−1 and 3000 cm
−1 Figure 3a–c.
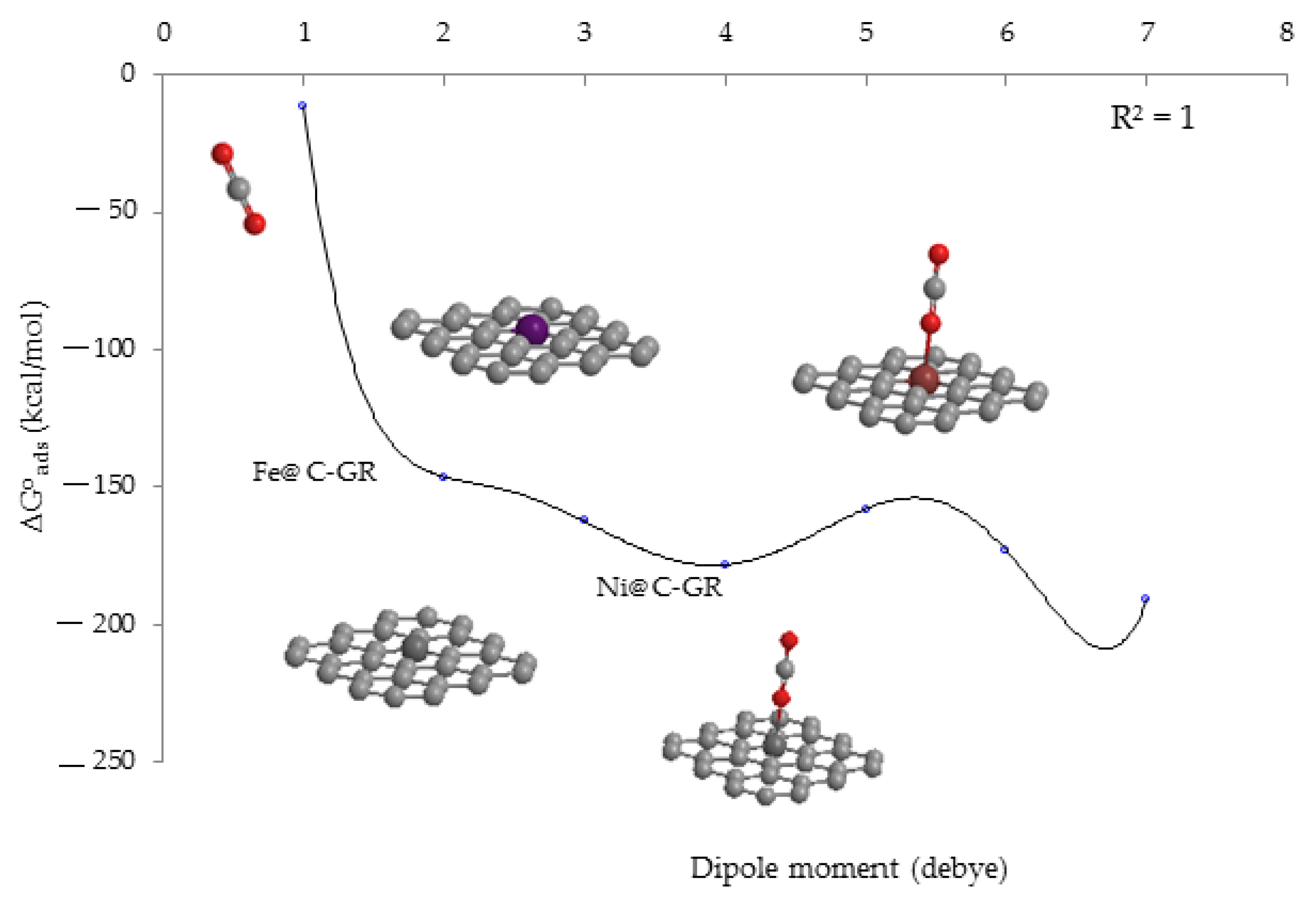
From
Figure 4, it could be understood that the maximum of the Langmuir adsorbing isotherm plots related to
versus a dipole moment may depend on the interactions between the CO
2 and TM-embedded C-nanographene. The order of Gibbs free energy changes for clusters of gas→TM@ C- GR is
˃
˃
.
The change of energy band gaps for the most stable structure of CO
2 molecules adsorbed on the GR sheet doped with TM has been graphed in respect of the corresponding pure sheet due to the thermodynamic reported data in
Table 3 (
Figure 4).
The adsorptive capacity of CO
2 on the TM-embedded C-nanographene is approved by the
amounts:
On the basis of data in
Table 3, it is predicted that the adsorption of CO
2 on the TM-embedded graphene nanosheet must be physico-chemical attributes. As seen in
Figure 4,
(−1.2787 × 10
4, kcal/mol) has the largest gap of Gibbs free energy adsorption with a dipole moment which defines the alterations between the Gibbs free energies of initial compounds (
and
) and product compound (
) through polarizability. In fact, TM-embedded C-nanographene can possess enough efficiency for the adsorption of the toxic gases carbon dioxide and nitrogen dioxide through charge transfer from oxygen atoms to transition metals.
The electric dipole moments from the computations have been summarized in
Table 3. They display that the dipole moment diffused by the transitions metals of Fe, Ni and Zn has been augmented. After the doping of transition metals on the GR nanosheet, the distribution of electrons will influence electric dipole moments, causing long-range interactions between the CO
2 molecules adsorbing on the GR nanosheet concerning the effect of transition metals on the zone growth (
Figure 4).
3.4. Frontier Molecular Orbital’s of HOMO, LUMO and UV-VIS Analysis
The lowest unoccupied molecular orbital (LUMO) energy is generated by ionization and the highest occupied molecular orbital (HOMO) energy is observed by the electron affinity. These parameters have been evaluated for adsorption of carbon dioxide on the (Fe, Ni, Zn) embedding of nanographene as the gas detector in
Table 4. The HOMO (ev), LUMO (ev), and band energy gap (∆E = E
LUMO − E
HOMO) (ev) have exhibited the pictorial explanation of the frontier molecular orbitals and their respective positive and negative areas, which are a significant parameter for discovering the molecular properties of efficient compounds in adsorption of CO
2 on the TM-embedded nanographene surface (
Table 4).
Moreover, for getting more conclusive approving in identifying the compound characteristics of adsorption complexes of CO
2 on the (Fe, Ni, Zn) embedding of C-nanographene surfaces, a series of chemical reactivity parameters, such as chemical potential (µ), electronegativity (χ), hardness (η), softness (ζ) and electrophilicity index (ψ), have been carried out (
Table 4) [
53,
54,
55].
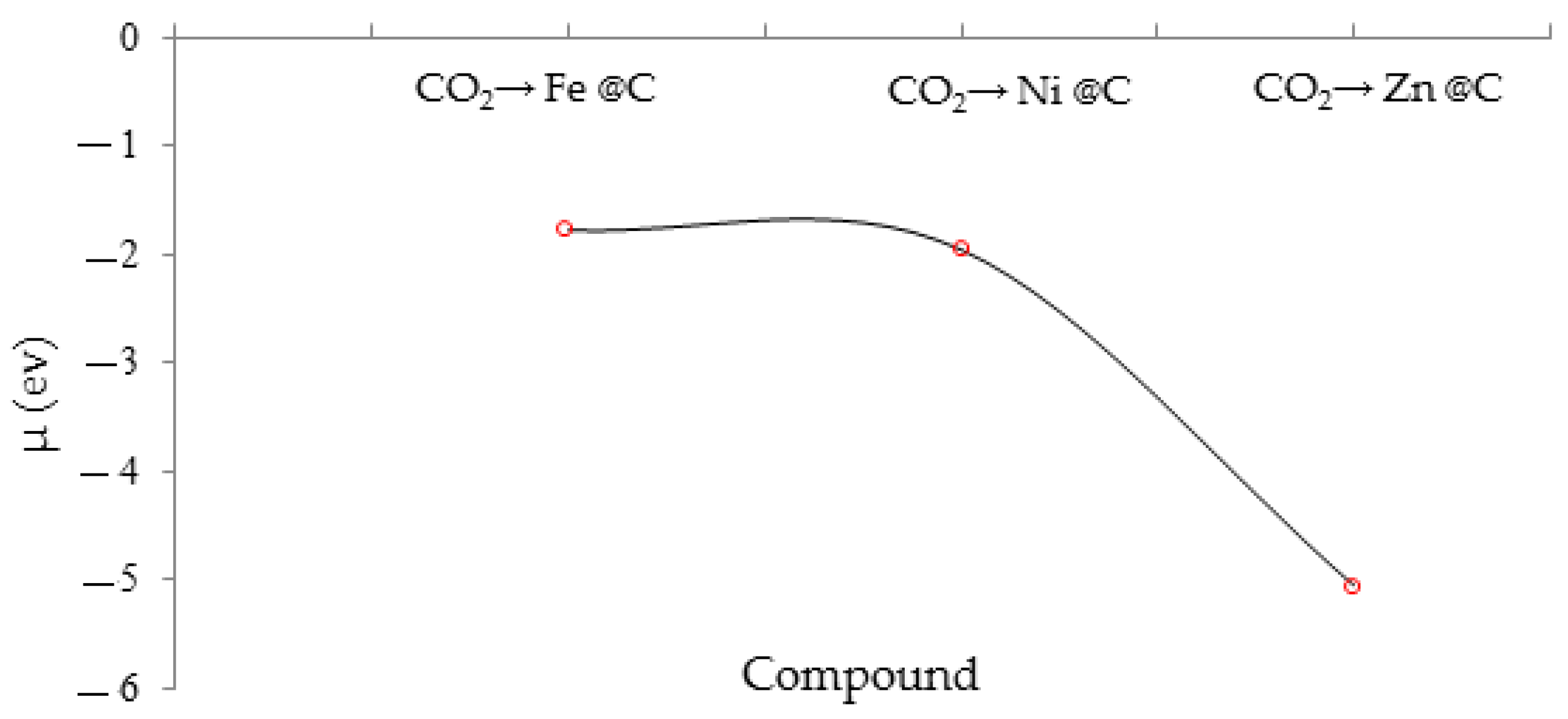
Figure 5 has drawn that chemical potential (µ) for CO
2 adsorption on the surface of a zinc embedding of carbon-nanographene has a considerable minimum potential well. The negative content of the chemical potential (μ) and the positive contents of other factors have remarked an admissible efficiency of scavenging CO
2 by zinc-embedded carbon-nanographene. In fact, the chemical potential defined the increase of CO
2 molecules to the crystal of Zn@C- GR while the number of other particles and the number of unoccupied lattice locations kept constant. As a matter of fact, enhancement of the gas molecules of CO
2 thus involves the simultaneous increasing of a lattice site or unit cell to the crystal of the Zn@C- GR surface. This procedure leads to an augmentation in surface region and, thereby, energy must be spent in generating a new surface of nanographene. In addition, the alliance of particles alters the mass of the crystal and the process is also accomplished versus the mechanical powers.
From
Table 3, the Zn@C- GR surface has indicated the considerable value of stabilized energy compared to Fe@C- GR and Ni@C- GR. In addition, the parameter of chemical potential (μ) has approved the activity of zinc atoms that might form the bonds with the oxygen atoms of CO
2 from functional groups towards the covalent bond of optimized coordination, performing like grapnel sites for increasing the sensitivity and selectivity of the GR nanosheet (
Figure 5).
In this work, the energy gap establishes how toxic gases of CO
2 can be adsorbed on the (Fe, Ni, Zn) embedding of nanographene as the gas sensors with the CAM-B3LYP/LANL2DZ, 6-311+G (2d, p) quantum approach. In addition, frontier molecular orbitals perform an essential function in the optical and electrical factors like ultraviolet and visible spectra [
56].
The energy gap between LUMO and HOMO has recognized the qualifications of molecular electrical transport [
57].
Furthermore, TD-DFT/LANL2DZ, 6-31+G (d,p) calculations with a CAM-B3LYP functional have been accomplished to discern the low-lying excited states of CO
2 can be adsorbed on the (Fe, Ni, Zn) embedding of nanographene. The consequences contain the vertical stimulation energies, oscillator strength and wavelength, which have been introduced in
Figure 6a–c.
Figure 6a–c have shown UV-VIS spectra for CO
2→Fe @C- GR, CO
2→Ni @C- GR, CO
2→Zn @C- GR with maximum adsorption bands between 1000–5000 nm. Moreover, a sharp peak around 2500 nm for CO
2 →TM@C- GR using a CAM-B3LYP functional has been observed (
Figure 6a–c).
4. Conclusions
This article has reported the trends for toxic carbon dioxide (CO2) adsorption on transition metals of iron, nickel and zinc embedding of carbon-nanographene surface.
In particular, the energetic, structural and infrared adsorption characteristics of linearly (atop) CO2 adsorbed on (Fe, Ni, Zn) embedding of C- GR have been discussed. Spin-unrestricted density functional theory (DFT) calculations were applied to verdict the tendency of CO2 adsorption energy of (CO2→ Fe-, CO2→Ni-, CO2→Zn-) embedded on the nanographene sheet and normal mode vibrational frequencies (νCO2) of for clusters composed of Fe, Ni and Zn.
Moreover, the adsorption of CO2 molecules has indicated the spin polarization in GR nanosheets with a magnetic moment of transition metals, exhibiting that the magnetic properties of the TM-doped GR nanosheet has changed.
The effects of the transition metal electronic structure onto the adsorption energy of CO2 toxic gas and how these chemical factors might be related to the catalytic activity of transition-supported metal catalysts that deal with adsorption, and surface diffusion, have been investigated.
Author Contributions
F.M.: Conceptualization and idea, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Writing—original draft preparation, Visualization, Supervision, Project administration. M.M.: Methodology, Software, Formal analysis, Investigation, Data Curation, Writing—review and editing, Visualization, Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
In successfully completing this paper and its research, the authors are grateful to Kastamonu University for their support through the library, the laboratory and scientific websites.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, C.; Wang, Z.; Zhang, S.; Zhang, J.; Li, K. Ab Initio Investigation of the Adsorption of CO2 Molecules on Defect Sites of Graphene Surfaces: Role of Local Vacancy Structures. Materials 2023, 16, 981. [Google Scholar] [CrossRef] [PubMed]
- Mihet, M.; Dan, M.; Lazar, M.D. CO2 Hydrogenation Catalyzed by Graphene-Based Materials. Molecules 2022, 27, 3367. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Deng, W.; Kong, S. Preparation and Adsorption Properties of Graphene-Modified, Pitch-Based Carbon Foam Composites. Polymers 2022, 14, 4455. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Ghani, N.A. CO2 Adsorption Performance on Surface-Functionalized Activated Carbon Impregnated with Pyrrolidinium-Based Ionic Liquid. Processes 2022, 10, 2372. [Google Scholar] [CrossRef]
- Duan, T.; Li, H.; Daukiya, L.; Simon, L.; Leifer, K. Enhanced Ammonia Gas Adsorption through Site-Selective Fluorination of Graphene. Crystals 2022, 12, 1117. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, W.; Wang, J.; Li, X.; Wang, L. Adsorption and Sensing Properties of Formaldehyde on Chemically Modified Graphene Surfaces. Crystals 2022, 12, 553. [Google Scholar] [CrossRef]
- Lisovski, O.; Piskunov, S.; Bocharov, D.; Zhukovskii, Y.F.; Kleperis, J.; Knoks, A.; Lesnicenoks, P. CO2 and CH2 Adsorption on Copper-Decorated Graphene: Predictions from First Principle Calculations. Crystals 2022, 12, 194. [Google Scholar] [CrossRef]
- Yan, H.; Ku, P.-C.; Gan, Z.-Y.; Liu, S.; Li, P. Strain Effects in Gallium Nitride Adsorption on Defective and Doped Graphene: First-Principles Calculations. Crystals 2018, 8, 58. [Google Scholar] [CrossRef]
- Shahriari, S.; Mollaamin, F.; Monajjemi, M. Increasing the Performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)] O2}, xLi2MnO3, yLiCoO2 Composites as Cathode Material in Lithium-Ion Battery: Synthesis and Characterization. Micromachines 2023, 14, 241. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, J.; Jin, Z.; Zhang, D.; Shi, J.; Wei, S. Electric-Field Induced Doping Polarity Conversion in Top-Gated Transistor Based on Chemical Vapor Deposition of Graphene. Crystals 2022, 12, 184. [Google Scholar] [CrossRef]
- Montejo-Alvaro, F.; Martínez-Espinosa, J.A.; Rojas-Chávez, H.; Navarro-Ibarra, D.C.; Cruz-Martínez, H.; Medina, D.I. CO2 Adsorption over 3d Transition-Metal Nanoclusters Supported on Pyridinic N3-Doped Graphene: A DFT Investigation. Materials 2022, 15, 6136. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.-Z.; Hupalo, M.; Lin, H.-Q.; Ho, K.-M.; Tringides, M.C. Metals on Graphene: Interactions, Growth Morphology, and Thermal Stability. Crystals 2013, 3, 79–111. [Google Scholar] [CrossRef]
- Vinogradov, K.Y.; Bulanova, A.V.; Shafigulin, R.V.; Tokranova, E.O.; Zhu, H. Quantum-Chemical Modeling of the Catalytic Activity of Graphene Doped with Metal Phthalocyanines in ORR. Catalysts 2022, 12, 786. [Google Scholar] [CrossRef]
- Canales, M.; Ramírez-De-Arellano, J.M.; Arellano, J.S.; Magaña, L.F. Ab Initio Study of the Interaction of a Graphene Surface Decorated with a Metal-Doped C30 with Carbon Monoxide, Carbon Dioxide, Methane, and Ozone. Int. J. Mol. Sci. 2022, 23, 4933. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, G.K.; Casillas, N.; Astudillo-Sánchez, P.D.; Flores-Moreno, R. Role of Defects on Regioselectivity of Nano Pristine Graphene. J. Phys. Chem. A 2016, 120, 9101–9108. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.G.; van Duin, A.C.T.; Ganesh, P. Development of a ReaxFF Potential for Carbon Condensed Phases and Its Application to the Thermal Fragmentation of a Large Fullerene. J. Phys. Chem. A 2015, 119, 571–580. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Wang, B.; Yang, T.; Yang, B.; Xie, G.; Zhou, Y.; Zhang, S.; Tai, H.; Cai, Z.; et al. Alveolus-Inspired Active Membrane Sensors for Self-Powered Wearable Chemical Sensing and Breath Analysis. ACS Nano 2020, 14, 6067–6075. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Li, X.; He, C.; Lu, Z.; Lu, Z.; Yang, Z.; Wang, Y. C3N monolayers as promising candidates for NO2 sensors. Sens. Actuators B Chem. 2018, 266, 664–673. [Google Scholar] [CrossRef]
- Pacheco, M.; Pacheco, J.; Valdivia, R.; Santana, A.; Tu, X.; Mendoza, D.; Frias, H.; Medina, L.; Macias, J. Green Applications of Carbon Nanostructures produced by Plasma Techniques. MRS Adv. 2017, 2, 2647–2659. [Google Scholar] [CrossRef]
- Joel, E.F.; Lujaniene, G. Progress in Graphene Oxide Hybrids for Environmental Applications. Environments 2022, 9, 153. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O′Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Pikhitsa, P.V.; Jiang, H.; Brown, D.P.; Krasheninnikov, A.V.; Anisimov, A.S.; Queipo, P.; Moisala, A.; Gonzalez, D.; Lientschnig, G.; et al. A novel hybrid carbon material. Nat. Nanotechnol. 2007, 2, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Moisala, A.; Nasibulin, A.G.; Shandakov, S.D.; Jiang, H.; Kauppinen, E.I. On-line detection of single-walled carbon nanotube formation during aerosol synthesis methods. Carbon 2005, 43, 2066–2074. [Google Scholar] [CrossRef]
- Delgado, J.L.; Herranz, M.; Martín, N. The nano-forms of carbon. J. Mater. Chem. 2008, 18, 1417–1426. [Google Scholar] [CrossRef]
- Falcao, E.H.; Wudl, F. Carbon allotropes: Beyond graphite and diamond. J. Chem. Technol. Biotechnol. 2007, 82, 524–531. [Google Scholar] [CrossRef]
- Langenhorst, F.; Campione, M. Ideal and real structures of different forms of carbon, with some remarks on their geological significance. J. Geol. Soc. 2018, 176, 337–347. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent advances in carbon material-based NO2 gas sensors. Sensors Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Jayaprakash, G.K. Pre-post redox electron transfer regioselectivity at the alanine modified nano graphene electrode interface. Chem. Phys. Lett. 2021, 789, 139295. [Google Scholar] [CrossRef]
- Ramirez-De-Arellano, J.M.; Canales, M.; Magaña, L.F. Carbon Nanostructures Doped with Transition Metals for Pollutant Gas Adsorption Systems. Molecules 2021, 26, 5346. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Ghadiri, M.; Chrzanowski, W.; Gan, Y. Scalable Surface Area Characterization by Electrokinetic Analysis of Complex Anion Adsorption. Langmuir 2014, 30, 15143–15152. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Dube, I.; Fedorov, G.; Paranjape, M.; Barbara, P. Gas sensing mechanism of carbon nanotubes: From single tubes to high-density networks. Carbon 2014, 69, 417–423. [Google Scholar] [CrossRef]
- Zhao, J.; Buldum, A.; Han, J.; Lu, J.P. Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar] [CrossRef]
- Mollaamin, F.; Monajjemi, M. Molecular modelling framework of metal-organic clusters for conserving surfaces: Langmuir sorption through the TD-DFT/ONIOM approach. Mol. Simul. 2022, 49, 365–376. [Google Scholar] [CrossRef]
- Bakhshi, K.; Mollaamin, F.; Monajjemi, M. Exchange and Correlation Effect of Hydrogen Chemisorption on Nano V(100) Surface: A DFT Study by Generalized Gradient Approximation (GGA). J. Comput. Theor. Nanosci. 2011, 8, 763–768. [Google Scholar] [CrossRef]
- Mollaamin, F.; Shahriari, S.; Monajjemi, M.; Khalaj, Z. Nanocluster of Aluminum Lattice via Organic Inhibitors Coating: A Study of Freundlich Adsorption. J. Clust. Sci. 2022, 1–16. [Google Scholar] [CrossRef]
- Roderick, A.F.; Kideok, D.K.; Sridhar, K.; James, D.K.; Karl, T.M. Solid-State NMR and Computational Chemistry Study of Mononucleotides Adsorbed to Alumina. Langmuir 2006, 22, 9281–9286. [Google Scholar]
- Yanai, T.; Tew, D.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Lehtola, S. A review on non-relativistic, fully numerical electronic structure calculations on atoms and diatomic molecules. Int. J. Quantum Chem. 2019, 119, e25968. [Google Scholar] [CrossRef]
- Svensson, M.; Humbel, S.; Froese, R.D.J.; Matsubara, T.; Sieber, S.; Morokuma, K. ONIOM: A Multilayered Integrated MO + MM Method for Geometry Optimizations and Single Point Energy Predictions. A Test for Diels−Alder Reactions and Pt(P(t-Bu)3)2 + H2 Oxidative Addition. J. Phys. Chem. 1996, 100, 19357–19363. [Google Scholar] [CrossRef]
- Brandt, F.; Jacob, C.R. Systematic QM Region Construction in QM/MM Calculations Based on Uncertainty Quantification. J. Chem. Theory Comput. 2022, 18, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, S.; Zhang, X.; Xia, Y.; Li, J. Extended Line Defect Graphene Modified by the Adsorption of Mn Atoms and Its Properties of Adsorbing CH4. Nanomaterials 2022, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 3, 785–789. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Ciprian, R.; Torelli, P.; Giglia, A.; Gobaut, B.; Ressel, B.; Vinai, G.; Stupar, M.; Caretta, A.; Ninno, G.; Pincelli, T.; et al. New strategy for magnetic gas sensing. RSC Adv. 2016, 6, 83399–83405. [Google Scholar] [CrossRef]
- Matatagui, D.; Kolokoltsev, O.V.; Qureshi, N.; Mejía-Uriarte, E.V.; Saniger, J.M. A magnonic gas sensor based on magnetic nanoparticles. Nanoscale 2015, 7, 9607–9613. [Google Scholar] [CrossRef]
- Matatagui, D.; Kolokoltsev, O.V.; Qureshi, N.; Mejía-Uriarte, E.V.; Saniger, J.M. Magnonic sensor array based on magnetic nanoparticles to detect, discriminate and classify toxic gases. Sens. Actuators B Chem. 2017, 240, 497–502. [Google Scholar] [CrossRef]
- Tahan, A.; Mollaamin, F.; Monajjemi, M. Thermochemistry and NBO analysis of peptide bond: Investigation of basis sets and binding energy. Russ. J. Phys. Chem. A 2009, 83, 587–597. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Lu, C.; Su, F.; Zeng, W.; Chen, W. Thermodynamics and regeneration studies of CO2 adsorption on multiwalled carbon nanotubes. Chem. Eng. Sci. 2010, 65, 1354–1361. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density Functional Theory of Electronic Structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Politzer, P.; Abu-Awwad, F. A comparative analysis of Hartree-Fock and Kohn-Sham orbital energies. Theor. Chem. Accounts 1998, 99, 83–87. [Google Scholar] [CrossRef]
- Aihara, J.-I. Reduced HOMO−LUMO Gap as an Index of Kinetic Stability for Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 1999, 103, 7487–7495. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric Identification of Organic Compounds, 5th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1981. [Google Scholar]
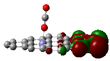
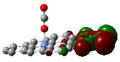
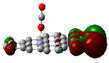
Scheme 1.
IR spectra of molecules which adsorb on transition metal (TM) embedding of C-nanographene.
Scheme 1.
IR spectra of molecules which adsorb on transition metal (TM) embedding of C-nanographene.
Figure 1.
The fluctuation of charge distribution versus atom number for adsorbing CO2 on the (Fe, Ni, Zn)@C- GR.
Figure 1.
The fluctuation of charge distribution versus atom number for adsorbing CO2 on the (Fe, Ni, Zn)@C- GR.
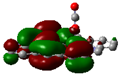
Scheme 2.
Langmuir adsorbing of CO2 as the toxic gas pollutant onto (Fe, Ni, Zn) embedding of C-nanographene graphene on optimized structure due to three-degree layered of high, medium and low levels of ONIOM model.
Scheme 2.
Langmuir adsorbing of CO2 as the toxic gas pollutant onto (Fe, Ni, Zn) embedding of C-nanographene graphene on optimized structure due to three-degree layered of high, medium and low levels of ONIOM model.
Figure 2.
Occupancy fluctuation extracted of NBO method for bond lengths of C-O, C-TM (Fe, Ni, Zn) through adsorption of CO2 TM@C-NG surfaces.
Figure 2.
Occupancy fluctuation extracted of NBO method for bond lengths of C-O, C-TM (Fe, Ni, Zn) through adsorption of CO2 TM@C-NG surfaces.
Figure 3.
Alterations of frequency (cm−1) in the IR spectra for (a) →Fe @C- GR, (b) →Ni @C- GR, (c) →Zn @C- GR as the selective gas sensors.
Figure 3.
Alterations of frequency (cm−1) in the IR spectra for (a) →Fe @C- GR, (b) →Ni @C- GR, (c) →Zn @C- GR as the selective gas sensors.
Figure 4.
The changes of Gibbs free energy (kcal/mol) versus dipole moment (Debye) for adsorption of CO2 on the (Fe, Ni, Zn) embedding of C-nanographene surfaces.
Figure 4.
The changes of Gibbs free energy (kcal/mol) versus dipole moment (Debye) for adsorption of CO2 on the (Fe, Ni, Zn) embedding of C-nanographene surfaces.
Figure 5.
The chemical potential (µ) of CO2 adsorption onto the crystal of Zn@C- GR.
Figure 5.
The chemical potential (µ) of CO2 adsorption onto the crystal of Zn@C- GR.
Figure 6.
UV-VIS spectra for (a) →Fe @C- GR, (b) →Ni @C- GR, and (c) →Zn @C- GR as the selective gas sensors.
Figure 6.
UV-VIS spectra for (a) →Fe @C- GR, (b) →Ni @C- GR, and (c) →Zn @C- GR as the selective gas sensors.
Table 1.
Calculated NMR chemical shielding tensors and Mullikan charge (Q) for some atoms in the active site of CO2 gas adsorption on the (Fe, Ni, Zn)-embedded C-nanographene.
Table 1.
Calculated NMR chemical shielding tensors and Mullikan charge (Q) for some atoms in the active site of CO2 gas adsorption on the (Fe, Ni, Zn)-embedded C-nanographene.
| CO2→Fe @C- GR | | CO2→Ni@C- GR | | CO2→Zn@C- GR | |
|---|
| Atom | σiso | σaniso | Q | Atom | σiso | σaniso | Q | Atom | σiso | σaniso | Q |
|---|
| C1 | 148.52 | 196.31 | 0.5999 | C1 | 178.24 | 145.52 | 0.5848 | C1 | 123.10 | 234.73 | 1.1957 |
| O2 | 321.65 | 183.04 | −0.2528 | O2 | 398.64 | 64.18 | −0.2501 | O2 | 295.29 | 190.97 | −0.6366 |
| C8 | 100.76 | 347.30 | −0.4878 | C8 | 296.58 | 574.94 | −0.4852 | C8 | 273.90 | 3225.40 | −0.8329 |
| C10 | 237.59 | 173.64 | −0.1115 | C10 | 41.45 | 461.74 | −0.0808 | C10 | 292.83 | 1382.14 | −0.3038 |
| C14 | 240.60 | 485.27 | 0.0250 | C14 | 68.33 | 371.25 | 0.0338 | C14 | 463.91 | 927.12 | 0.0904 |
| C15 | 237.40 | 341.12 | −0.5742 | C15 | 94.84 | 252.27 | −0.5501 | C15 | 534.07 | 1981.05 | −0.9951 |
| C16 | 183.26 | 383.67 | −0.5461 | C16 | 25.48 | 160.68 | −0.6144 | C16 | 1163.51 | 3304.01 | −0.9772 |
| Fe 17 | 21,368.96 | 19,790.99 | 1.8981 | Ni17 | 7265.00 | 43,068.15 | 1.7629 | Zn 17 | 1374.46 | 528.33 | 3.2211 |
| C18 | 40.19 | 130.77 | −0.0049 | C18 | 304.39 | 798.78 | 0.0153 | C18 | 233.95 | 593.95 | −0.2791 |
| C19 | 739.86 | 1981.10 | −0.0750 | C19 | 1620.91 | 4749.68 | −0.0591 | C19 | 5571.71 | 12,698.28 | 0.1942 |
| C23 | 93.25 | 179.03 | −0.0816 | C23 | 68.71 | 216.09 | −0.0767 | C23 | 127.04 | 228.84 | −0.2287 |
| C25 | 267.56 | 385.81 | 0.0005 | C25 | 130.48 | 138.07 | 0.0295 | C25 | 630.19 | 1178.08 | 0.0828 |
| O33 | 284.15 | 153.28 | −0.1321 | O33 | 343.76 | 111.69 | −0.1407 | O33 | 293.15 | 160.22 | −0.4784 |
Table 2.
NBO analysis for adsorbing CO2 on the (Fe, Ni, Zn)@C- GR and (Mn, Co, Cu)@C- GR, respectively.
Table 2.
NBO analysis for adsorbing CO2 on the (Fe, Ni, Zn)@C- GR and (Mn, Co, Cu)@C- GR, respectively.
| NO→TM-Embedded/Gr Nanosheet | Bond Orbital | Occupancy | Hybrids |
|---|
| →Fe @C- GR | BD (1) C1–O2 | 1.9895 | 0.6388 (sp1.03) C + 0.7694 (sp3.01) O |
| BD (1) C1–O3 | 1.9975 | 0.6503 (sp0.97) C + 0.7597 (sp2.93) O |
| BD (1) C8–Fe17 | 1.9573 | 0.8077 (sp1.70) C + 0.5895 (sp0.31 d3.23) Fe |
| BD (1) C15–Fe17 | 1.9528 | 0.8178 (sp1.40) C + 0.5756 (sp0.36 d3.29) Fe |
| BD (1) C16–Fe17 | 1.9613 | 0.8196 (sp1.46) C + 0.5730 (sp0.4 d4.24) Fe |
| →Ni @C- GR | BD (1) C1–O2 | 1.9894 | 0.6430 (sp1.02) C + 0.7659 (sp3.25) O |
| BD (1) C1–O3 | 1.9974 | 0.6498 (sp0.98) C + 0.7601 (sp2.91) O |
| BD (1) C8–Ni17 | 1.9682 | 0.8015 (sp1.58) C + 0.5980 (sp0.34 d1.91) Ni |
| BD (1) C15–Ni17 | 1.9682 | 0.8098 (sp1.38) C + 0.5868 (sp0.38 d2.13) Ni |
| BD (1) C16–Ni17 | 1.9745 | 0.8219 (sp1.44) C + 0.5697 (sp0.67 d4.65) Ni |
| →Zn @C- GR | BD (1) C1–O2 | 1.9948 | 0.5712 (sp0.99) C + 0.8208 (sp1.51) O |
| BD (1) C1–O3 | 1.9979 | 0.5802 (sp1.02) C + 0.8145 (sp1.50) O |
| BD (1) C8–Zn17 | 1.9643 | 0.6481 (sp1.39) C + 0.7616 (sp0.37 d2.47) Zn |
| BD (1) C15–Zn17 | 1.9592 | 0.7035 (sp1.19) C + 0.7107 (sp0.33 d3.15) Zn |
| BD (1) C16–Zn17 | 1.9287 | 0.7038 (sp1.25) C + 0.7104 (sp0.62 d4.67) Zn |
Table 3.
The thermodynamic attributes of CO2 adsorbed on the (Fe, Ni, Zn)@C- GR as the selective gas sensor.
Table 3.
The thermodynamic attributes of CO2 adsorbed on the (Fe, Ni, Zn)@C- GR as the selective gas sensor.
| Compound | ∆Eo × 10−4
(kcal/mol) | ∆Ho × 10−4
(kcal/mol) | ∆Go × 10−4
(kcal/mol) | ∆Goads × 10−4
(kcal/mol) | So
(Cal/K.mol) | Dipole Moment
(Debye) |
|---|
| −11.6121 | −11.6121 | −11.6136 | - | 51.378 | 0.0000 |
| Fe@ C- GR | −146.2783 | −146.2782 | −146.2816 | - | 111.175 | 2.3199 |
| Ni@ C- GR | −162.4794 | −162.4793 | −162.4828 | - | 116.150 | 13.6226 |
| Zn@ C- GR | −178.2031 | −178.2030 | −178.2066 | - | 120.533 | 1.7301 |
| → Fe @C- GR | −157.8893 | −157.8893 | −157.8932 | 0.002 | 130.634 | 14.1988 |
| → Ni @C- GR | −173.0324 | −173.0323 | −173.0360 | 1.0604 | 122.276 | 12.5830 |
| → Zn @C- GR | −191.0952 | −191.0951 | −191.0989 | −1.2787 | 127.012 | 2.0963 |
Table 4.
The LUMO (ev), HOMO (ev), band energy gap (∆E/ev) and other qualifications (ev) for adsorption of CO2 on the (Fe, Ni, Zn) embedding of C-nanographene surfaces using CAM-B3LYP/LANL2DZ, 6-31+G (d,p).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).