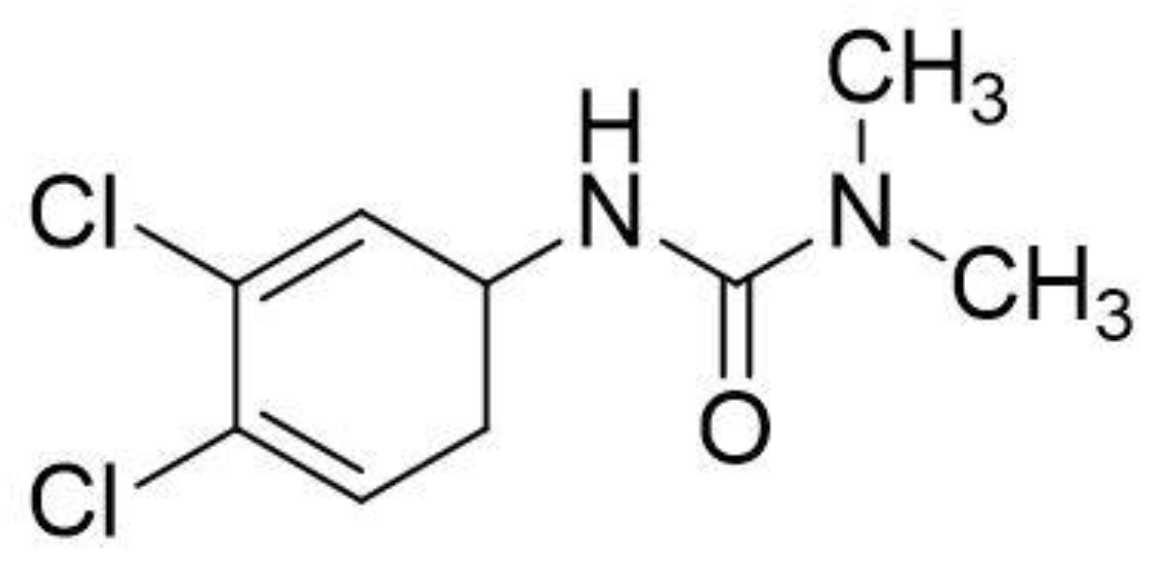
Thermal- and MnO2-Activated Peroxydisulfate for Diuron Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. MnO2 Analysis
2.3. Diuron Degradation Experiments
3. Results and Discussion
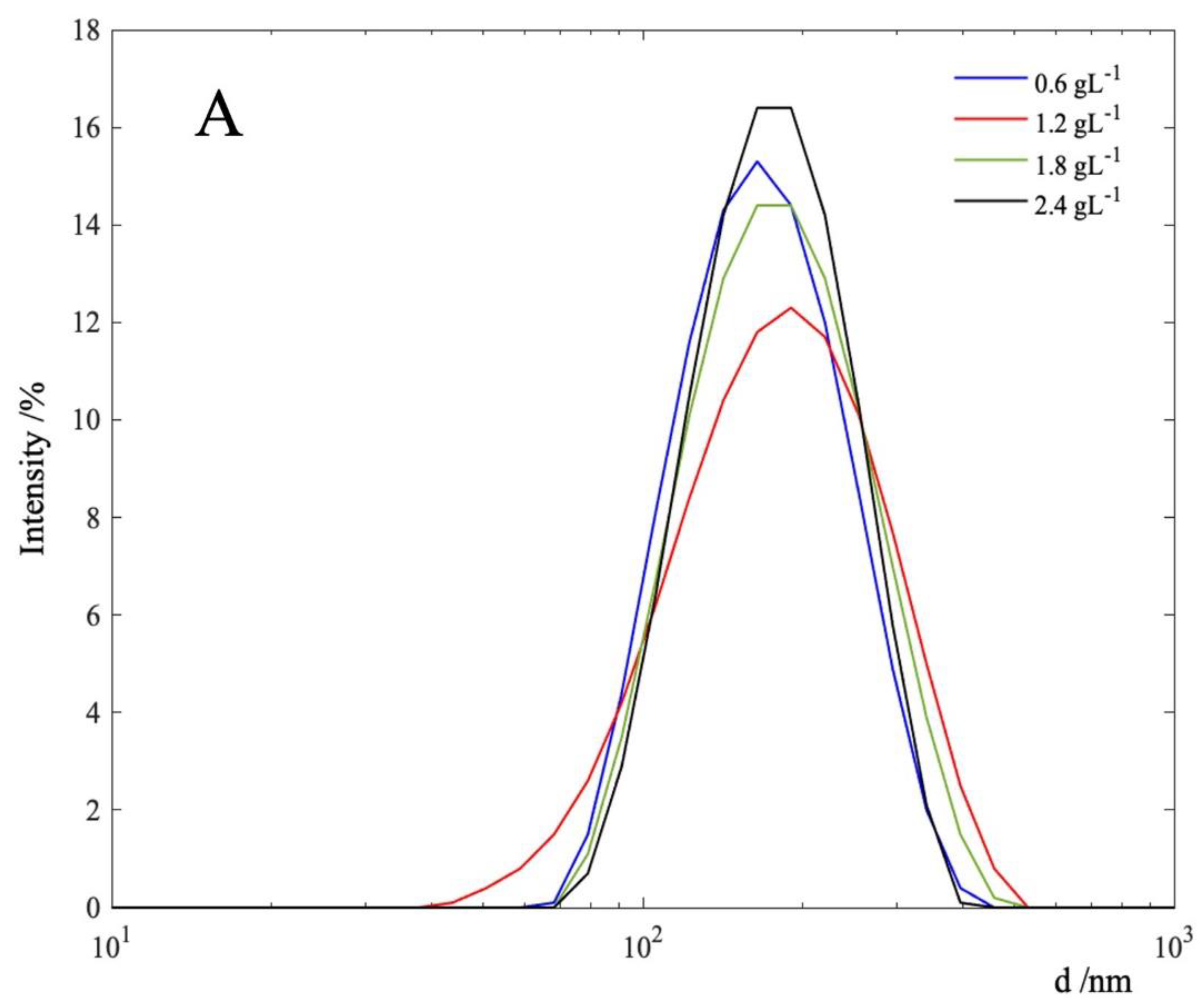
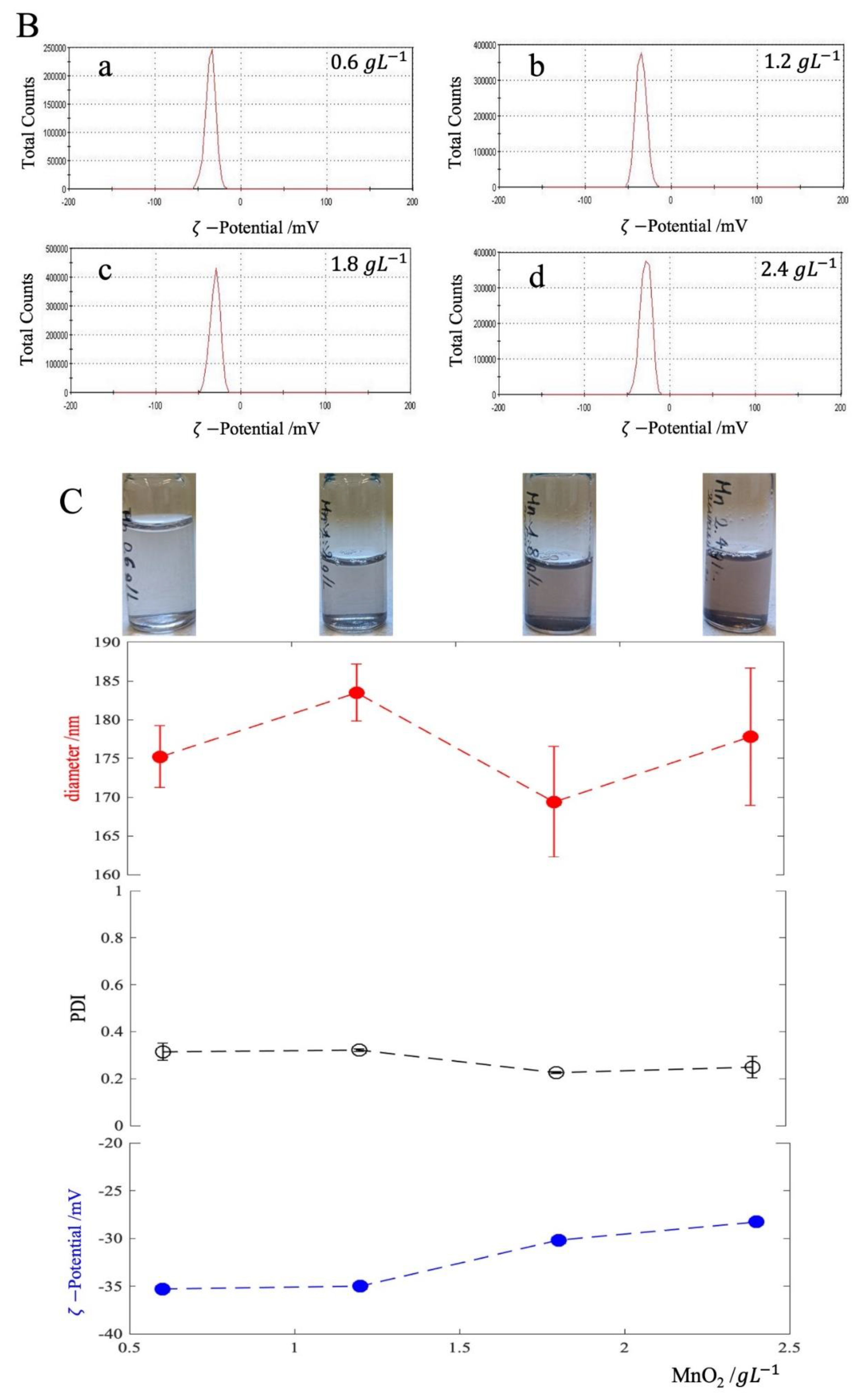
3.1. MnO2 Characterization
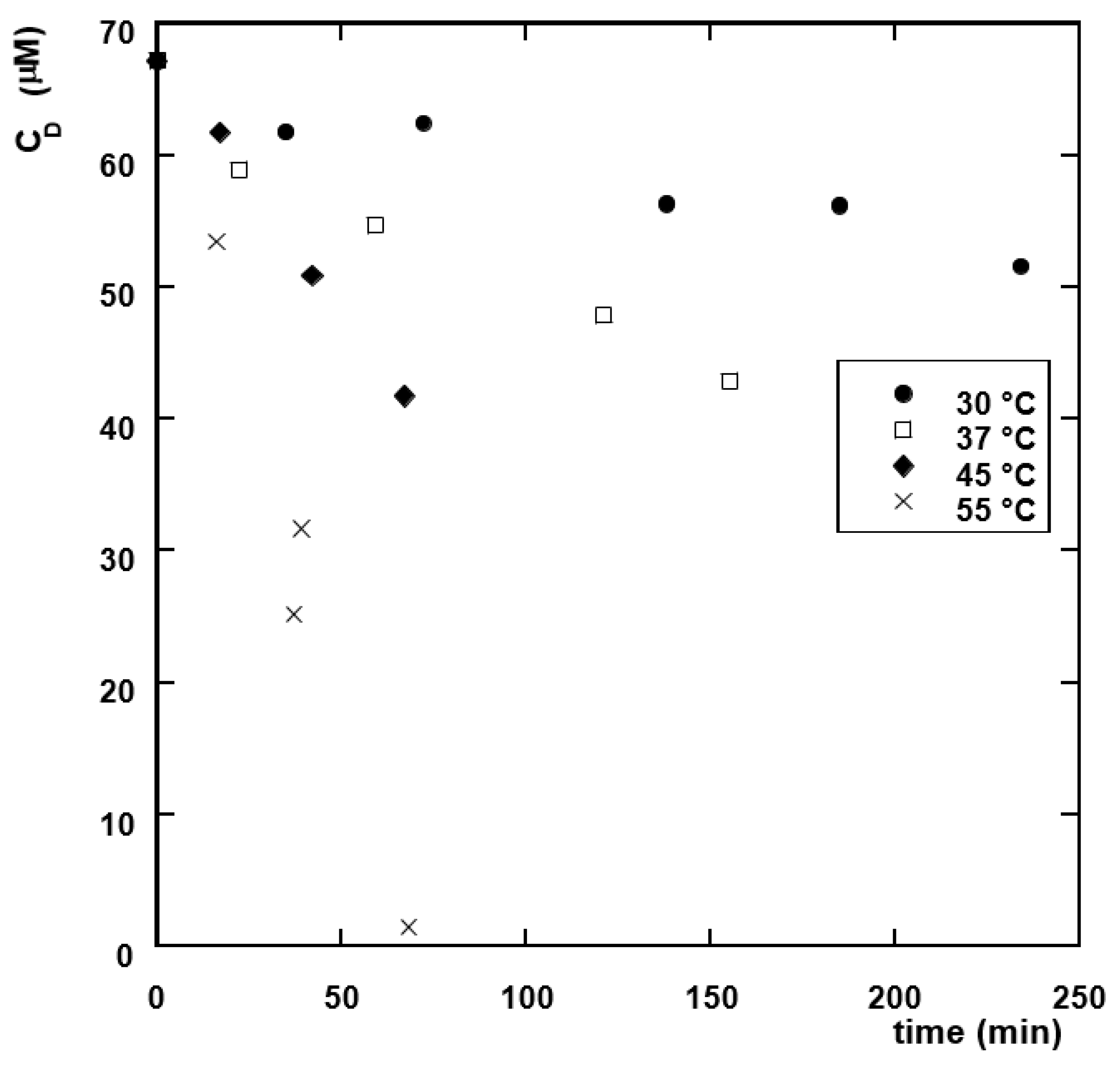
3.2. Diuron Degradation Kinetics
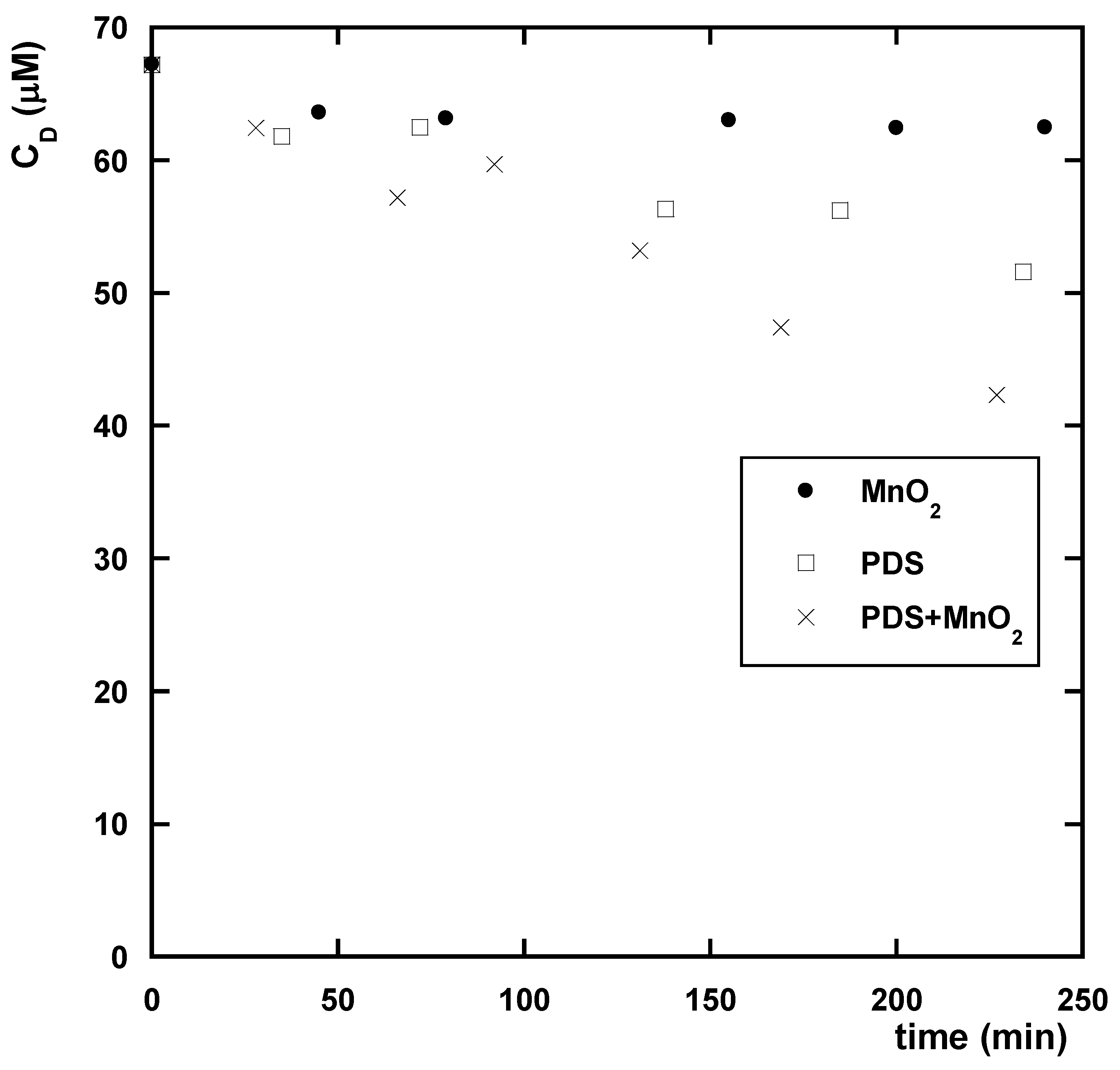
3.2.1. Effect of the Presence of MnO2
3.2.2. Effect of the Presence of PDS
3.2.3. Effect of the Co-Presence of MnO2 and PDS
3.2.4. Kinetic Modeling
3.3. Diuron Degradation Mechanism
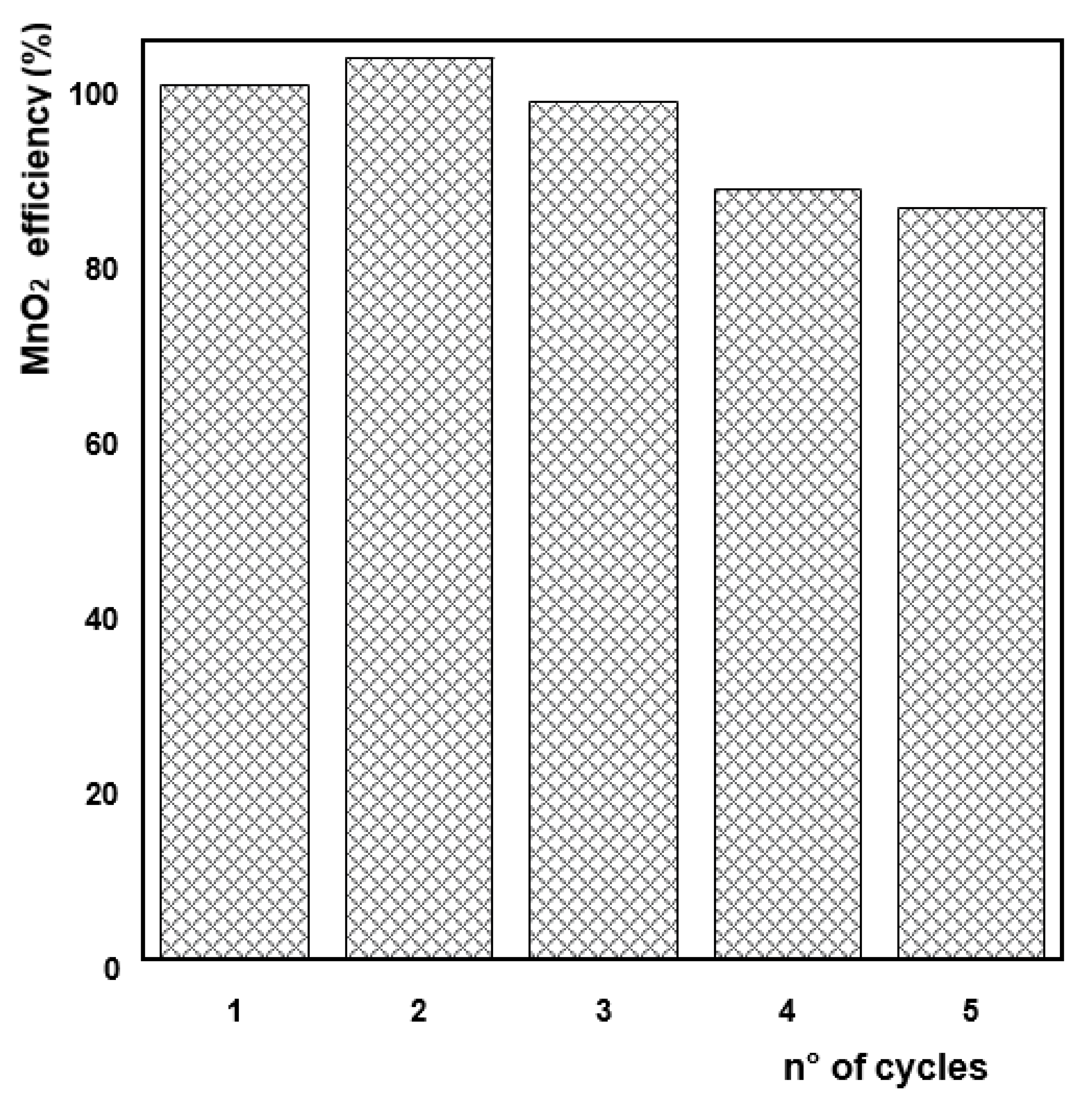
3.4. Reusability of MnO2
3.5. Operational Cost
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Wang, M.; Lv, J.; Deng, H.; Liu, Q.; Liang, S. Occurrence and Removal of Triazine Herbicides during Wastewater Treatment Processes and Their Environmental Impact on Aquatic Life. Int. J. Environ. Res. Public Health 2022, 19, 4557. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, X.; Yang, J.; Tang, W.; Zhu, Y.; Wang, Y.; Zhang, Y.; Zhang, Y.; Duan, J.; Li, W. Degradation of the typical herbicide atrazine by UV/persulfate: Kinetics and mechanisms. Environ. Sci. Pollut. Res. 2022, 29, 43928–43941. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Tian, F.X.; Xu, B.; Ye, W.K.; Gao, Y.Q.; Chen, C.; Xing, H.B.; Wang, B.; Xie, M.J.; Hu, X.J. A comparative study on the degradation of phenylurea herbicides by UV/persulfate process: Kinetics, mechanisms, energy demand and toxicity evaluation associated with DBPs. Chem. Eng. J. 2022, 428, 132088. [Google Scholar] [CrossRef]
- Bhowmick, T.; Sen, G.; Mukherjee, J.; Das, R. Assessing the effect of herbicide diuron on river biofilm: A statistical model. Chemosphere 2021, 282, 131104. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, S.; Bu, L.; Shi, Z.; Zhu, S. Degradation of Diuron by Electrochemically Activated Persulfate. Water Air. Soil Pollut. 2016, 227, 1–9. [Google Scholar] [CrossRef]
- Salvestrini, S. Diuron herbicide degradation catalyzed by low molecular weight humic acid-like compounds. Environ. Chem. Lett. 2013, 11, 359–363. [Google Scholar] [CrossRef]
- Rodriguez, S.; Santos, A.; Romero, A.; Vicente, F. Kinetic of oxidation and mineralization of priority and emerging pollutants by activated persulfate. Chem. Eng. J. 2012, 213, 225–234. [Google Scholar] [CrossRef]
- Brillas, E. Recent development of electrochemical advanced oxidation of herbicides. A review on its application to wastewater treatment and soil remediation. J. Clean. Prod. 2021, 290, 125841. [Google Scholar] [CrossRef]
- de Camargo Lima Beluci, N.; Tonial dos Santos, T.R.; Marcuzzo, J.S.; Bergamasco, R. Facile filtration system to remove Diuron in aqueous solutions. J. Hazard. Mater. 2021, 404, 124163. [Google Scholar] [CrossRef]
- Qi, H.; Sun, X.; Sun, Z. Porous graphite felt electrode with catalytic defects for enhanced degradation of pollutants by electro-Fenton process. Chem. Eng. J. 2021, 403, 126270. [Google Scholar] [CrossRef]
- Salvestrini, S.; Jovanović, J.; Adnadjević, B. Comparison of adsorbent materials for herbicide diuron removal from water. Desalin. Water Treat. 2016, 57, 22868–22877. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, L.; Jegatheesan, V.; Gao, B. Coagulation and nano-filtration: A hybrid system for the removal of lower molecular weight organic compounds (LMWOC). Desalination Water Treat. 2012, 11, 23–31. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, L.; Jegatheesan, V.; Gao, B. Removal and adsorption of diuron through nanofiltration membrane: The effects of ionic environment and operating pressures. Sep. Purif. Technol. 2010, 74, 236–241. [Google Scholar] [CrossRef]
- Fujioka, T.; Kodamatani, H.; Yujue, W.; Yu, K.D.; Wanjaya, E.R.; Yuan, H.; Fang, M.; Snyder, S.A. Assessing the passage of small pesticides through reverse osmosis membranes. J. Memb. Sci. 2020, 595, 117577. [Google Scholar] [CrossRef]
- Bumroongsakulsawat, P.; Khongthon, W.; Pavarajarn, V. Degradation of diuron in water by electrochemical advanced oxidation in a microreactor: Effects of anion contamination on degradation and toxicity. J. Environ. Chem. Eng. 2020, 8, 103824. [Google Scholar] [CrossRef]
- Zheng, Z.; Deletic, A.; Toe, C.Y.; Amal, R.; Zhang, X.; Pickford, R.; Zhou, S.; Zhang, K. Photo-electrochemical oxidation herbicides removal in stormwater: Degradation mechanism and pathway investigation. J. Hazard. Mater. 2022, 436, 129239. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Electro-Oxidation of Humic Acids Using Platinum Electrodes: An Experimental Approach and Kinetic Modelling. Water 2020, 12, 2250. [Google Scholar] [CrossRef]
- Fenti, A.; Jin, Y.; Rhoades, A.J.H.; Dooley, G.P.; Iovino, P.; Salvestrini, S.; Musmarra, D.; Mahendra, S.; Peaslee, G.F.; Blotevogel, J. Performance testing of mesh anodes for in situ electrochemical oxidation of PFAS. Chem. Eng. J. Adv. 2022, 9, 100205. [Google Scholar] [CrossRef]
- Sugihartono, V.E.; Mahasti, N.N.N.; Shih, Y.J.; Huang, Y.H. Photo-persulfate oxidation and mineralization of benzoic acid: Kinetics and optimization under UVC irradiation. Chemosphere 2022, 296, 133663. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Lin, Y.; Jiang, L.; Zhou, Y.; Huang, A.; Yang, J.; Luo, L. Remediation of persistent organic pollutants in aqueous systems by electrochemical activation of persulfates: A review. J. Environ. Manag. 2020, 260, 110125. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Zhou, S.; Huang, R.; Huang, S.; Kuang, H.; Zeng, X.; Zhao, S. Activation of persulfate by MnOOH: Degradation of organic compounds by nonradical mechanism. Chemosphere 2021, 272, 129629. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; He, L.; Sun, X.; Li, X.; Li, M. The transformation of Benzophenone-3 in natural waters and AOPs: The roles of reactive oxygen species and potential environmental risks of products. J. Hazard. Mater. 2022, 427, 127941. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lv, G.; He, L.; Sun, X. New insight into photodegradation mechanisms, kinetics and health effects of p-nitrophenol by ozonation in polluted water. J. Hazard. Mater. 2021, 403, 123805. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, J.A.; Khan, H.M.; Boccelli, D.L.; Dionysiou, D.D. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017, 318, 135–142. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Gabet, A.; Métivier, H.; de Brauer, C.; Mailhot, G.; Brigante, M. Hydrogen peroxide and persulfate activation using UVA-UVB radiation: Degradation of estrogenic compounds and application in sewage treatment plant waters. J. Hazard. Mater. 2021, 405, 124693. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, X.; Zhao, Y.; Wang, H. In-situ soil remediation via heterogeneous iron-based catalysts activated persulfate process: A review. Chem. Eng. J. 2022, 431, 133833. [Google Scholar] [CrossRef]
- Rajabi, S.; Nasiri, A.; Hashemi, M. Enhanced activation of persulfate by CuCoFe2O4@MC/AC as a novel nanomagnetic heterogeneous catalyst with ultrasonic for metronidazole degradation. Chemosphere 2022, 286, 131872. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Ji, Z.Y.; Zhao, Y.Y.; Liu, J.; Li, F.; Yuan, J.S. Treatment of wastewater containing 2-methoxyphenol by persulfate with thermal and alkali synergistic activation: Kinetics and mechanism. Chem. Eng. J. 2020, 380, 122411. [Google Scholar] [CrossRef]
- Hou, X.; Zhan, G.; Huang, X.; Wang, N.; Ai, Z.; Zhang, L. Persulfate activation induced by ascorbic acid for efficient organic pollutants oxidation. Chem. Eng. J. 2020, 382, 122355. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; An, N.; Deng, J. Heat-activated persulfate oxidation of diuron in water. Chem. Eng. J. 2012, 203, 294–300. [Google Scholar] [CrossRef]
- Ren, W.; Huang, X.; Wang, L.; Liu, X.; Zhou, Z.; Wang, Y.; Lin, C.; He, M.; Ouyang, W. Degradation of simazine by heat-activated peroxydisulfate process: A coherent study on kinetics, radicals and models. Chem. Eng. J. 2021, 426, 131876. [Google Scholar] [CrossRef]
- Lebik-Elhadi, H.; Frontistis, Z.; Ait-Amar, H.; Madjene, F.; Mantzavinos, D. Degradation of pesticide thiamethoxam by heat–activated and ultrasound–activated persulfate: Effect of key operating parameters and the water matrix. Process Saf. Environ. Prot. 2020, 134, 197–207. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J.; Zhou, Q. Heat-activated persulfate oxidation of atrazine: Implications for remediation of groundwater contaminated by herbicides. Chem. Eng. J. 2015, 263, 45–54. [Google Scholar] [CrossRef]
- Oh, W.D.; Lim, T.T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: An overview. Chem. Eng. J. 2019, 358, 110–133. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, S.; Jin, X.; Chong, Y.; Li, Y.; Li, A.; Lin, J.; Qiu, Y.; Ye, D. Acid-activated layered δ-MnO2 promotes VOCs combustion. Appl. Surf. Sci. 2022, 574, 151707. [Google Scholar] [CrossRef]
- Fang, C.; Gujarati, H.; Osinaga, F.; Hsia, V.; Cheney, M.A.; Kharel, M.K. Optimization of the catalytic activity of manganese dioxide (MnO2) nanoparticles for degradation of environmental pollutants. Res. Chem. Intermed. 2021, 47, 3673–3690. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Chen, Y.; He, A.; Li, J.; Sheng, G.D. Metal inhibition on the reactivity of manganese dioxide toward organic contaminant oxidation in relation to metal adsorption and ionic potential. Chemosphere 2017, 170, 95–103. [Google Scholar] [CrossRef]
- Vanore, P.; Coppola, E.; Iovino, P.; Leone, V.; Salvestrini, S.; Capasso, S. Sorption thermodynamics of organic pollutants onto zeolitic tuff: Isosteric and standard enthalpy. J. Water Chem. Technol. 2017, 39, 228–232. [Google Scholar] [CrossRef]
- Salvestrini, S. A modification of the Langmuir rate equation for diffusion-controlled adsorption kinetics. React. Kinet. Mech. Catal. 2019, 128, 571–586. [Google Scholar] [CrossRef]
- Lei, Y.J.; Tian, Y.; Fang, C.; Zhan, W.; Duan, L.C.; Zhang, J.; Zuo, W.; Kong, X.W. Insights into the oxidation kinetics and mechanism of diesel hydrocarbons by ultrasound activated persulfate in a soil system. Chem. Eng. J. 2019, 378, 122253. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Diclofenac sorption from synthetic water: Kinetic and thermodynamic analysis. J. Environ. Chem. Eng. 2020, 8, 104105. [Google Scholar] [CrossRef]
- Fenti, A.; Salvestrini, S. Analytical solution of the Langmuir-based linear driving force model and its application to the adsorption kinetics of boscalid onto granular activated carbon. React. Kinet. Mech. Catal. 2018, 125, 1–13. [Google Scholar] [CrossRef]
- Salvestrini, S.; Canzano, S.; Iovino, P.; Leone, V.; Capasso, S. Modelling the biphasic sorption of simazine, imidacloprid, and boscalid in water/soil systems. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2014, 49, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Du, J.; Wang, Z.; Xiao, G.; Luo, L.; Faheem, M.; Ling, H.; Bao, J. Degradation of sulfamethoxazole by MnO2/heat-activated persulfate: Kinetics, synergistic effect and reaction mechanism. Chem. Eng. J. Adv. 2022, 9, 100200. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-Merit for the Technical Development and Application of Advanced Oxidation Processes. J. Adv. Oxid. Technol. 1996, 1, 13–17. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of peroxodisulfate and peroxymonosulfate by ultrasound with different frequencies: Impact on ibuprofen removal efficient, cost estimation and energy analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]





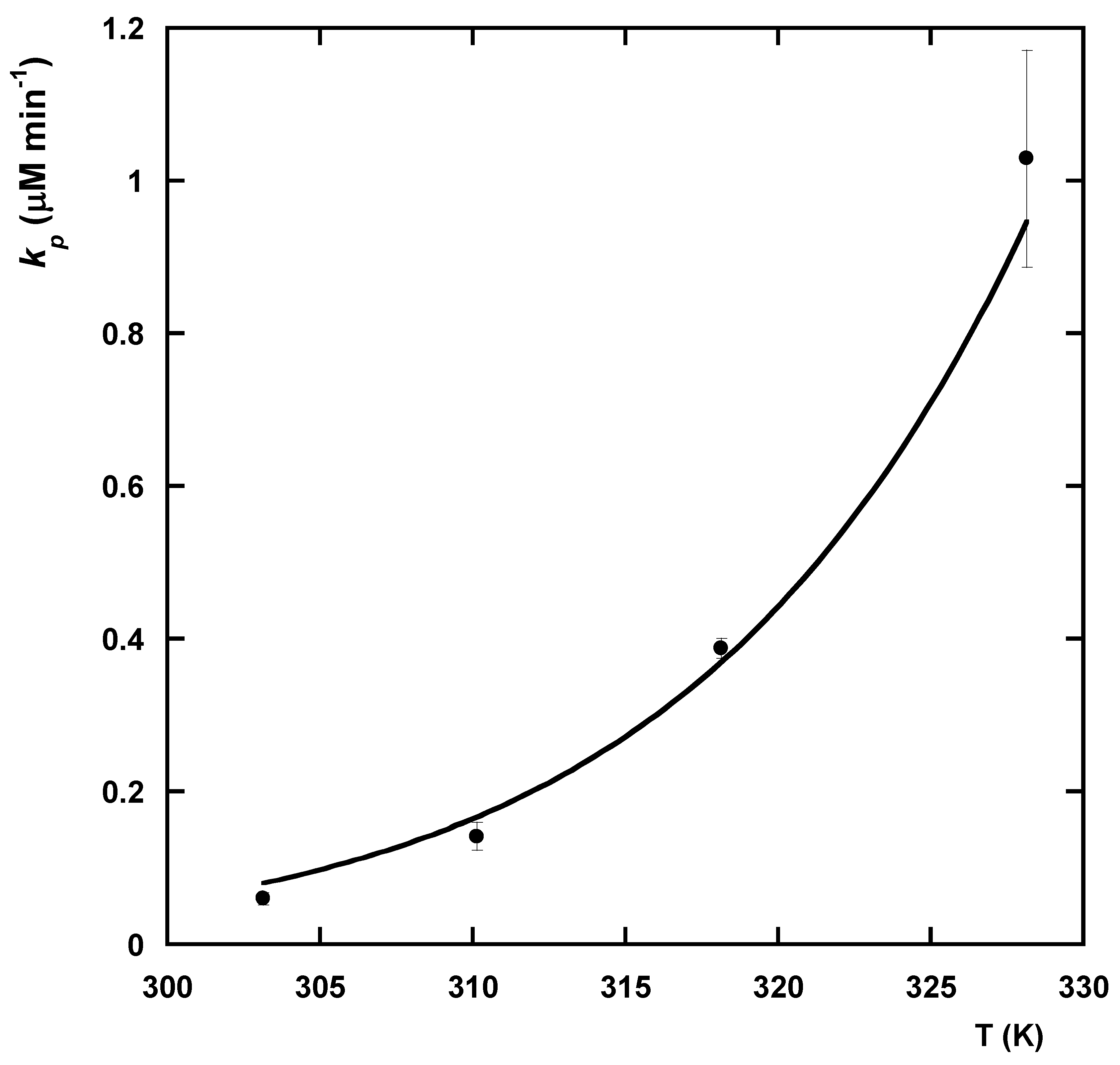


| T (K) | (μM−1 min−1) | (μM min−1) | (μM min−1) |
|---|---|---|---|
| 303.15 | (7.9 ± 3.9) × 10−4 | (5.98 ± 0.79) × 10−2 | (7.20 ± 0.17) × 10−2 |
| 310.15 | (7.9 ± 3.9) × 10−4 | (14.1 ± 1.8) × 10−2 | (13.6 ± 0.1) × 10−2 |
| 318.15 | (7.9 ± 3.9) × 10−4 | (38.7 ± 1.3) × 10−2 | (48.9 ± 12.4) × 10−2 |
| 328.15 | (7.9 ± 3.9) × 10−4 | (103 ± 14) × 10−2 | (109 ± 14) × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenti, A.; Minò, A.; Salvestrini, S. Thermal- and MnO2-Activated Peroxydisulfate for Diuron Removal from Water. Clean Technol. 2022, 4, 1071-1087. https://doi.org/10.3390/cleantechnol4040065
Fenti A, Minò A, Salvestrini S. Thermal- and MnO2-Activated Peroxydisulfate for Diuron Removal from Water. Clean Technologies. 2022; 4(4):1071-1087. https://doi.org/10.3390/cleantechnol4040065
Chicago/Turabian StyleFenti, Angelo, Antonio Minò, and Stefano Salvestrini. 2022. "Thermal- and MnO2-Activated Peroxydisulfate for Diuron Removal from Water" Clean Technologies 4, no. 4: 1071-1087. https://doi.org/10.3390/cleantechnol4040065
APA StyleFenti, A., Minò, A., & Salvestrini, S. (2022). Thermal- and MnO2-Activated Peroxydisulfate for Diuron Removal from Water. Clean Technologies, 4(4), 1071-1087. https://doi.org/10.3390/cleantechnol4040065









