1. Introduction
Human society has an urgent need for liquid fuels, in a world which is almost entirely dependent on petroleum in the present scenario. Continued use of petroleum fuels is unsustainable due to depleting supplies, and they are the source of air pollutants that emit NO
2, CO, SO
2 and CO
2 gases. These gases show a hazardous impact on the environment, which results in global warming, ozone layer depletion, smog and acid rain. Furthermore, these gases also cause numerous health problems in humans, including asthma and cancer [
1]. The environmental problems caused by the emission of harmful gases can only be eradicated by replacing petroleum fuel with an efficient and economically viable renewable and sustainable fuel such as biodiesel [
2]. Bioenergy research involves finding a suitable feedstock to turn into biofuel, and discovering environmentally friendly, commercially feasible, long-term biodiesel and coproducts supplies [
3]. Microalgae were analysed and found to be very useful for bioenergy production [
4,
5]. Microalgal biomass has emerged as an efficient alternative raw material for the production of biodiesel, reflecting its ability to grow exponentially with the accumulation of large quantities of stored lipids in the form of triacylglycerides (TAG) [
6,
7,
8,
9,
10].
Nutrient stress such as that from nitrogen and phosphorous alters certain algae’s biochemical pathways toward TAG (triglycerides) accumulation [
11]. These TAGs can serve as a feedstock for biodiesel production by reaction with methanol using a base-catalyzed transesterification reaction [
12]. The average percentage of lipid accumulated inside the cells varies from 10% to 30% of the total dry weight; in some exceptional cases, it can even reach up to 75% [
8,
13]. Lipids exist in two forms, saturated and unsaturated fatty acids. Microalgae with a higher amount of these lipids are suitable for producing good quality biodiesel [
14]. Microalgal lipids with a lower degree of unsaturation are more suitable for biofuel [
13,
15]. Therefore, to produce higher biomass with the most increased microalgal lipid production in the form of triglycerides, it is essential to identify the appropriate growth medium for the particular microalgal species [
16].
Various biotic and abiotic conditions influence the growth of microalgae and the accumulation of lipid content. The ingredients of autotrophic growth media such as nitrogen, phosphorus, carbon and other salts are the essential components, so increasing or decreasing their concentration can influence the amount of biomass and lipid productivity [
3]. The critical factors, such as productivity of biomass and lipid yield, must be optimized for the commercial viability of microalgal-based biodiesel. The biomass content and the quantity of biomolecule can be altered. Especially, the accumulation of fatty acids in the form of triacylglycerides (TAG) can be optimized by changing the cultivation procedures and growth criteria such as the nutrient composition of the growth medium, and alteration in the environmental parameters and growth stages of microalgae [
17]. The most important environmental conditions for microalgal cultivation such as pH, light intensity, photoperiods, temperature, and even CO
2 supplementation, help to improve biomass productivity. Another critical strategy to enhance the productivity of the desired compound is change in the type of cultivation and alteration in metabolism (e.g., phototrophic, heterotrophic, and mixotrophic) [
13,
18]. Therefore, due to the above information, it is believed that the growth medium composition has a significant impact on the amount of biomass generation and accumulation of lipids in the microalga.
The members of the Scenedesmaceae family are recognized as a prominent feedstock for biofuel production [
13,
18,
19]. The microalga
Scenedesmus rotundus-MG910488 was selected in this experiment due to its higher biomass productivity, maximum lipid synthesis and potential to grow in various kinds of wastewater. It is easy to harvest owing to its large cell size and higher adaptive capacity over a wide range of nutrition and pH, which has encouraged extensive selection and assessment of this promising strain for biodiesel production [
20]. It is advantageous to understand that the optimization of lipid yield is essential for producing biodiesel from microalgae.
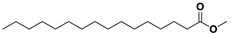
In this study, the impact of different autotrophic growth media such as BBM, CHU-10, Fogg’s, BG-11, CFTRI and Zarrouk’s on the morphology and synthesis of the lipid of newly isolated microalga Scenedesmus rotundus-MG910488 was evaluated. This strain is well characterized for growth and lipid content, and while utilizing BG-11 medium without optimizing any biotic or abiotic conditions, it synthesized 33.30 ± 1.21% of lipid content on dry cell weight, which indicates its potential for large-scale cultivation. The fatty acid composition determines the quality of the fuel, so the characterization of fatty acid methyl esters was performed by GC-MS, FT-IR and NMR spectroscopy. Presence of C16:0, C18:0, C18:1 and C18:2 in the lipids obtained from this microalga shows its effectiveness as a feedstock for good quality biodiesel production.
2. Materials and Methods
2.1. Microalga
Axenic culture of
Scenedesmus rotundus-MG910488 isolate of Central India was used as an experimental organism. According to the literature,
Scenedesmus species is a promising strain of microalgae, which is more suitable for producing higher biomass that can be utilized as food, feed and fuel. It can also be helpful for wastewater treatment. The isolated microalga was purified by serial dilution, and the streak plate was used as a standard microbiological method to obtain an axenic form. The microalga culture was maintained in BG-11 medium (pH 7.5) [
21]. It was identified as
Scenedesmus sp based on microscopic observation of cell morphology. The molecular classification was performed for the confirmation of the microalga strain. The microalga strain was identified as
Scenedesmus rotundus, which was 98% identical to
Scenedesmus rotundus strain BCP-SEV2VF49 after submission of 28S ribosomal gene sequence to the NCBI, and was allotted MG910488 as an accession number for this strain [
3].
2.2. Experimental Setup
This experiment aims to find out the most appropriate and economically viable growth medium to enhance biomass productivity and high lipid accumulation. The microalga
Scenedesmus rotundus-MG910488 was grown in six different autotrophic growth media to ascertain the most favourable medium for biomass and lipid production. The optimization of growth media is one of the critical criteria for evaluating the productivity of desirable products from the selected strain. Media was selected based on nutrient composition and the concentration of specific nutrients that were essential for microalgal growth. As a general culture medium, BG-11 was used. All the experiments performed in triplicates in 1L sterilized Erlenmeyer flasks. In these flasks, 700 mL of sterile autotrophic growth media such as Bold Basal Medium (BBM) [
22], CHU-10 [
23], Fogg’s [
24], BG-11 [
21], CFTRI [
25] and Zarrouk’s Medium (ZM) [
26] were inoculated with 10% of fully grown (stationary phase) culture of microalga. The medium composition is shown in
Table 1. The batch culture of
Scenedesmus rotundus-MG910488 was incubated for 30 days and grown photo-autotrophically in an artificial culture room at 25 ± 1 °C at 16:08 h light-dark cycle, under 60 μmol m
2 s
−1 intensity of light. The experimental flasks were shaken twice a day at 100 rpm for 30 min in the orbital shaker to provide adequate dispersion of light, air, and nutrients available to the culture and avoid the adherence of the cells to the glass walls of the Erlenmeyer flask. To estimate growth parameters of microalga, the culture of various growth medium were taken on every 3rd day of the study.
2.3. Morphological Analysis
Cellular morphological analysis of Scenedesmus rotundus-MG910488 was carried out by using a compound microscope Olympus-CH20iBIMF, India, at 40× magnification. To observe the changes in length and width of microalgal cells, 1 mL of microalgal sample was taken from the different growth medium and harvested by centrifugation at 10,000 rpm for 10 min using a centrifuge (Remi-CPR-24, Mumbai, India). The cells were washed with distilled water to remove media components. The alga cells were mixed with the appropriate amount of sterile double distilled water. Later, one drop of culture was shifted on the slide under a microscope, placed with a coverslip, and examined under a compound microscope.
2.4. Calculation of Growth Kinetics
Growth of this microalgae was observed by taking the optical density (OD) at 750 nm using a Spectrophotometer (Systronics-305, India). To determine the growth rate of microalga, the cell count was performed through direct microscopic observation using Neubour hemocytometer with the help of a compound microscope (Olympus-CH20iBIMF).
Specific Growth Rate (µ): The specific growth rate was calculated by applying the following equation [
27]
where, Nt = Total number of cells at the end of the log phase, N0 is the total number of cells starting a log phase, Tt is the last day of the log phase, and T0 is the first day of a log phase.
2.5. Determination of Photosynthetic Pigments
The impact of various growth media on the growth of the microalga can also be estimated by the content of photosynthetic pigments: Chlorophyll a, b and carotenoid. To evaluate photosynthetic pigments, a known amount of microalgal culture (3 mL) was taken out from each experimental flask and centrifuged (Remi-PR-24, India) at 10,000 rpm for 5 min at 4 °C. The supernatant containing growth medium was discarded, and cells of microalga in the pellet were rinsed thrice with deionized water to take off the chemical ingredients. The washed microalgae cells were re-dissolved in the same amount of 99.99% methanol (
v/
v) in a centrifuge tube and mixed well followed by keeping at 60 °C for 15 min in a thermostatic water bath (Taco-PLT 109, India). Then, extract of all pigments in the methanol was cooled at room temperature and centrifuged at 5000 rpm for 5 min. The absorbance of the supernatant was carried out at 665 nm, 652 nm and 470 nm by using a Spectrophotometer (Systronics-305, Mumbai, India). The pigment content was determined according to the following equations as mentioned by Patel et al. [
16]:
2.6. Determination of Biomass Content
To estimate the dry cell weight (DCW) and productivity, the fully grown culture of microalga was taken and harvested at 10,000 rpm at 4 °C for 10 min by centrifugation (Remi-PR-24, Mumbai, India). The supernatant was withdrawn, and the cells in the pellet were dried overnight at 60 °C in the hot air oven. The biomass productivity (mg L
−1 day
−1) of microalga was gravimetrically estimated by using the following equation: [
3,
18].
where X2 and X1 are the concentration of dry cell weight of microalga (mg L
−1) at time t2 (harvesting day of stationary phase culture) and t1 (start day of the experiment), respectively.
2.7. Determination of Lipid Content
The lipid was extracted from the dry biomass of
Scenedesmus rotundus-MG910488 by using Bligh and Dyer’s method [
20]. In brief, the stationary phase of the culture was taken, and the cells were harvested by centrifugation at 10,000 rpm at 4 °C for 10 min. The pellet was rinsed twice with double distilled water, and the water discarded by re-centrifugation, after which the pellet was dried in an oven for 24 h at 60 °C. Mortar and pestle were used for the grinding of dry biomass. For the extraction of lipid, approximately 200 mg of biomass powder was taken in 50 mL of glass beaker containing 20 mL of chloroform:methanol (1:2,
v/
v) and all vortexes together for 30 s, and ultrasonicated for 20 min at room temperature. 10 mL of chloroform was added and again ultrasonicated for 10 min. The mixture was kept for 24 h at 4 °C, and then 10 mL of double distilled water was added to the sample and again vortexed for 5 min. The lipid was segregated by centrifugation at 5000 rpm for 5 min. Lipid content in the lower organic layer was transferred in the pre-weighed glass vial (V1), and the same method was repeated thrice with the pellet. The extracted lipid in the vial was dried in a hot air oven at 60 °C for one hour to vaporize the water and solvents. The lipid weight in the vial was recorded as V2; the total lipid content was measured gravimetrically by subtracting V1 from V2 and expressed as % dry cell weight.
2.8. Transesterification of Lipid
Extracted lipid was converted into fatty acid methyl esters (FAME) by transesterification [
28,
29]. FAMEs were produced by using extracted lipids mixed with methanol (5 mL) and 12% solution of saturated NaOH prepared in methanol. This mixture was kept on a magnetic stirrer and heated at 75 °C; the reaction time was 60 min. After the first reaction, the sample was again mixed with 5% HCl in methanol and heated at the same temperature for another 15 min. This mixture was cooled at room temperature and mixed with 2 mL of deionized water. In the later stage of the reaction, the FAME was segregated by centrifugation at 10,000 rpm at 4 °C for 10 min. The FAMEs were extracted as an upper organic layer, and cautiously transferred into the new clean glass vial. Fatty acid methyl esters were heated at 100 °C for 10 min to remove water and excess solvents. After the transesterification reaction, the fatty acid methyl esters (biodiesel) had been produced from the lipid of
Scenedesmus rotundus-MG910488. The lipids profile was employed to evaluate biofuel quality, so the characterization of fatty acid methyl esters was estimated by GCMS, FTIR, and NMR techniques to assess the quality of the fatty acid profile of microalga.
2.9. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Biodiesel
The fatty acid methyl esters composition in biodiesel produced after transesterification [
9] of
Scenedesmus rotundus-MG910488 lipid was examined by Gas Chromatography-Mass Spectroscopy (GC-MS). Fatty acid profile analysis was carried out using hexane (1 mL), added to 1 mL filtered esterified lipid, and the product was shaken gently for 1 min. Gas-Chromatography Mass Spectrometry (GC-MS) analysis was performed using Shimadzu GCMS-QP-2010 Ultra (Shimadzu Corporation, Kyoto, Japan), equipped with RTx-5 MS column (30 m × 0.25 mm id × 0.25 film thickness), used for the investigation of fatty acid methyl ester composition. The mode operation of the column was as follows: oven temperature program from 80–210 °C at 4 °C/min with a hold time of 2 min and from 210–300 °C at 15 °C/min with a hold time of 5 min, and the final temperature was maintained for 20 min. The temperature in the injector was maintained at 270 °C, carrier gas helium (99.999% pure) was utilized, and 0.3 μL of FAME sample was injected. The pressure 85.4 kPa, total flow 76.8 mL/min, column flow 1.21 mL/min, linear velocity 40.5 cm/s, purge flow 3.0 mL/min, split ratio: 60.0; ion source temperature 230 °C; scan mass range of
m/
z 40–600 and interface line temperature 280 °C. The profiling of fatty acid methyl ester compounds was performed by evaluating their mass spectra from the information available in the NIST08 (National Institute of Standards and Technology, USA, Wiley 08) libraries.
2.10. ATR-FT-IR Analysis of Biodiesel
The chemical profiling of the fatty acid methyl ester compounds was analysed by Attenuated Total Reflectance Fourier Transform Infra-Red (ATR-FT/IR-4600A type FTIR Spectrophotometer (Jasco, Europe) coupled with a TCS detector. Scanning was performed at wave numbers ranging from 4000–500 cm
−1 with a resolution of 4.0 cm
−1. To assist the diamond tip in touching the material equally, 4 mg of FAME was combined with chloroform and dispersed across the plate’s surface. Before the assessment, chloroform was vaporized at room temperature. The spectral estimation and stacking of spectra were obtained with the help of standard software provided with the FT/IR-4600A instrument. The scan results were evaluated and compared with published FT-IR spectra of fatty acid methyl esters of microalgae [
30,
31].
2.11. NMR Analysis of Biodiesel
The 1H NMR spectra have been recorded on a Bruker 500 MHz NMR Spectrometer equipped with a broad band probe (BB) and inverse probe (BBI). The concentration of the sample was made by dissolving about 20 mg sample of fatty acid methyl esters in 0.7 mL of chloroform (CDCl3) solvent, and tetra-methyl-silane (TMS) was used as an internal reference. The operating procedure of NMR was as follows; Spectral width (SW) = 5000 Hz (0.0–12.0 ppm chemical shift range), spectral size = 16 K, P1 (90° PW) = 10.90 μs, relaxation delay (D1) = 1.0 s, and number of scans (NS) = 16. The 13C NMR spectra were recorded on a Bruker 500 MHz NMR equipped with a broad band probe (BBO) in a CDCl3 solution of algal fatty acid methyl esters (20 mg/0.7 mL of CDCl3), containing internal standard TMS in the inverse gated mode. 13C NMR recordings with a maximum number of scans of 2048 with a relaxation delay time of 2.0 s, PW of 16.80 μs, and sweep width of 0–240 ppm were carried out.
2.12. Statistical Analysis
All the experiments were performed in three independent biological replicates, and results are expressed as values of Means ± SD (standard deviation) with error bars of using Microsoft Office Excel 2010 (Microsoft, Albuquerque, NM, USA).
3. Results and Discussion
3.1. Effects of Different Autotrophic Growth Media on Cell Morphology of Scenedesmus rotundus-MG910488
The growth of microalga is affected by the composition of macro and micronutrients in the growth media and their concentration available to the cells. The present investigation was conducted to maximize biomass and lipid accumulation in
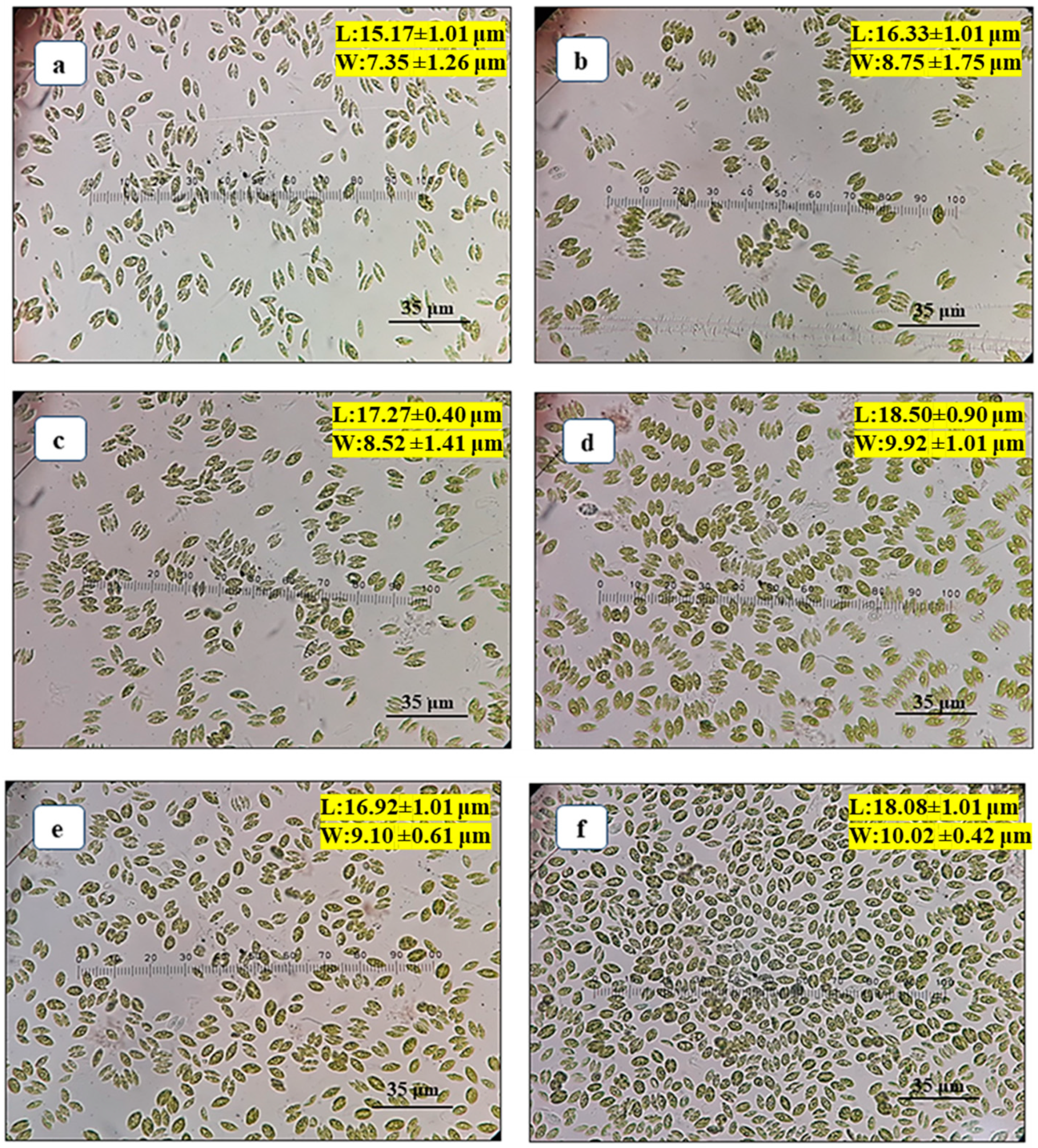
Scenedesmus rotundus-MG910488 using different autotrophic growth media such as BBM, CHU-10, Fogg’s BG-11, CFTRI and ZM. The morphology of the cells was altered due to the presence of a different concentration of the chemical ingredient in the growth medium used for the cultivation of microalga, primarily carbon, nitrogen and phosphorus concentrations. Studies have reported that the shape and size of the microalgal cells become abnormal under adverse conditions. The length (L) and width (W) of microalga reduced after the formation of unicell to coenobia (2 to 4 cells) in CHU-10, Fogg’s and BG-11.
Figure 1a–f show that the length and width of the cells of
Scenedesmus rotundus-MG910488 were changed in different media after 15 days of inoculation, as represented in
Table S1. The decrease in cell size of microalga was observed (16.33 ± 1.01 μm long and 8.75 ± 1.75 μm wide) when cells were grown in CHU-10 medium which was 1.13 times less compared to its average size found in the BG-11 medium. A similar result was found when the culture was grown in CFTRI medium, and the L and W of the cell were recorded as 16.92 ± 1.01 µm and 9.10 ± 0.61 µm, respectively.
Furthermore, the decrease in length and width of cells (15.17 ± 1.01 μm and 7.35 ± 1.26 μm wide) was also seen in BBM, most likely due to lower concentrations of nitrogen in the growth medium. The observation in the previous report found that the size of cells reduced with the reduction in the amount of nitrogen in the growth medium [
3].
The normal size of cell morphology was found in the culture grown in BG-11 medium (18.50 ± 0.90 μm in length and 9.92 ± 1.01 μm in width), whereas, in Zarrouk’s medium, cells were oval with the unicellular structure. The higher concentration of sodium bicarbonate in the Zarrouk’s growth medium resulted in oval-shaped morphology, with the length and width of cells as 18.08 ± 1.01 μm and 10.02 ± 0.42 μm, respectively. Note that the width of the cell is more in the case of Zarrouk’s medium (10.02 ± 0.42 μm) as compared to BG-11 medium (9.92 ± 1.01 μm), and the cells are also unicellular.
3.2. Effect of Different Growth Medium on the Growth of Scenedesmus rotundus-MG910488
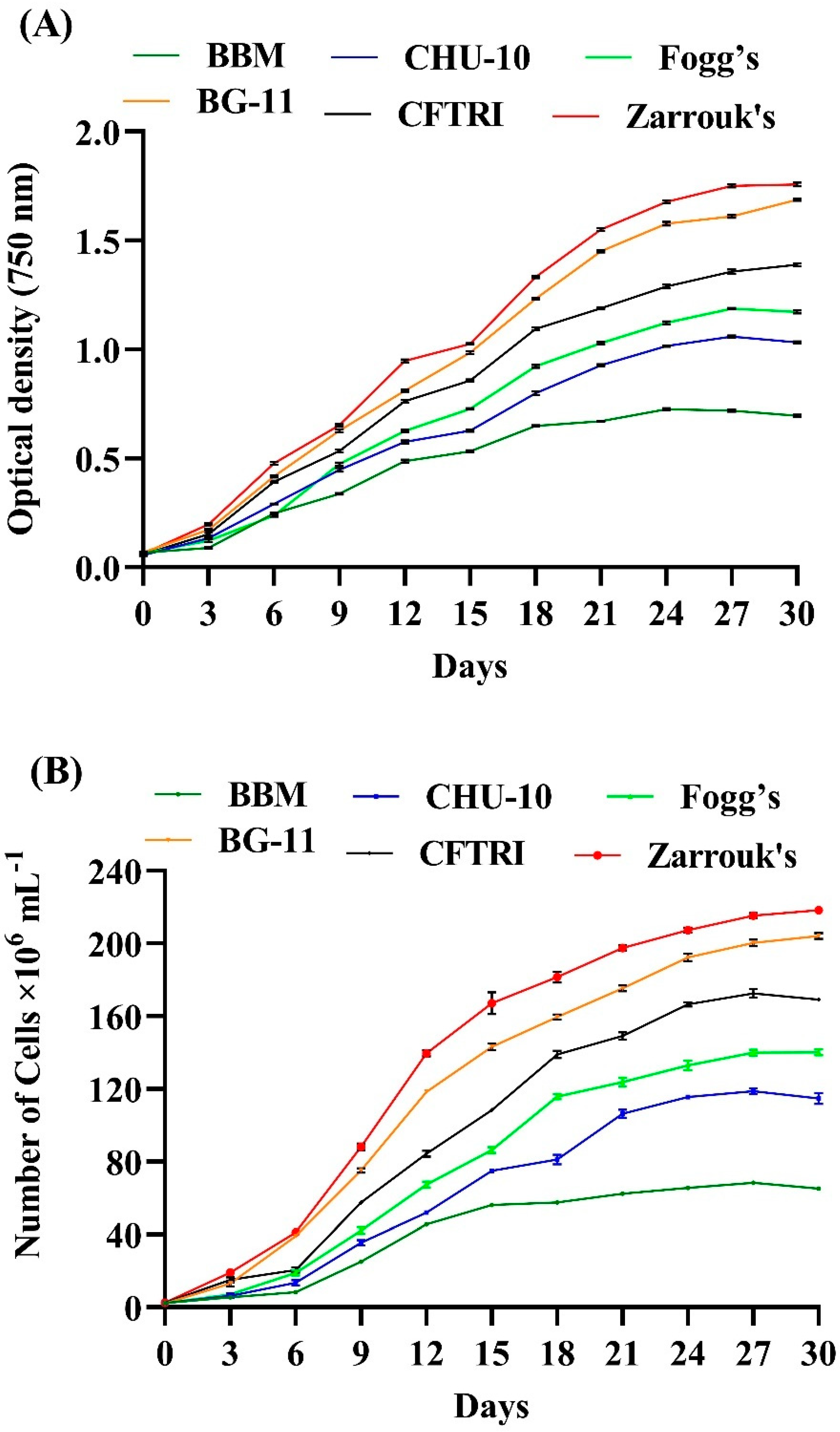
The growth of this microalga was monitored every third day by taking absorbance of culture density at 750 nm using a Spectrophotometer for 30 days, shown in
Figure 2A. The different growth medium was used to evaluate the impact on the growth behaviour of microalga. The study revealed that the optimum microalga growth was observed in Zarrouk’s and BG-11 medium. Zarrouk’s medium shows maximum growth compared with other media used to cultivate microalgae such as BBM, CHU-10, Fogg’s, BG-11 and CFTRI. The possible reason behind the higher growth of
Scenedesmus rotundus-MG910488 in Zarrouk’s medium might be a higher concentration of NaHCO
3 and NaNO
3 (16.8 g L
−1 and 2.5 g L
−1) in the composition. NaHCO
3 and NaNO
3 induce microalga metabolism, which leads to cell division.
Gour et al. studied the impact of CHU-10, BBM, F/2 media, and modified BG-11 medium on the growth of
Scenedesmus dimorphus,
Scenedesmus quadricauda and
Chlorella sp. The highest specific growth rate and cell division per day were found in modified BG-11 medium [
32]. The significant variation in cell number and growth kinetics after cultivating in various media was also observed in the present case (
Tables S2 and S3).
Figure 2B represents the effect of different media on the cell count. In favourable conditions, most microalgae have a generation time of 24 to 48 h [
33].
Table 2 presents the growth kinetics data, where the specific growth rate (μ) was highest in Zarrouk’s and BG-11 media, for example 0.312 ± 0.003 and 0.289 ± 0.008, respectively, probably due to higher nitrate concentration (2.5 g L
−1 and 2.5 g L
−1 respectively). In contrast, the lowest specific growth rate was seen in BBM (μ =0.196 ± 0.002). The presence of a lower concentration of nitrate (NaNO
3, 0.25 g L
−1) in the composition of BBM is likely to be responsible for a slower growth rate and low productivity in it. Therefore, nitrogen deficiency inhibits the growth kinetics of microalgae and decreases the growth rate and the productivity of biomass content. The highest specific growth rate achieved was also supported by higher cell density (218.37 ± 1.04 × 106 cells mL
−1) in Zarrouk’s medium, and (204.02 ± 1.74 × 106 cells mL
−1) in the BG-11 medium, and lower in BBM (68.29 ± 0.09 × 106 cells mL
−1). Similarly, Pancha et al. also found that the growth medium supplemented with sodium bicarbonate significantly increased DCW and biomass productivity of the microalgae
Scenedesmus sp. CCNM 1077, which suggests that the addition of sodium bicarbonate improves the cell division and metabolic process in microalga [
34].
3.3. Effect of Different Growth Media on the Photosynthetic Pigments of Scenedesmus rotundus-MG910488
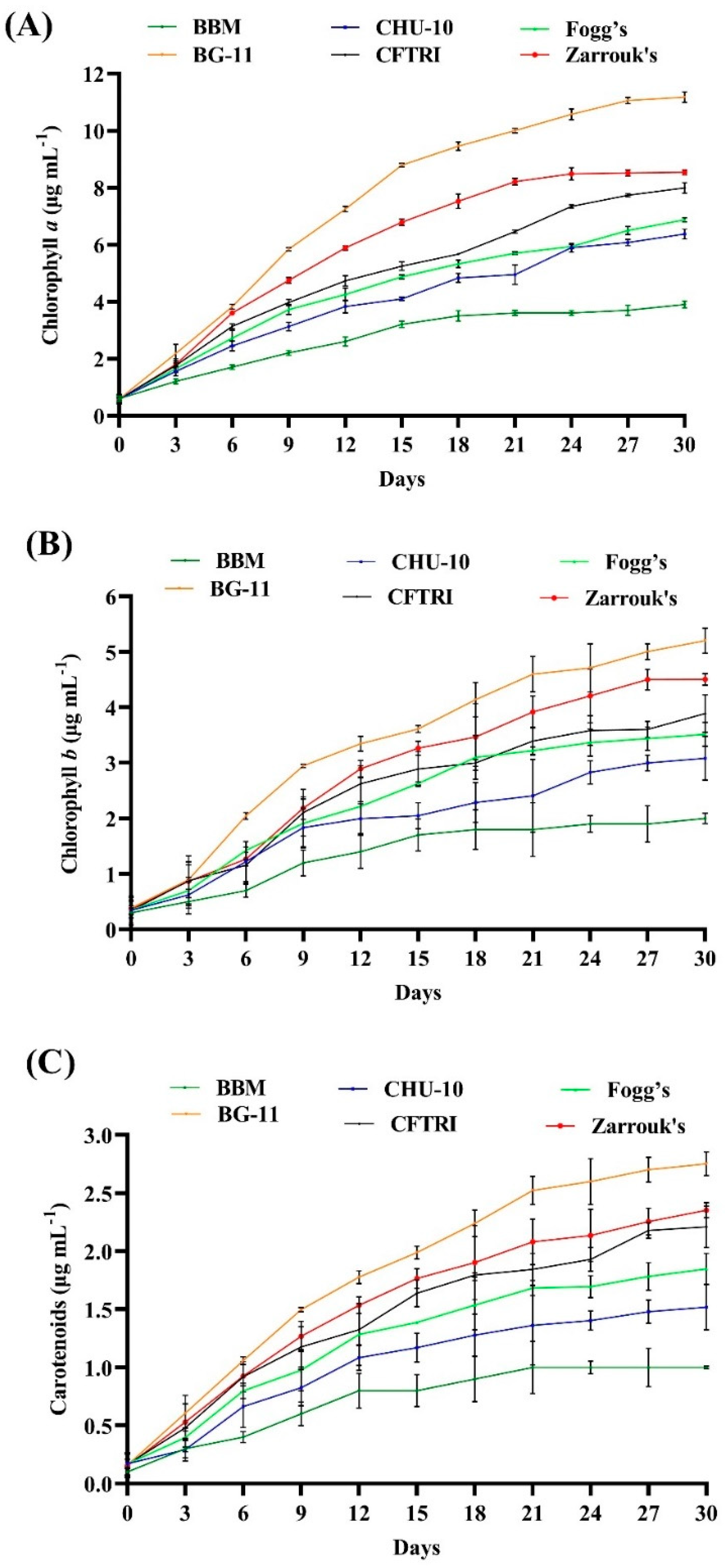
Different autotrophic media were prepared by altering the specific nutrients to encourage microalgae growth, which was determined by the amount of photosynthetic pigments. The growth of microalga
Scenedesmus rotundus-MG910488 in the different autotrophic media was estimated in the term of photosynthetic pigments by utilizing the spectrophotometric measurement of photosynthetic pigments (
Figure 3A–C and
Table S4).
The highest chlorophyll contents, i.e., Chl-a, Chl-b and carotenoids, were observed in the cells grown in BG-11 medium (11.174 ± 0.181 μg mL−1, 5.202 ± 0.229 μg mL−1 and 2.753 ± 0.103 μg mL−1, respectively) followed by Zarrouk’s medium (8.540 ± 0.089 μg mL−1, 4.506 ± 0.106 μg mL−1 and 2.352 ± 0.065 μg mL−1, respectively) and CFTRI medium (7.987 ± 0.182, 3.889 ± 0.338 μg mL−1 and 2.210 ± 0.179 μg mL−1, respectively). A lesser amount was found in CHU-10 and Fogg’s medium, which were (6.375 ± 0.174 μg mL−1, 3.082 ± 0.395 μg mL−1, 1.518 ± 0.195 μg mL−1), (6.876 ± 0.072 μg mL−1 3.515 ± 0.214 μg mL−1 1.847 ± 0.135 μg mL−1), respectively. The lowest amounts of pigments were found in the cells grown in BBM (3.857 ± 0.120 μg mL−1 Chl-a, 2.013 ± 0.093 μg mL−1 Chl-b and 1.022 ± 0.013 μg mL−1 carotenoids), which indicates the lower photosynthetic activity of microalga. Obtained results show that the amounts of chlorophyll pigments are significantly affected by the different growth media.
The content of pigments may be influenced by the variation of chemical ingredients and their different concentrations in the growth medium. Among six other media, BG-11 and Zarrouk’s were the most suitable growth media for the growth of photosynthetic pigments in
Scenedesmus rotundus-MG910488. Similarly, George et al. [
35] and Chokshi et al. [
18] also found BG-11 and Zarrouk’s as the most suitable media.
3.4. Effect of Different Growth Medium on the Dry Cell Weight and Biomass Productivity of Scenedesmus rotundus-MG910488
The growth medium is one of the crucial aspects to be examined to increase the productivity of microalgae biomass [
36]. Microalgae have been recorded to endure in all possible environments. Hence the impact of specific growth medium on the biomass content and biomass productivity of
Scenedesmus rotundus-MG910488 has been explored in the current experiment (
Table S5). To study the effects of different media ingredients on the biomass content and its productivity, the freshwater microalga
Scenedesmus rotundus-MG910488 was inoculated in the other growth media, i.e., Zarrouk’s, BBM, CHU-10, Fogg’s, BG-11 and CFTRI, and cultivated in batch culture for up to 30 days of inoculation.
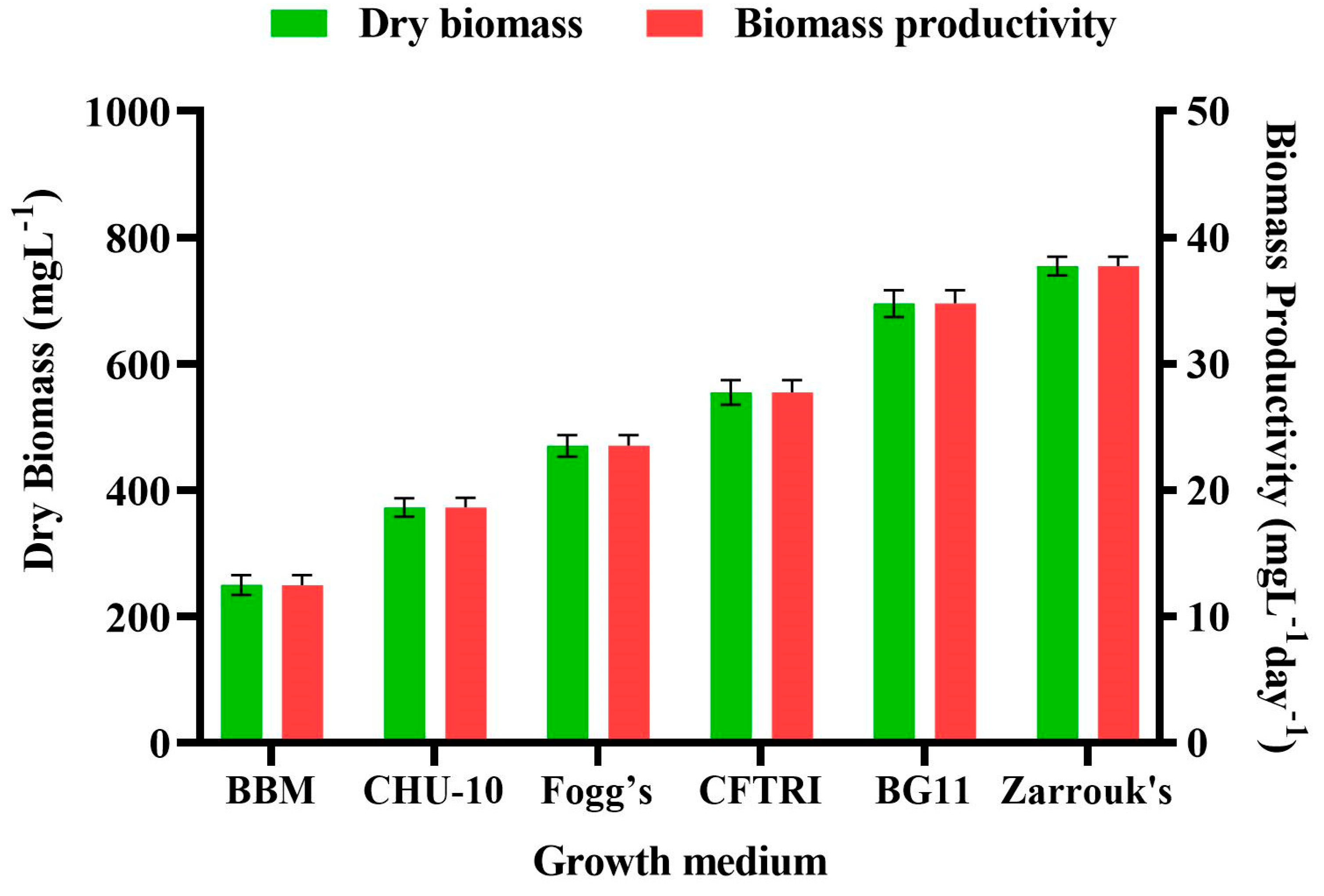
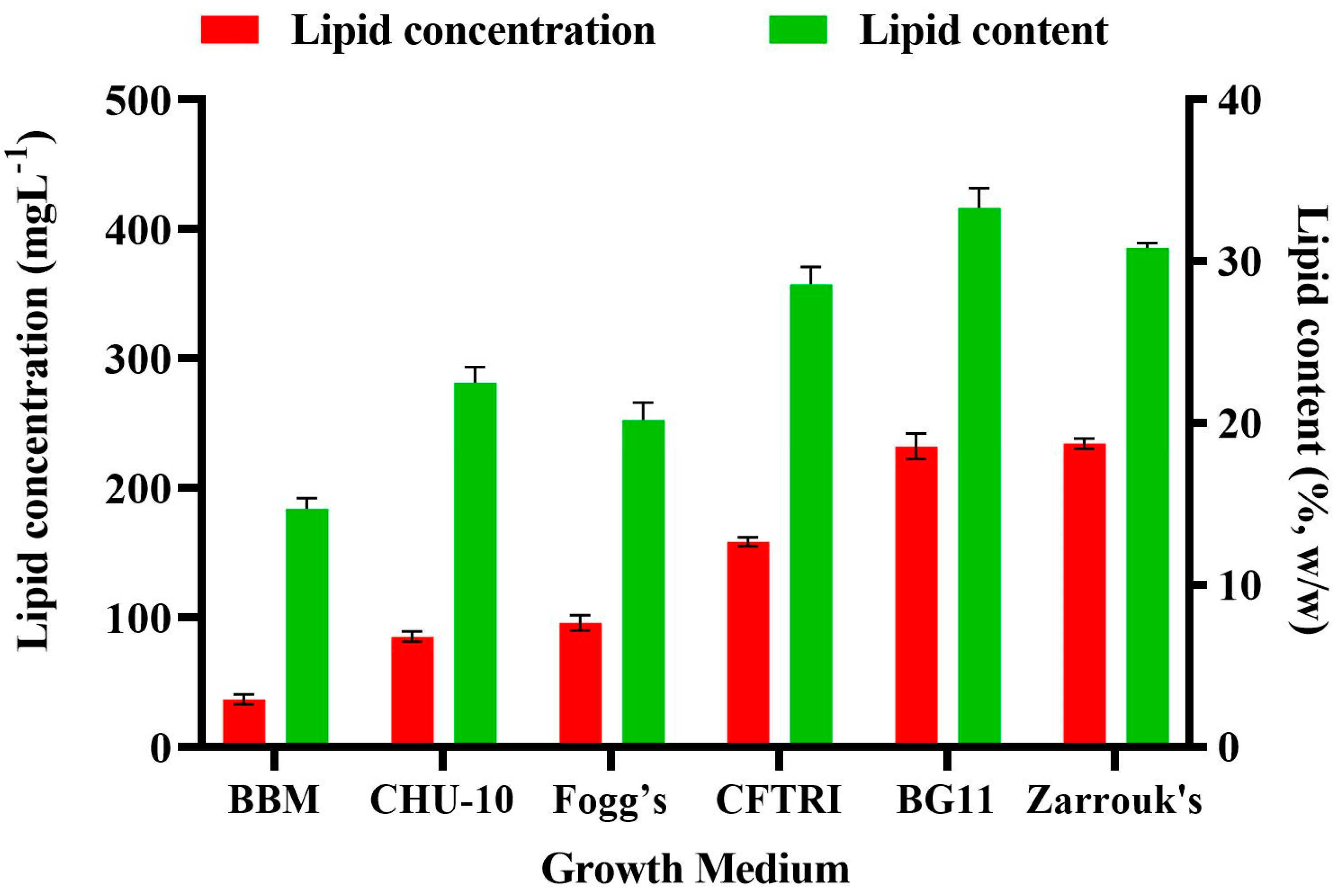
Figure 4 shows changes in biomass content and biomass productivity (BP) of
Scenedesmus rotundus-MG910488 cultivated in different culture media, which illustrated that the components of the growth medium and their different concentration affect the metabolic process and biomass productivity of the microalga.
The highest amount of dry cell weight and the biomass productivities were obtained in Zarrouk’s medium (754.56 ± 14.80 mg L
−1 and 37.73 ± 0.74 mg L
−1, respectively), and the dry cell weight of 695.15 ± 21.32 mg L
−1 and the biomass productivity of 34.76 ± 1.07 mg L
−1 were found in the BG-11 medium. Bayona et al. found the highest biomass production and highest growth kinetic in BG-11 medium for the green microalgae
Botryococcus braunii [
37]. Similarly, Sharma et al. evaluated BG-11, BBM, Fogg’s and M4N medium for the biomass and lipid content; obtained results indicated that the BG-11 was the most economical and effective growth medium for
Chlorella species to produce maximum biomass and lipid content [
38].
The lowest biomass content of 249.49 ± 15.81 mg L
−1 with the biomass productivity of 12.47 ± 0.79 mg L
−1 was found in the culture grown using BBM. The reason for the highest DCW in Zarrouk’s and the lowest in BBM is most likely the nitrate content, which is 10 times higher in Zarrouk’s medium than in the BBM [
18] as carbon is also one of the crucial elements responsible for the growth of microalgae [
38]. The highest dry cell weight and the biomass productivities were obtained in ZM, whereas the lowest biomass along the biomass productivity was recorded in BBM. So, the presence of vitamins in modified CHU-10 medium showed 372.74 ± 14.64 mg L
− 1 dry cell weight; and 18.64 ± 0.73 mg L
−1 biomass productivity, which made it better than BBM. Fogg’s medium also did not show significant biomass content (470.09 ± 16.94 mg L
−1) and productivity of biomass (23.50 ± 0.85 mg L
−1) due to lack of carbon ingredients in their composition [
38]. Due to a smaller amount of chemical ingredients in BG-11 medium and the production of maximum biomass, it was recorded as the most suitable growth medium for the growth and biomass productivity of
Scenedesmus rotundus-MG910488.
3.5. Effect of Different Growth Medium on the Lipid Content of Scenedesmus rotundus-MG910488
The medium chosen for the growth of microalgal cultures plays an essential role in optimizing the growth and lipid content of microalgae. The composition of biomolecules of microalga is significantly affected by the ingredient of macro and micronutrients in the growth media and their concentration available to the cells [
32]. Changing media components may trigger some biochemical changes in microalgae due to stress, thereby promoting the synthesis of protein, lipid and carbohydrates of microalgae [
36].
The impact of different growth media on the lipid accumulation in
Scenedesmus rotundus-MG910488 microalga is presented in
Figure 5. High availability of carbon has been reported to result in higher activity of Ribulose-1 5-bisphosphate carboxylase/oxygenase (RuBisCO), which is a crucial enzyme in plants and microalgae for the conversion of 3 phosphoglycerates; it is a substrate for the biosynthesis of carbohydrate and fatty acid [
39]. The lipid content was maximum in BG-11 (33.30 ± 1.21%), followed by Zarrouk’s (30.82 ± 0.32%) whereas, in BBM, it was 14.81 ± 0.67% (
Table S6). Citric acid was present as an additional element in the BG-11 medium and iron (Fe) in the form of ferric ammonium citrate. Citric acid helps to solubilize the salts, which prevents precipitation of another chemical ingredient of the growth media. Thus, it increases their abundance in the cells and contributes to a higher growth rate. These findings are supported by the observations of Gour et al. [
32], where the highest biomass and lipid accumulation was found in the modified BG-11 medium over the other media selected for the growth and lipid estimation of
Scenedesmus dimorphus. Rios et al. found similar observations in growth and lipid accumulation of
Desmodesmus sp. in BG-11 medium over the Guillard F/2 media [
40].
Chokshi et al. reported similar results—that BG-11 was the most economical and efficient growth medium for the microalga
Acutodesmus dimorphus among the tested media [
18]. Sharma et al. also reported that the BG-11 medium was the most economical and suitable growth medium for the cultivation of Chlorella sp. when compared with the other autotrophic growth media used [
31]. The results confirm that for the culture of
Scenedesmus rotundus-MG910488, BG-11 is an appropriate growth medium. It also supports that medium ingredient significantly impact the microalgae growth and content of biomolecules. The evaluation of chemical profiling of microalgal biomass is necessary to understand the chemistry of neutral and polar lipids to look into their potential to produce biodiesel and valuable product. It also helps predict the quality of physio-chemical properties and yield of microalgal derived biofuels. Hence, NMR, GC-MS and FT-IR analysis are used to characterize and determine the quality of biodiesel and their composition of fatty acids [
41].
3.6. Fatty Acid Methyl Esters Profiling by Gas Chromatography-Mass Spectrometry (GC-MS)
Fatty acids are known for their reasonable balance of better fuel properties [
42]. The chain length of fatty acids lies between C16, C18 and C19 to produce high-quality biofuel [
43]. Biodiesel is mainly enriched with five C14-C18 fatty acids: C14:0 (myristic acid), C16:0 (palmitic acid), C18:0 (stearic acid), C18:1 (oleic acid), C18:2 (linoleic acid), and C18:3 (linolenic acid) [
44].
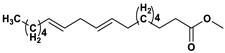
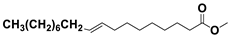
The GC-MS analysis of
Scenedesmus rotundus-MG910488 biodiesel showed the six types of major fatty acid methyl ester components. Oleic acid methyl ester (43.4%), linoleic acid methyl ester (19.5%) and palmitic acid methyl ester (12.93%), are the significant fatty acid methyl esters present in the microalgal sample, along with stearic acid methyl ester and palmitoleic acid methyl ester in lesser amounts relative to total fatty acid methyl esters. Apart from these components, heptacene, henicosyl formate, ergost-7-en-3-ol and stigmasta-7 are the other components with the least amount, along with phytol. The chromatogram of GC-MS shows each peak of a fatty acid at a certain retention time as well as the corresponding amount of fatty acids, respectively (
Figure S1). The fatty acid methyl esters at the retention time (RT) 12.818 min, 18.013 min, 22.624 min and 22.819 min shows the base peak at
m/
z 57.10, 55.05, 67.05 and 55.10, respectively, while at retention time 18.619 min. and 23.381 min. shows a similar base peak at
m/
z 74.05, as shown in the
Supplementary Materials (Figure S2a–f) [
45]. The fatty acid methyl esters were confirmed via examining their mass spectra with the data present in NIST08 (National Institute of Standards and Technology, US, WILEY 08) library. The GCMS data of the biodiesel sample shows the presence of 9-Octadecenoic acid methyl ester (oleic acid methyl ester) in the highest percentage, which was followed by the methyl octadeca-9,12-dienoate (linoleic acid methyl ester) and hexa-decanoic acid methyl ester (palmitic acid methyl ester); notice the chromatographic peak intensity, retention time and area percentage in
Table 3.
Excellent biodiesel quality could be achieved, when microalgal-lipid contains a higher amount of mono-unsaturated fatty acids such as palmitoleic acid (16:1) and oleic acid (18:1), and their presence offers a balance between oxidative stability, viscosity, lubricity, combustion heat and cold filter plugging point (CFPP) [
44,
46]. So, from this GC-MS study, it can be noticed that
Scenedesmus rotundus-MG910488 microalga has a remarkable quantity of C16 and C18 fatty acid methyl esters, which helps to produce high-quality biodiesel.
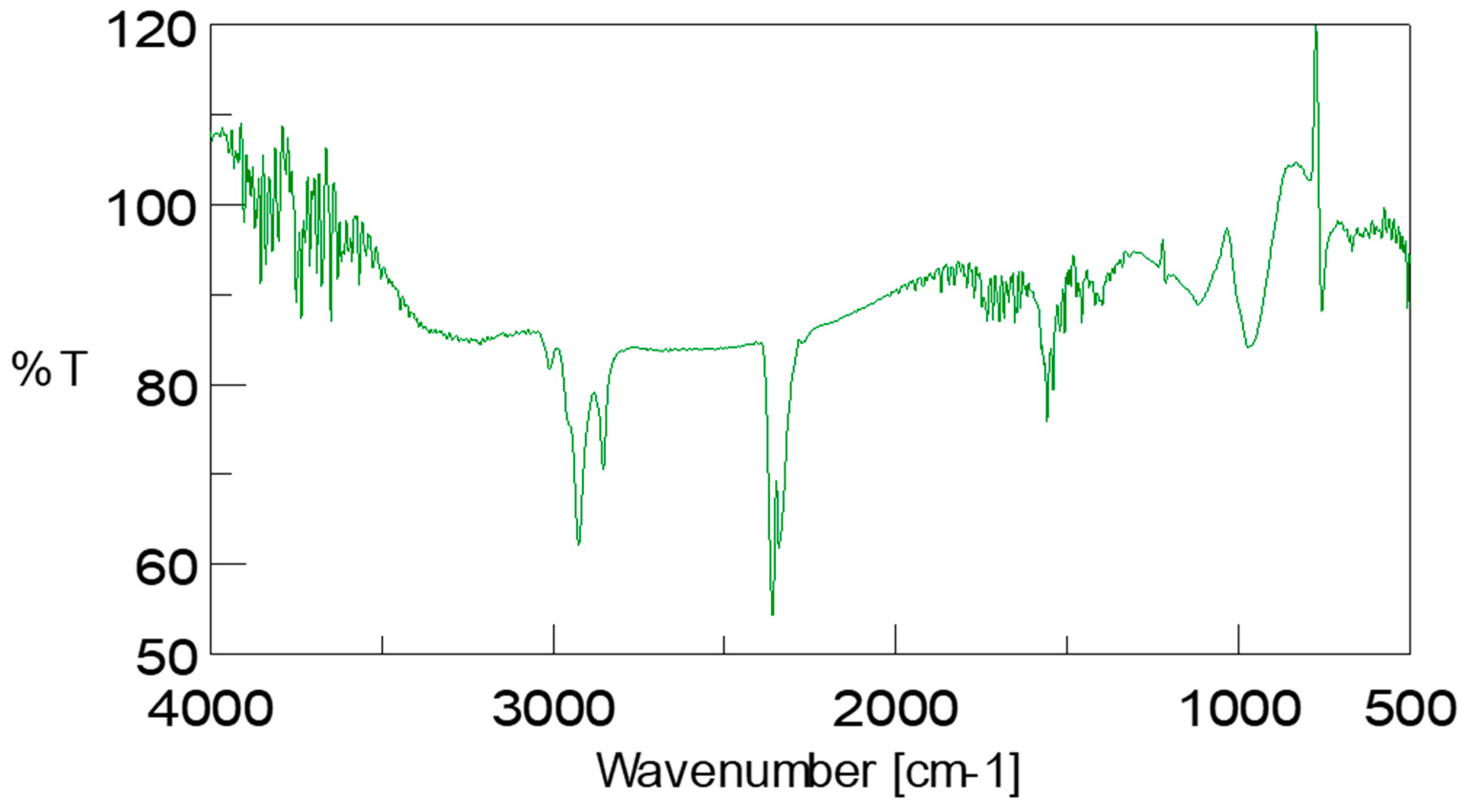
3.7. Confirmation of Biodiesel by Assignment of Bands Found in ATR-FTIR Spectrum of Scenedesmus rotundus-MG910488 Fatty Acid Methyl Esters
The biodiesel sample was further analysed using Fourier Transform-Infrared Spectroscopy (FTIR) with attenuated total reflectance (ATR) accessory. FTIR spectra of
Scenedesmus rotundus-MG910488 biodiesel were measured in the wavelength range of 500–4000 cm
−1 shown in
Figure 6. The IR bands were tentatively identified [
47,
48]. The obtained IR result from the biodiesel sample shows the (O-H) stretching band of the hydroxyl group at 3211.85 cm
−1. C-H stretching band can be observed at wavelength 2854.18 cm
−1, 2925.48 cm
−1 and 3011.30 cm
−1. The presence of the carbonyl group (C=O bond) and C-O in the biodiesel sample is confirmed by peaks in the range of 1733.60 cm
−1 and 1118.51 cm
−1. Two alkane peaks attribute the bending mode of methyl (-CH
3) and methylene (-CH
2) group at 1456.96 and 1397.17 cm
−1, respectively. The alkane C-C stretching band was observed at 1652.7 cm
−1 for CH
3 and CH
2 at 1456.96 cm
−1 and 1397.17 cm
−1 respectively, ether group at 1733.60 cm
−1 alcoholic group O-H at 3211.86 cm
−1 and 3643.66 cm
−1. These functional groups show the presence of fatty acid methyl ester in the
Scenedesmus rotundus-MG910488 biodiesel (
Figure S3).
Based on the IR results reported, the peak at 2855–3000 cm
−1 indicates the methylene and methyl stretching, which shows lipid content. The peak of FTIR at 3011.30 cm
−1 shows the (-CH=CH-) vibrational band from the unsaturated fatty acid and the peak at 1733.60 cm
−1 shows the presence of carbonyl (C=O) group of triglycerides, and the (O-H) stretching band indicate the hydroxyl group at 3211.85 cm
−1. C-H stretching band is confirmed by 2854.18 cm
−1 2925.48 cm
−1 and 3011.30 cm
−1. Peaks at 1715 ± 100 cm
−1, as reported in the literature, demonstrate the existence of the C=O bond (carbonyl group) [
30,
31,
49,
50,
51].
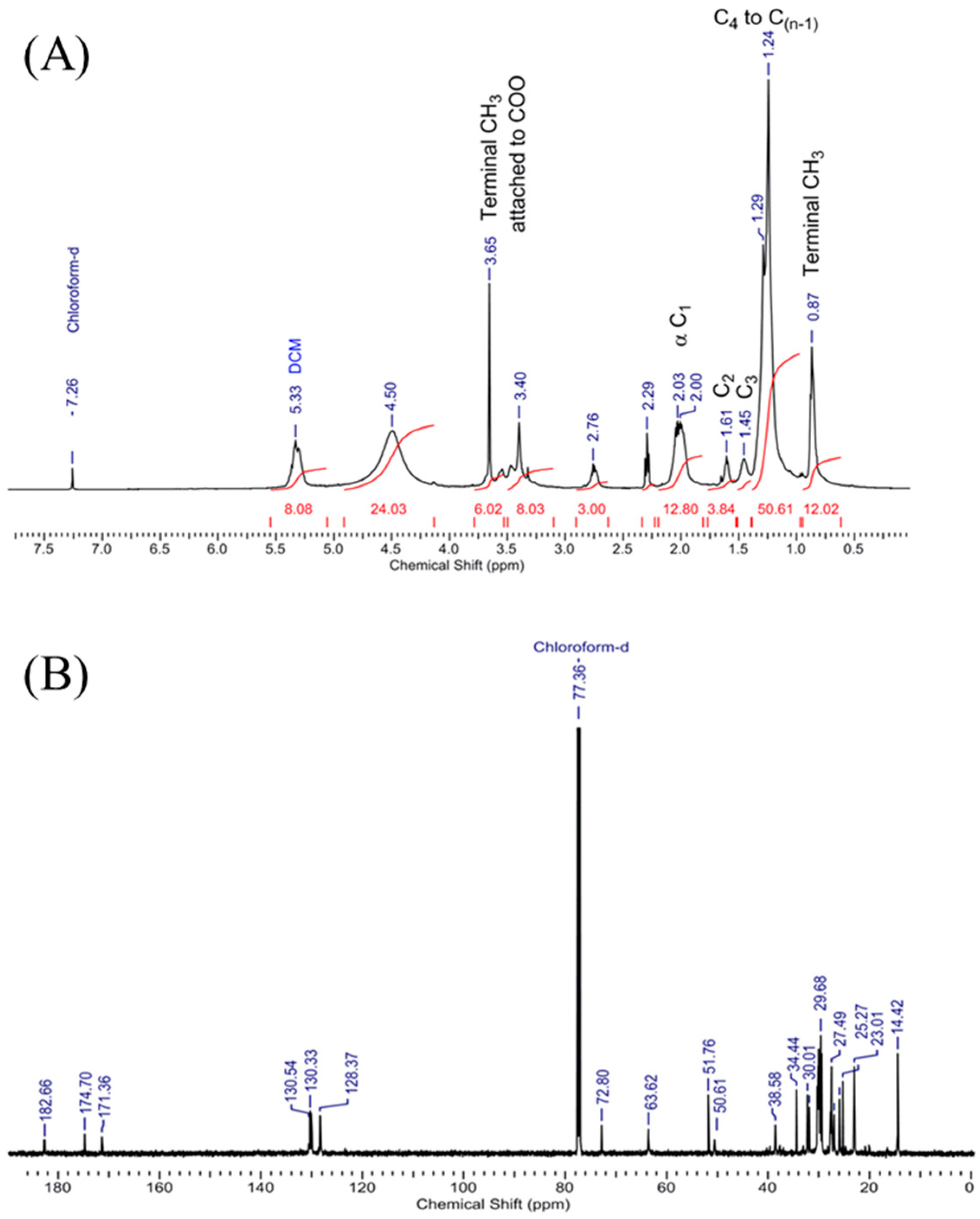
3.8. Confirmation of Biodiesel by Assignment of Peaks Obtained in 1H NMR and 13C NMR Spectrum of Scenedesmus rotundus-MG910488 Fatty Acid Methyl Esters
1H NMR (500 MHz, CDCl
3) was measured and analysed, keeping the reference chemical shift peak at δ 7.26 for CDCl
3, which shows the presence of OH proton at δ 8.49. The dried sample had no peak at δ the 7–8 region, which ruled out the existence of an aromatic ring in the sample. An intense peak at δ 1.482–1.317 confirms the presence of C-H of alkanes. Peaks at δ 2.0, 2.32 and 2.76 confirm the alpha CH
2 near the functional group carbonyl of fatty acid methyl ester, whereas the presence of δ 3.29–3.68 peaks confirms the terminal methoxy group presence for different esters (-OCH
3). Peaks at δ 4.5 and 5.4 are for dichloromethane and ethyl acetate in the sample (
Figure 7A). The existence of the characteristic peaks in the microalga sample illustrates the conversion of triglycerides to methyl esters of fatty acids [
52].
13C NMR (100 MHz, CDCl
3) was also measured, and analysed by keeping reference chemical shift peak at δ 77.36 for CDCl
3; the peaks at 182.7, 174.7 and 171.4 confirm the presence of three types of CO carbon of three different fatty acid methyl ester [
41] (biodiesel), and the peaks at 72.8, 63.6 and 51.7 confirm the terminal OCH
3 carbon in the sample. In contrast, the peaks ranging from 14 to 40 ensure the alkyl chains carbons in the biodiesel (
Figure 7B). So, the
1H and
13C NMR confirms the presence of fatty acid methyl ester in the
Scenedesmus rotundus-MG910488 biodiesel successfully. The assignments of peaks obtained by
1H NMR and
13C NMR spectrum confirm the mixture of the sample was biodiesel. The peaks of 1H NMR indicate the presence of OH proton, C-H of alkanes, alpha CH
2 near to carbonyl of fatty acid methyl ester and terminal methoxy group presence for different esters (-OCH
3).
13C NMR peak shows the presence of CO carbon of three other fatty acid methyl esters. Some peaks confirm the existence of terminal OCH
3 carbon in the sample, whereas the peaks found between 14 to 40 confirm the presence of alkyl chains carbons. Sarpal et al. [
41] also reported the importance of NMR, FT-IR, and GC-MS techniques to estimate the composition of fatty acid methyl ester derived from the different strains of microalgae [
14].
4. Conclusions
The study focuses on the identification of the most suitable and economically viable growth medium to produce higher biomass along with the high lipid yields from the newly isolated strain of microalga Scenedesmus rotundus-MG910488. The evaluation of fatty acid methyl esters of this strain was performed and they were found as a potential candidate for biodiesel application, since it also has an inherited quality of fast growth, capability to produce a sufficient amount of biomass and high lipid accumulation, without optimizing any growth conditions or sparging of air and CO2. The different autotrophic growth media were studied, and the results reveal that the BG-11 is the best growth media to generate higher biomass and the lipid content from the MG910488 strain. However, for further improvement in biomass and lipid production, cultivation conditions can be optimized with other parameters such as nutrients tress, supplementation of CO2, temperature, light intensity, mixing of culture, etc.
The qualitative analysis of the obtained product from Scenedesmus rotundus-MG910488 was performed using FT-IR, 1H NMR and 13C NMR, which confirms the chemical-structural aspects of the biodiesel sample. The GC-MS analysis showed the presence of linoleic acid methyl ester and oleic acid methyl ester, which is also significant in making it a suitable candidate for producing biodiesel.