1. Introduction
In our daily lives, the existence and use of tires is considered natural, without taking into account, among other problems, their end-of-life and disposal [
1,
2]. After serving their purpose, tires are commonly disposed of and used for energy recovery. This is usually performed either by direct combustion in incineration units or by co-incineration in cement kilns [
3,
4,
5]. During the process, in addition to energy recovery and in the case of industrial tires, structural iron and sulfur from rubber vulcanization are both reused, as substitutes for pyrite, as raw material for clinker production [
6,
7]. However, given the enormous number of vehicles used worldwide and the consequent volume of waste accumulated at a global scale, a very significant proportion of end-of-life tires are just stockpiled, without any control, landfilled or burned. Despite the environmental impact caused by such disposal, concern about its potential use for energy recovery and application in the production of new industrial rubber-based products is still scarce [
8,
9,
10]. However, the composition of tires is complex, and several problems can be associated with the management of such a group of compounds, as presented in
Table 1.
The use of thermochemical conversion processes for the valorization of waste tires has been previously documented in the scientific literature and is available through several works, where different options are presented. For example, Fini et al. presented a work on the synthesis and characterization of modified rubber asphalt, which combines the sustainable management of waste tires and swine manure in the solution presented. The authors describe the production process of a biobinder through the use of a thermochemical conversion process, which combines the subsequent mixture of the biobinder with crumb rubber and an asphalt binder to produce biomodified rubber asphalt [
12]. However, despite the existing alternatives, energy recovery seems to be the preferred option for most of the studies, especially when analyzing the studies carried out in recent years. These studies are also in line with the investigations carried out for the characterization and valorization of urban solid waste, where a particular focus was placed on the fraction of plastics. Indeed, in studies such as the one presented by Shi et al., the energy recovery from municipal solid waste is carried out using thermochemical conversion processes. This work modelled the evolution of the heating value of these materials, to define the most efficient processes. The authors resorted to thermogravimetric analysis to simulate two thermochemical processes, namely, pyrolysis and torrefaction, in order to study the thermal properties of the residual materials [
13]. Other authors, such as Kordoghli et al., applied thermochemical conversion processes to the rubber of waste tires [
14]. These authors also used thermogravimetric analysis to assess the kinetic and thermodynamic parameters of thermochemical transformations. The authors then used a small laboratory-scale reactor to produce samples in which they analyzed the correlation between the influence of catalysts on waste tire pyrolysis. More recently, Calado et al. studied the possibility of energy recovery by the thermal conversion of waste tires as a potential feedstock for combustion and gasification processes [
15]. These authors consider it necessary to use these materials in co-firing and co-gasification systems because they assume difficulties in using these residues in isolation. In this perspective, the authors carried out the tests using a mixture of waste tires and biomass and concluded that gasification is a perfectly adjusted technology for the valuing of waste tires, allowing its transformation into fuel for energy recovery from waste that would not be possible to recover in any other way. In addition, having as motto the growing problem of waste tires generation worldwide, Policella et al. studied its energy recovery through thermochemical conversion processes and presented CO
2 gasification as a possibility for tire recovery [
16]. These authors analyzed the quality of the syngas produced, concluding that this methodology, in addition to presenting a valid solution for solving the environmental problem of the deposition of waste tires, can also be a direct pathway for reducing greenhouse gas emissions.
The use of catalytic hydropyrolysis for waste tires was also another thermochemical conversion process studied. Wang et al. studied the use of this process for the production of gasoline fuel [
17]. The authors analyzed the effects of variables influencing the catalytic hydropyrolysis process, such as the type and concentration of the catalysts, the operation temperature, residence time and pressure. They concluded that catalytic hydropyrolysis is an alternative option to produce high-quality fuels from waste tires.
The relationship between waste tires and plastic waste continued to be addressed from a joint recovery perspective. Carmo-Calado et al. considered that given the similar properties between these two types of waste, it would make perfect sense to reuse them together [
18]. These authors also understood that it is possible to mix these materials with biomass, considering the thermal conversion difficulties of these residues. The results obtained demonstrated the viability of the gasification process up to a certain limit of waste mix.
Regarding other forms of recycling waste tires, they have only recently started to be addressed, with the objective of finding solutions for solving the huge environmental problems caused by the remaining high volumes of this type of waste. Thus, Xu et al. analyzed the possibility of producing carbon black from thermochemical conversion processes [
19]. These authors, unlike other studies that mainly focused on the liquid phase, devoted special attention to the solid character, from where pyrolysis black carbon can be recovered. This is a very important achievement for the tire industry due to its wide application and high value utilization in a circular economy perspective. In this study, the authors investigated (i) the influence of process temperature and residence time, (ii) the catalysts to be used and (iii) the reactor type. However, in the conclusions of this work, the authors also referred that it is necessary to adjust the procedures of the production process to the specific characteristics of the raw materials, which need to be combined with more experimental results to realize its full feasibility.
The possibility of recycling tires and reusing/recovering some materials that could be incorporated into different production processes (namely that of producing new tires), presents itself in a perspective of circularizing the economy and enhance the sustainability of this industry of extreme importance [
20,
21]. The recycling of tires would benefit the reduction in operational costs from the recovered material and largely solve the environmental issues and impact linked to their huge accumulation and disposal problem [
22]. The use of new processes, such as thermochemical conversion technologies, which have a long history of use in other sectors (e.g., biomass), may present as an efficient alternative to traditional technologies [
23]. This process allows for the recovery of materials, namely carbon, which can be used in the production of carbon black, one of the raw materials for tire production [
24]. Carbon black is a paracrystalline material obtained from the incomplete combustion of heavy petroleum products [
25]. The resulting material is then added to the rubber as a reinforcing filler [
26]. Furthermore, the condensation of gases resulting from the pyrolysis leads to the formation of a liquid usually called pyrolytic oil. In this sense, the recovery of carbon from the rubber of an end-of-life tire, as well as the pyrolytic oil, can give rise to new raw materials to be used in the production of carbon black [
27,
28,
29].
Tire recycling has become a need that has been identified since the 1980s, mainly due to the existence of huge tires piles accumulated and dispersed all over the world, which represent a serious environmental problem needing a solution. Roy et al. (1990) presents one of the first works where the possibility of recycling scrap tires to oil and carbon black using pyrolysis is possible, since, as the authors refer, scrap tires represent an immense source of energy and chemical products [
30]. In other words, as sustained in the work, the thermochemical decomposition of rubber, occurring at low pressure, allows the recovery of various compounds, which can be reused and valued. This project managed to separate several compounds, with very interesting percentages reaching 55% for oil yield and reaching 25% for carbon black. The authors concluded that the process can be viable, especially if economies of scale are obtained and if there are uses for all fractions available and resulting from the process, namely, the gaseous fraction and recovered metals. It was verified, at this stage, that the quality of recovered carbon black favorably compared with the low standard grades and may find an application in low grade rubber goods but need to be followed by further research and development.
As this and other projects proved to deserve more attention, thermochemical conversion processes continued to be the means through which several researchers addressed the problem of valuing scrap tires. In fact, pyrolysis was used in several projects, with the objective of finding the most efficient way to achieve, on the one hand, the recovery of a waste, while on the other hand, finding a solution for the large volume of existing and growing waste. Roy et al. (1999) went a little further, taking advantage of the experience acquired in previous projects, also obtaining oil, gas and carbon black from the pyrolysis of scrap tires [
31]. This time, the authors investigated several commercial applications for the different products obtained during the pyrolysis process. The carbon black obtained, which the authors designated as pyrolytic carbon black (CBp) has a surface chemistry and activity like those presented by available commercial carbon black and, therefore, presents a potential to replace commercial carbon black grades in certain rubber applications. The remaining fractions obtained, such as gas and pyrolytic oil, were also tested, and the authors reported several possibilities of valuation, including for the heavy fraction, which presented the production of coke as the best use, which can be incorporated in road pavements.
A different approach was used by Piskorz et al. [
32], who used a hydrogenation process specifically developed to recover carbon black from rubber crumbs prepared from scrap tires. In this process, the rubber was placed in an autoclave, with the hydrogen being continuously bubbled through a slurry of rubber crumbs and parafinic dissolution oil under pressures of 300 to 1500 psig and temperatures around 400 °C. This process led to the dissolution of the rubber, allowing carbon black particles to escape from the polymer matrix. The authors reported a yield of carbon black (in association with other inorganic assets) of approximately 36%, 1–2% gas, and 8% naphtha. The remaining 56% is made up of oil that can be recycled as a dissolution agent. The carbon black obtained by this liquefaction process, after being filtered and dried, presented properties very close to those of the various grades added during tire manufacturing, as part of the inorganic contaminants could be removed with the use of a simple acid wash. The authors also refer to the fact that carbon black has a higher value than any of the other products obtained, and may, therefore, be the determining factor for the economic viability of a large-scale process. Developments continued, with several authors presenting other possibilities for the recovery of carbon black, as was the case with the process described by Tang and Huang [
33], which describes the recovery of carbon black and also other products in the form of gases, using thermal nitrogen plasma pyrolysis of used tires. In this study, the authors report the recovery of a yield that can vary between 30 and 35% CBp if steam is not injected during the process, while with steam injection, the carbon black yield was of about 23%. As tires have, on average, 25–30% virgin carbon black in their composition, it was found that when obtaining a yield of 30–35% there must be some chemical process during the pyrolysis which enhances the formation of more carbon black, indicating a good possibility to find an alternative path to produce carbon black without the need to resort to the use of fossil fuels.
The recovery of carbon black seems to have also aroused interest due to the capacity that this material has for the adsorption of chemical elements, as demonstrated by Smith et al. [
34], who investigated the adsorption of an aqueous mixture of light rare earth elements (Y, La, Ce, Nd and Sm) by carbon black derived from pyrolyzed scrap tires. The authors concluded that the use of CBp as a sorbent material for the extraction of aqueous rare earth elements can be attractive due to its inexpensive cost and utilization of a recycled material stream, giving strength to the circular economy approach of this industry. In fact, this study later came to see its results confirmed by another one, presented by Sugatri et al. (2018), which confirms an improvement in the adsorbent capacities of carbon black, as it appears that the increase in the temperature of the pyrolysis process could activate surface function groups, such as C–O stretching in the carboxyl group in carbon black recycled at 410 °C to resemble standard carbon black N660. As a measure for the improvement of the final product, the use of an acid wash to remove inorganic impurities, as previously mentioned in the study by Piskorz et al. [
32], was presented, this time using hydrochloric acid 1 M for 2 h.
With the advent of decarbonization processes in the economy, the search for processes that can replace all fossil fuels uses acquires even more importance. However, carbon black, which consists of a material that can be obtained through the partial combustion of heavy petroleum feedstock, is one of the products for which it is necessary to find an alternative. However, the preparation of carbon black requires sophisticated equipment, chemical pre-treatment, and a set of complex separation and purification techniques, so the use of carbon black recovery from scrap tires remains a current issue, as demonstrated by the work presented by Gómez-Hernández et al. [
35]. In the work carried out, these authors stated that although the method is possible and widely known, the yields obtained are modest and the process is technically complex, a fact that led these researchers to invest in the development of a simple and inexpensive method for the preparation of carbon black from scrap tires. This work reports, for optimal conditions, a carbon black recovery of 81%, with the material showing good thermal stability and conductivity, with a chemical composition presenting values for carbon, sulfur, and oxygen of 84.9%, 10.21%, and 4.9%, respectively.
The sequence of work carried out in this period points to a wide range of uses for recovered carbon black, since more and more fields of possibilities are pointed out for this recovered carbon black. Xu et al. [
19], in the review they carried out on the high-value utilization of scrap tires, focused precisely on modified carbon black from pyrolysis, and present the pyrolysis of carbon black as an alternative to commercial carbon black in rubber manufacturing, as activated carbon in pollution control, and as biochar for soil amendment. Along the same lines, Dwivedi et al. [
36] classify the recycling of scrap tires for pyrolysis as an environmentally friendly alternative reinforcing filler for natural rubber compounds. These authors used the recovered carbon black in rubber compounds of general-purpose conveyor belts to investigate its practical feasibility as a substitute for commercial-grade N330 carbon black, demonstrating a good equivalence between the recovered products and the standard commercial products.
However, despite the various developments achieved regarding the possibility of obtaining recovered carbon black, all the works, even if indirectly, point to the need to implement purification processes for the products resulting from pyrolysis or from the other processes used and described above. Cardona-Uribe et al. [
37] applied purification and demineralization processes to improve the properties of the recovered carbon black. These authors consider that the experience gained provides an important contribution to deem the recovered carbon black as an alternative to virgin carbon black and paves the way to choose the scrap tire recycling loop using both pyrolysis and chemical upgrading strategies. For example, the study of Roy et al. [
31], using vacuum pyrolysis methods, approaches the topic of carbon black and bio-oil recycling. At the time, the authors highlighted the need to deal with the disposal of the huge existing piles of scrap tires observed everywhere. In 1999, the topic was again raised by these same authors. However, no additional developments to the previous study were presented. Zabaniotou and Stavropoulos [
38] also resorted to pyrolysis for the valuation of end-of-life tires, with the prospect of finding ways of valuing the resulting char.
Nonetheless, although this approach was proposed so long ago, the path followed was a different one. Even when recycling was the central theme of the studies, e.g., Shu and Huang (2014), its end-use would be the incorporation of rubber from tires into asphalt or concrete [
39]. Guo et al. [
40] also followed this path, developing several types of modified concrete with the incorporation of tire rubber. Recently, Gheni et al. [
41] developed cement-based mortars where different percentages of rubber-fiber powder (RFP) were incorporated to determine the different degrees of durability. There are also several other studies that addressed the adsorbent properties of char produced from tire rubber, namely, for the removal of phenolic compounds, as presented by Makrigianni et al. [
42]; in the recovery of gold from acidic solutions; or even in the removal of bisphenol [
42,
43,
44].
The objective of this study was therefore to initiate a more comprehensive project, aimed at the reconversion of scrap tires, through the recovery of compounds using thermochemical conversion technologies, such as pyrolysis. Additionally, we studied the reuse of the resulting compounds, namely, rubber char, to produce carbon black, which can be used in the production of new tires or other types of rubber products for industrial use.
3. Results and Discussion
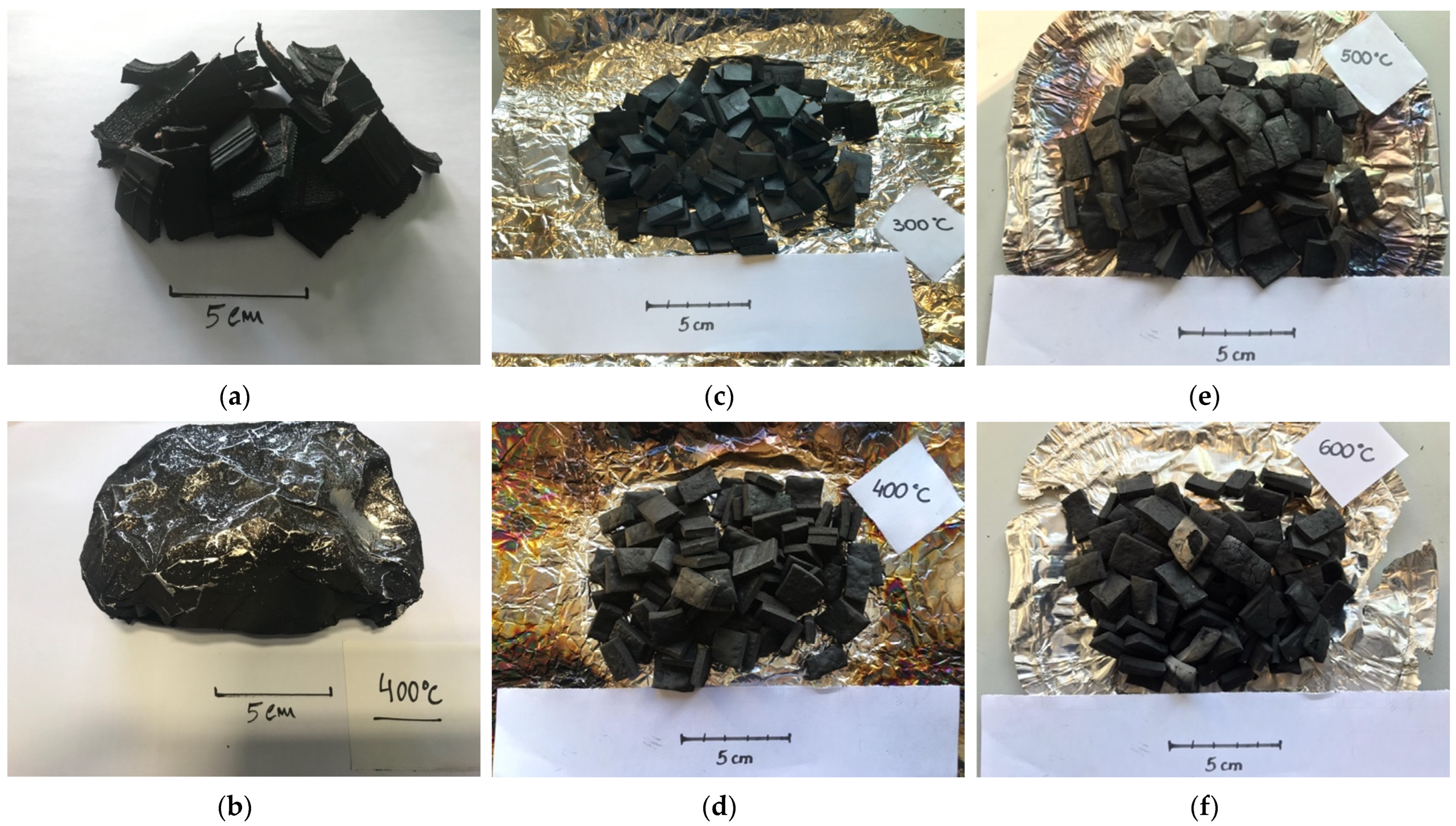
The samples were subjected to a torrefaction (300 °C) and carbonization process at different temperatures (400–600 °C), according to the methodology presented in
Section 2. After this step, the samples were analyzed from the point of view of its elemental and proximate composition. The results obtained are shown in
Table 2,
Table 3 and
Table 4 and in
Figure 2.
The first aspect that deserves attention is related to the loss of mass observed for samples processed at 300 °C and 400 °C. As can be seen in the results for samples processed at 300 °C, the mass loss reached 63.42 ± 2.41%, while for samples processed at 400 °C, the mass loss reached 67.56 ± 3.26% (
Table 1). On the other hand, for samples processed at 500 °C and 600 °C, the mass loss seems to be less significant, with results of 70.82 ± 4.07% and 72.17 ± 4.21%. This decreasing acceleration of mass loss is related to the minimal amount of volatile compounds and moisture present in samples processed at these higher temperatures. These mass losses indicate a high potential for the yield to shift to the gaseous fraction, but mainly to the liquid fraction. These results are in line with the results obtained in the works presented previously by Wang et al. [
47] or by Ramirez-Canon et al. [
48]. In any case, further support that the simple use of thermochemical conversion processes already shows a high potential for reducing the volumes of available waste, provided that forms of energy recovery that justify the viability of the processes are found, as stated previously by Oliveira Neto et al. (2019) [
49].
As can be seen, the results presented in
Table 1 indicate that during the thermochemical conversion process carried out for each of the temperatures, the most significant mass loss occurs right in the roasting stage, carried out at 300 °C. In the following stages, already within the pyrolysis spectrum, although there are progressively higher mass losses, these are not as significant as those observed in the range up to 300 °C, and it is in this range that, most likely, the degradation of most carbon-based materials occurs.
Figure 2 shows the mass loss verified for each of the different temperature levels for tire samples and for biomass samples (A1 grade ENPlus
® wood pellets produced using maritime pine wood).
Thus, in the torrefaction process (300 °C), there is a mass loss of more than 63%, most likely corresponding to the volatilization of the carbon-based materials that make up the tire. In the following steps, there was a progressive mass loss, relatively to the previous step, of 8%, 4% and 2% for the temperature levels of 400 °C, 500 °C and 600 °C, respectively. This decreasing percentage of mass loss compared to the previous level is related to the availability and type of carbon-based material still existing in the material and the way they react to temperature. This behavior only differs from the behavior presented by the biomass processed using the same procedure described in
Section 2 due to the moisture contents, which, as can also be seen in
Figure 2, are the origin of a more significant loss of initial mass than what happens with the tire. However, mass loss only occurs when the temperature exceeds 350 °C. This situation is due to the fact that, as mentioned by Nunes et al. [
50], in the range 230–320 °C, the degradation of hemicellulose occurs, and only when this temperature is exceeded does the degradation of cellulose and lignin begin.
The standard commercially available carbon black, which is normally produced through controlled combustion or thermal decomposition, from acetylene, natural gas, coal tar residues, or petroleum oils, presents an approximate elemental carbon content >97%. Of this percentage of elemental carbon, 1–2% may still be organic carbon; H normally has a concentration of <0.008% and an H/C ratio of 0.006 [
51]. From the results obtained, it appears that there is still a significant difference between the values of the present work and the values presented by Long et al. (2013), with the values showing a difference. With the increase in the process temperature from 300 °C to 600 °C, the concentrations of C also increased from 76.70% of the initial rubber to 80.80%, 82.30%, 86.49% and 90.32%, demonstrating a clear concentration of carbon. On the other hand, H follows the opposite path, and its concentration decreases from the initial 8.15% found in non-thermally treated rubber to, 2.69% at 300 °C, 1.62% at 400 °C, 1.52% at 500 °C and 1.07% at 600 °C. This drop is an important expected result, since hydrogenated compounds are tendentially eliminated during the torrefaction (300 °C) and pyrolysis processes (400–600 °C) [
52].
As this is still a preliminary approach to the problem, an expeditious way to determine the potential for the recovery of carbon black from scrap tires could be through the analysis of the H/C ratio. It seems that this comparative approach had its beginnings in the work of Medalia et al. [
53], who used the H/C ratio to differentiate between carbon black with soot. Long et al. (2013) presents this criterion as a comparative element between carbon black and other carbonaceous products. For the results obtained, the evolution of the H/C ratio presented the values that can be seen in
Table 5.
As can be seen, the H/C ratio decreases in the respective proportion of the increase in the C content and the decrease in the H content, indicating that to reach an H/C ratio identical to that presented by Long et al. (2013) for carbon black, which was 0.006, everything points to the need to change the parameters of the pyrolysis process, specifically by increasing the temperature or by increasing the residence time. This methodology follows on from the work by Hong et al. [
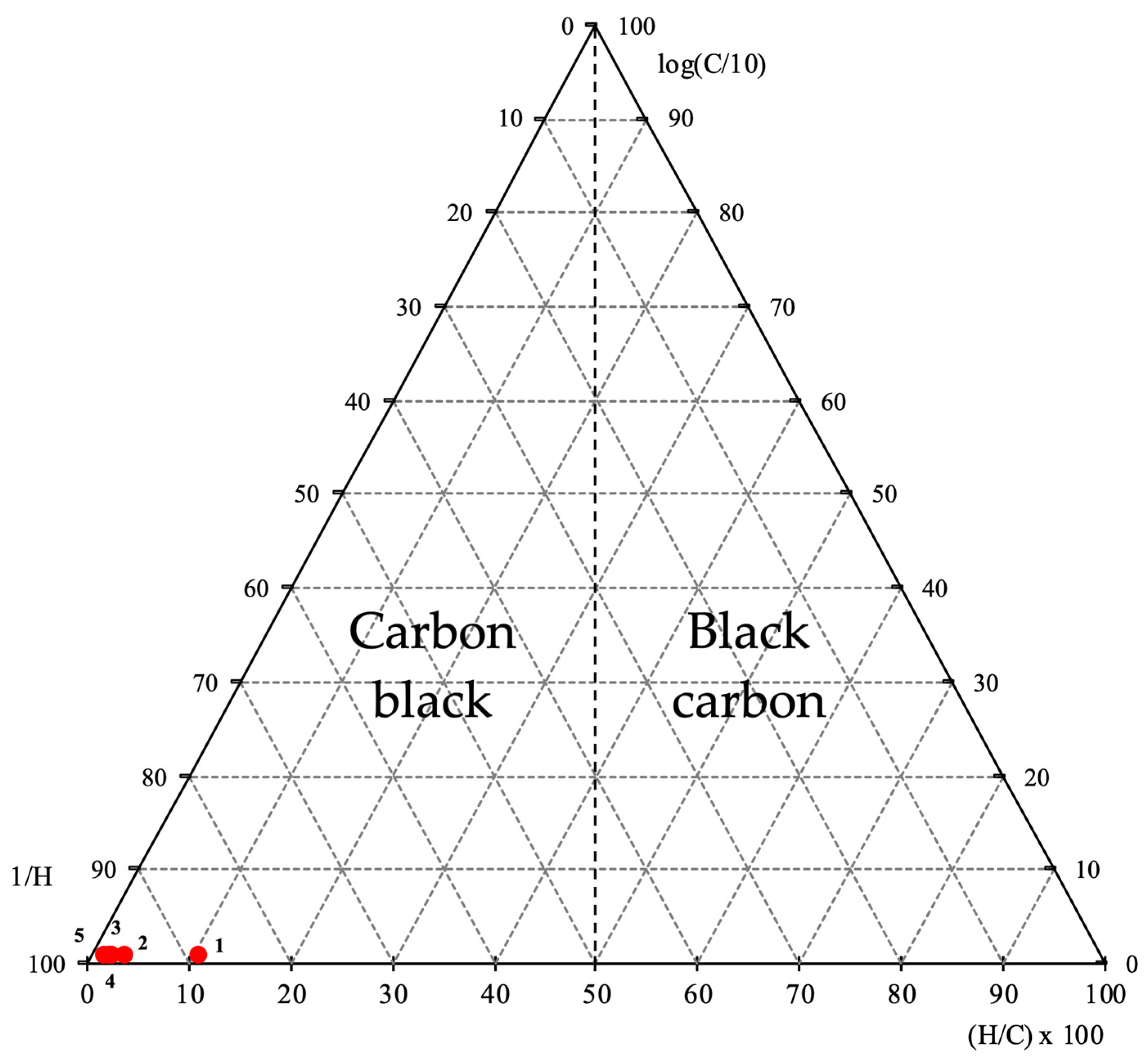
54], who resorted to the analysis of the ratio between H and C to differentiate carbon black from black carbon using a ternary plot based on elemental analysis, defining as the axes of the ternary plot the ratios H/C × 100-log(C/10)-1/H. With this procedure, the authors divided the triangle precisely at 50% of the lower axis, with the carbon black samples located on the left side of the diagram, and the black carbon samples located on the right side of the diagram. The projection of the samples of rubber, RC 300 °C, RC 400 °C, RC 500 °C and RC 600 °C are shown in
Figure 3.
As can be seen from the projection of the samples on the ternary diagram, all samples are placed on the carbon black side, as this is even part of the initial composition of the scrap tire. In other words, what the pyrolysis process could have achieved was the release of the previously existing carbon black particles by removing the remaining material, leaving only the carbon black and an inorganic fraction, which is composed of the ash and the metallic tire fraction. In fact, this can be seen by the percentage of mass loss in each of the tests, as well as by the percentage attributed to the ash, which also includes the metallic fraction. Cataldo [
55], in his work on the characterization of carbon black from tire pyrolysis, through a TGA-DTA analysis, demonstrated that the composition of commercially available pyrolytic carbon black produced from the pyrolysis of scrap tires can be presented as being 70% carbon black, while about 14% are ashes and about 16% are rubber pyrolytic residues adsorbed on the surface. As can be seen, the value obtained for the ash content for the sample processed at 400 °C, although a little lower than that presented by Cataldo, is already very close to the 16% referred to by the author. Regarding the use of the classification method proposed by Hong et al. [
54], these authors used 37 samples, while in the present work, because it is a preliminary approach, the number of samples for each phase, 3 + 3 + 3, respectively, for the non-thermally processed rubber, for the processed rubber at 300 °C and for rubber processed at 400 °C, it must be analyzed carefully, and more samples must be produced and characterized to allow the confirmation of the results and its repeatability to confirm the hypothesis here defended.
An interesting and noteworthy result is the increase in the fixed carbon concentration, which reached values >75% for samples subjected to temperatures of 300 °C and values >83% for samples subjected to temperatures of 400 °C (
Table 2). The obtained results are in accordance with previous studies that addressed the production of char from scrap tire rubber as potential adsorbent of Remazol Yellow dye [
44]. In that study, the authors showed, for example, an increase in the fixed carbon content close to 70%, while the ash content reached 12.9% [
56]. In our study, the mass loss reached 63.42% at 300 °C and 67.56% at 400 °C (
Table 2), which also meets the results of the tests carried out by Nogueira et al. (2019). According to them, the TGA curves indicate a mass loss slightly higher than 60% for the temperature range between 380 and 450 °C [
56]. In conclusion, the high content of fixed carbon verified in the samples produced at 400 °C shows that the potential of this material to produce carbon black is very promising. Although previous studies have shown similar processes to the ones identified in this study, such as the work of Dewivedi et al. [
36]. The differences in methodology and the resulting material show a much more promising technique sustaining further studies. For example, the process in the present study shows a higher black carbon recovery potential (76.7–80.8%) compared to the previous work (22–49.9%).
The TGA curve performed with non-heat-treated rubber proves the different stages and which materials are involved in the mass loss at each moment of the process, as shown in
Figure 4.
As can be seen, the mass loss that occurs up to 450 °C is related to the depolymerization of the rubber and can represent a mass loss of approximately 60% of the total mass of the tire. This value of seems to be significantly above the amount of rubber normally used in the composition of light-duty tires, which, according to Fragassa [
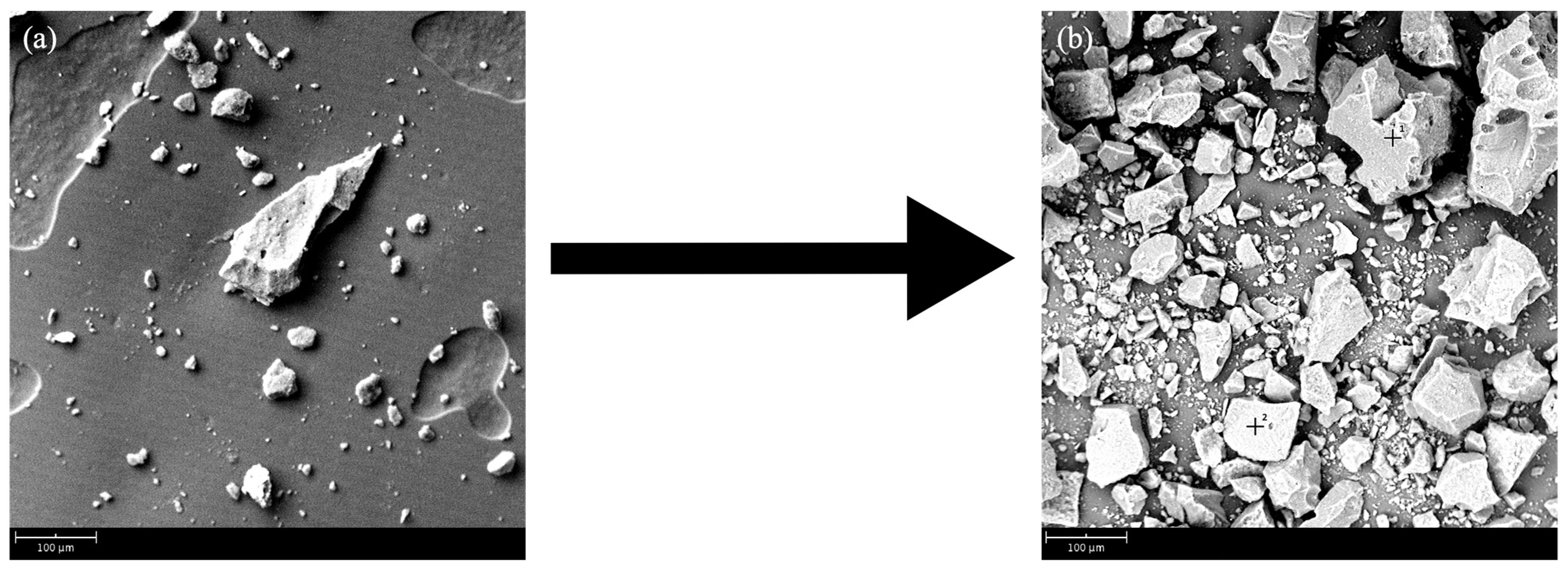
11], present average values for rubber/elastomers of 47%. However, the same author presents the total carbon-based materials as 74%, so it is very likely that in this temperature range the volatilization of other constituent materials, such as textiles (5.5%) and even some carbon black (21.5%), which already in the final phase of the heating ramp, approaching 450 °C, may have some fraction reacting to the temperature. In the temperature range 450–600 °C, carbon fillers volatilize, including carbon black, corresponding to about 20% of the mass loss. The remaining 20% correspond to inert fillers, such as silica, which remain unchanged at these temperatures and contribute to the accumulation of ash. This situation can even be confirmed through the SEM images shown in
Figure 5.
As seen in
Figure 5a, the inert fillers are dispersed in the matrix made up of carbon-based materials, which are essentially rubber.
Figure 5b shows an apparent greater abundance of inert filler materials, as the rubber has degraded. If this inert filler is combined with metallic particles, this can deeply influence the carbon particles’ surface. In fact, these metallic elements can create bounds directly with the surface of these same particles, and this combination may alter the superficial texture of the carbon particles and change its properties. For example, it can change the adsorption capacity if the carbon is intended to be used for filters, or it can simply change the metallic elements content of a tire produced with recycled carbon.
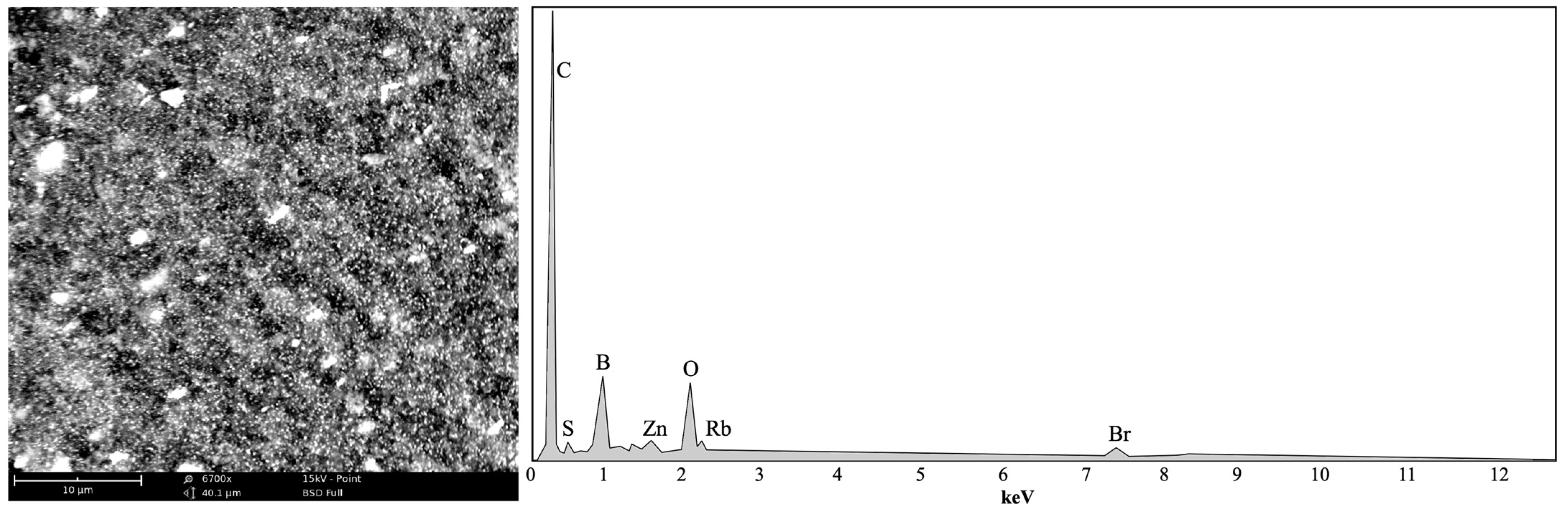
Table 2 presents a column with the aggregated results corresponding to other elements which were not identified. Using SEM analysis, a cut-out of a map and elemental mapping were performed to present a preliminary approach to the composition of these remaining materials. The preliminary results are shown in
Figure 6.
The presence of sulfur had already been evidenced in
Table 2, and its presence is now confirmed again. This fact, as would be expected, is associated with the vulcanization process of rubber, as well as the presence of zinc, since zinc oxide is also part of the additives used in the production of tires. However, the list of elements still seems to be very short, so more analysis of the materials will be necessary so that all the constituent elements can be identified and thus assess potential impacts depending on the use to be given to the final product.
There are several works that present studies on the sustainability and feasibility of projects for the recovery and recovery of waste from the tire industry. However, it appears that most works found in bibliographic databases refer to development projects of pilot-plants for pyrolysis of scrap tires to obtain carbon black and other byproducts. As an example, the work presented by Oliveira Neto et al. [
49], where the authors present an economic, environmental and social benefits analysis of the adoption of pyrolysis process of tires as a feasible and ecofriendly mode to reduce the impacts in Brazil. In this study, the authors obtained a carbon black composed of 90% carbon and envisaged the energetic valorization of pyrolytic oils, since they were shown to have high quality and to be marketable. In addition, Ali et al. [
57] reported that the development and production of carbon black using pyrolysis is sustainable, as well as Mora et al. [
58], who even presented and supported the valuation of scrap tires from a CO
2 mitigation assessment perspectivel or Araujo-Morera et al. [
59], who included the component of the treatment given to scrap tires in the definition of sustainable mobility. Perhaps the identification of a more impactful use, from a social point of view, is presented by Gharaibeh et al. [
60]. In this work, the authors present the use of pyrolytic carbon black as an alternative source of heating in refugee camps in Jordan and concluded that the economic aspects of this use showed to be the most significant advantage.
The heating value of the tire without heat treatment is already high, as can be seen in the results obtained for the heating value shown in
Figure 7.
The energy recovery of materials obtained using thermochemical conversion processes seems to be a good option since the heating values are high. For example, by comparison with biomass fuels, the tire has a low heating value (LHV), almost twice that of the A1 grade ENPlus® wood pellets. However, the recovery of the tire or rubber chars can cause significant environmental problems, namely caused by the gaseous emissions generated in the combustion. As previously noted, sulfur is an element present in the composition of tires, so the formation of large amounts of SOx is expected. However, other harmful compounds may form, such as NOx, since, in this study, the constituent materials of the ash were not characterized. Thus, energy recovery using direct combustion processes is not the most suitable.
As can be seen in the analyzed bibliography, the processes studied seem to all follow the same direction, which is to follow the path of sustainability in all its aspects. However, as presented by Das et al. [
26], the tire industry is basically a slow-changing industry, at a time when the number of environmental and sustainability-related issues are impacting the tire industry to a large extent. In this perspective, the approaches presented, specifically the use of thermochemical conversion processes as a tool for valuing scrap tires, is a possibility that deserves to be further explored, always with a view to valuing the largest number of fractions and not just carbon black, although it can be understood that this material may be the one with the highest potential for recovery, mainly because it can be part of the transition of the entire tire industry to the circular economy.