Key Targets for Improving Algal Biofuel Production
Abstract
:1. Introduction
2. Algal Taxonomy
3. Growing Algae—Requirements and Cost Implications
3.1. Macroalgae Production
3.2. Microalgae Production
3.3. Economics of Microalgae Production
3.4. Potential Areas for Mass Algal Production for Global Biofuels Production
4. Harvesting, Dewatering, and Drying of Microalgae
4.1. Centrifugation
4.2. Flocculation
4.3. Froth Flotation
4.4. Physical Methods
5. Added and High-Value Products from Algae
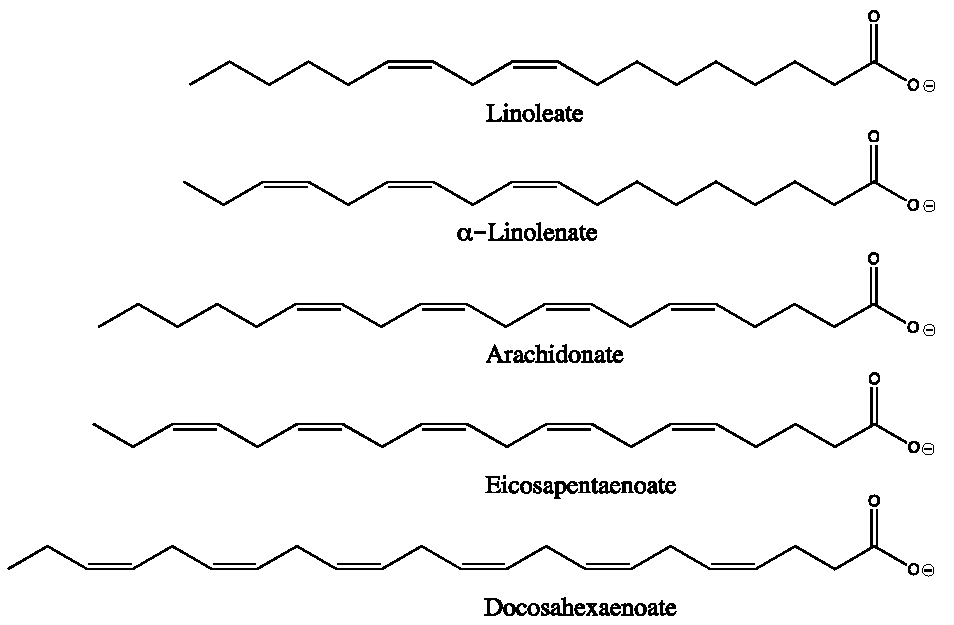
5.1. Omega-3 Fatty Acids
5.2. Squalene
5.3. Pigments
5.4. Hydrocolloids
5.5. Phlorotannins
6. Types of Biofuel from Algae
6.1. Bioethanol
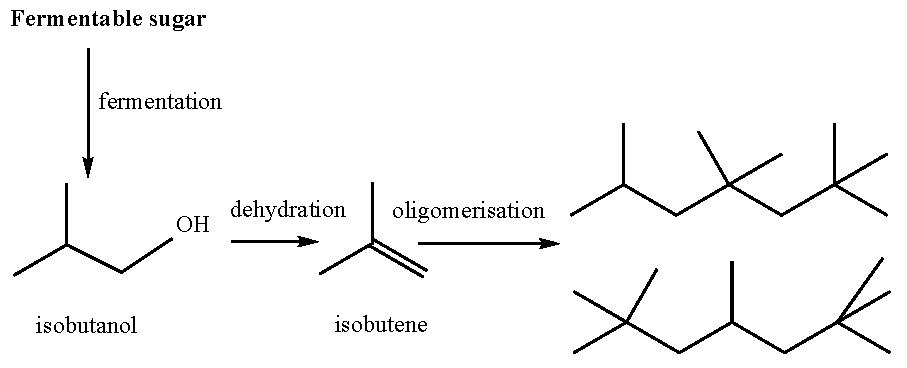
6.2. Conversion of Sugars to Paraffins
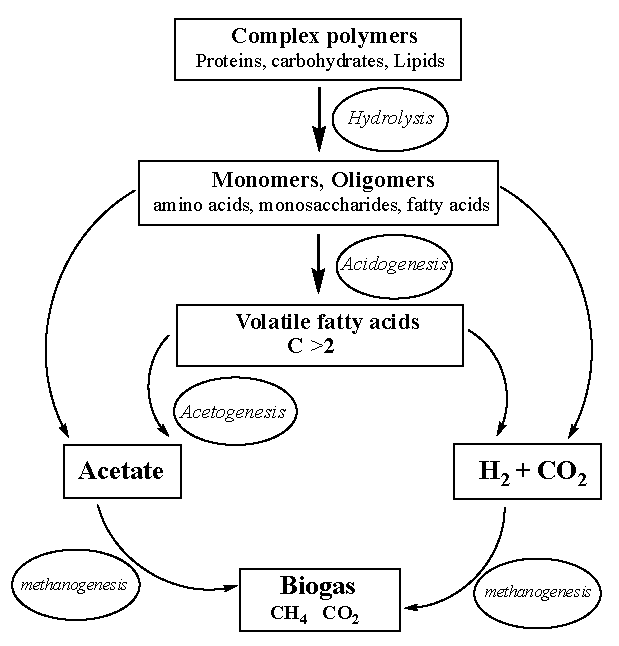
6.3. Anaerobic Fermentation
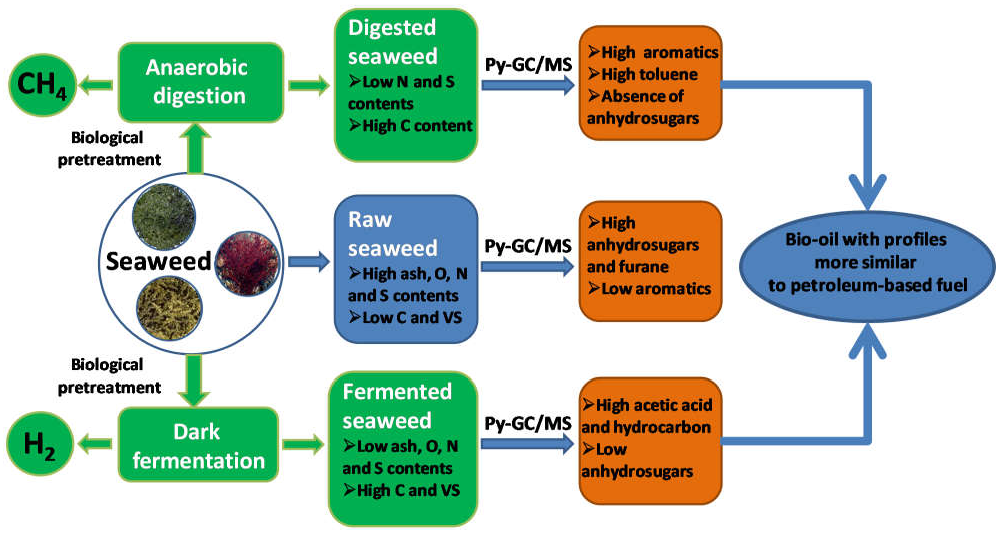
6.4. AD of Macroalgae
6.5. AD of Microalgae
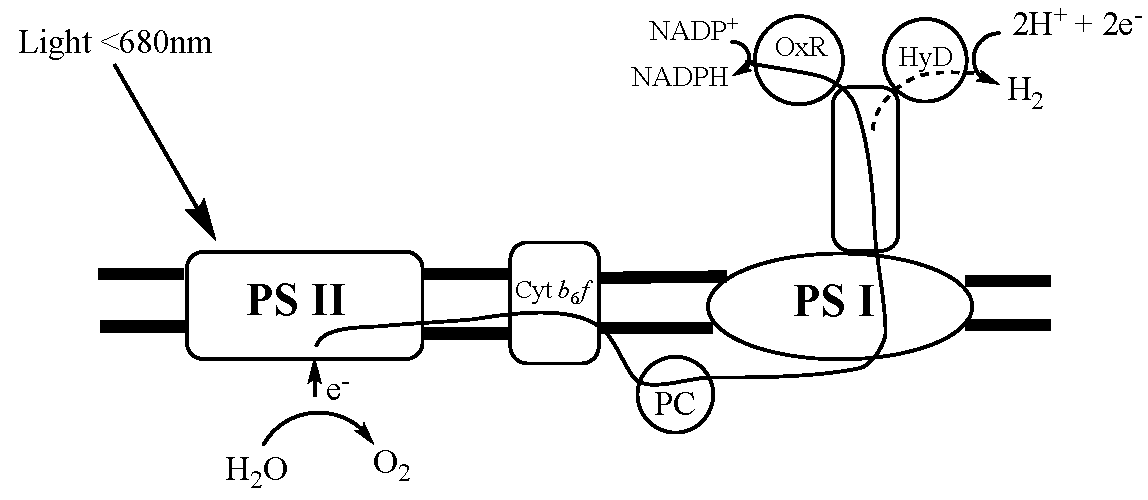
6.6. Biohydrogen—Manipulation of Photosynthesis for Hydrogen Generation
6.7. Fast Pyrolysis
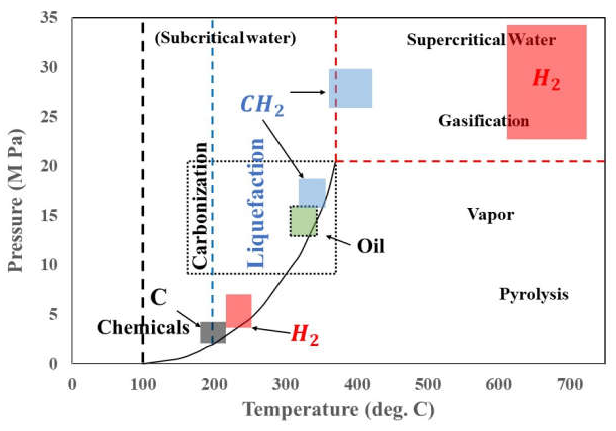
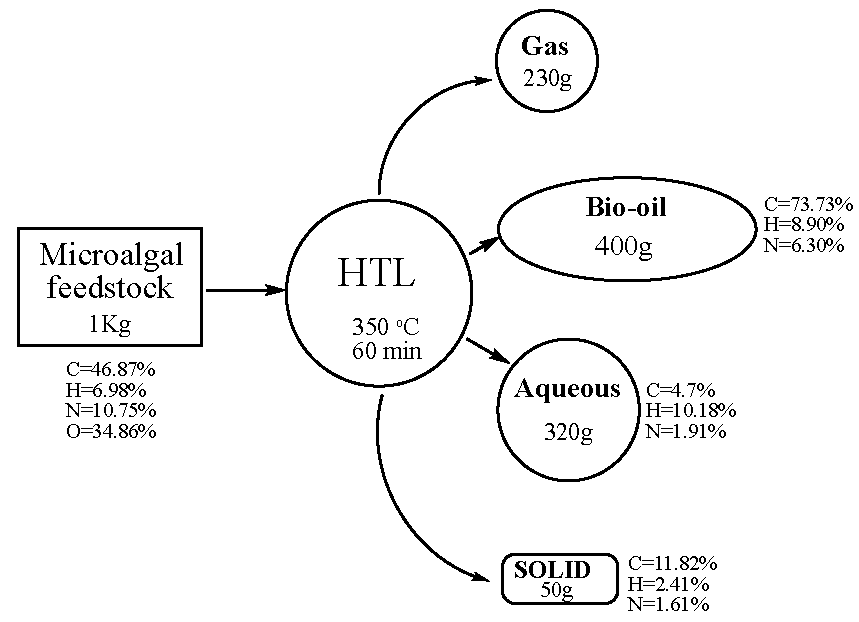
6.8. Hydrothermal Liquefaction
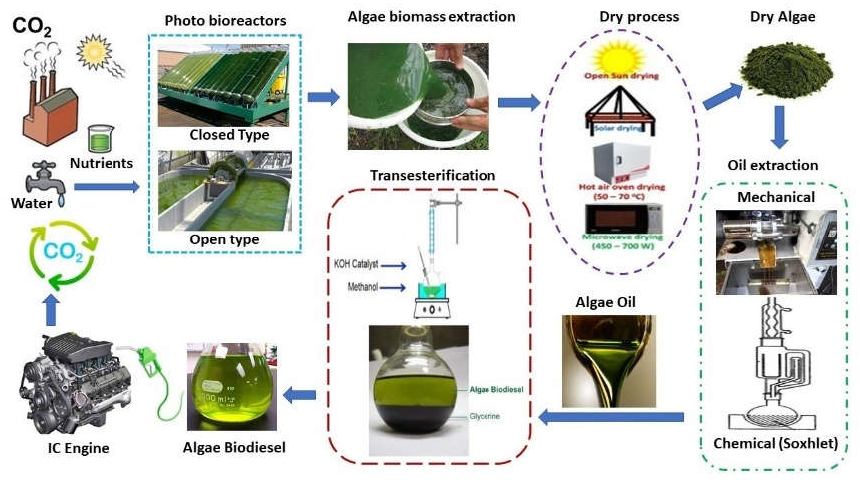
7. Algal Oils and Biodiesel Production
7.1. Terpenoid ‘Oils’
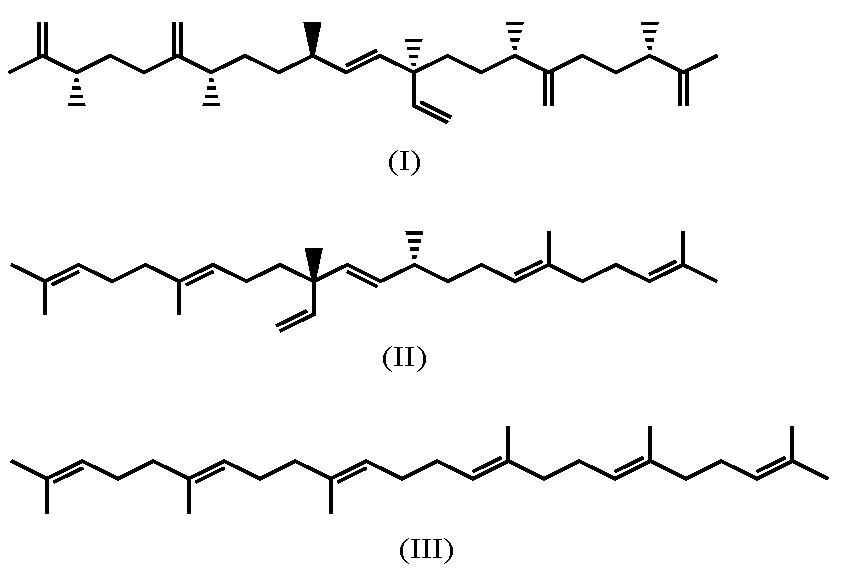
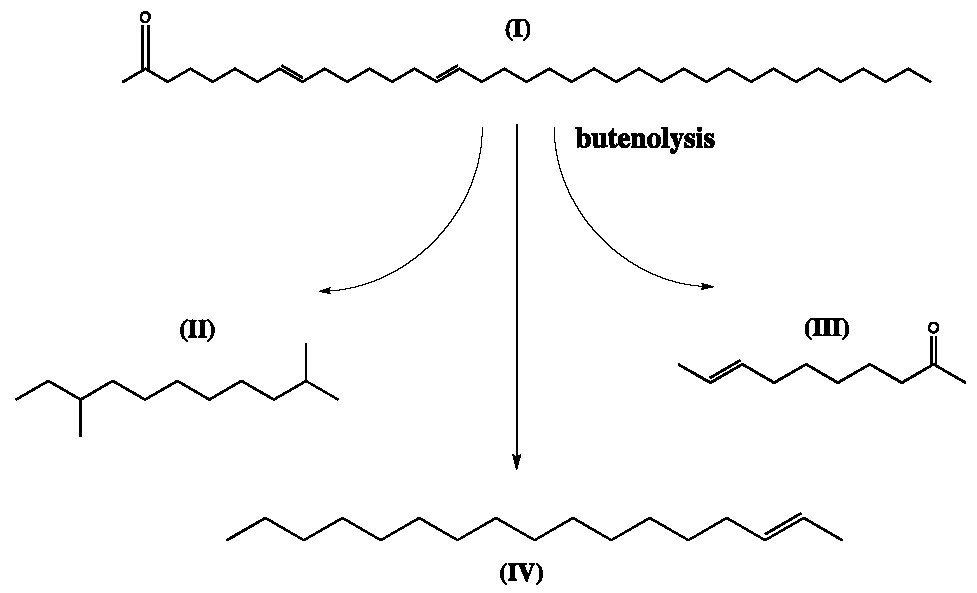
7.2. Alkenones
7.3. Biodiesel Fuel Characteristics
7.4. Algae Biodiesel Engine performance
7.5. Algae Biodiesel Engine Emissions
7.6. Algal Biodiesel Cost
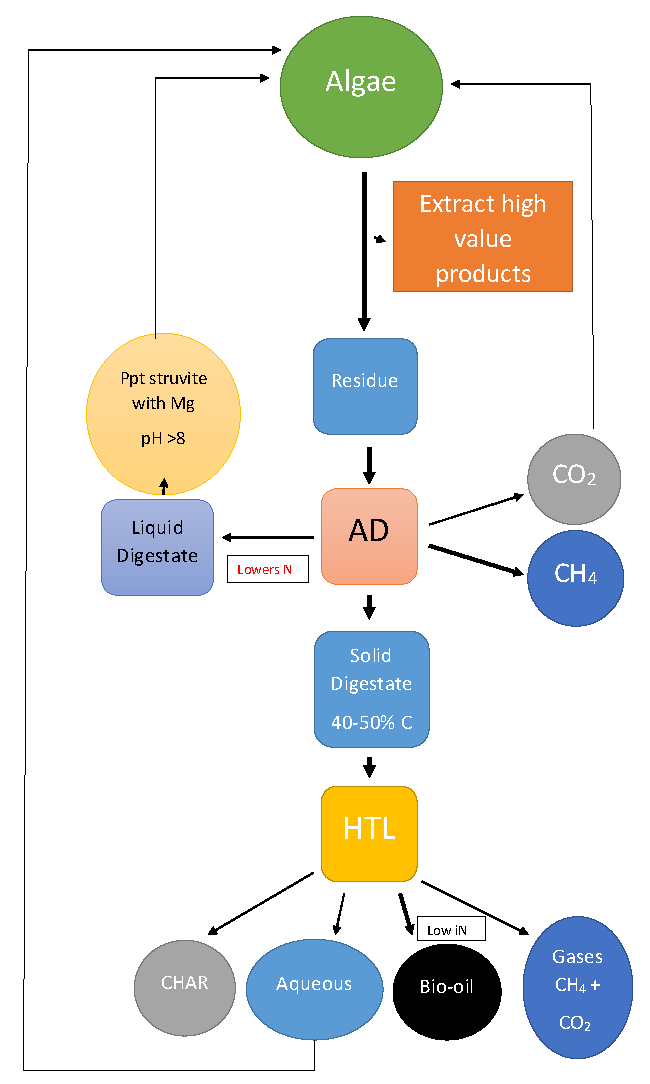
8. Integrated Biorefinery Approaches
9. Conclusions
10. Webpage Links
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AA | arachidonic acid |
| AD | Anaerobic digestion |
| Asx | astaxanthin |
| B100 | 100% biodiesel |
| BMP | biomethane potential |
| BTE | Brake thermal efficiency |
| Cyt b6f | cytochrome b6f complex |
| DHA | docosahexaenoic acid |
| DAG | diacylglycerol |
| EGR | exhaust gas re-circulation |
| EPA | eicosapentaenoic acid |
| FFA | free fatty acid |
| FP | fast pyrolysis |
| FAME | fatty acid methyl ester |
| GM | genetic modification |
| HTL | hydrothermal liquefaction |
| HyD | hydrogenase |
| LCFA | long chain fatty acid |
| NADP+ | nicotinamide adenine dinucleotide phosphate |
| OxR | oxidoreductase |
| PA | phosphatidic acid |
| PC | plastocyanin |
| PCh | phosphatidylcholine |
| PQ | plastoquinone |
| PS I | photosystem I |
| PS II | photosystem II |
| PUFA | polyunsaturated fatty acid |
| SEQHTL | Two stage sequential hydrothermal liquefaction |
| TAG | triacylglycerol |
| TCA | tricarboxylic acid |
References
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; Van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res.-Biomass Biofuels Bioprod. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Hebbale, D. Bioethanol from macroalgae: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 117, 109479. [Google Scholar] [CrossRef]
- Manoyana, J.; Gabrielyana, L.; Kozelb, N.; Trchouniana, A. Regulation of biohydrogen production by protonophores in novel green microalgae Parachlorella kessleri. J. Photochem. Photobiol. B Biol. 2019, 199, 111597. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Nielsen, B.V.; Maneein, S.; Harvey, P.J. A Brief Review of Anaerobic Digestion of Algae for Bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef] [Green Version]
- Paula, T.; Sinharoy, A.; Baskaran, D.; Kannan, P.; Pugazhenthi, G.; Lens, P.N.L. Bio-oil production from oleaginous microorganisms using hydrothermal liquefaction: A biorefinery approach. Crit. Rev. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Katam, G.B.; Murthy, M.; Warkhade, G.S. Review on algae for biodiesel fuel production, its characteristics comparison with other and their impact on performance, combustion and emissions of diesel engine. World J. Eng. 2017, 14, 127–138. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Manoylov, K.M. Taxonomic identification of algae (morphological and molecular): Species concepts, methodologies, and their implications for ecological bioassessment. J. Phycol. 2014, 50, 409–424. [Google Scholar] [CrossRef]
- Leliaert, F.; Verbruggen, H.; Vanormelingen, P.; Steen, F.; Lopez-Bautista, L.M.; Zuccarello, G.C.; De Clerck, O. DNA-based species delimitation in algae. Eur. J. Phycol. 2014, 49, 179–196. [Google Scholar] [CrossRef] [Green Version]
- Brodie, J.; Chan, C.X.; De Clerck, O.; Cock, J.M.; Coelho, S.M.; Gachon, C.; Grossman, A.R.; Mock, T.; Raven, J.A.; Smith, A.G.; et al. The Algal Revolution. Trends Plant Sci. 2017, 22, 726–738. [Google Scholar] [CrossRef]
- Bringloe, T.T.; Samuel, S.; Rachael, M.W.; Christophe, V.; Hiroshi, K.; Olivier, D.C.; Mark, J.C.; Susana, M. Phylogeny and Evolution of the Brown Algae. Crit. Rev. Plant Sci. 2020, 39, 281–321. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis-Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef] [PubMed]
- Bosnjakovic, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- Zheng, Y.; Jin, R.; Zhang, X.; Wang, Q.; Wu, J. The considerable environmental benefits of seaweed aquaculture in China. Stoch. Environ. Res. Risk Assess. 2019, 33, 1203–1221. [Google Scholar] [CrossRef]
- Kim, J.K.; Charles, Y.; Eun, K.H.; Miseon, P.; Youngdae, K. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zuniga-Jara, S.; Contreras, C. An economic valuation of the commercial cultivation ofAgarophyton chilensisin northern Chile. J. Appl. Phycol. 2020, 32, 3233–3242. [Google Scholar] [CrossRef]
- Mantri, V.A.; Ganesan, M.; Gupta, V.; Krishnan, P.; Siddhanta, A.K. An overview on agarophyte trade in India and need for policy interventions. J. Appl. Phycol. 2019, 31, 3011–3023. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, A.J.; Hayashi, L.; Vásquez, J.A.; Paul, K. Seaweeds: An opportunity for wealth and sustainable livelihood for coastal communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopin, T.; Tacon, A.G.J. Importance of Seaweeds and Extractive Species in Global Aquaculture Production. Rev. Fish. Sci. Aquac. 2021, 29, 139–148. [Google Scholar] [CrossRef]
- Liang, Y.N.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef]
- Zheng, Y.; Chi, Z.; Lucker, B.; Chen, S. Two-stage heterotrophic and phototrophic culture strategy for algal biomass and lipid production. Bioresour. Technol. 2012, 103, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Awad, N.; Griffiths, G. Effect of ornithine decarboxylase and norspermidine in modulating cell division in the green alga Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2018, 123, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, N.; Vega-Estevez, S.; Griffiths, G. Salicylic acid and aspirin stimulate growth of Chlamydomonas and inhibit lipoxygenase and chloroplast desaturase pathways. Plant Physiol. Biochem. 2020, 149, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Saavedra, M.; Sauceda-Carvajal, D.; Castro-Ochoa, F.Y.; Molina-Cárdenas, C.A. The Use of Light Spectra to Improve the Growth and Lipid Content of Chlorella vulgaris for Biofuels Production. Bioenergy Res. 2020, 13, 487–498. [Google Scholar] [CrossRef]
- Dahlin, L.R.; Gerritsen, A.T.; Henard, C.A.; Wychen, A.V.; Linger, J.G. Development of a high-productivity, halophilic, thermotolerant microalga Picochlorum renovo. Commun. Biol. 2019, 2, 388–391. [Google Scholar] [CrossRef]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, D.; Rocha, A.C.; Pereira, L.; Verdelhos, T. Microalgae Water Bioremediation: Trends and Hot Topics. Appl. Sci. 2020, 10, 1886. [Google Scholar] [CrossRef] [Green Version]
- Craggs, R.J.; Heubeck, S.; Lundquist, T.J.; Benemann, J.R. Algal biofuels from wastewater treatment high rate algal ponds. Water Sci. Technol. 2011, 63, 660–665. [Google Scholar] [CrossRef]
- Cheng, D.; Li, X.; Yuan, Y.; Zhao, Q. Kinetic model for effects of simulated flue gas onto growth profiles of Chlorella sp. AE10 and Chlorella sp. Cv. Biotechnol. Appl. Biochem. 2020, 67, 783–789. [Google Scholar] [CrossRef]
- Veronesi, D.; D’Imporzano, G.; Menin, B.; Salati, S.; Adani, F. Organic wastes/by-products as alternative to CO2 for producing mixotrophic microalgae enhancing lipid production. Bioprocess Biosyst. Eng. 2020, 43, 1911–1919. [Google Scholar] [CrossRef]
- Saxena, A.; Marella, T.K.; Singh, P.K.; Tiwari, A. Indoor mass cultivation of marine diatoms for biodiesel production using induction plasma synthesized nanosilica. Bioresour. Technol. 2021, 332, 125098. [Google Scholar] [CrossRef]
- Roy, U.K.; Nielsen, B.V.; Milledge, J.J. Antioxidant Production in Dunaliella. Appl. Sci. 2021, 11, 3959. [Google Scholar] [CrossRef]
- Figueroa-Torres, G.M.; Pittman, J.K.; Theodoropoulos, C. Optimisation of microalgal cultivation via nutrient-enhanced strategies: The biorefinery paradigm. Biotechnol. Biofuels 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Patlakas, P.; Stathopoulos, C.; Flocas, H.; Kalogeri, C.; Kallos, G. Regional Climatic Features of the Arabian Peninsula. Atmosphere 2019, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Mupambwa, H.A.; Hausiku, M.K.; Nciizah, A.D.; Dube, E. The unique Namib desert-coastal region and its opportunities for climate smart agriculture: A review. Cogent Food Agric. 2019, 5. [Google Scholar] [CrossRef]
- Ghose, D.S. Pradhan, and Shabbiruddin, Development of model for assessment of renewable energy sources: A case study on Gujarat, India. Int. J. Ambient. Energy 2019. [Google Scholar] [CrossRef]
- Mobin, S.M.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications—A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Apandi, N.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Kassim, A.H.M. Microalgal biomass production through phycoremediation of fresh market wastewater and potential applications as aquaculture feeds. Environ. Sci. Pollut.Res. 2019, 26, 3226–3242. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Wang, L.G.; Schenk, P.M. Effective harvesting of low surface-hydrophobicity microalgae by froth flotation. Bioresour. Technol. 2014, 159, 437–441. [Google Scholar] [CrossRef]
- Kebelmann, K.; Hornung, A.; Karsten, U.; Griffiths, G. Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 2013, 49, 38–48. [Google Scholar] [CrossRef]
- Yin, Z.; Chu, R.; Zhu, L.; Li, S.; Mo, F.; Hu, D.; Liu, C. Application of chitosan-based flocculants to harvest microalgal biomass for biofuel production: A review. Renew. Sustain. Energy Rev. 2021, 145, 111159. [Google Scholar] [CrossRef]
- Lee, D.J.; Liao, G.Y.; Chang, Y.R.; Chang, J.S. Coagulation-membrane filtration of Chlorella vulgaris. Bioresour. Technol. 2012, 108, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.L.; Gao, Z.; Yin, J.; Tang, X.; Ji, X.; Huang, H. Harvesting of microalgae by flocculation with poly (gamma-glutamic acid). Bioresour. Technol. 2012, 112, 212–220. [Google Scholar] [CrossRef]
- Ogbonna, C.N.; Nwoba, E.G. Bio-based flocculants for sustainable harvesting of microalgae for biofuel production. A review. Renew. Sustain. Energy Rev. 2021, 139, 110690. [Google Scholar] [CrossRef]
- Ray, A.; Banerjee, S.; Das, D. Microalgal bio-flocculation: Present scenario and prospects for commercialization. Environ. Sci. Pollut. Res. 2021, 28, 26294–26312. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G. Jasmonates: Biosynthesis, perception and signal transduction. Essays Biochem. 2020, 64, 501–512. [Google Scholar] [CrossRef]
- Mohseni, F.; Zenooz, A.M. Flocculation of Chlorella vulgaris with alum and pH adjustment. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Phasey, J.; Vandamme, D.; Fallowfield, H.J. Harvesting of algae in municipal wastewater treatment by calcium phosphate precipitation mediated by photosynthesis, sodium hydroxide and lime. Algal Res.-Biomass Biofuels Bioprod. 2017, 27, 115–120. [Google Scholar] [CrossRef]
- Xia, L.; Yinta, L.; Rong, H.; Shaoxian, S. Effective harvesting of microalgae by coagulation-flotation. R. Soc. Open Sci. 2017, 4, 170867. [Google Scholar] [CrossRef] [Green Version]
- Almomani, F. Algal cells harvesting using cost-effective magnetic nano-particles. Sci. Total. Environ. 2020, 720, 12. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, M.G.; Dardavila, M.M.; Georgiopoulou, I.; Louli, V.; Stamatis, H.; Kekos, D.; Voutsas, E. Optimization of Microalga Chlorella vulgaris Magnetic Harvesting. Nanomaterials 2021, 11, 1614. [Google Scholar] [CrossRef]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of Microalgae by Flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef] [Green Version]
- Van Dael, P. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: Review of recent studies and recommendations. Nutr. Res. Pract. 2021, 15, 137–159. [Google Scholar] [CrossRef]
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Cigliano, A.R.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Prog. Lipid Res. 2019, 76, 101007. [Google Scholar] [CrossRef]
- Gohil, N.; Bhattacharjee, G.; Khambhati, K.; Braddick, D.; Singh, V. Engineering Strategies in Microorganisms for the Enhanced Production of Squalene: Advances, Challenges and Opportunities. Front. Bioeng. Biotechnol. 2019, 7, 50. [Google Scholar] [CrossRef]
- Morrison, G.C.; Eftekhari, A.; Majluf, F.; Krechmer, J.E. Yields and Variability of Ozone Reaction Products from Human Skin. Environ. Sci. Technol. 2021, 55, 179–187. [Google Scholar] [CrossRef]
- Santos, D.N.; Silva, F.S.; Verde, A.B.; Bittencourt, G.M.; Oliveira, A.L. Determination of functional compounds in blue shark (Prionace glauca) liver oil obtained by green technology. Grasas Y Aceites 2020, 71, 354. [Google Scholar] [CrossRef]
- Mikrou, T.; Pantelidou, E.; Parasyri, N.; Papaioannou, A.; Kapsokefalou, M.; Gardeli, C.; Mallouchos, A. Varietal and Geographical Discrimination of Greek Monovarietal Extra Virgin Olive Oils Based on Squalene, Tocopherol, and Fatty Acid Composition. Molecules 2020, 25, 3818. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Jeon, Y.J. Squalene isolated from marine macroalgae Caulerpa racemosa and its potent antioxidant and anti-inflammatory activities. J. Food Biochem. 2018, 42, e12628. [Google Scholar] [CrossRef]
- Bourdon, L.; Jensen, A.A.; Kavanagh, J.M.; McClure, D.D. Microalgal production of zeaxanthin. Algal Res.-Biomass Biofuels Bioprod. 2021, 55, 102266. [Google Scholar]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H-pluvialis and beta-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, A.S. Dietary administration of astaxanthin improves feed utilization, growth performance and survival of Asian seabass, Lates calcarifer (Bloch, 1790). Aquac. Nutr. 2019, 25, 1410–1421. [Google Scholar] [CrossRef]
- Gargouch, N.; Karkouch, I.; Elleuch, J.; Elkahoui, S.; Michaud, P.; Abdelkafi, S.; Laroche, C.; Fendri, I. Enhanced B-phycoerythrin production by the red microalga Porphyridium marinum: A powerful agent in industrial applications. Int. J. Biol. Macromol. 2018, 120, 2106–2114. [Google Scholar] [CrossRef]
- García, A.B.; Longo, E.; Murillo, M.C.; Bermejo, R. Using a B-Phycoerythrin Extract as a Natural Colorant: Application in Milk-Based Products. Molecules 2021, 26, 297. [Google Scholar] [CrossRef]
- Sui, S.F. Structure of Phycobilisomes. Annu. Rev. Biophys. 2021, 50, 53–72. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.H.; Xue, C.H. Recent advances in carrageenan-based delivery systems for bioactive ingredients: A review. Trends Food Sci. Technol. 2021, 112, 348–361. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2019, 31, 779–786. [Google Scholar] [CrossRef]
- Pradyawong, S.; Juneja, A.; Sadiq, M.B.; Noomhorm, A.; Singh, V. Comparison of Cassava Starch with Corn as a Feedstock for Bioethanol Production. Energies 2018, 11, 3476. [Google Scholar] [CrossRef] [Green Version]
- Ran, W.; Wang, H.; Liu, Y.; Qi, M.; Xiang, Q.; Yao, C.; Zhang, Y.; Lan, X. Storage of starch and lipids in microalgae: Biosynthesis and manipulation by nutrients. Bioresour. Technol. 2019, 291, 121894. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dagle, V.L.; Lopez, J.S.; Cooper, A.; Luecke, J.; Swita, M.; Dagle, R.A.; Gaspar, D. Production and fuel properties of iso-olefins with controlled molecular structure and obtained from butene oligomerization. Fuel 2020, 277, 118147. [Google Scholar] [CrossRef]
- O’Neil, G.W.; Culler, A.R.; Williams, J.R.; Burlow, N.P. Production of Jet Fuel Range Hydrocarbons as a Coproduct of Algal Biodiesel by Butenolysis of Long-Chain Alkenones. Energy Fuels 2015, 29, 922–930. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Wang, Q.; He, Z.; Abomohra, A.E.F.; Cao, B. Influence of torrefaction pretreatment on the pyrolysis characteristics of seaweed biomass. Cellulose 2019, 26, 8475–8487. [Google Scholar] [CrossRef]
- Allen, E.; Wall, D.M.; Herrmann, C.; Xia, A.; Murphy, J.D. What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Mhatre, A.; Gore, S.; Mhatre, A.; Trivedi, N.; Sharma, M.; Pandit, R.; Anil, A.; Lali, A. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renew. Energy 2019, 132, 742–751. [Google Scholar] [CrossRef]
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel Production in Ireland-An Approach to 2020 Targets with a Focus on Algal Biomass. Energies 2013, 6, 6391–6412. [Google Scholar] [CrossRef]
- Rajendran, K.; Browne, J.D.; Murphy, J.D. What is the level of incentivisation required for biomethane upgrading technologies with carbon capture and reuse? Renew. Energy 2019, 133, 951–963. [Google Scholar] [CrossRef]
- Gao, G.; Burgess, J.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Roberts, K.P.; Heaven, S.; Banks, C.J. Comparative testing of energy yields from micro-algal biomass cultures processed via anaerobic digestion. Renew. Energy 2016, 87, 744–753. [Google Scholar] [CrossRef] [Green Version]
- Klassen, V.; Blifernez-Klassen, O.; Bax, J.; Kruse, O. Wastewater-borne microalga Chlamydomonas sp.: A robust chassis for efficient biomass and biomethane production applying low-N cultivation strategy. Bioresour. Technol. 2020, 315, 123825. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kumar, P.; Mehariya, S.; Purohit, H.J.; Lee, J.; Vipin, C.; Kalia, V.C. Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int. J. Hydrogen Energy 2014, 39, 14663–14668. [Google Scholar] [CrossRef]
- Oey, M.; Sawyer, A.L.; Ross, I.L.; Hankamer, B. Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol. J. 2016, 14, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Medisetty, V.M.; Kumar, R.; Ahmadi, M.H.; Vo, D.V.N.; Ochoa, A.A.V.; Solanki, R. Overview on the Current Status of Hydrogen Energy Research and Development in India. Chem. Eng. Technol. 2020, 43, 613–624. [Google Scholar] [CrossRef]
- Chai, S.; Zhang, G.; Li, G.; Zhang, Y. Industrial hydrogen production technology and development status in China: A review. Clean Technol. Environ. Policy 2021, 23, 1931–1946. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Obulisamy, P.K.; Verma, P. Advanced microalgae-based renewable biohydrogen production systems: A review. Bioresour. Technol. 2021, 320, 124301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, L.J.; Wang, W.; Yan, Q.; Kuang, T.; Qin, X.; Shen, J.R. Structure of plant photosystem I-light harvesting complex I supercomplex at 2.4 angstrom resolution. J. Integr. Plant Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Guo, Y.; Govindjee, G. Photosynthesis: Basics, history and modelling. Ann. Bot. 2020, 126, 511–537. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production - A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef] [PubMed]
- Picone, N.; Mohammadi, S.S.; Waajen, A.C.; Alen, V.T.A.; Jetten, M.S.M.; Pol, A.; Op-den Camp, H.J.M. More Than a Methanotroph: A Broader Substrate Spectrum for Methylacidiphilum fumariolicum SolV. Front. Microbiol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Wang, G.Y.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Lee, X.J.; Onga, H.C.; Gan, Y.Y.; Chen, W.H.; Meurah, T.; Mahlia, I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, B.; Zhang, Y.; Huang, Y.; Xu, M. Investigation on Pyrolysis of Low Lipid Microalgae Chlorella vulgaris and Dunaliella salina. Energy Fuels 2014, 28, 95–103. [Google Scholar] [CrossRef]
- Miao, X.L.; Wu, Q.Y.; Yang, C.Y. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.; Chen, L.; Qin, L.; Wang, F.; Xu, Y.; Peigao Duan, P. Progress in Hydrothermal Liquefaction of Algal Biomass and Hydrothermal Upgrading of the Subsequent Crude Bio-Oil: A Mini Review. Energy Fuels 2020, 34, 11723–11751. [Google Scholar] [CrossRef]
- Zheng, Y.; Jida Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Activity and selectivity of Ni-Cu bimetallic zeolites catalysts on biomass conversion for bio-aromatic and bio-phenols. J. Energy Inst. 2021, 97, 58–72. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C. Comparative Evaluation of Thermochemical Liquefaction and Pyrolysis for Bio-Oil Production from Microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Martinez-Fernandez, J.S.; Pang, N.; Fu, X.; Chen, S. Recent development of hydrothermal liquefaction for algal biorefinery. Renew. Sustain. Energy Rev. 2020, 121, 10970–10977. [Google Scholar] [CrossRef]
- Latif, N.; Ong, M.Y.; Nomanbhay, S. Hydrothermal liquefaction of Malaysia’s algal biomass for high-quality bio-oil production. Eng. Life Sci. 2019, 19, 246–269. [Google Scholar] [CrossRef] [Green Version]
- Raikova, S.; Allen, M.J.; Chuck, C.J. Hydrothermal liquefaction of macroalgae for the production of renewable biofuels. Biofuels Bioprod. Biorefin. 2019, 13, 1483–1504. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A. Sustainable saline microalgae co-cultivation for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2017, 78, 356–368. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Beisson, F.; Riekhof, W. Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J. 2015, 82, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Geleta, M.; Gustafsson, C.; Lager, I.; Hofvander, P.; Löfstedt, C.; Cahoon, E.B.; Minina, E.; Bozhkov, P.; Stymne, S. Oil crops for the future. Curr. Opin. Plant Biol. 2020, 56, 181–189. [Google Scholar] [CrossRef]
- Griffiths, G.; Morse, N. Clinical Applications of C-18 and C-20 Chain Length Polyunsaturated Fatty Acids and Their Biotechnological Production in Plants. J. Am. Oil Chem. Soc. 2006, 73, 171–185. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Hamouda, R.A. Lipids extraction from the green alga Ankistrodesmus falcatus using different methods. Rend. Lincei-Sci. Fis. E Nat. 2016, 27, 589–595. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
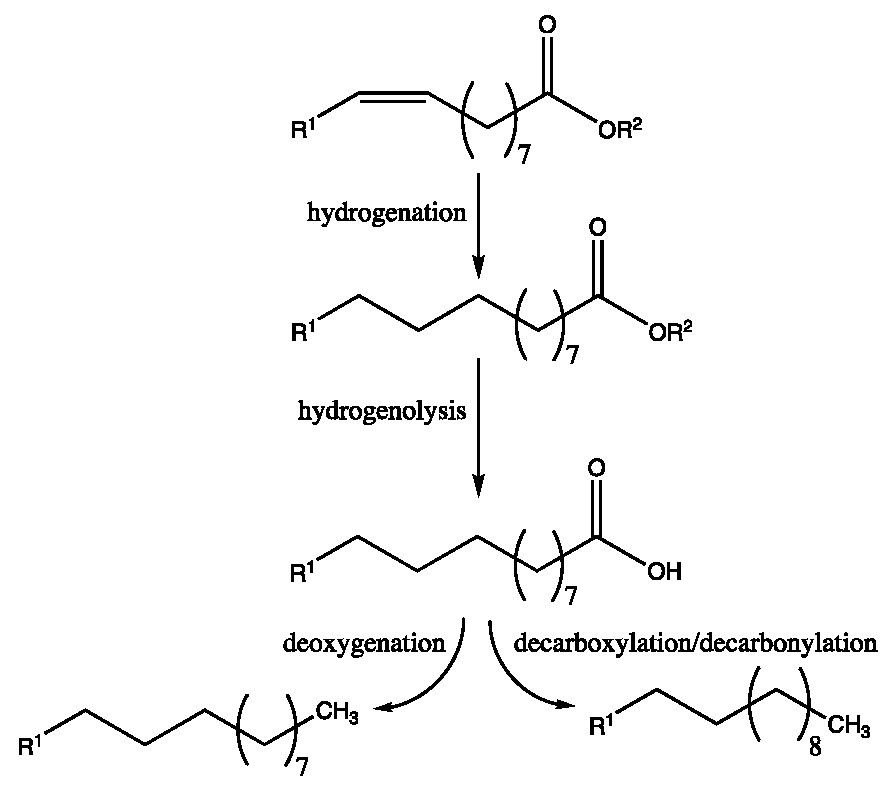
- Kruger, J.S.; Knoshaug, E.P.; Dong, T.; Hull, T.C.; Pienkos, P.T. Catalytic Hydroprocessing of Single-Cell Oils to Hydrocarbon Fuels Converting microbial lipids to fuels is a promising approach to replace fossil fuels. Johns. Matthey Technol. Rev. 2021, 65, 227–246. [Google Scholar] [CrossRef]
- Zheng, Y.; Dillon, J.T.; Zhang, Y.; Huang, Y. Discovery of Alkenones with Variable Methylene-Interrupted Double Bonds: Implications for the Biosynthetic Pathway. J. Phycol. 2016, 52, 1037–1050. [Google Scholar] [CrossRef]
- Cañavate, J.; Hachero-Cruzado, I.; Pérez-Gavilán, C.; Fernández-Díaz, C. Lipid dynamics and nutritional value of the estuarine strainIsochrysis galbanaVLP grown from hypo to hyper salinity. J. Appl. Phycol. 2020, 32, 3749–3766. [Google Scholar] [CrossRef]
- Al-Lwayzy, S.H.; Yusaf, T. Chlorella protothecoides Microalgae as an Alternative Fuel for Tractor Diesel Engines. Energies 2013, 6, 766–783. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Jacob, A.; Ashok, B.; Alagumalai, A.; Chyuan, O.H.; Le, P.T.K. Critical review on third generation micro algae biodiesel production and its feasibility as future bioenergy for IC engine applications. Energy Convers. Manag. 2021, 228, 113655. [Google Scholar] [CrossRef]
- Sharma, V.; Duraisamy, G. Production and characterization of bio-mix fuel produced from the mixture of raw oil feedstock, and its effects on performance and emission analysis in DICI diesel engine. Environ. Sci. Pollut. Res. 2019, 26, 16742–16761. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Kalaimurugan, K.; Prathima, A. Quality analysis studies on biodiesel production of neochloris oleoabundans algae. Energy Sour. Part A Recovery Util. Environ. Eff. 2018, 40, 439–445. [Google Scholar] [CrossRef]
- Subramaniam, M.; Solomon, J.M.; Nadanakumar, V.; Anaimuthu, S.; Sathyamurthy, R. Experimental investigation on performance, combustion and emission characteristics of DI diesel engine using algae as a biodiesel. Energy Rep. 2020, 6, 1382–1392. [Google Scholar] [CrossRef]
- Rajak, U.; Nashine, P.; Verma, T.N. Effect of spirulina microalgae biodiesel enriched with diesel fuel on performance and emission characteristics of CI engine. Fuel 2020, 268, 117305. [Google Scholar] [CrossRef]
- Rajak, U.; Nashine, P.; Verma, T.N.; Pugazhendhi, A. Performance and emission analysis of a diesel engine using hydrogen enriched n-butanol, diethyl ester and Spirulina microalgae biodiesel. Fuel 2020, 271, 117645. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Prathima, A. Environmental effect of CI engine using microalgae methyl ester with doped nano additives. Transp. Res. Part D Transp. Environ. 2017, 50, 385–396. [Google Scholar] [CrossRef]
- Al-Lwayzy, S.H.; Yusaf, T. Diesel engine performance and exhaust gas emissions using Microalgae Chlorella protothecoides biodiesel. Renew. Energy 2017, 101, 690–701. [Google Scholar] [CrossRef]
- Mathimani, T.; Kumar, T.S.; Chandrasekar, M.; Uma, L.; Prabaharan, D. Assessment of fuel properties, engine performance and emission characteristics of outdoor grown marine Chlorella vulgaris BDUG 91771 biodiesel. Renew. Energy 2017, 105, 637–646. [Google Scholar] [CrossRef]
- Hossain, F.M.; Nabi, M.N.; Brown, R.J. Investigation of diesel engine performance and exhaust emissions of microalgae fuel components in a turbocharged diesel engine. Energy Convers. Manag. 2019, 186, 220–228. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Lee, T.I.; Doane, A.J.; Butterfield, A.E.; McLennan, J.D.; Mohanty, A.K.; Pease, L.F. Periodic symmetry defined bioreactors enhance algae growth. Environ. Sci. Water Res. Technol. 2019, 5, 1037–1045. [Google Scholar] [CrossRef]
- Yan, Q.; Pfleger, B.F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 2020, 58, 35–46. [Google Scholar] [CrossRef]
- Koyande, A.K.; Show, P.L.; Guo, R.; Tang, B.; Ogino, C.; Chang, J.S. Bio-processing of algal bio-refinery: A review on current advances and future perspectives. Bioengineered 2019, 10, 574–592. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Knoshaug, E.P.; Davis, R.; Laurens, L.M.L.; Wychen, S.V.; Philip, T.; Pienkos, P.T.; Nagle, N. Combined algal processing: A novel integrated biorefinery process to produce algal biofuels and bioproducts. Algal Res.-Biomass Biofuels Bioprod. 2016, 19, 316–323. [Google Scholar] [CrossRef] [Green Version]
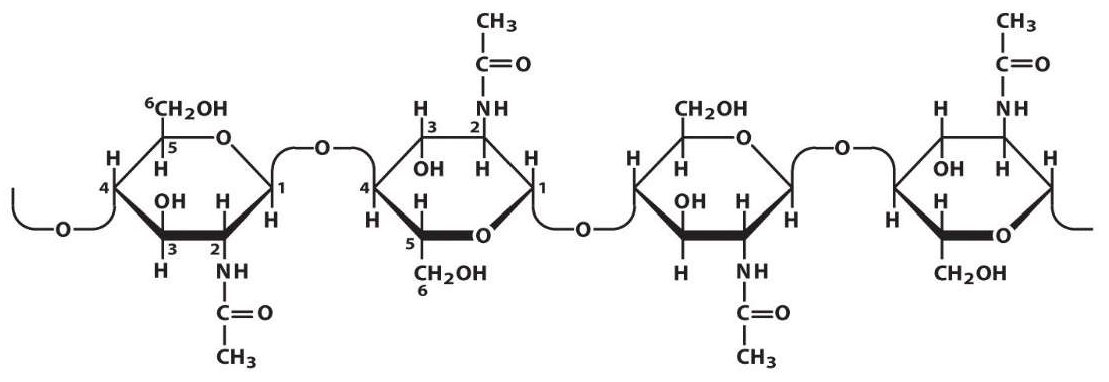

| Cyanophyta, blue-green algae or cyanobacteria. Chlorophyta-green algae Charaphyta Rhodophyta, red algae Cryptophyta, cryptomonads Dinophyta, dinoflagellates Heterokontophyta, heterokonts which includes: Phaephyceae (Brown algae), Bacilliarophyceae (Diatoms) Chrysophyceae (Golden algae) Haptophyta, haptophytes Chlorarachniophyta, chlorarachniophytes Euglenophyta, euglenoids |
| Country | Seaweed Production (Million Tonnes) | World Production (%) |
|---|---|---|
| China | 18,506 | 57.3 |
| Indonesia | 9320 | 28.9 |
| Republic of Korea | 1711 | 5.3 |
| Phillipines | 1478 | 4.6 |
| DPR Korea | 553 | 1.7 |
| Japan | 89 | 1.2 |
| Malaysia | 174 | 0.5 |
| Zanzibar/Tanzania | 103 | 0.3 |
| Chile | 21 | <0.1 |
| Vietnam | 19 | <0.1 |
| India | 5 | <0.02 |
| Russian Federation | 5 | <0.02 |
| Others | 21 | <0.1 |
| Species | % World Production | Major Product Harvested |
|---|---|---|
| Eucheuma sp | 34 | Carrageenan |
| Laminaria japonica | 28 | Human food |
| Gracilaria sp | 14 | Agar |
| Undaria pinnatifida | 9 | Human food |
| Porphyria sp | 7 | Human food |
| Kapphaphycus alvarezii | 6 | Carrageenan |
| Sargassum fusiforme | 1 | Human food |
| Others Macrocystis pyrifera Enteromorpha clathrate Codium fragile Monostroma nitidum Chondracanthus chamissoi | <1 | Alginate Bioactive Skin care Human food Human food |
| Species | Phylum | Product | Application |
|---|---|---|---|
| Arthrospira platensis | Cyanophyta | Phycocyanin, biomass | Health food, cosmetics |
| Aphanizomenon flos-aquae | Cyanophyta | Protein. Essential fatty acids, ꞵ-carotene | Health food, food supplement |
| Lyngbya majuscule | Cyanophyta | Immune modulators | Pharmaceuticals, nutrition |
| Chlorella spp | Chlorophyta | Biomass, carbohydrate | Animal nutrition, food supplement |
| Dunaliella salina | Chlorophyta | carotenoids | Health foods |
| Haematococcus pluvialis | Chlorophyta | astaxanthin | Pharmaceuticals, feeds |
| Scenedesmus spp | Chlorophyta | Protein | Aquaculture, human nutrition |
| Odontella aurita | Heterokonta- | EPA | Pharmaceuticals, cosmetics |
| Phaedactylum tricomutum | Heterokonta | Lipids, fatty acids | Nutrition, fuel production |
| Schizochytrium spp | Heterokonta | DHA and EPA | Food supplement, beverage |
| Nannochloropsis oculata | Heterokonta | biomass | Larval/juvenile marine fish food |
| Nannochloropsis spp | Heterokonta | EPA | Pharmaceuticals food supplement |
| Porphyridium cruentum | Rhodophyta | polysaccharides | Pharmaceuticals, cosmetics |
| Isochrysis galbana | Haptophyta | Fatty acids | Animal nutrition |
| Crypthecodinium cohnii | Dinoflagellata | DHA | Infant health and nutrition, brain development |
| Country | Production 2019 (Billions L) |
|---|---|
| USA | 15.78 |
| Brazil | 8.57 |
| EU | 1.44 |
| China | 0.90 |
| Canada | 0.50 |
| Rest of the World | 1.84 |
| Group | Species | Ethanol Yield g·g−1 Sugar | Theoretical Yield (%) |
|---|---|---|---|
| Chlorophyta | Enteromorpha intestinalis | 0.21 | 42 |
| Ulva fasciata | 0.45 | 88 | |
| Ulva lactuca | 0.47 | 92 | |
| Ulva pertusa | 0.47 | 91 | |
| Heterokontophyta | |||
| A. crassifolia | 0.38 | 75 | |
| L. hyperborea | 0.43 | 84 | |
| S. sagamianum | 0.35 | 69 | |
| S. japonica | 0.41 | 81 | |
| U. pinnatifida | 0.14 | 28 | |
| Rhodophyta | G. elegans | 0.38 | 74 |
| G. amansii | 0.47 | 92 | |
| G. verrucose | 0.43 | 84 | |
| K. alverzii | 0.25 | 49 | |
| P. palmata | 0.17 | 34 |
| Genus | Methane Yield (L·g·VS−1) |
|---|---|
| Gracillaria | 0.28–0.40 |
| Laminaria | 0.23–0.30 |
| Sargassum | 0.06–0.19 |
| Macrocystis | 0.14–0.40 |
| Ulva | 0.31 |
| Elemental Composition | HTL | Pyrolysis |
|---|---|---|
| C (wt %) | 73 | 58 |
| H (wt %) | 8 | 6 |
| O (wt %) | 16 | 36 |
| S (wt %) | <45 | 29 |
| Moisture | 5.1 | 24.8 |
| HHV (MJ Kg−1) | 35.7 | 22.6 |
| Viscosity (cPs) | 15,000 | 59 |
| Algal Species | Viscosity (cst) at 40 °C | Specific Gravity | Cloud Point (°C) | Pour Point (°C) | Flash Point (°C) | Cetane Number | Calorific Value MJ·kg |
|---|---|---|---|---|---|---|---|
| Chlorella protothecoides | 2.8 | 0.867 | −27 | −11 | 124 | 52 | 40 |
| Tolypothrix | 4.1 | 0.857 | 7.38 | 1.19 | - | 58 | - |
| Dunaliella salina | 2.40 | 0.8513 | 0 | −6 | 129 | 50 | 34 |
| Spirogyra | 4.4 | 0.884 | 3 | −7 | 78 | - | 13.62 |
| Botryococcus braunii | 5.35 | 0.853 | - | - | 138 | - | 50 |
| Chlorella vulgaris | 4.8 | 0.870 | 0 | −11 | 140 | 60 | 17.44 |
| Cladophora | 3.8 | 0.892 | - | −12 | 110 | 60 | 17.44 |
| Kirchneriella lunari | 4.15 | 0.882 | - | - | - | 51 | 41.50 |
| Nannochloropsis oculata | 5.76 | 0.854 | 3.39 | −4 | 180 | 46 | 16.80 |
| Entromorpha | 3.12 | 0.862 | −1 | −6 | 194 | 50 | 39.760 |
| Stoechospermum marginatum | 4.84 | 0.890 | - | - | 128 | 63 | 42.05 |
| Neochloris oleoabundans | 5.54 | 0.887 | −10 | −12 | 126 | 55 | 39.76 |
| Crypthecodinium cohnii | 5.06 | 0.912 | 16.1 | 95 | 46 | 39.86 | |
| Fossil diesel | 2.3 | 0.830 | - | - | 60 | 56 | 43.25 |
| ASTM standard | 1.9–6.0 | 0.86–0.9 | - | - | >52 | 40 min |
| Algae Types | Performance | Emissions |
|---|---|---|
| Botryococcus braunii |
|
|
| Spirulina |
|
|
| Chlorellaprotothecoides |
|
|
| Chlorella vulgaris |
|
|
| Scenedesmus sp. |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffiths, G.; Hossain, A.K.; Sharma, V.; Duraisamy, G. Key Targets for Improving Algal Biofuel Production. Clean Technol. 2021, 3, 711-742. https://doi.org/10.3390/cleantechnol3040043
Griffiths G, Hossain AK, Sharma V, Duraisamy G. Key Targets for Improving Algal Biofuel Production. Clean Technologies. 2021; 3(4):711-742. https://doi.org/10.3390/cleantechnol3040043
Chicago/Turabian StyleGriffiths, Gareth, Abul Kalam Hossain, Vikas Sharma, and Ganesh Duraisamy. 2021. "Key Targets for Improving Algal Biofuel Production" Clean Technologies 3, no. 4: 711-742. https://doi.org/10.3390/cleantechnol3040043
APA StyleGriffiths, G., Hossain, A. K., Sharma, V., & Duraisamy, G. (2021). Key Targets for Improving Algal Biofuel Production. Clean Technologies, 3(4), 711-742. https://doi.org/10.3390/cleantechnol3040043







