Comparative Life Cycle Assessment of EPA and DHA Production from Microalgae and Farmed Fish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goal and Scope
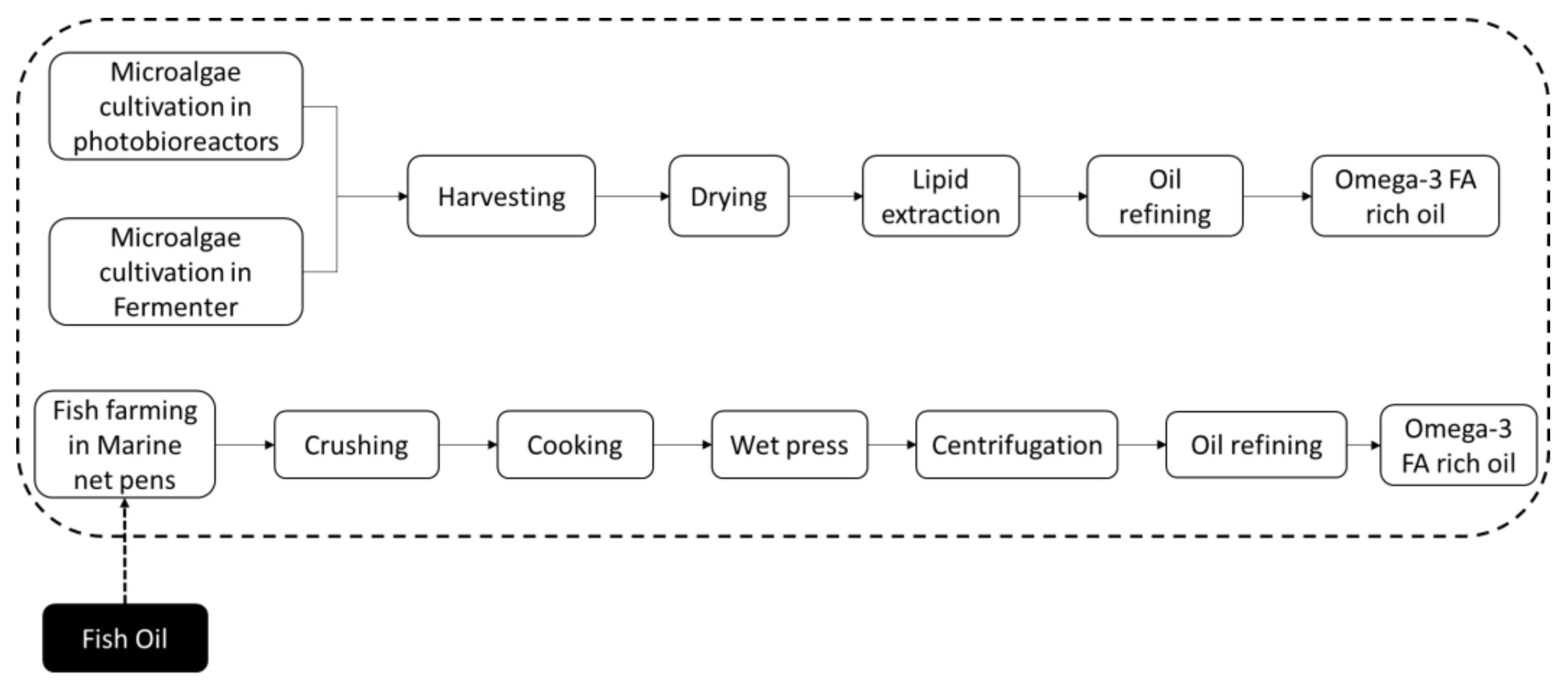
2.2. Life Cycle Inventory Data
2.3. Life Cycle Impact Assessment
3. Results and Discussion
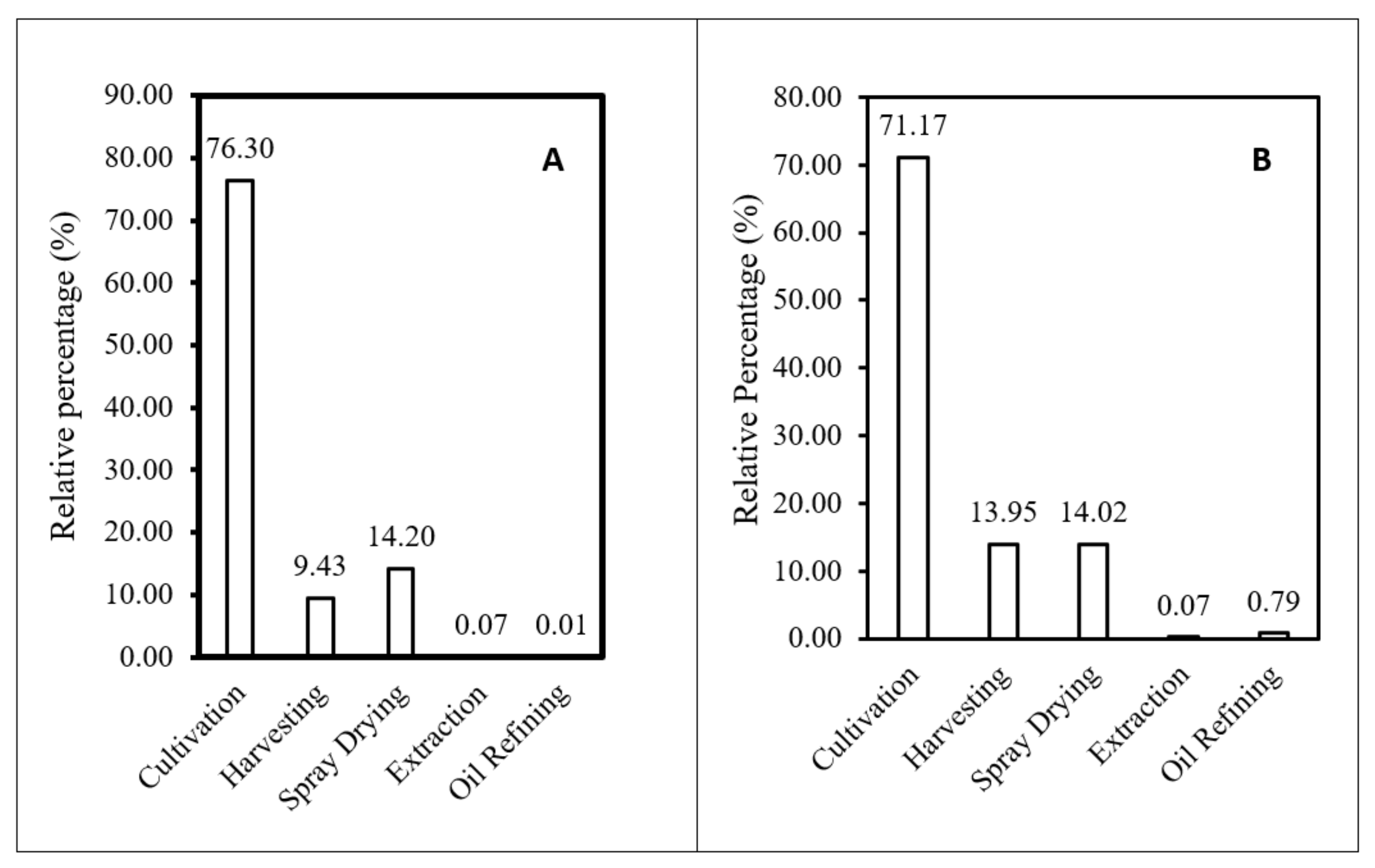
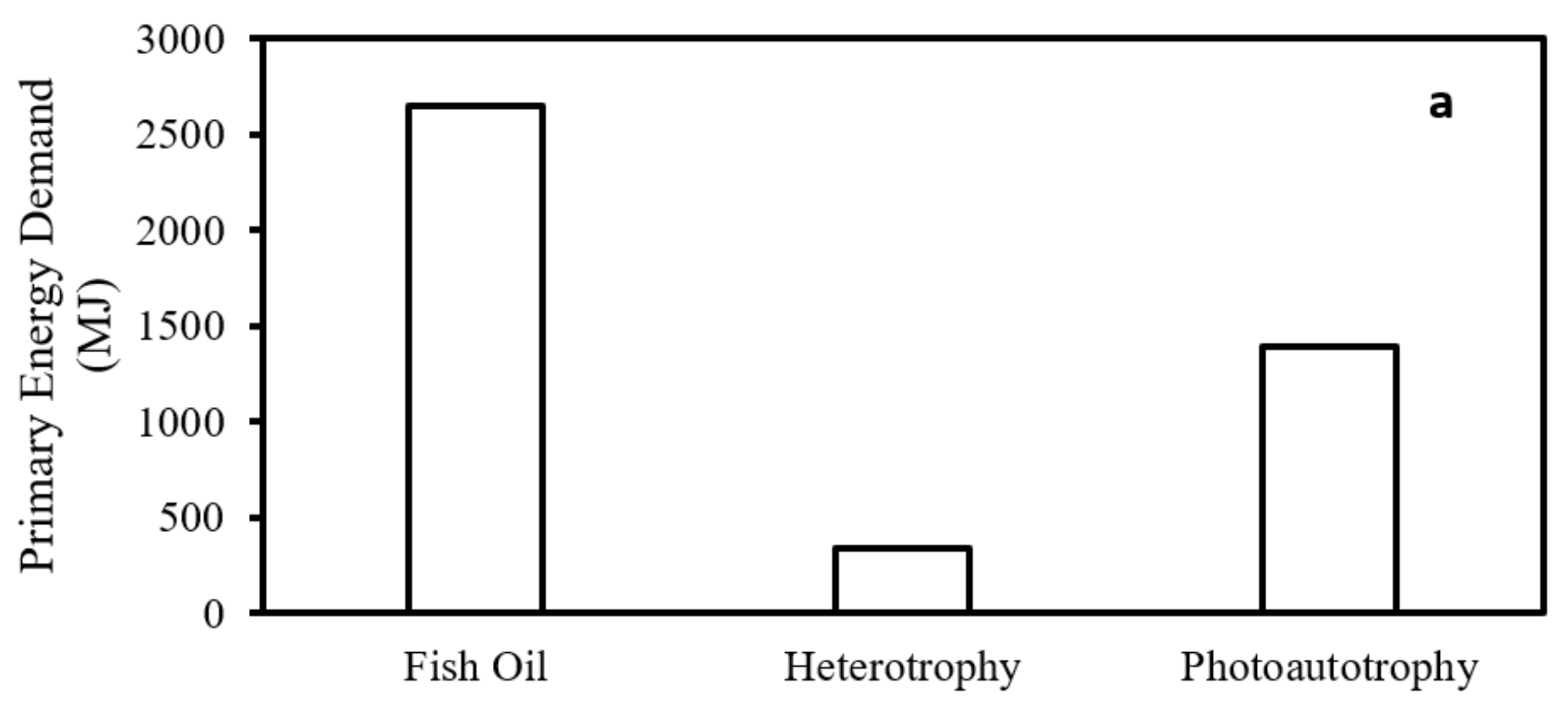
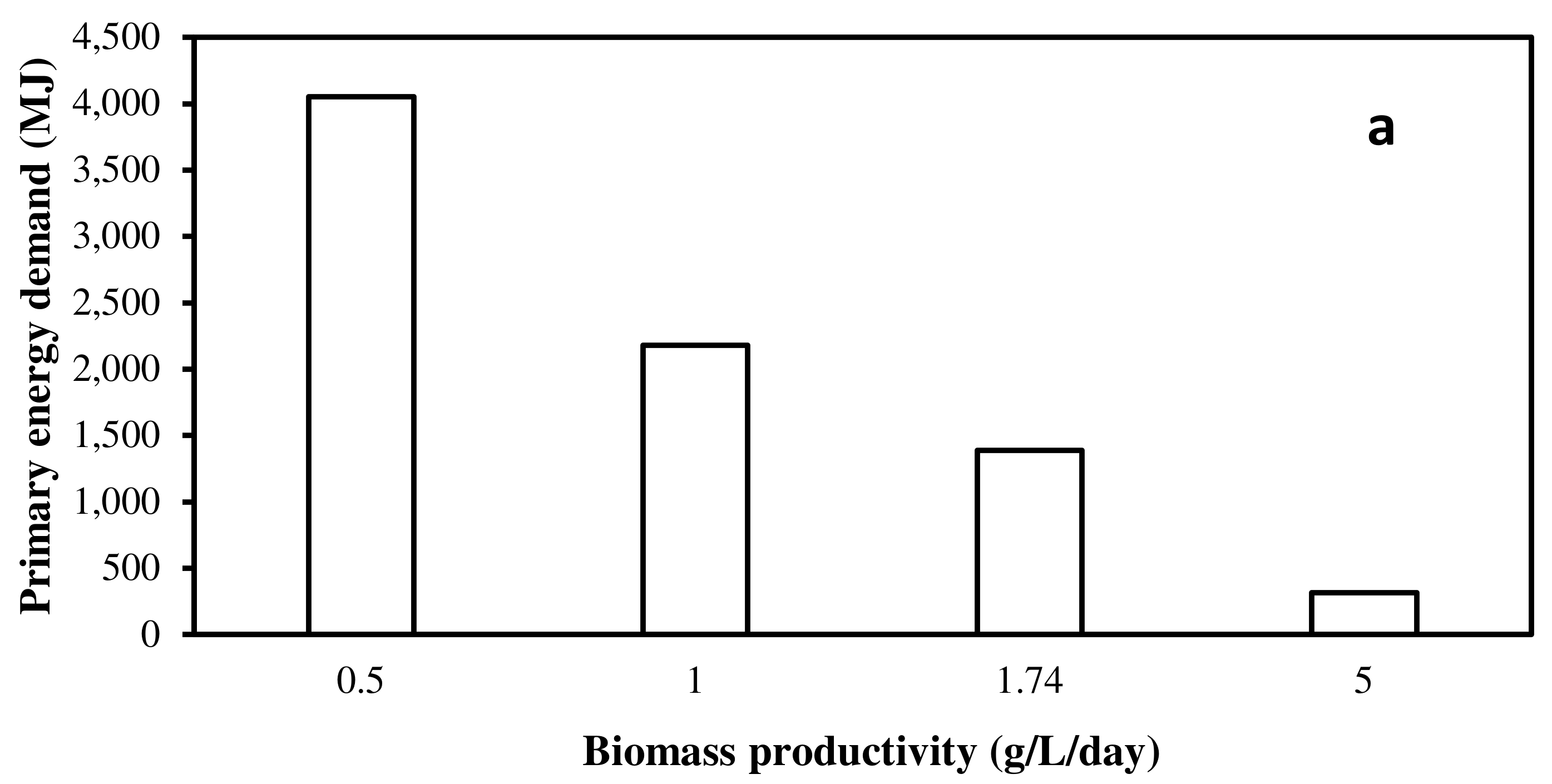
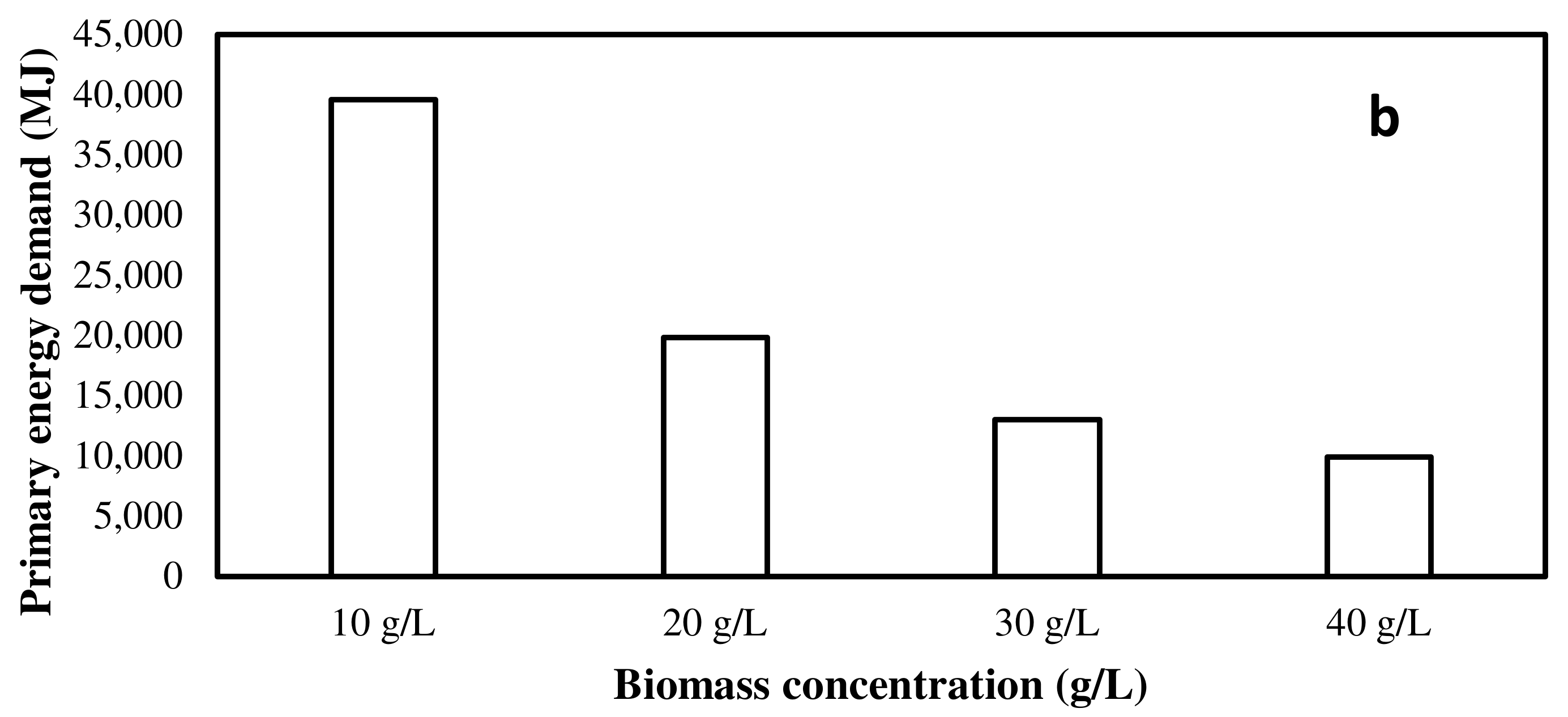
3.1. EPA+DHA Production from Photoautotrophic and Heterotrophic Microalgae
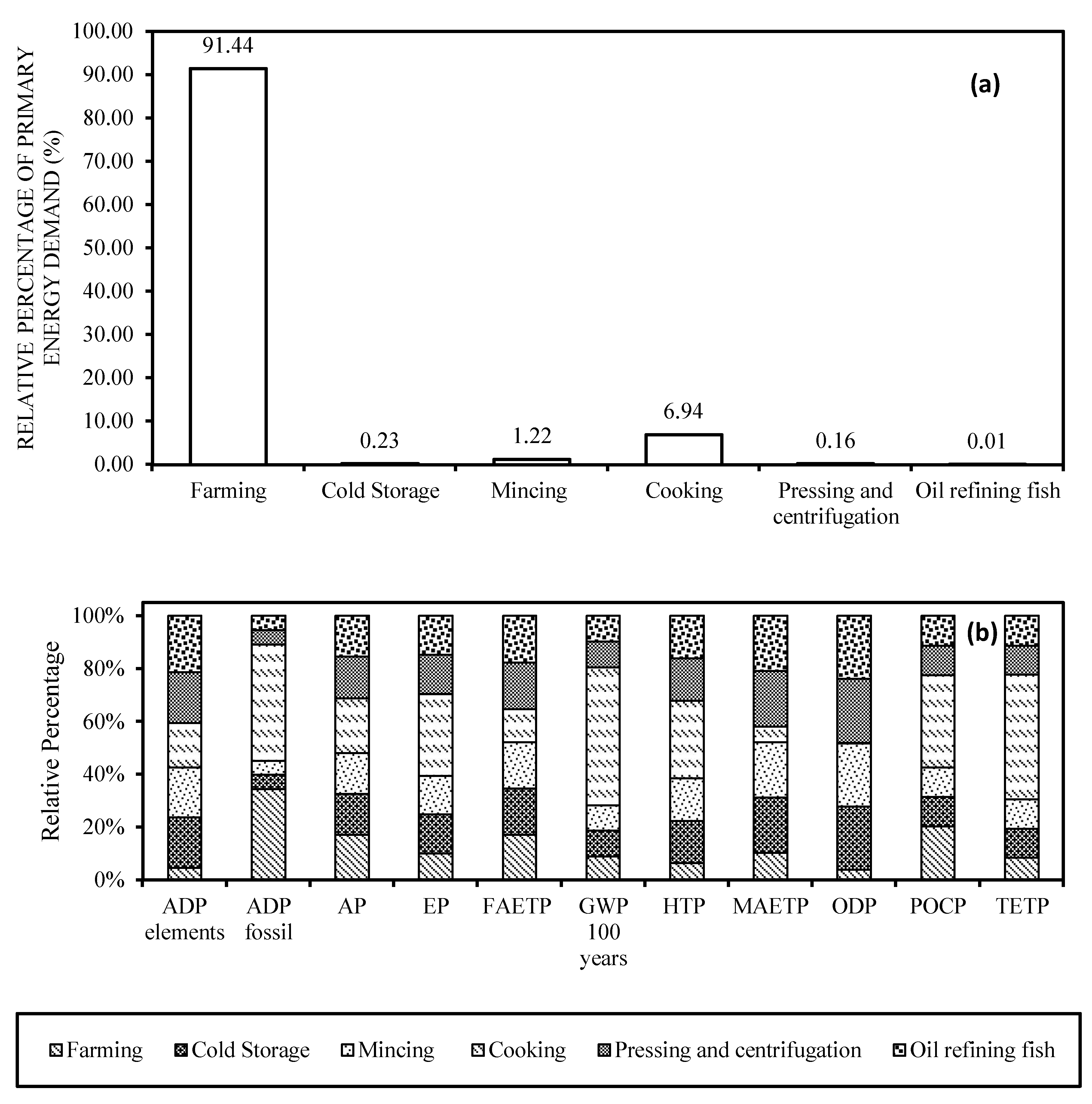
3.2. EPA+DHA Production from Farmed Fish
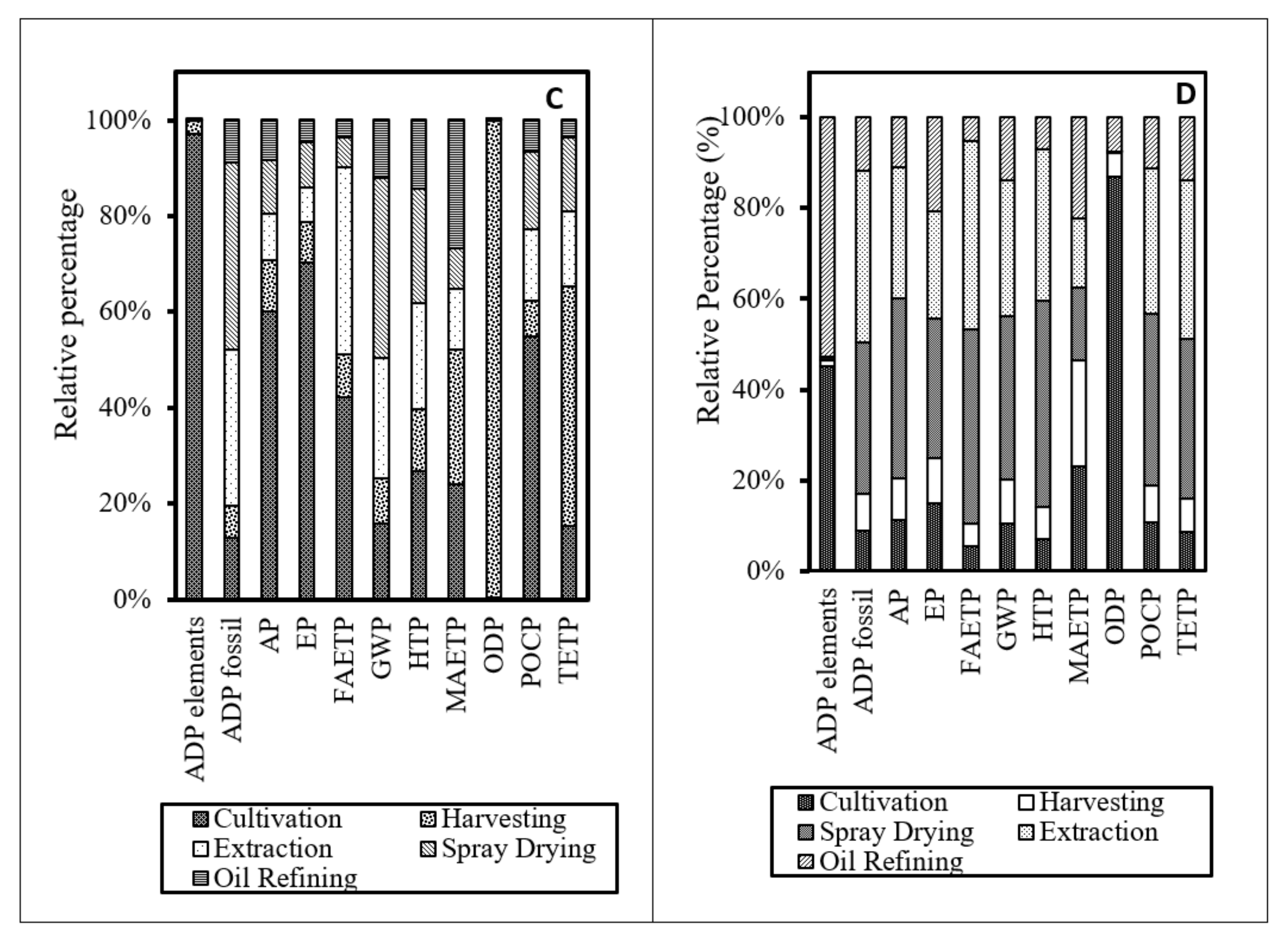
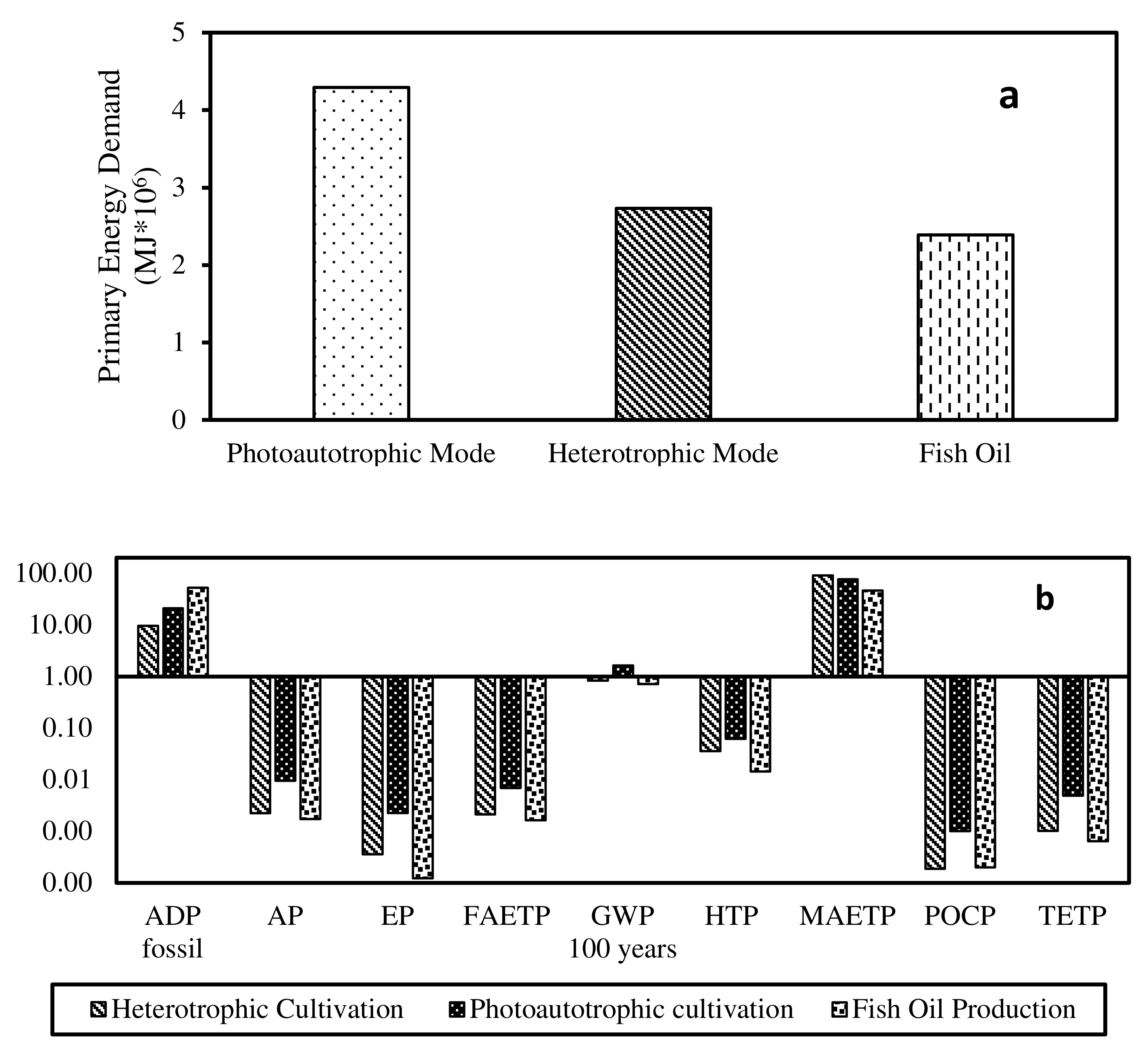
3.3. Life Cycle Impact Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grand View Research. Omega 3 Supplement Market Analysis by Source (Fish Oil, Krill Oil), by Application (Infant Formula, Food & Beverages, Nutritional Supplements, Pharmaceutical, Animal Feed, Clinical Nutrition), and Segment Forecasts, 2018–2025. 2017. Available online: https://www.grandviewresearch.com/ (accessed on 18 May 2021).
- Global Recommendations for EPA and DHA Intake (Rev. 19 November 2014). Available online: https://www.issfal.org/assets/globalrecommendationssummary19nov2014landscape_-3-.pdf (accessed on 18 October 2020).
- FAO. The State of World Fisheries and Aquaculture 2010. Rome: Fisheries and Aquaculture Department, Food and Agriculture Organisation of the United Nations. 2010. Available online: http://www.fao.org/docrep/013/i1820e/i1820e.pdf (accessed on 21 May 2021).
- Jackson, A. Fishmeal and Fish Oil and Their Role in Sustainable Aquaculture. International Aquafeed. 2012. Available online: https://effop.org/wp-content/uploads/2019/01/andrew-jackson.pdf (accessed on 21 May 2021).
- Callejón, M.J.J.; Medina, A.R.; Moreno, P.A.G.; Cerdán, L.E.; Guillén, S.O.; Grima, E.M. Simultaneous extraction and fractionation of lipids from the microalga Nannochloropsis sp. for the production of EPA-rich polar lipid concentrates. J. Appl. Phycol. 2020, 32, 1–12. [Google Scholar]
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousão-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; et al. Lipid composition and some bioactivities of 3 newly isolated microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquac. Int. 2020, 28, 711–727. [Google Scholar] [CrossRef]
- Steinrücken, P.; Prestegard, S.K.; de Vree, J.H.; Storesund, J.E.; Pree, B.; Mjøs, S.A.; Erga, S.R. Comparing EPA production and fatty acid profiles of three Phaeodactylum tricornutum strains under western Norwegian climate conditions. Algal Res. 2018, 30, 11–22. [Google Scholar] [CrossRef]
- Meng, Y.; Jiang, J.; Wang, H.; Cao, X.; Xue, S.; Yang, Q.; Wang, W. The characteristics of TAG and EPA accumulation in Nannochloropsis oceanica IMET1 under different nitrogen supply regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.P.; Chuang, L.T.; Chen, C.N.N. Production of long chain omega-3 fatty acids and carotenoids in tropical areas by a new heat-tolerant microalga Tetraselmis sp. DS3. Food Chem. 2016, 192, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.K.; Yu, A.; Oh, B.R.; Park, J.M.; Kim, C.H.; Sohn, J.H.; Kondo, A.; Seo, J.W. Large-scale production of microalgal lipids containing high levels of docosahexaenoic acid upon fermentation of Aurantiochytrium sp. KRS101. Food Nutr. Sci. 2013, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Karnaouri, A.; Chalima, A.; Kalogiannis, K.G.; Varamogianni-Mamatsi, D.; Lappas, A.; Topakas, E. Utilization of lignocellulosic biomass towards the production of omega-3 fatty acids by the heterotrophic marine microalga Crypthecodinium cohnii. Bioresour. Technol. 2020, 303, 122899–122907. [Google Scholar] [CrossRef]
- Chalima, A.; Taxeidis, G.; Topakas, E. Optimization of the production of docosahexaenoic fatty acid by the heterotrophic microalga Crypthecodinium cohnii utilizing a dark fermentation effluent. Renew. Energy 2020, 152, 102–109. [Google Scholar] [CrossRef]
- Sahin, D.; Tas, E.; Altindag, U.H. Enhancement of docosahexaenoic acid (DHA) production from Schizochytrium sp. S31 using different growth medium conditions. Amb. Express 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Barclay, W.; Weaver, C.; Metz, J.; Hansen, J. Development of a docosahexaenoic acid production technology using Schizochytrium: Historical perspective and update. In Single Cell Oils; AOCS Press: Urbana, IL, USA, 2010; pp. 75–96. [Google Scholar]
- Hu, X.C.; Ren, L.J.; Chen, S.L.; Zhang, L.; Ji, X.J.; Huang, H. The roles of different salts and a novel osmotic pressure control strategy for improvement of DHA production by Schizochytrium sp. Bioprocess Biosyst. Eng. 2015, 38, 2129–2136. [Google Scholar] [CrossRef]
- Barr, W.J.; Landis, A.E. Comparative life cycle assessment of a commercial algal multiproduct biorefinery and wild caught fishery for small pelagic fish. Int. J. Life Cycle Assess. 2018, 23, 1141–1150. [Google Scholar] [CrossRef]
- Chang, K.J.L.; Rye, L.; Dunstan, G.A.; Grant, T.; Koutoulis, A.; Nichols, P.D.; Blackburn, S.I. Life cycle assessment: Heterotrophic cultivation of thraustochytrids for biodiesel production. J. Appl. Phycol. 2015, 27, 639–647. [Google Scholar] [CrossRef]
- Depra, M.C.; Severo, I.A.; dos Santos, A.M.; Zepka, L.Q.; Jacob-Lopes, E. Environmental impacts on commercial microalgae-based products: Sustainability metrics and indicators. Algal Res. 2020, 51, 102056–102070. [Google Scholar] [CrossRef]
- Bartek, L.; Strid, I.; Henryson, K.; Junne, S.; Rasi, S.; Eriksson, M. Life cycle assessment of fish oil substitute produced by microalgae using food waste. Sustain. Prod. Consum. 2021, 27, 2002–2021. [Google Scholar] [CrossRef]
- Lu, Y.; Mu, D.; Xue, Z.; Xu, P.; Li, Y.; Xiang, W.; Burnett, J.; Bryant, K.; Zhou, W.; Zhou, W. Life cycle assessment of industrial production of microalgal oil from heterotrophic fermentation. Algal Res. 2021, 58, 102404. [Google Scholar] [CrossRef]
- ISO. ISO 14040:2006. In Environmental Management-Life Cycle Assesment-Principles and Framework; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- ISO. ISO 14044:2006. In Environmental Management-Life Cycle Assesment-Requirement and Guidelines; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: Research challenges and possibilities. Aquaculture 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Chandra, T.S.; Kumar, M.M.; Mukherji, S.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Comparative life cycle assessment of microalgae-mediated CO2 capture in open raceway pond and airlift photobioreactor system. Clean Technol. Environ. Policy 2018, 20, 2357–2364. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P. Feeding farmed salmon: Is organic better? Aquaculture 2007, 272, 399–416. [Google Scholar] [CrossRef]
- Ayer, N.W.; Tyedmers, P.H. Assessing alternative aquaculture technologies: Life cycle assessment of salmonid culture systems in Canada. J. Clean. Prod. 2009, 17, 362–373. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and refinement of omega-3 rich oils from processing by-products of farmed fish species. Foods 2019, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Akdemir, S. Energy consumption of an Experimental cold storage. Bulg. J. Agric. Sci. 2012, 18, 991–996. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Ghamkhar, R.; Hicks, A. Comparative environmental impact assessment of aquafeed production: Sustainability implications of forage fish meal and oil free diets. Resour. Conserv. Recycl. 2020, 161, 104849. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Oliviero, M.; Sacchi, R.; Masi, P. Sustainable production of food grade omega-3 oil using aquatic protists: Reliability and future horizons. New Biotechnol. 2021, 62, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Beal, C.M.; Gerber, L.N.; Thongrod, S.; Phromkunthong, W.; Kiron, V.; Granados, J.; Archibald, I.; Greene, C.H.; Huntley, M.E. Marine microalgae commercial production improves sustainability of global fisheries and aquaculture. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Woods, J.S.; Veltman, K.; Huijbregts, M.A.; Verones, F.; Hertwich, E.G. Towards a meaningful assessment of marine ecological impacts in life cycle assessment (LCA). Environ. Int. 2016, 89, 48–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, L.; Lehmann, A.; Finogenova, N.; Finkbeiner, M. Including biodiversity in life cycle assessment–State of the art, gaps and research needs. Environ. Impact Assess. Rev. 2017, 67, 88–100. [Google Scholar] [CrossRef]
- Asselin, A.; Rabaud, S.; Catalan, C.; Leveque, B.; L’Haridon, J.; Martz, P.; Neveux, G. Product Biodiversity Footprint—A novel approach to compare the impact of products on biodiversity combining Life Cycle Assessment and Ecology. J. Clean. Prod. 2020, 248, 119262. [Google Scholar] [CrossRef]
- Avadí, A.; Vázquez-Rowe, I.; Symeonidis, A.; Moreno-Ruiz, E. First series of seafood datasets in ecoinvent: Setting the pace for future development. Int. J. Life Cycle Assess. 2020, 25, 1333–1342. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togarcheti, S.C.; Padamati, R.B. Comparative Life Cycle Assessment of EPA and DHA Production from Microalgae and Farmed Fish. Clean Technol. 2021, 3, 699-710. https://doi.org/10.3390/cleantechnol3040042
Togarcheti SC, Padamati RB. Comparative Life Cycle Assessment of EPA and DHA Production from Microalgae and Farmed Fish. Clean Technologies. 2021; 3(4):699-710. https://doi.org/10.3390/cleantechnol3040042
Chicago/Turabian StyleTogarcheti, Sarat Chandra, and Ramesh Babu Padamati. 2021. "Comparative Life Cycle Assessment of EPA and DHA Production from Microalgae and Farmed Fish" Clean Technologies 3, no. 4: 699-710. https://doi.org/10.3390/cleantechnol3040042
APA StyleTogarcheti, S. C., & Padamati, R. B. (2021). Comparative Life Cycle Assessment of EPA and DHA Production from Microalgae and Farmed Fish. Clean Technologies, 3(4), 699-710. https://doi.org/10.3390/cleantechnol3040042






