Arsenic Behavior in Paddy Soils: Sorption Capacity and the Role of Algal Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Characterization
2.2. Arsenic Adsorption Assay
2.3. Empirical Adsorption Models
2.4. Effect of Algae Application on Soil pH-Eh Conditions and As Speciation
3. Results and Discussion
3.1. Physicochemical Quality of Paddy Soils
3.2. The Soil Adsorption Capacity of As and the Effect of Algae Application
3.3. Effect of Algae Application on pH-Eh Conditions and As Speciation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.G.; Williams, P.N.; Meharg, A.A. Exposure to inorganic arsenic from rice: A global health issue? Environ. Pollut. 2008, 154, 169–171. [Google Scholar] [CrossRef]
- Su, Y.H.; McGrath, S.P.; Zhao, F.J. Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 2010, 328, 27–34. [Google Scholar] [CrossRef]
- Lin, S.C.; Chang, T.K.; Huang, W.D.; Lur, H.S.; Shyu, G.S. Accumulation of arsenic in rice plant: A study of an arsenic-contaminated site in Taiwan. Paddy Water Environ. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Naveed, S.; Dong, B.; Zhang, C.; Ge, Y. Microalgae and their effects on metal bioavailability in paddy fields. J. Soils Sediments 2018, 18, 936–945. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha, N. Arsenic Uptake, Toxicity, Detoxification, Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Zhu, Q.; Wang, C.; Tang, X. Review on arsenic environment behaviors in aqueous solution and soil. Chemosphere 2023, 333, 138869. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Wei, X.; Peng, H.; Hu, L.; Zhu, X. Migration, transformation of arsenic, and pollution controlling strategies in paddy soil-rice system: A comprehensive review. Sci. Total Environ. 2024, 951, 175500. [Google Scholar] [CrossRef]
- Abedin, M.D.J.; Cresser, M.S.; Meharg, A.; Feldmann, J.; Cotter-Howells, C. Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ. Sci. Technol. 2002, 36, 962–968. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Li, C.; Parikh, S.J.; Linquist, B.A. Irrigation management for arsenic mitigation in rice grain: Timing and severity of a single soil drying. Sci. Total Environ. 2019, 649, 300–307. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Wang, P.; Kretzschmar, R.; Zhao, F.J. Control of arsenic mobilization in paddy soils by manganese and iron oxides. Environ. Pollut. 2017, 231, 37–47. [Google Scholar] [CrossRef]
- Upadhyay, A.K.; Singh, N.K.; Singh, R.; Rai, U.N. Amelioration of arsenic toxicity in rice: Comparative effect of inoculation of Chlorella vulgaris and Nannochloropsis sp. on growth, biochemical changes and arsenic uptake. Ecotoxicol. Environ. Saf. 2016, 124, 68–73. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/465 of 3 March 2023 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Arsenic in Certain Foods. In Official Journal of the European Union. 6.3.2023; L 68/51; European Commission: Brussels, Belgium, 2023; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0465 (accessed on 1 May 2025).
- Hussain, M.M.; Bibi, I.; Niazi, N.K.; Shahid, M.; Iqbal, J.; Shakoor, M.B.; Ahmad, A.; Shah, N.S.; Bhattacharya, P.; Mao, K.; et al. Arsenic biogeochemical cycling in paddy soil-rice system: Interaction with various factors, amendments and mineral nutrients. Sci. Total Environ. 2021, 773, 145040. [Google Scholar] [CrossRef]
- Aschonitis, V.G.; Lekakis, E.H.; Petridou, N.C.; Koukouli, S.G.; Pavlatou-Ve, A. Nutrients fixation by algae and limiting factors of algal growth in flooded rice fields under semi-arid Mediterranean conditions: Case study in Thessaloniki plain in Greece. Nutr. Cycl. Agroecosyst. 2013, 96, 1–13. [Google Scholar] [CrossRef]
- Oh, H.M.; Rhee, G.Y. A comparative study of microalgae isolated from flooded rice paddies: Light-limited growth, C fixation, growth efficiency and relative N and P requirement. J. Appl. Phycol. 1991, 3, 211–220. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, Y.; Chen, Z.; Yin, X.; Sun, G. Arsenic mobilization and speciation during iron plaque decomposition in a paddy soil. J. Soils Sediments 2012, 12, 402–410. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Rafiq, M.; Bakhat, H.F.; Imran, M.; Abbas, T.; Bibi, I.; Dumat, C. Arsenic behaviour in soil-plant system: Biogeochemical reactions and chemical speciation influences. In Enhancing Cleanup of Environmental Pollutants; Anjum, N., Gill, S., Tuteja, N., Eds.; Springer: Berlin, Germany, 2017; pp. 97–140. [Google Scholar]
- Spencer, D.F.; Linquist, B.A. Reducing rice field algae and cyanobacteria abundance by altering phosphorus fertilizer applications. Paddy Water Environ. 2014, 12, 147–154. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Ok, Y.S.; Meharg, A.A.; Bundschuh, J. Mitigation of arsenic accumulation in rice: An agronomical, physico-chemical, and biological approach—A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 31–71. [Google Scholar] [CrossRef]
- Roy, A.; Manjaiah, K.M.; Datta, S.P.; Rakshit, D.; Barman, M.; Ray, P.; Golui, D.; Raza, M.B.; Tigga, P.; Mondal, S.; et al. Effect of low-molecular-weight organic acids and silicon on arsenic adsorption and desorption in a paddy soil of bengal delta plain: Insights from thermodynamics and equilibrium modeling. Water Air Soil. Pollut. 2025, 236, 344. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Alves, N.M.d.S.; Pimentel, N.; Silva, D.B.d.; Inácio, M.; Cunha, A.G.; Freitas, M.d.C. Analysis of land use changes in the Sado estuary (Portugal) from the 19th to the 21st Century, based on historical maps, fieldwork, and remote sensing. Sustainability 2024, 16, 5798. [Google Scholar] [CrossRef]
- Guitián, F.; Carballas, T. Técnicas de Análisis de Suelos; Pico Sacro: Santiago de Compostela, Spain, 1976; p. 288. [Google Scholar]
- Springer, U.; Klee, J. Prüfung der Leistungsf¨ahigkeit von einigen wichtigeren Verfahren zur Bestimmung des Kohlen-stoffs mittels Chromschwefels aure sowie Vorschlag einer neuen Schnellmethode. Z. Für Pflanzenern Ahrung Düngung Bodenkd. 1954, 64, 1–26. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soils by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. EPA Method 3050B: Acid digestion of sediments, sludges, and soils. In Selected Analytical Methods for Environmental Reme-Diation and Recovery (SAM); U.S. Environmental Protection Agency: Washington, DC, USA, 1996; p. 723. [Google Scholar]
- Buján, E.; García-Arrese, A.; Velasco-Molina, M.; Macías, F. La disolución del suelo en comunidades de Erica andevalensis del entorno de las minas de Riotinto (Huelva, SO España). Rev. Ciênc. Agrár. 2010, 33, 111–118. [Google Scholar] [CrossRef]
- Bascomb, C.L. Distribution of pyrophosphate-extractable iron and organic carbon in soils of various groups. Eur. J. Soil Sci. 1968, 19, 251–268. [Google Scholar] [CrossRef]
- Holmgren, G.G.S. A rapid citrate-dithionite extractable iron procedure. Soil Sci. Soc. Am. J. 1967, 31, 210–211. [Google Scholar] [CrossRef]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Extractable iron, aluminum and silicon. In Methods for Chemical Analysis of Soils New Zealand Soil Bureau Scientific Report 10A; Blakemore, L.C., Searle, P.L., Daly, B.K., Eds.; DISR: Lower Hutt, New Zealand, 1987; pp. 71–76. [Google Scholar]
- Chao, T.T. Selective dissolution of manganese oxides from soils and sediments with acidified hydroxylamine hydrochloride. Soil Sci. 1972, 36, 764–768. [Google Scholar] [CrossRef]
- Arán, D.; Antelo, J.; Fiol, S.; Macías, F. Immobilization of phosphate by a Technosol spolic silandic: Kinetics, equilibrium and dependency on environmental variables. J. Soils Sediments 2018, 18, 2914–2923. [Google Scholar] [CrossRef]
- Arán, D.; Antelo, J.; Fiol, S.; Macías, F. Influence of feedstock on the copper removal capacity of waste-derived biochars. Bioresour. Technol. 2016, 212, 199–206. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wang, H.; Tang, H.; Liu, Z.; Zhang, X.; Hao, Z.; Liu, Z. Removal of cobalt(II) ion from aqueous solution by chitosan–montmorillonite. J. Environ. Sci. 2014, 26, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, J.; Ji, J.; Liu, Y. Arsenate adsorption on different fractions of iron oxides in the paddy soil from the Karst Region of China. Bull. Environ. Contam. Toxicol. 2021, 106, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dai, Z.; Sun, R.; Zhao, Z.; Dong, Y.; Hong, Z.; Xu, R. Evaluation of ferrolysis in arsenate adsorption on the paddy soil derived from an Oxisol. Chemosphere 2017, 179, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; Book 6, Chapter A43; U.S. Geological Survey: Denver, CO, USA, 2013. [Google Scholar]
- De Varennes, A. Productividade dos Solos e Ambiente; Escolar Editora: Lisbon, Portugal, 2003. [Google Scholar]
- Veloso, A.; Sempiterno, C.; Calouro, F.; Rebelo, F.; Pedra, F.; Castro, I.V.; Gonçalves, M.C.; Marcelo, M.E.; Pereira, P.; Fareleira, P.; et al. Manual de Fertilização das Culturas, 3rd ed.; Instituto Nacional de Investigação Agrária e Veterinária, I.P.—INIAV: Lisbon, Portugal, 2022. [Google Scholar]
- Tiberg, C.; Sjöstedt, C.; Eriksson, A.K.; Klysubun, W.; Gustafsson, J.P. Phosphate competition with arsenate on poorly crystalline iron and aluminum (hydr)oxide mixtures. Chemosphere 2020, 255, 126937. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P.; Antelo, J. Competitive arsenate and phosphate adsorption on ferrihydrite as described by the CD-MUSIC model. ACS Earth Space Chem. 2022, 6, 1397–1406. [Google Scholar] [CrossRef]
- Sato, K.; Hama, T.; Tanaka, R.; Wakita, R.; Nakamura, K. The effects of phosphate and pH on arsenate adsorption on allophanic Andosols in Miyazaki. J. Soil Sci. Plant Nutr. 2023, 69, 151–162. [Google Scholar] [CrossRef]
- APA—Agência Portuguesa do Ambiente. Solos Contaminados—Guia Técnico: Valores de Referência Para Solos (Revisão 3); Agência Portuguesa do Ambiente: Amadora, Portugal, 2022; p. 72. [Google Scholar]
- Signes-Pastor, A.J.; Carey, M.; Carbonell-Barrachina, A.A.; Moreno-Jiménez, E.; Green, A.J.; Meharg, A.A. Geographical variation in inorganic arsenic in paddy field samples and commercial rice from the Iberian Peninsula. Food Chem. 2016, 202, 356–363. [Google Scholar] [CrossRef]
- Gama, M.S.; Portela, L.; Patinha, C.; Durães, N. Assessing trace elements in soils and rice: Insights from the Baixo Vouga Lagunar (Portugal). Environ. Geochem. Health 2025, 47, 96. [Google Scholar] [CrossRef]
- Hafeznezami, S.; Zimmer-Faust, A.G.; Dunne, A.; Tran, T.; Yang, C.; Lam, J.R.; Reynolds, M.D.; Davis, J.A. Adsorption and desorption of arsenate on sandy sediments from contaminated and uncontaminated saturated zones: Kinetic and equilibrium modeling. Environ. Pollut. 2016, 215, 290–301. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.L.; Gu, J.D. Oxidation of As(III) by MnO2 in the absence and presence of Fe(II) under acidic conditions. Geochim. Cosmochim. Acta 2011, 75, 368–379. [Google Scholar] [CrossRef]

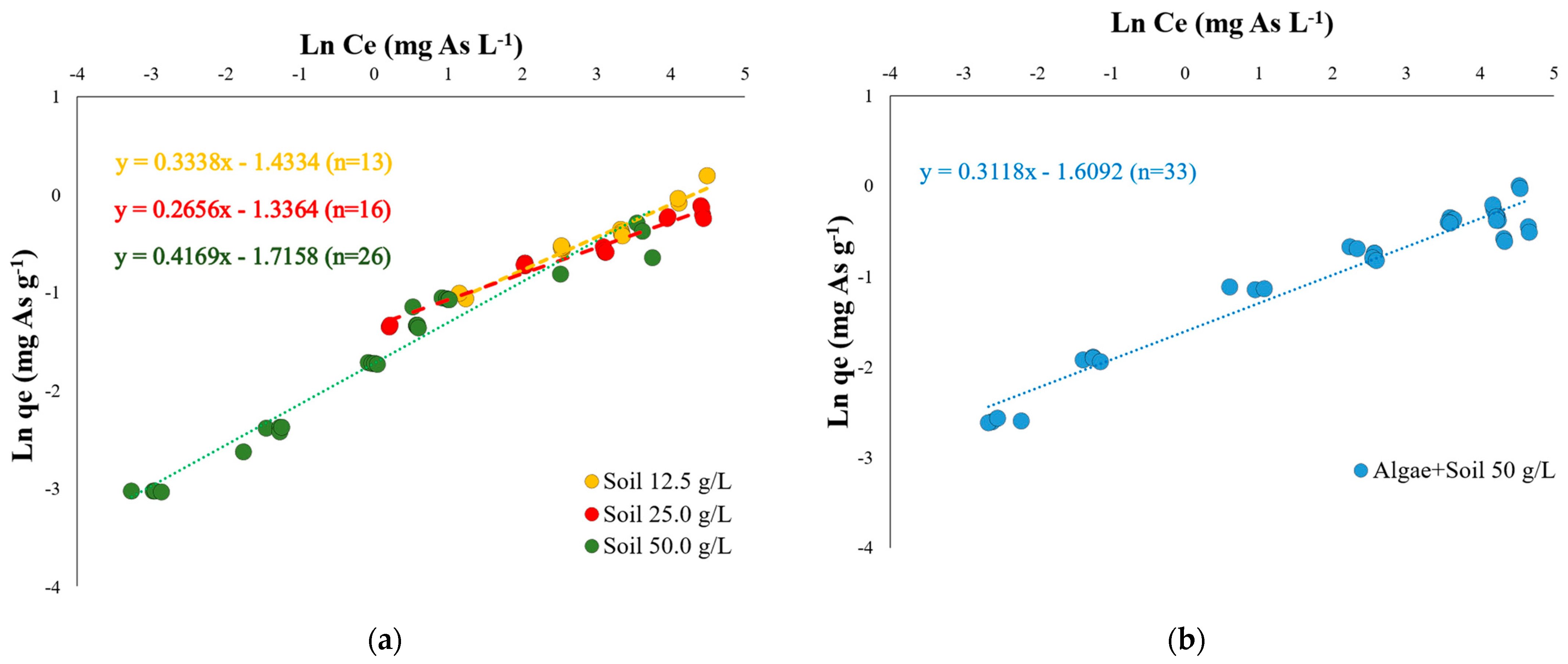
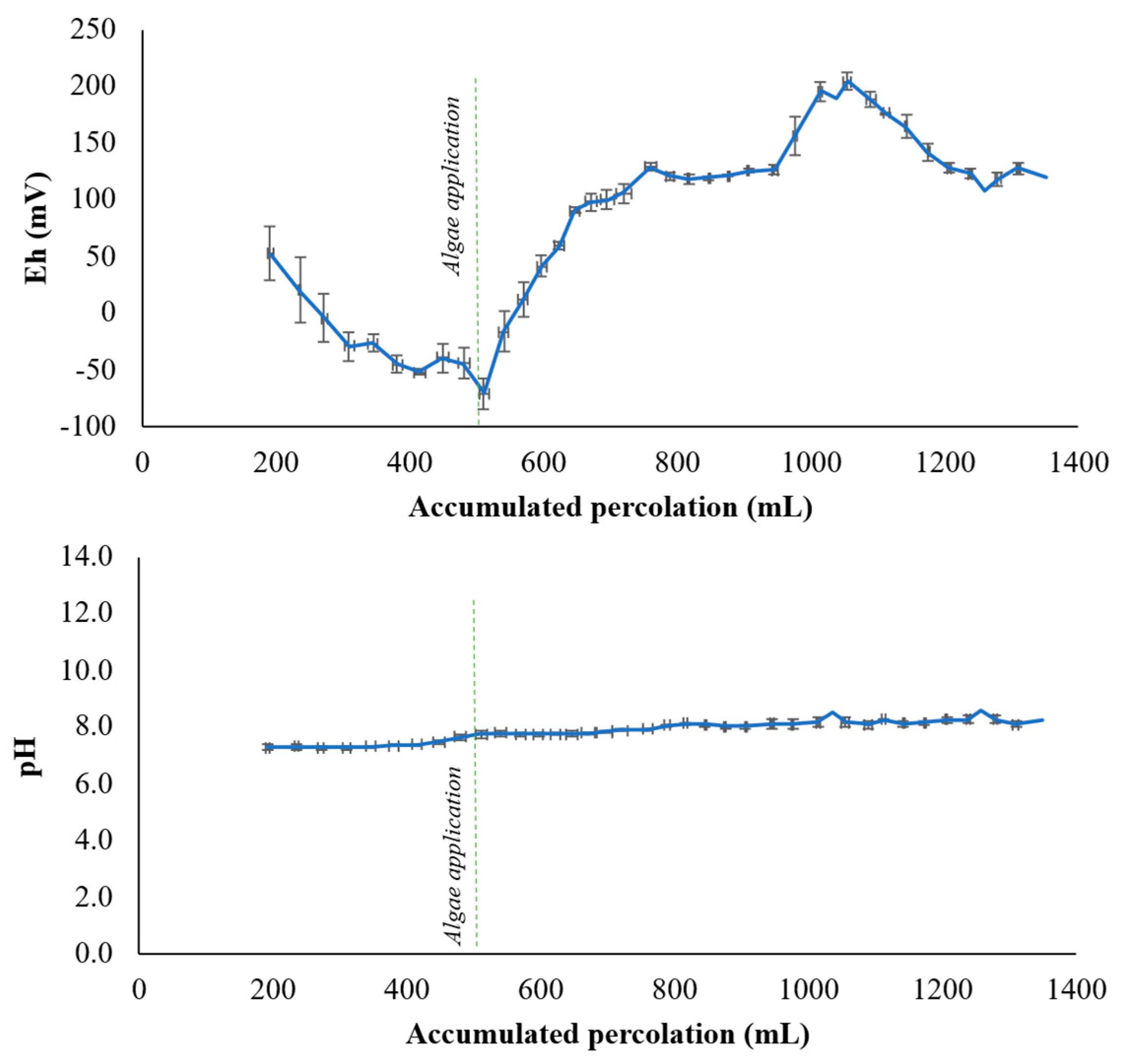

| Elements | Total | Available | MAV |
|---|---|---|---|
| mg·kg−1 | |||
| As | 16.4 ± 6.72 | <2.0 | 11 |
| Sb | 0.36 ± 0.21 | <0.2 | 1 |
| Cd | 0.37 ± 0.34 | <0.2 | 1 |
| Cu | 72.5 ± 11.5 | 0.46 ± 0.25 | 62 |
| Zn | 152 ± 96.8 | 4.85 ± 5.64 | 290 |
| Ni | 36.2 ± 4.88 | <0.6 | 37 |
| Pb | 31.3 ± 1.63 | <1.0 | 45 |
| Co | 17.95 ± 5.16 | <0.10 | 19 |
| Cr | 53.50 ± 3.54 | <2.0 | 67 |
| Hg | 0.17 ± 0.04 | Nd | 0.16 |
| Sorption Model | Adsorption Parameter | Relation Soil–Solution (g·L−1) | |||
|---|---|---|---|---|---|
| Soil | Soil + Algae | ||||
| 12.5 (n = 13) | 25.0 (n = 16) | 50.0 (n = 26) | 50.0 (n = 33) | ||
| Langmuir model | Qmax (mg·g−1) | 0.1770 | 0.3270 | 0.4538 | 1.0717 |
| KL (L·mg−1) | 4.8486 | 2.6757 | 0.9250 | 0.4792 | |
| RL | 0.002–0.026 | 0.003–0.046 | 0.010–0.219 | 0.014–0.3610 | |
| R2 | 0.9041 | 0.9257 | 0.9575 | 0.9408 | |
| SSR | 0.4050 | 0.7737 | 12.543 | 30.645 | |
| Freundlich model | KF (L·g−1) | 0.2385 | 0.2628 | 0.1798 | 0.2000 |
| n | 2.9958 | 3.7651 | 2.3987 | 3.2072 | |
| R2 | 0.9619 | 0.9665 | 0.9525 | 0.9502 | |
| SSR | 0.0551 | 0.0713 | 0.8480 | 1.0529 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arán, D.; Abreu, M.M.; Martins, L.L.; Mourato, M.P.; Santos, E.S. Arsenic Behavior in Paddy Soils: Sorption Capacity and the Role of Algal Addition. Soil Syst. 2025, 9, 106. https://doi.org/10.3390/soilsystems9040106
Arán D, Abreu MM, Martins LL, Mourato MP, Santos ES. Arsenic Behavior in Paddy Soils: Sorption Capacity and the Role of Algal Addition. Soil Systems. 2025; 9(4):106. https://doi.org/10.3390/soilsystems9040106
Chicago/Turabian StyleArán, Diego, Maria Manuela Abreu, Luisa Louro Martins, Miguel Pedro Mourato, and Erika S. Santos. 2025. "Arsenic Behavior in Paddy Soils: Sorption Capacity and the Role of Algal Addition" Soil Systems 9, no. 4: 106. https://doi.org/10.3390/soilsystems9040106
APA StyleArán, D., Abreu, M. M., Martins, L. L., Mourato, M. P., & Santos, E. S. (2025). Arsenic Behavior in Paddy Soils: Sorption Capacity and the Role of Algal Addition. Soil Systems, 9(4), 106. https://doi.org/10.3390/soilsystems9040106










