In Vitro Evaluation of Enhanced Efficiency Nitrogen Fertilizers Using Two Different Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Ammonia Volatilization Study
2.3. Statistical Analysis
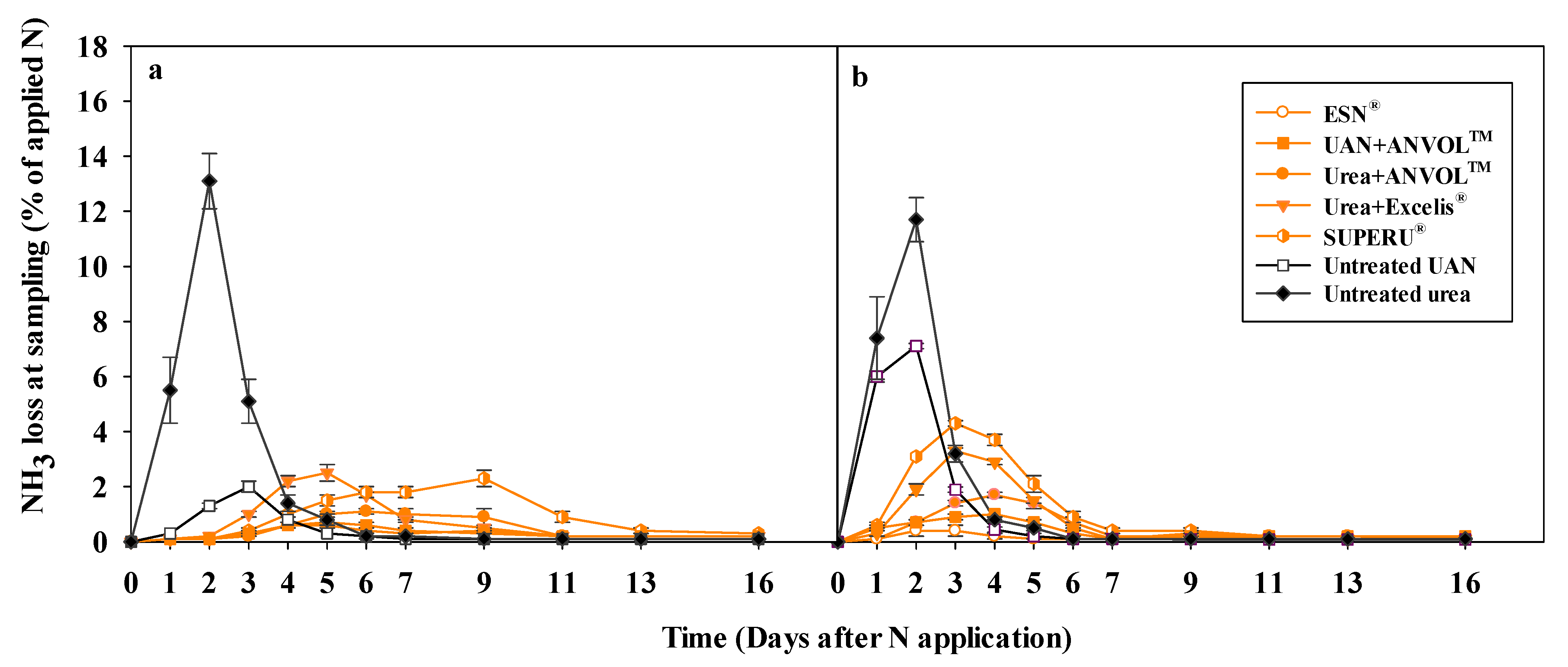
3. Results
3.1. Ammonia Loss Flux
3.1.1. Ammonia Volatilization Flux
3.1.2. Ammonia Loss Rate
3.1.3. Cumulative Ammonia Loss
3.1.4. Comparing Initial and Maximum Ammonia Volatilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NUE | Nitrogen use efficiency |
| UAN | Urea ammonium nitrate |
| EENF | Enhanced efficiency nitrogen fertilizer |
| NBPT | N-(butyl) thiophosphoric triamide |
| DCD | Dicyandiamide |
| ESN | Environmentally smart nitrogen |
| SOM | Soil organic matter |
| DAF | Days after fertilizer application |
References
- Chuong, T.; Plant, R.; Linquist, B.A. Fertilizer source and placement influence ammonia volatilization losses from water-seeded rice systems. Soil Sci. Soc. Am. J. 2020, 84, 784–797. [Google Scholar] [CrossRef]
- Fageria, N.K. Nitrogen Management in Crop Production; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Li, S.; Zheng, X.; Zhang, W.; Han, S.; Deng, J.; Wang, K.; Wang, R.; Yao, Z.; Liu, C. Modeling ammonia volatilization following the application of synthetic fertilizers to cultivated uplands with calcareous soils using an improved DNDC biogeochemistry model. Sci. Total. Environ. 2019, 660, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Adotey, N.; Kongchum, M.; Li, J.; Whitehurst, G.B.; Sucre, E.; Harrell, D.L. Ammonia Volatilization of Zinc Sulfate-Coated and NBPT-Treated Urea Fertilizers. Agron. J. 2017, 109, 2918–2926. [Google Scholar] [CrossRef]
- Palma, R.M.; Saubidet, M.I.; Rimolo, M.; Utsumi, J. Nitrogen losses by volatilization in a corn crop with two tillage systems in the Argentine Pampa. Commun. Soil Sci. Plant Anal. 1998, 29, 2865–2879. [Google Scholar] [CrossRef]
- Lasisi, A.A.; Akinremi, O.O.; Kumaragamage, D. Nitrification inhibitor reduces the inhibitory effect of N-(n-butyl) thiophosphoric triamide (NBPT) on the hydrolysis of urea. Soil Sci. Soc. Am. J. 2020, 84, 1782–1794. [Google Scholar] [CrossRef]
- Frame, W.H.; Alley, M.M.; Whitehurst, G.B.; Whitehurst, B.M.; Campbell, R. In vitro evaluation of coatings to control ammonia volatilization from surface-applied urea. Agron. J. 2012, 104, 1201–1207. [Google Scholar] [CrossRef]
- Adams, C.B.; Thapa, S.B.; Fan, Y.; Park, S. Agronomic and economic effects of two enhanced-efficiency urea fertilizer technologies on Southern Great Plains winter wheat. Agron. J. 2018, 110, 1097–1102. [Google Scholar] [CrossRef]
- Dillon, K.A.; Walker, T.W.; Harrell, D.L.; Krutz, L.J.; Varco, J.J.; Koger, C.H.; Cox, M.S. Nitrogen sources and timing effects on nitrogen loss and uptake in delayed flood rice. Agron. J. 2012, 104, 466–472. [Google Scholar] [CrossRef]
- Frame, W. Ammonia volatilization from urea treated with NBPT and two nitrification inhibitors. Agron. J. 2017, 109, 378–387. [Google Scholar] [CrossRef]
- Ruark, M.D.; Soratto, R.P.; Rosen, C.J. Merits and limitations of enhanced efficiency fertilizers. In Soil Nitrogen Uses and Environmental Impacts; CRC Press: Boca Raton, FL, USA, 2018; pp. 289–314. [Google Scholar]
- Awale, R.; Chatterjee, A. Enhanced efficiency nitrogen products influence ammonia volatilization and nitrous oxide emission from two contrasting soils. Agron. J. 2017, 109, 47–57. [Google Scholar] [CrossRef]
- Junejo, N.; Khanif, M.Y.; Hanfi, M.M.; Yunus, W.W.; Dharejo, K.A. Role of inhibitors and biodegradable material in mitigation of nitrogen losses from fertilized lands. Afr. J. Biotechnol. 2011, 10, 3504–3514. [Google Scholar]
- Trenkel, M.E. Slow-and Controlled-Releasee and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, 2nd ed.; International Fertilizer Industry Association: Paris, France, 2010; pp. 14–69. [Google Scholar]
- Peng, X.; Maharjan, B.; Yu, C.; Su, A.; Jin, V.; Ferguson, R.B. A laboratory evaluation of ammonia volatilization and nitrate leaching following nitrogen fertilizer application on a coarse-textured soil. Agron. J. 2015, 107, 871–879. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Menegale, M.L.d.C. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Watson, C.J.; Akhonzada, N.A.; Hamilton, J.T.G.; Matthews, D.I. Rate and mode of application of the urease inhibitor N-(n-butyl) thiophosphoric triamide on ammonia volatilization from surface-applied urea. Soil Use Manag. 2008, 24, 246–253. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.; Blennerhassett, J.; Quin, B. Reducing NH3, N2O and NO3-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol. Fertil. Soils 2008, 44, 693–705. [Google Scholar] [CrossRef]
- Al-Kanani, T.; MacKenzie, A.F.; Barthakur, N.N. Soil water and ammonia volatilization relationships with surface-applied nitrogen fertilizer solutions. Soil Sci. Soc. Am. J. 1991, 55, 1761–1766. [Google Scholar] [CrossRef]
- San Francisco, S.; Urrutia, O.; Martin, V.; Peristeropoulos, A.; Garcia-Mina, J.M. Efficiency of urease and nitrification inhibitors in reducing ammonia volatilization from diverse nitrogen fertilizers applied to different soil types and wheat straw mulching. J. Soc. Food Agric. 2011, 91, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Sunderlage, B.; Cook, R.L. Soil property and fertilizer additive effects on ammonia volatilization from urea. Soil Sci. Soc. Am. J. 2018, 82, 253–259. [Google Scholar] [CrossRef]
- Jantalia, C.P.; Halvorson, A.D.; Follett, R.F.; Alves, B.J.R.; Polidoro, J.C.; Urquiaga, S. Nitrogen source effects on ammonia volatilization as measured with semi-static chambers. Agron. J. 2012, 104, 1595–1603. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture, Official Soil Series Descriptions. Available online: https://www.nrcs.usda.gov/conservation-basics/natural-resource-concerns/soil/citing-our-databases (accessed on 5 October 2024).
- Sikora, F.J.; Hardy, M. Soil organic matter. In Soil Test Methods from the Southeastern United States, 2nd ed.; Sikora, F.J., Moore, K.P., Eds.; Southern Cooperative Series Bulletin No. 419; Southern Extension and Research Activity Information Exchange Group-6 (SERA-IEG-6): Kentucky, KY, USA, 2014; pp. 48–53. [Google Scholar]
- Rosales, A.P. Kinetics of ammonium volatilization in the form of ammonia in soils through a textural gradient. Agro Prod. 2024, 17, 23–33. [Google Scholar]
- Damodar Reddy, D.; Sharma, K. Effect of amending urea fertilizer with chemical additives on ammonia volatilization loss and nitrogen-use efficiency. Biol. Fertil. Soils 2000, 32, 24–27. [Google Scholar] [CrossRef]
- Griggs, B.R.; Norman, R.J.; Wilson, C.E.; Slaton, N.A. Ammonia volatilization and nitrogen uptake for conventional and conservation tilled dry-seeded, delayed-flood rice. Soil Sci. Soc. Am. J. 2007, 71, 745–751. [Google Scholar] [CrossRef]
- Kissel, D.E.; Cabrera, M.L.; Ferguson, R.B. Reactions of ammonia and urea hydrolysis products with soil. Soil Sci. Soc. Am. J. 1988, 52, 1793–1796. [Google Scholar] [CrossRef]
- Silva, A.G.B.; Sequeira, C.H.; Sermarini, R.A.; Otto, R. Urease inhibitor NBPT on ammonia volatilization and crop productivity: A meta-analysis. Agron. J. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Rochette, P.; MacDonald, J.D.; Angers, D.A.; Chantigny, M.H.; Gasser, M.; Bertrand, N. Banding of urea increased ammonia volatilization in a dry acidic soil. J. Environ. Qual. 2009, 38, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Manunza, B.; Deiana, S.; Pintore, M.; Gessa, C. The binding mechanism of urea, hydroxamic acid and N-(N-butyl)-phosphoric triamide to the urease active site. A comparative molecular dynamics study. Soil Biol. Biochem. 1999, 31, 789–796. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, L.; Van Cleemput, O.; Wang, Z. Fate of urea-15N in a soil-wheat system as influenced by urease inhibitor hydroquinone and nitrification inhibitor dicyandiamide. Plant Soil 2000, 220, 261–270. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Y.; Zhou, L.; Huang, G.; Cleemput, O. Effect of dicyandiamide and hydroquinone on the transformation of urea-nitrogen-15 in soil cropped to wheat. Biol. Fertil. Soils 2001, 34, 286–290. [Google Scholar] [CrossRef]
- Gioacchini, P.; Nastri, A.; Marzadori, C.; Giovannini, C.; Antisari, L.V.; Gessa, C. Influence of urease and nitrification inhibitors on N losses from soils fertilized with urea. Biol. Fertil. Soils 2002, 36, 129–135. [Google Scholar] [CrossRef]


| EENF | Type of EENF | Company | Name and Conc. (%) of Active Ingredients | Application Rate (L/kg) |
|---|---|---|---|---|
| ANVOLTM | Nitrogen stabilizer | Koch Industries, Wichita, KS, USA | NBPT (10–20%); Duromide (20–30%); N-methyl-2-pyrrolidone (<10%) | 0.0014 (Urea) 0.0007 (UAN) |
| Excelis® | Nitrogen stabilizer | Timac Agro, Reading, PA, USA | NBPT (12%); DCD (2%); Other extracts (0.8%) | 0.0019 (Urea) 0.0021 (UAN) |
| ESN® | Slow-release | Agrium U.S. Inc., Loveland, CO, USA | Urea (>95%); Castor oil (4%); Imidodicarbonic diamide (<1%) | N/A |
| SUPERU® | Nitrogen stabilizer | Koch Industries, Wichita, KS, USA | Urea (60–100%); DCD (0.85%); NBPT (0.06%); N-Methyl-2-pyrrolidone (<0.1%) | N/A |
| Soil Property | Value | |
|---|---|---|
| Loring | Grenada | |
| Soil pH | 5.8 | 7.0 |
| Soil Organic Matter (g/kg) | 30 | 4.2 |
| Estimated Nitrogen Release (# N/ha) | 80 | 68 |
| Mehlich 3 extractable nutrients | ||
| Sulfur (ppm) | 19 | 9.0 |
| Phosphorus (mg/kg) | 90 | 97 |
| Calcium (mg/kg) | 1404 | 1999 |
| Magnesium (mg/kg) | 120 | 267 |
| Potassium (mg/kg) | 155 | 225 |
| Sodium (mg/kg) | 10.0 | 11 |
| Boron (mg/kg) | 0.8 | 0.6 |
| Iron (mg/kg) | 296.0 | 170 |
| Manganese (mg/kg) | 106.0 | 91 |
| Copper (mg/kg) | 1.1 | 1.7 |
| Zinc (mg/kg) | 5.4 | 5.2 |
| Aluminum (mg/kg) | 401 | 508 |
| Clay (g/kg) | 140 | 180 |
| Sand (g/kg) | 360 | 330 |
| Silt (g/kg) | 500 | 490 |
| Field capacity (g/kg) | 300 | 320 |
| Soil | N Source | Sampling Time (Days After Fertilizer Application) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 9 | 11 | 13 | 16 | ||
| -------------------------------------------% of Applied N d−1---------------------------------------------- | ||||||||||||
| Loring | Untreated urea | 5.5 | 13.1 | 5.1 | 1.4 | 0.8 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Untreated UAN | 0.3 | 1.3 | 1.9 | 0.8 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| SUPERU® | 0.1 | 0.1 | 0.4 | 1.0 | 1.5 | 1.8 | 1.8 | 2.2 | 0.9 | 0.4 | 0.3 | |
| Urea+Excelis® | 0.1 | 0.2 | 1.0 | 2.2 | 2.5 | 1.7 | 0.8 | 0.5 | 0.2 | 0.2 | 0.2 | |
| Urea+ANVOLTM | 0.1 | 0.1 | 0.2 | 0.7 | 1.0 | 1.2 | 1.0 | 1.0 | 0.2 | 0.2 | 0.1 | |
| UAN+ANVOLTM | 0.1 | 0.2 | 0.3 | 0.6 | 0.7 | 0.7 | 0.4 | 0.3 | 0.3 | 0.2 | 0.2 | |
| ESN® | 0.1 | 0.1 | 0.3 | 0.6 | 0.6 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.2 | |
| Grenada | Untreated urea | 7.3 | 11.7 | 3.2 | 0.8 | 0.5 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Untreated UAN | 6.0 | 7.1 | 1.9 | 0.4 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| SUPERU® | 0.6 | 3.1 | 4.3 | 3.7 | 2.1 | 0.9 | 0.4 | 0.4 | 0.2 | 0.2 | 0.1 | |
| Urea+Excelis® | 0.3 | 1.9 | 3.3 | 2.9 | 1.5 | 0.5 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Urea+ANVOLTM | 0.1 | 0.7 | 1.5 | 1.7 | 1.4 | 0.7 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | |
| UAN+ANVOLTM | 0.5 | 0.7 | 0.9 | 1.0 | 0.7 | 0.3 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | |
| ESN® | 0.1 | 0.4 | 0.4 | 0.2 | 0.1 | 0.1 | 0.1 | 0.3 | 0.2 | 0.2 | 0.2 | |
| Soil | N Source | 1 DAF † | 3 DAF | 7 DAF | 16 DAF | DIFF ¥ |
|---|---|---|---|---|---|---|
| --------------------------------% of Applied N ----------------------------------------- | ||||||
| Loring | Untreated urea | 5.49 b | 23.61 a | 26.22 a | 26.49 a | 21.00 a |
| Untreated UAN | 0.26 c | 3.52 e | 4.85 fg | 5.24 de | 4.98 d–f | |
| SUPERU® | 0.11 c | 0.59 f | 6.62 ef | 10.43 c | 10.32 c | |
| Urea+Excelis® | 0.10 c | 1.35 f | 8.53 de | 9.58 c | 9.48 c | |
| Urea+ANVOLTM | 0.10 c | 0.40 f | 4.18 f–h | 5.65 de | 5.55 de | |
| UAN+ANVOLTM | 0.10 c | 0.58 f | 2.88 g–i | 3.83 ef | 3.73 d–f | |
| ESN® | 0.10 c | 0.53 f | 2.28 hi | 3.28 ef | 3.18 fg | |
| Grenada | Untreated urea | 7.35 a | 22.32 a | 23.87 b | 24.12 a | 16.77 b |
| Untreated UAN | 6.00 a | 15.00 b | 15.85 c | 16.17 b | 10.17 c | |
| SUPERU® | 0.59 c | 8.11 c | 15.36 c | 16.34 b | 15.75 b | |
| Urea+Excelis® | 0.25 c | 5.45 d | 10.50 d | 11.03 c | 10.78 c | |
| Urea+ANVOLTM | 0.13 c | 2.21 ef | 6.20 ef | 6.67 d | 6.54 d | |
| UAN+ANVOLTM | 0.48 c | 2.07 ef | 4.23 f–h | 4.93 de | 4.45 d–f | |
| ESN® | 0.11 c | 0.88 f | 1.38 i | 2.18 f | 2.07 g | |
| N Treatment | Loring | Grenada | ||
|---|---|---|---|---|
| Ammonia Loss | Reduction | Ammonia Loss | Reduction | |
| % of Applied N | % † | % of Applied N | % † | |
| Untreated UAN | 5.2 | - | 16.2 | - |
| Untreated urea | 26.5 | - | 24.1 | - |
| UAN+ANVOLTM | 3.8 | 27 * | 4.9 | 70 * |
| SUPERU® | 10.4 | 61 | 16.3 | 32 |
| Urea+Excelis® | 9.6 | 64 | 11.0 | 54 |
| Urea+ANVOLTM | 5.7 | 79 | 6.7 | 72 |
| ESN® | 3.3 | 88 | 2.2 | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okai, S.; Yin, X.; Duncan, L.A.; Yoder, D.; Saha, D.; Walker, F.; Logwood, S.; Akuaku, J.; Adotey, N. In Vitro Evaluation of Enhanced Efficiency Nitrogen Fertilizers Using Two Different Soils. Soil Syst. 2025, 9, 80. https://doi.org/10.3390/soilsystems9030080
Okai S, Yin X, Duncan LA, Yoder D, Saha D, Walker F, Logwood S, Akuaku J, Adotey N. In Vitro Evaluation of Enhanced Efficiency Nitrogen Fertilizers Using Two Different Soils. Soil Systems. 2025; 9(3):80. https://doi.org/10.3390/soilsystems9030080
Chicago/Turabian StyleOkai, Samuel, Xinhua Yin, Lori Allison Duncan, Daniel Yoder, Debasish Saha, Forbes Walker, Sydney Logwood, Jones Akuaku, and Nutifafa Adotey. 2025. "In Vitro Evaluation of Enhanced Efficiency Nitrogen Fertilizers Using Two Different Soils" Soil Systems 9, no. 3: 80. https://doi.org/10.3390/soilsystems9030080
APA StyleOkai, S., Yin, X., Duncan, L. A., Yoder, D., Saha, D., Walker, F., Logwood, S., Akuaku, J., & Adotey, N. (2025). In Vitro Evaluation of Enhanced Efficiency Nitrogen Fertilizers Using Two Different Soils. Soil Systems, 9(3), 80. https://doi.org/10.3390/soilsystems9030080






