Impact of Different Soil Tillage Practices on Microplastic Particle Abundance and Distribution
Abstract
:1. Introduction
2. Materials and Methods
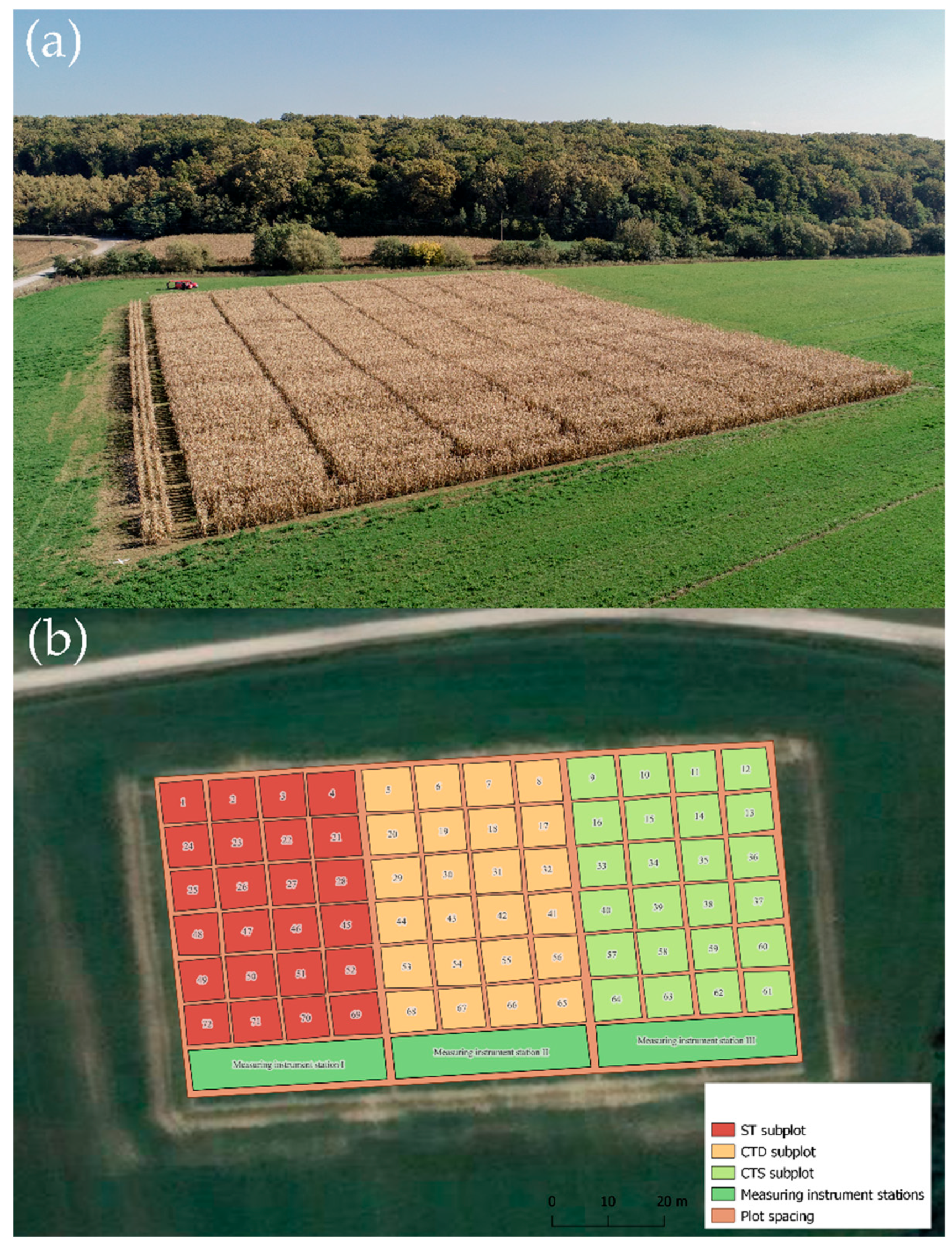
2.1. Site Description, Experimental Design, and Treatments
2.2. Soil Sampling and Sample Preparation
2.3. Microplastics Extraction
2.4. Data Analysis
3. Results
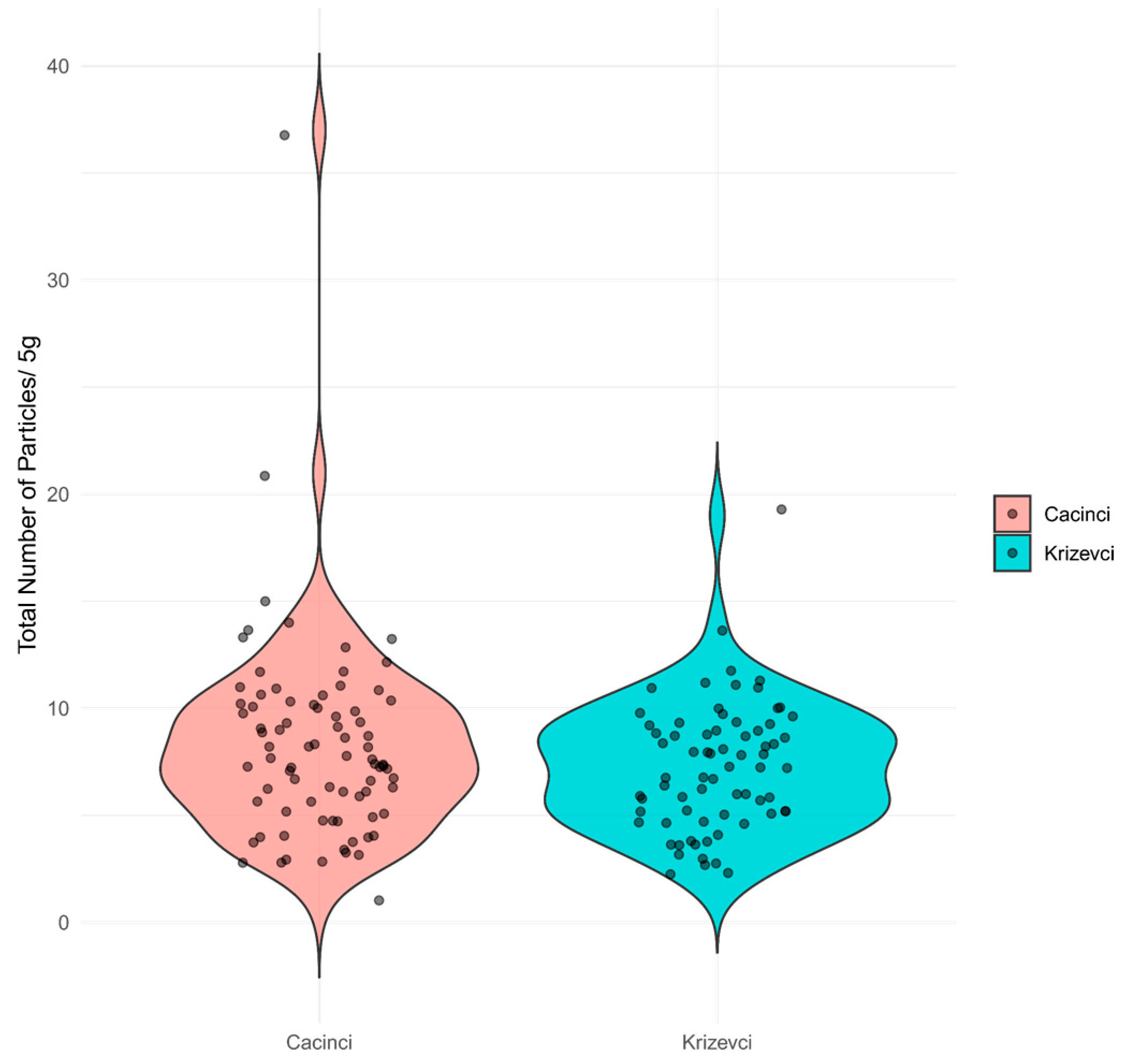
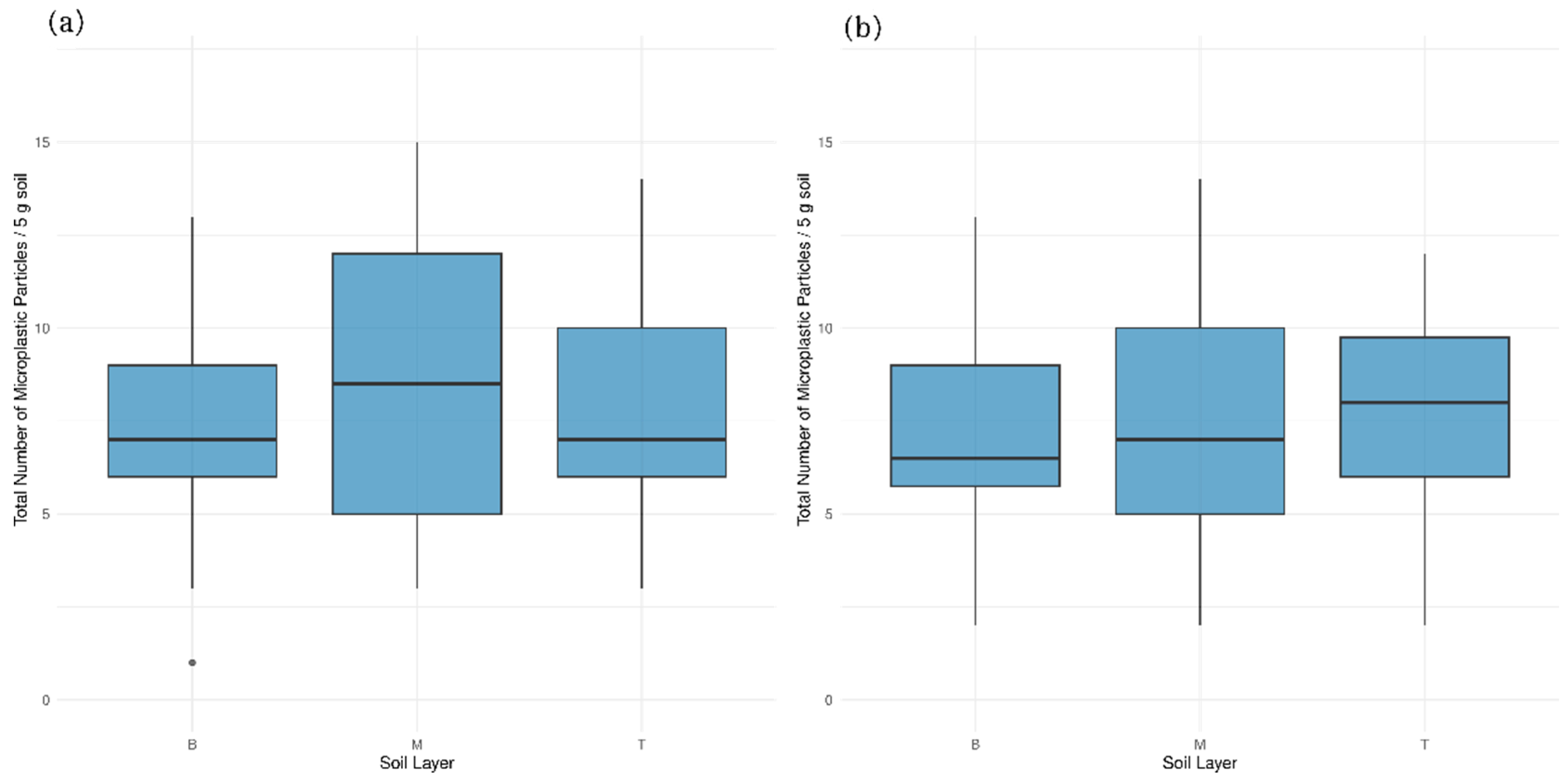
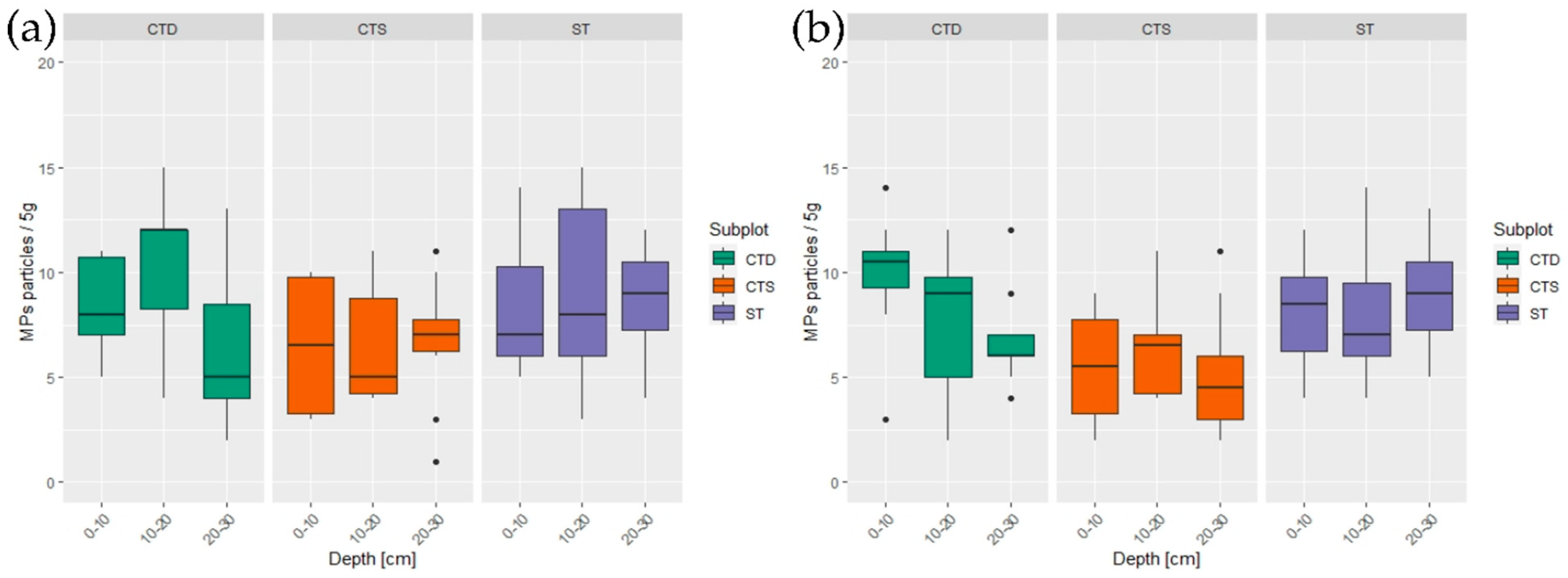
3.1. Effect of Site, Soil Characteristics, and Vertical Distribution of Microplastic Particles
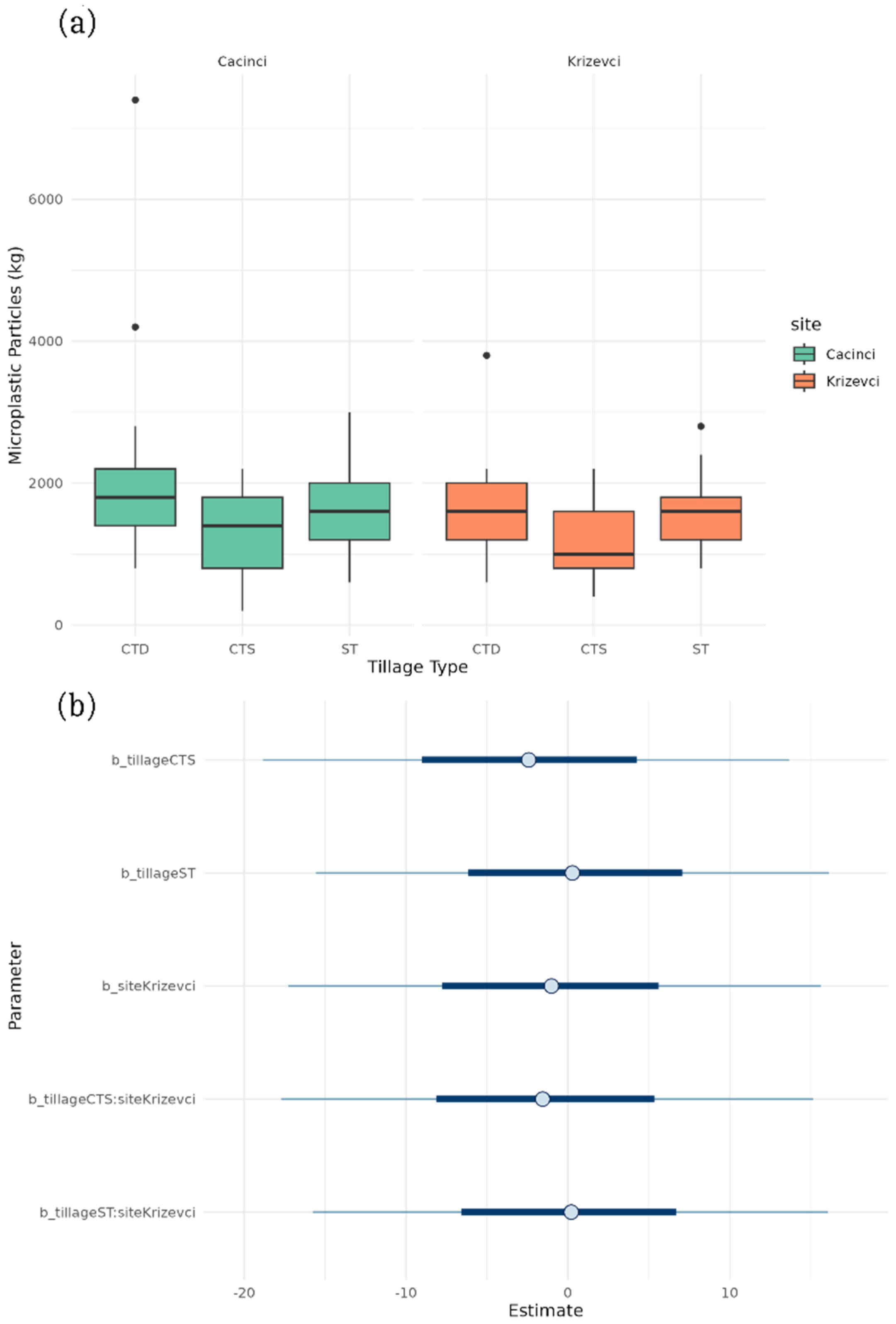
3.2. Tillage Effect on Microplastics
3.3. Fertilization Effect on Microplastics
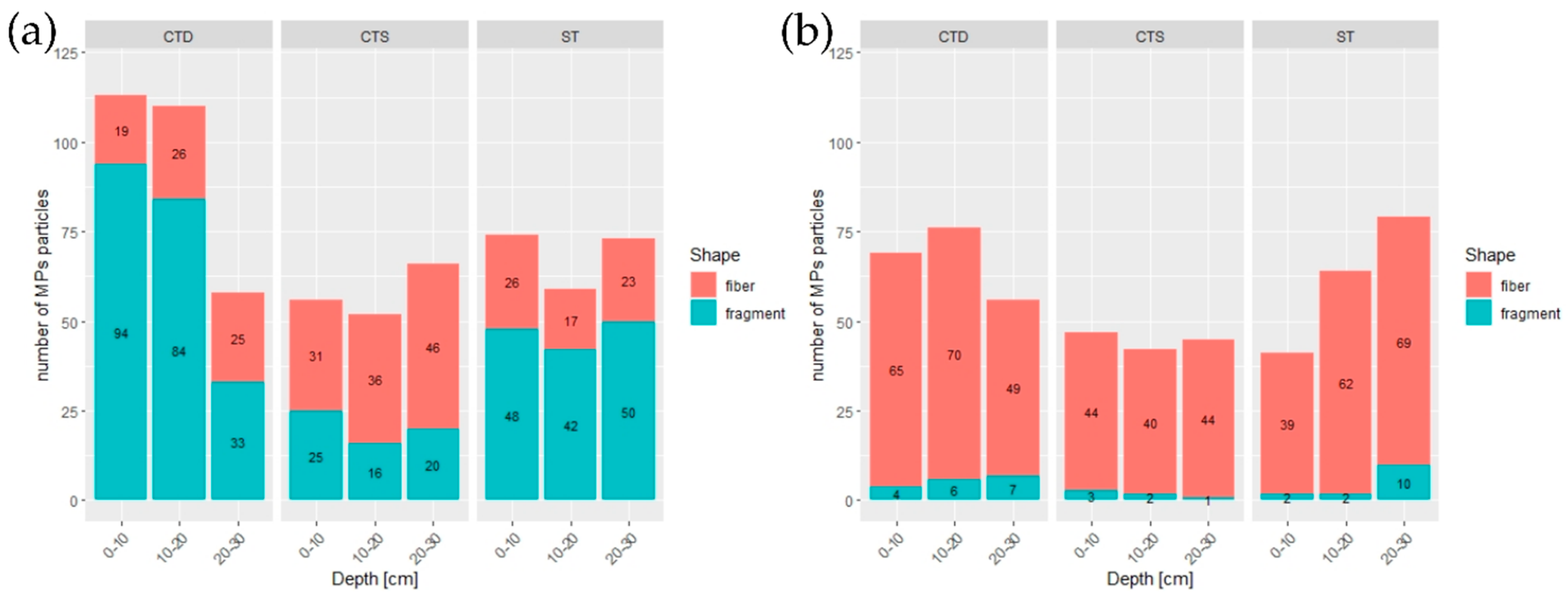
3.4. Shape of Microplastic Particles
4. Discussion
4.1. Tillage and Microplastics Distribution
4.2. Fertilization and Microplastics Abundance
4.3. Particle Shape and Vertical Stratification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; David, S.; Elma, L.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Reineccius, J.; Waniek, J.J. Critical reassessment of microplastic abundances in the marine environment. Sci. Total Environ. 2024, 954, 176449. [Google Scholar] [CrossRef] [PubMed]
- De Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- Yu, Y.; Flury, M. Current understanding of subsurface transport of micro- and nanoplastics in soil. Vadose Zone J. 2021, 20, e20108. [Google Scholar] [CrossRef]
- Mbachu, O.; Jenkins, G.; Kaparaju, P.; Pratt, C. The rise of artificial soil carbon inputs: Reviewing microplastic pollution effects in the soil environment. Sci. Total Environ. 2021, 780, 146569. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between microplastics and organic pollutants: Effects on toxicity, bioaccumulation, degradation, and transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef]
- Aghilinasrollahabadi, K.; Salehi, M.; Fujiwara, T. Investigate the influence of microplastics weathering on their heavy metals uptake in stormwater. J. Hazard. Mater. 2021, 408, 124439. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zeng, A.; Jiang, X.; Gu, X. Are microplastics correlated to phthalates in facility agriculture soil? J. Hazard. Mater. 2021, 412, 125164. [Google Scholar] [CrossRef]
- Mammo, F.K.; Amoah, I.D.; Gani, K.M.; Pillay, L.; Ratha, S.K.; Bux, F.; Kumari, S. Microplastics in the environment: Interactions with microbes and chemical contaminants. Sci. Total Environ. 2020, 743, 140518. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Huang, X.P.; Xiang, L.; Wang, Y.Z.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Klimek, B.; Grzyb, D.; Łukiewicz, B.; Niklińska, M. Microplastics increase soil respiration rate, decrease soil mesofauna feeding activity and change enchytraeid body length distribution in three contrasting soils. Appl. Soil Ecol. 2024, 201, 105463. [Google Scholar] [CrossRef]
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef]
- Büks, F.; Kaupenjohann, M. Global concentrations of microplastics in soils—A review. Soil 2020, 6, 649–662. [Google Scholar] [CrossRef]
- Fan, W.; Qiu, C.; Qu, Q.; Hu, X.; Mu, L.; Gao, Z.; Tang, X. Sources and identification of microplastics in soils. Soil Environ. Health 2023, 1, 100019. [Google Scholar] [CrossRef]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, S.; Li, P.; Wang, L.; Hou, R.; Wu, W.M.; Luo, J.; Hou, D. Vertical migration of microplastics in porous media: Multiple controlling factors under wet-dry cycling. J. Hazard. Mater. 2021, 419, 126413. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, X.; Wang, S.; Zheng, B.; Xu, Y. Vertical migration of microplastics along soil profile under different crop root systems. Environ. Pollut. 2021, 278, 116833. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; De Souza Machado, A.A. Microplastic incorporation into soil in agroecosystems. Front. Plant Sci. 2017, 8, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Brandes, E.; Henseler, M.; Kreins, P. Identifying hot-spots for microplastic contamination in agricultural soils—A spatial modelling approach for Germany. Environ. Res. Lett. 2021, 16, 104041. [Google Scholar] [CrossRef]
- ISO 10390; International Organization for Standardization ISO 10390; Soil Quality—Determination of pH-An Instrumental Method for the Routine Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 14235; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. International Organization for Standardization: Geneva, Switzerland, 1998.
- Pribyl, W.D. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- ISO 11277; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. International Organization for Standardization: Geneva, Switzerland, 2009.
- Jug, D.; Jug, I.; Radočaj, D.; Wilczewski, E.; Đurđević, B.; Jurišić, M.; Zsembeli, J.; Brozović, B. Spatio-Temporal Dynamics of Soil Penetration Resistance Depending on Different Conservation Tillage Systems. Agronomy 2024, 14, 2168. [Google Scholar] [CrossRef]
- Shelton, D.P.; Jasa, P.J. Estimating Percent Residue Cover Using the Line-Transect Method; G1931; University of Nebraska–Lincoln Extension: Lincoln, NE, USA, 2009; Available online: https://www.researchgate.net/publication/242579305_G93-1133_Estimating_Percent_Residue_Cover_Using_the_Line-Transect_Method (accessed on 22 January 2025).
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Vermeiren, P.; Muñoz, C.; Ikejima, K. Microplastic identification and quantification from organic rich sediments: A validated laboratory protocol. Environ. Pollut. 2020, 262, 114298. [Google Scholar] [CrossRef]
- Norén, F. Small Plastic Particles in Coastal Swedish Waters; KIMO Report: Lysekil, Sweden, 2007. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Beckingham, B.; Apintiloaiei, A.; Moore, C.; Brandes, J. Hot or not: Systematic review and laboratory evaluation of the hot needle test for microplastic identification. Micropl. Nanopl. 2023, 3, 8. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 February 2025).
- Zhang, X.; Chen, Y.; Li, X.; Zhang, Y.; Gao, W.; Jiang, J.; Mo, A.; He, D. Size/shape-dependent migration of microplastics in agricultural soil under simulative and natural rainfall. Sci. Total Environ. 2022, 815, 152507. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Salem, H.M.; Valero, C.; Muñoz, M.Á.; Gil-Rodríguez, M. Effect of integrated reservoir tillage for in-situ rainwater harvesting and other tillage practices on soil physical properties. Soil Tillage Res. 2015, 151, 50–60. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, P.; Liu, K.; Hayat, K.; Liu, W. Depth distribution of nano-and microplastics and their contribution to carbon storage in Chinese agricultural soils. Sci. Total Environ. 2024, 913, 169709. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef]
- Cusworth, S.J.; Davies, W.J.; McAinsh, M.R.; Gregory, A.S.; Storkey, J.; Stevens, C.J. Agricultural fertilisers contribute substantially to microplastic concentrations in UK soils. Commun. Earth Environ. 2024, 5, 7. [Google Scholar] [CrossRef]
- Heinze, W.M.; Steinmetz, Z.; Klemmensen, N.D.R.; Vollertsen, J.; Cornelis, G. Vertical distribution of microplastics in an agricultural soil after long-term treatment with sewage sludge and mineral fertiliser. Env. Pollut. 2024, 356, 124343. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, R.; Zhou, Q.; Li, L.; Li, Y.; Tu, C.; Zhao, X.; Xiong, K.; Christie, P.; Luo, Y. Abundance and morphology of microplastics in an agricultural soil following long-term repeated application of pig manure. Environ. Pollut. 2021, 272, 116028. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Leifheit, E.F.; Gerdawischke, M.; Rillig, M.C. Microplastics have shape- and polymer-dependent effects on soil aggregation and organic matter loss—An experimental and meta-analytical approach. Microplastics Nanoplastics 2021, 1, 7. [Google Scholar] [CrossRef]
| Site | Sand [%] | Silt [%] | Clay [%] | st | FC [%vol] | ρb [g cm−3] | ci [cm h−1] | sty | SOC [%] | SOM [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| Cacinci | 12.07 | 53.35 | 34.58 | Silty Clay Loam | 34.91 | 1.64 | 57.33 | Stagnosol | 1.42 | 2.84 |
| Krizevci | 7.30 | 82.60 | 10.10 | Silt | 44.59 | 1.39 | 80.04 | Gleysol | 1.18 | 2.36 |
| Factor | Cacinci | Krizevci |
|---|---|---|
| Effect of soil layer | No significant effect | Moderate effect: greater variability in upper layers |
| Soil texture (sand, silt, clay) | No significant correlation | Possible influence (p ≈ 0.048) |
| Soil pH | No significant effect | No significant effect |
| Soil organic carbon (SOC) | No significant effect | Significant negative correlation with particle variability |
| Layer | Cacinci (Mean ± SD) | Krizevci (Mean ± SD) |
|---|---|---|
| B | 1.17 ± 0.506 | 1.13 ± 0.318 |
| M | 1.14 ± 0.462 | 1.10 ± 0.270 |
| T | 1.05 ± 0.449 | 1.16 ± 0.383 |
| Cacinci | Krizevci | |||
|---|---|---|---|---|
| Fertilization Treatment | MP/kg | Sample Size (n) | MP/kg | Sample Size (n) |
| FD | 1317 ± 313 | 12 | 1417 ± 522 | 12 |
| FR | 2233 ± 1534 | 18 | 1492 ± 700 | 24 |
| GFD | 1492 ± 639 | 39 | 1350 ± 593 | 24 |
| GFR | 1636 ± 742 | 11 | 1655 ± 391 | 11 |
| Cacinci | Krizevci | |||||
|---|---|---|---|---|---|---|
| Difference | 95% CI | p-Value | Difference | 95% CI | p-Value | |
| FR–FD | 916.67 | (31.69, 1801.65) | 0.039 | 75.00 | (−481.38, 631.38) | 0.9845 |
| GFD–FR | −741.03 | (−1417.68, −64.37) | 0.026 | −66.67 | (−623.04, 489.71) | 0.9890 |
| GFR–FR | −596.97 | (−1505.77, 311.83) | 0.318 | 237.88 | (−419.01, 894.77) | 0.7757 |
| GFD–FD | 175.64 | (−608.26, 959.54) | 0.935 | −141.67 | (−595.95, 312.61) | 0.8440 |
| GFR–FD | 319.70 | (−671.54, 1310.93) | 0.832 | 162.88 | (−410.11, 735.87) | 0.8768 |
| GFR–GFD | 144.06 | (−666.64, 954.75) | 0.966 | 304.55 | (−268.44, 877.53) | 0.5036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćaleta, B.; Hackenberger Kutuzović, B.; Jug, D.; Jug, I.; Hackenberger Kutuzović, D. Impact of Different Soil Tillage Practices on Microplastic Particle Abundance and Distribution. Soil Syst. 2025, 9, 63. https://doi.org/10.3390/soilsystems9020063
Ćaleta B, Hackenberger Kutuzović B, Jug D, Jug I, Hackenberger Kutuzović D. Impact of Different Soil Tillage Practices on Microplastic Particle Abundance and Distribution. Soil Systems. 2025; 9(2):63. https://doi.org/10.3390/soilsystems9020063
Chicago/Turabian StyleĆaleta, Bruno, Branimir Hackenberger Kutuzović, Danijel Jug, Irena Jug, and Davorka Hackenberger Kutuzović. 2025. "Impact of Different Soil Tillage Practices on Microplastic Particle Abundance and Distribution" Soil Systems 9, no. 2: 63. https://doi.org/10.3390/soilsystems9020063
APA StyleĆaleta, B., Hackenberger Kutuzović, B., Jug, D., Jug, I., & Hackenberger Kutuzović, D. (2025). Impact of Different Soil Tillage Practices on Microplastic Particle Abundance and Distribution. Soil Systems, 9(2), 63. https://doi.org/10.3390/soilsystems9020063










