Assessing the Effect of Organic and Inorganic Resources on Carbon Fractions in Soggy Sodic Soil at Sege in Ada West District, Ghana
Abstract
1. Introduction
- How do integrated organic and inorganic amendments influence labile organic carbon, soil carbon fractions, and total SOC in gleyic solonetz soils?
- What is the effect of these amendments on the Carbon Management Index (CMI)?
2. Materials and Methods
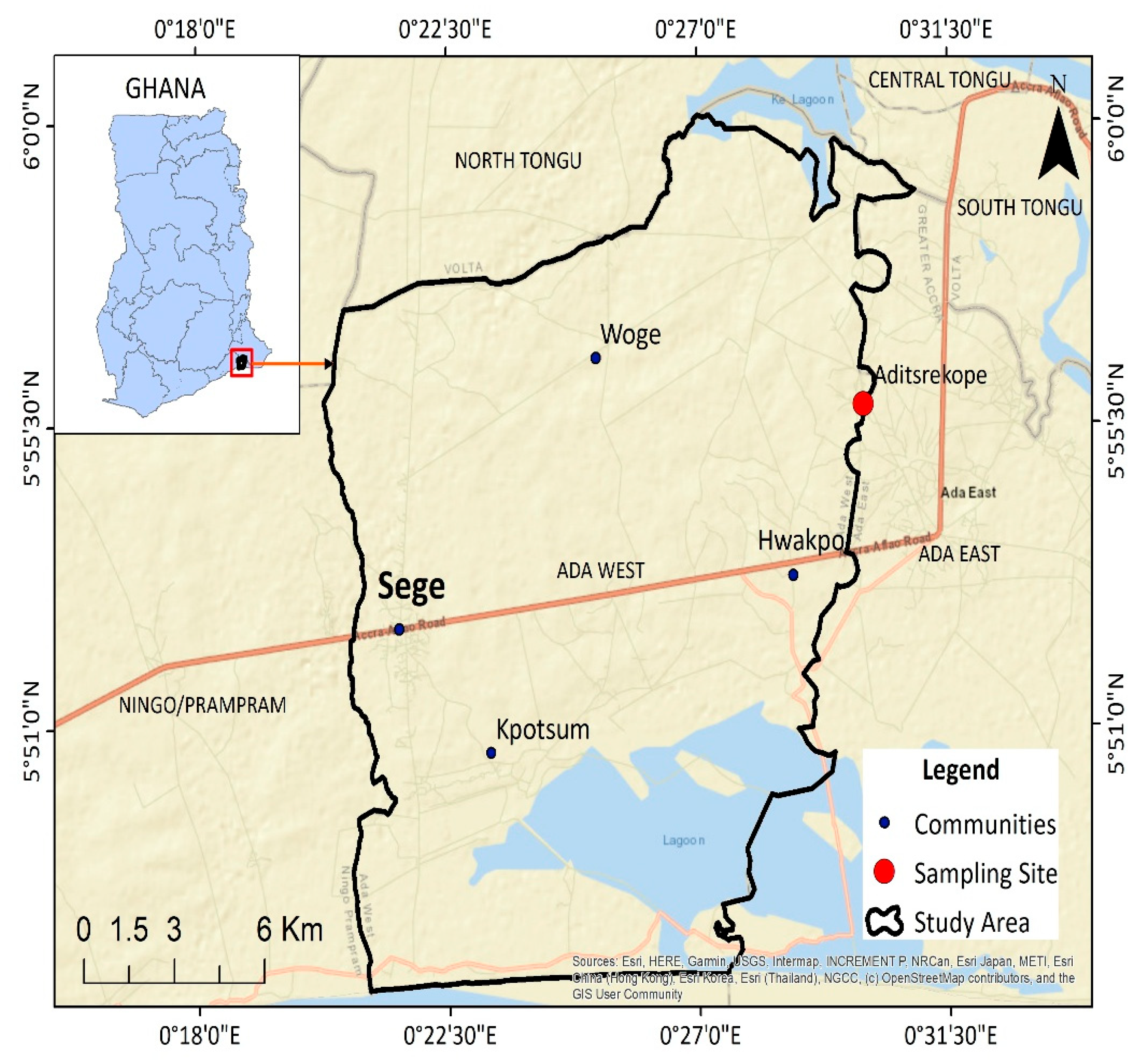
2.1. The Study Area
2.2. Experimental Design and Treatments
- MCfert [matured cow dung + sulfate of ammonia + triple superphosphate] MCbiofert [matured cow dung + sulfate of ammonia + biochar + triple superphosphate].
- CCfert [composted cow dung + sulfate of ammonia + triple superphosphate (TSP)].
- CCbiofert [composted cow dung + biochar + sulfate of ammonia + triple superphosphate].
- MC + CCfert [matured cow dung + composted cow dung + ammonium sulfate + triple superphosphate].
- NPK [15:15:15]—15% nitrogen (N), 15% Phosphorus (P), and 15% potassium (K).
- The reference soil was an uncultivated natural soil, which served as the control. The TSP (Ca(H2PO4)2·H2O) applied contains 46% P2O5 (phosphorus pentoxide) and ammonium sulfate ((NH4)2SO4) contains 21% nitrogen and 24% sulfur (S).
2.3. Soil Sampling and Measurements of Physical and Chemical Properties
2.4. Initial Soil Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Initial Chemical Properties of Soil, Biochar, Compost, and Cow Dung Before the Experiment
3.2. The Effect of Soil Amendments on Physical Properties of the Sodic Soggy Gleyic Solonetz Soil After Application of Treatment at Sege
3.3. The Effect of Soil Amendments on Chemical Characteristics of Gleyic Solonetz Soil at Sege
4. Discussion
4.1. The Effect of Combined Organic and Inorganic Amendment on Physicochemical Properties of Gleyic Soggy Sodic Soils at Sege
4.2. The Effect of the Organic and Inorganic Amendment on Soil Labile Carbon of the Gleyic Soggy Sodic Soils at Sege
4.3. The Influence of Combined Organic and Inorganic Amendment on the Carbon Management Index of the Gleyic Soggy Sodic Soils at Sege
4.4. The Influence of Combined Organic and Inorganic Soil Amendment on Total Soil Organic Carbon Stock of the Gleyic Soggy Sodic Soils at Sege
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction; Routledge: London, UK, 2015; pp. 1–13. [Google Scholar]
- Houssou, N.; Johnson, M.; Kolavalli, S.; Asante-Addo, C. Changes in Ghanaian Farming Systems: Stagnation or a Quiet Transformation? Agric. Hum. Values 2016, 35, 41–66. [Google Scholar] [CrossRef]
- Martey, E.; Kuwornu, J.K.M.; Adjebeng-Danquah, J. Estimating the effect of mineral fertilizer use on Land productivity and income: Evidence from Ghana. Land Use Policy 2019, 85, 463–475. [Google Scholar] [CrossRef]
- Doe, E.K.; Attua, E.M.; Obour, P.B.; Quaye, A.K.; Fosu-Mensah, B.Y. Soil health and synergy of ecological determinants of green cocoa productivity in different soil ecotypes in Ghana. Front. Sustain. Food Syst. 2023, 7, 1169015. [Google Scholar] [CrossRef]
- MacCarthy, D.S.; Darko, E.; Nartey, E.K.; Adiku, S.G.K.; Tettey, A. Integrating Biochar and Inorganic Fertilizer Improves Productivity and Profitability of Irrigated Rice in Ghana, West Africa. Agronomy 2020, 10, 904. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, Climate Change and Soil: A Review to Guide Future Research; CSIRO Land and Water Science Report 05/09; CSIRO: Canberra, Australia, 2009. [Google Scholar]
- Tovihoudji, P.G.; Akponikpè, P.I.; Adjogboto, A.; Djenontin, J.A.; Agbossou, E.K.; Bielders, C.L. Combining hill-placed manure and mineral fertilizer enhances maize productivity and profitability in northern Benin. Nutr. Cycl. Agroecosyst. 2018, 110, 375–393. [Google Scholar] [CrossRef]
- Dróżdż, D. Production and Use of Organic Soil Enhancers and Growing Media from Agro-Residues. Ph.D. Thesis, Faculty of Infrastructure and Environment, Czestochowa University of Technology, Częstochowa, Poland, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium, 2022. [Google Scholar]
- Gomez-Brandon, M.; Fernandez-Delgado, M.J.; Domiguez, J.; Insam, H. Animal manures: Recycling and management technologies. In Biomass Now—Cultivation and Utilization; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Akanni, D.I.; Ojeniyi, S.O. Residual effect of goat and poultry manures on soil properties, nutrient content, and yield of Amaranthus in Southwest Nigeria. Resh. J. Agron. 2008, 2, 44–47. [Google Scholar]
- Kapkiyai, J.J.; Karanja, N.K.; Woomer, P.; Qureshi, J.N. Soil organic carbon fractions in a long-term experiment and the potential for their use as a diagnostic assays in highland farming systems of central Kenya. Afr. Crop Sci. J. 1998, 6, 19–28. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Giller, K. Popular myths around soil fertility management in sub-Saharan Africa. Agric. Ecosyst. Environ. 2006, 116, 34–46. [Google Scholar] [CrossRef]
- Field, C.M. Combining Organic and Mineral Fertilizers for Integrated Soil Fertility Management in Smallholder Farming Systems of Kenya: Explorations Using the Crop-Soil Model FIELD. Agron. J. 2008, 100, 1511–1526. [Google Scholar] [CrossRef]
- Quaye, A.K.; Doe, E.K.; Amon-Armah, F.; Arthur, A.; Dogbatse, J.A.; Konlan, S. Predictors of integrated soil fertility management practice among cocoa farmers in Ghana. J. Agric. Food Res. 2021, 5, 100174. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural Systems. Aust. J. Soil Res. 1995, 46, 14591466. [Google Scholar] [CrossRef]
- Gentile, R.; Vanlauwe, B.; Chivenge, P.; Six, J. Soil Biology & Biochemistry Interactive effects from combining fertilizer and organic residue inputs on nitrogen transformations. Soil Biol. Biochem. 2008, 40, 2375–2384. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, S.; He, T.; Liu, L. Fresh carbon andnitrogen inputs alter organic carbon mineralization and microbial community in forest deep soil layers. Soil Biol. Biochem. 2014, 72, 145–151. [Google Scholar] [CrossRef]
- Saha, S.; Mina, B.L.; Gopinath, K.A.; Kundu, S.; Gupta, H.S. Organic amendments affect biochemical properties of a subtemperate soil of the Indian Himalayas. Nutr. Cycl. Agroecosyst. 2008, 80, 233–242. [Google Scholar] [CrossRef]
- Sher, A.; Adnan, M.; Sattar, A.; Ul-Allah, S.; Ijaz, M.; Hassan, M.U.; Manaf, A.; Qayyum, A.; Elesawy, B.H.; Ismail, K.A.; et al. Combined Application of Organic and Inorganic Amendments Improved the Yield and Nutritional Quality of Forage Sorghum. Agronomy 2022, 12, 896. [Google Scholar] [CrossRef]
- Ghana Statistical Service. 2010 Population and Housing Census. Summary Report of Final Results; GSS: Accra, Ghana, 2012. [Google Scholar]
- Doan, P.; Oduro, C.Y. Patterns of population growth in peri-urban Accra, Ghana. Int. J. Urban Reg. Res. 2012, 36, 1306–1325. [Google Scholar] [CrossRef]
- Owusu, G.; Yankson, P.W. Urbanization in Ghana. The Economy of Ghana Sixty Years After Independence; Oxford University Press: London, UK, 2017; pp. 23–38. [Google Scholar]
- FAO. The Euphrates Pilot Irrigation Project: Methods of Soil Analysis. Gabed Soil Laboratory (A Laboratory Manual); Food and Agriculture Organization: Rome, Italy, 1974. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Available online: https://www.isric.org/sites/default/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 8 July 2024).
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Day, P.R. Particle fractionation and particle-size analysis. In Methods of Soil Analysis, Part 1; Agronomy, M., Black, C.A., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Myers, R.J.K. Inclusion of Nitrate and Nitrite In The Kjeldahl Nitrogen Determination of Soils and Plant Materials Using Sodium Thiosulphate. Commun. Soil Sci. Plant Anal. 1984, 15, 1453–1461. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Sparling, G.P. Soil Quality Indicators. In Managing Soils and Terrestrial Systems; Taylor & Francis Group: Abingdon, UK, 2020; pp. 357–360. [Google Scholar] [CrossRef]
- Yadav, N.K.; Kumar, V.; Sharma, K.R.; Choudhary, R.S.; Butter, T.S.; Singh, G. Biochar and their impacts on soil properties and crop productivity: A review. J. Pharmacogn. Phytochem. 2018, 7, 49–54. [Google Scholar]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Mankasingh, U.; Choi, P.C.; Ragnarsdottir, V. Biochar application in a tropical, agricultural region: A plot scale study in Tamil Nadu, India. Appl. Geochem. 2011, 26, 218–221. [Google Scholar] [CrossRef]
- Ulyett, J.; Sakrabani, R.; Kibblewhite, M.; Hann, M. Impact of biochar addition on water retention, nitrification and carbon dioxide evolution from two sandy loam soils. Eur. J. Soil Sci. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Lal, R. Soils and food sufficiency. A review. Agron. Sustain. Dev. 2009, 29, 113–133. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Al-Wabel, M.I.; Usman, A.R.; Al-Omran, A.E. Effect of Conocarpus biochar application on the hydraulic properties of a sandy loam soil. Soil Sci. 2013, 178, 165–173. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Pan, G.; Liu, X.; Zhang, X.; Li, L.; Bian, R.; Cheng, K.; Jinwei, Z. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci. Total Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Raemaekers, H. Romain Crop Production in Tropical Africa; Directorate General for International Cooperation, Ministry of Foreign Affairs, External Trade and International Cooperation: Brussels, Belgium, 2001; pp. 403–407. [Google Scholar]
- Chintala, R.; Owen, R.; Kumar, S.; Schumacher, T.E.; Malo, D. Biochar impacts on denitrification under different soil water contents. In Proceedings of the 20th World Congress of Soil Science, Jeju, Republic of Korea, 8–13 June 2014; p. 157. [Google Scholar]
- Al-Wabe, M.I.; Usman, A.R.A.; Al-Farraj, A.S.; Ok, Y.S.; Abduljabbar, A.; Al-Faraj, A.; Sallam, A.S. Correction to: Date palm waste biochars alter a soil respiration, microbial biomass carbon, and heavy metal mobility in contaminated mined soil. Environ. Geochem. Health 2019, 41, 1809. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L.; Lesch, S.M. Apparent soil electrical conductivity measurements in agriculture. Comput. Electron. Agric. 2005, 46, 11–43. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramirez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zwieten, V.L.; Meszaros, I.; Dowine, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Davis, A.S.; Jacobs, D.F.; Wightman, K.E.; Birge, Z.K.D. Organic matter added to bareroot nursery beds influences soil properties and morphology of Fraxinus pennsylvanica and Quercus rubra seedlings. New For. 2006, 31, 293–303. [Google Scholar] [CrossRef]
- Olowoake, A.A.; Adeoye, G. O Influence of differently composted organic residues on the yield of maize and its residual effects on the fertility of an Alfisol in Ibadan, Nigeria. Intl. J. Agric. Environ. Biotech 2013, 6, 79–84. [Google Scholar]
- Sanni, K.O. Effect of compost, cow dung and NPK 15-15-15 fertilizer on growth and yield performance of Amaranth (Amaranthus hybridus); Department of Crop Production and Horticulture Lagos State Polytechnic: Ikorodu, Nigeria, 2016; pp. 76–81. [Google Scholar]
- Stevenson, F.J. Humic Chemistry, Genesis, Composition, Reactions; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Banger, K.; Kukal, S.; Toor, G.; Sudhir, K.; Hanumanthraju, T. Impact of long-term additions of chemical fertilizers and farmyard manure on carbon and nitrogen sequestration under rice-cowpea cropping system in semi-arid tropics. Plant Soil 2009, 318, 27–35. [Google Scholar] [CrossRef]
- Li, J.; Wen, Y.; Li, X.; Li, Y.; Yang, X.; Lin, Z.; Song, Z.; Cooper, J.M.; Zhao, B. Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil Tillage Res. 2018, 175, 281–290. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Li, F.; Zhang, J. Effects of Organic and Inorganic Fertilization on Soil Organic Carbon and Enzymatic Activities. Agronomy 2022, 12, 3125. [Google Scholar] [CrossRef]
- Dodor, D.E.; Amanor, Y.J.; Attor, F.T.; Adjadeh, T.A.; Neina, D.; Miyittah, M. Co-application of biochar and cattle manure counteract positive priming of carbon mineralization in a sandy soil. Environ. Syst. Res. 2018, 7, 5. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry, 2nd ed.; Academic Press: London, UK, 1996. [Google Scholar]
- Nyakatawa, E.Z.; Reddy, K.C.; Sistani, K.R. Tillage, covercropping, and poultry litter effects on selected soil chemical properties. Soil Till. Res. 2001, 58, 69–79. [Google Scholar] [CrossRef]
- Moreno, F.; Murillo, J.M.; Pelegrı’n, F.; Giro’n, I.F. Long-term impact of conservation tillage on stratification ratio of soil organic carbon and loss of total and active CaCO3. Soil Till. Res. 2006, 85, 86–93. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Yang, G.H.; Han, X.H.; Feng, Y.Z.; Ren, G.X. Stratification of carbon fractions and carbon management index in deep soil affected by the grain-to- green program in China. PLoS ONE 2014, 9, e99657. [Google Scholar] [CrossRef]
- Biederbeck, V.O.; Janzen, H.H.; Campbell, C.A.; Zentner, R.P. Labile soil organic matter as influenced by cropping practices in an arid environment. Soil Biol. Biochem 1994, 26, 1647–1656. [Google Scholar] [CrossRef]
- Conteh, A.; Lefroy, R.D.B.; Blair, G.J. Dynamics of organic matter in soil as determined by variations in 13C/12C isotopic ratios and fractionation by ease of oxidation. Soil Res. 1997, 35, 881–890. [Google Scholar] [CrossRef]
- Moharana, P.C.; Sharma, B.M.; Biswas, D.R.; Dwivedi, B.S.; Singh, R.V. Long-term effect of nutrient management on soil fertility and soil organic carbon pools under a 6-year-old pearl milletwheat cropping system in an incepticol of subtropical India. Field Crops Res. 2012, 136, 32–41. [Google Scholar] [CrossRef]
- Ng’ang’a, S.K.; Jalang’o, D.A.; Girvetz, E.H. Soil carbon enhancing practices: A systematic review of barriers and enablers of adoption. SN Appl. Sci. 2019, 1, 1726. [Google Scholar] [CrossRef]
- Xu, M.G.; Lou, Y.L.; Sun, X.H.; Wang, W.; Baniyamuddin, M.; Zhao, K. Effects of organic manure application with chemical fertilizers on nutrient absorption and yield of rice under long-term fertilization. Plant Soil 2011, 341, 427–438. [Google Scholar]
- Luo, Z.; Wang, E.; Sun, O.J. Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems: A review and synthesis. Geoderma 2017, 216, 226–233. [Google Scholar] [CrossRef]
- Leite, L.F.C.; Mendonga, E.S.; Machado, P.L.O.A. Influence of organic and mineral fertilization on organic matter fractions of a Brazilian Acrisol under maize/common bean intercrop. Aust. J. Soil Res. 2007, 45, 25–32. [Google Scholar] [CrossRef]
- Mtambanengwe, F.; Mapfumo, P. Smallholder farmer management impacts on particulate and labile carbon fractions of granitic sandy soils in Zimbabwe. Nutr. Cycl. Agroecosyst. 2008, 81, 1–15. [Google Scholar] [CrossRef]
- Cambardella, C.; Elliott, E. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P.; Townley-Smith, L. Light-fraction organic matter in soils from long-term crop rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 2015, 237, 149–158. [Google Scholar] [CrossRef]
- Gabarron-Galeote, M.A.; Trigalet, S.; Wesemael, B. Effect of land abandonment on soil organic carbon fractions along a Mediterranean precipitation gradient. Geoderma 2015, 249–250, 69–78. [Google Scholar]
- Christenson, L.M.; Lovett, G.M.; Weathers, K.C.; Arthur, M.A. The Influence of Tree Species, Nitrogen Fertilization, and Soil C to N ratio on Gross Soil Nitrogen Transformations. Soil Sci. Soc. Am. J. 2009, 73, 638–646. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Korschens, M.; Schulz, E. Long-term management impacts on soil C, N and physical fertility. Part II: Bad Lauchstadt static and extreme FYM experiments. Soil Till Res 2006, 91, 39–47. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2018, 312, 45–52. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Shen, J.; Li, L.; Zhang, F. Legacy of manure application history determines chemical and microbial responses of a temperate agricultural soil. Sci. Rep. 2015, 5, 16320. [Google Scholar] [CrossRef]
- Kalambukattu, J.G.; Singh, R.; Patra, A.K.; Arunkumar, K. Soil carbon pools and carbon management index under different land use systems in the Central Himalayan region. Acta Agric. Scan. Sect. B Soil Plant Sci. 2013, 63, 200–205. [Google Scholar] [CrossRef]
- Gami, S.K.; Lauren, J.G.; Duxbury, J.M. Influence of soil texture and cultivation on carbon and nitrogen levels in soils of the eastern Indo-Gangetic Plains. Geoderma 2009, 153, 304–311. [Google Scholar] [CrossRef]
| Soil Depth/ Unit | pH | EC | CEC | Ca | Mg | K | Na | Avail. P | OC | TN | C/N | OM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1:1) | dS/m | cmol | Exchangeable Bases (cmol/kg) | (cmol/kg) | (%) | (%) | % | |||||
| 0–15 cm | 4.9 | 0.08 | 8.1 | 0.43 | 0.36 | 0.06 | 0.4 | 14.48 | 0.17 | 0.05 | 1:03 | 0.29 |
| 15–30 cm | 5.2 | 0.05 | 7.7 | 0.36 | 0.23 | 0.04 | 0.2 | 14.06 | 0.11 | 0.04 | 1:6 | 0.19 |
| Chemical Properties | Biochar | Manure | Compost |
|---|---|---|---|
| pH | 6.8 | 8 | 7.4 |
| EC (dS/m) | 5.5 | 8.6 | 6.2 |
| CEC (cmol) | 82.1 | 27.9 | 21.4 |
| Ca (cmol/kg) | 3.0 | 6.5 | 4.2 |
| Mg (cmol/kg) | 7.4 | 8.1 | 6.4 |
| K (cmol/kg) | 0.64 | 1.0 | 0.74 |
| Na (cmol/kg) | 0.33 | 0.93 | 0.56 |
| Available P (%) | 66.1 | 91.0 | 77 |
| OC (%) | 37.8 | 8.0 | 7.6 |
| TN (%) | 0.49 | 0.69 | 0.55 |
| C/N | 77:1 | 12:1 | 11.1 |
| Treatments | Soil Bulk Density | % Sand | % Silt | % Clay | Texture |
|---|---|---|---|---|---|
| 0–15 cm | |||||
| MCfer | 1.56 ab | 89.2 a | 5.0 ab | 5.8 a | Sand |
| MCbiofert | 1.53 a | 88.3 a | 5.0 ab | 6.7 a | Loamy sand |
| CCfert | 1.54 a | 87.5 a | 5.8 b | 6.7 a | Loamy sand |
| CCbiofert | 1.51 a | 87.5 a | 5.0 ab | 7.5 a | Loamy sand |
| MC + CCfert | 1.56 ab | 88.3 a | 5.8 b | 5.8 a | Sand |
| NPK | 1.61 bc | 87.5 a | 5.0 ab | 7.5 a | Loamy sand |
| Uncultivated Soil Alone | 1.63 c | 89.2 a | 3.3 a | 7.5 a | Sand |
| L.S.D. (0.05) | 0.06 | 1.7 | 1.7 | 2.0 | |
| 15–30 cm | |||||
| MCfert | 1.65 a | 88.3 a | 3.3 a | 8.3 a | Loamy sand |
| MCbiofert | 1.60 a | 87.9 a | 3.3 a | 8.8 a | Loamy sand |
| CCfert | 1.61 a | 87.5 a | 3.8 a | 8.8 a | Loamy sand |
| CCbiofert | 1.63 a | 88.8 a | 3.8 a | 7.5 a | Loamy sand |
| MC + CCfert | 1.60 a | 87.5 a | 3.8 a | 8.8 a | Loamy sand |
| NPK | 1.63 a | 88.3 a | 3.3 a | 8.3 a | Loamy sand |
| Soil Alone | 1.63 a | 87.5 a | 4.2 a | 8.3 a | Loamy sand |
| L.S.D. (0.05) | 0.06 | 1.8 | 2.2 | 2.2 |
| 0–15 cm | 15–30 cm | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | OC (%) | Stock (c/ha) | N (%) | AV. P | OC (%) | Stock (c/ha) | N (%) | AV. P |
| MCfert | 0.29 c | 6.76 c | 0.04 a | 16.38 b | 0.15 ab | 7.57 c | 0.053 b | 15.03 ab |
| MCbiofert | 0.35 d | 8.08 d | 0.08 b | 16.32 b | 0.16 c | 7.64 c | 0.121 c | 15.11 ab |
| CCfert | 0.37 d | 8.58 d | 0.14 c | 19.15 c | 0.15 ab | 7.07 bc | 0.120 c | 15.73 bc |
| CCbiofert | 0.36 d | 8.14 d | 0.18 d | 18.62 c | 0.15 ab | 7.14 bc | 0.050 ab | 16.41 c |
| MC + CCfert | 0.19 ab | 4.34 ab | 0.05 a | 16.91 b | 0.15 ab | 7.32 bc | 0.053 b | 14.35 a |
| NPK | 0.20 b | 4.82 b | 0.12 c | 16.38 b | 0.12 ab | 5.84 b | 0.070 b | 14.27 a |
| Soil Alone | 0.15 a | 3.57 a | 0.04 a | 14.33 a | 0.09 a | 4.24 a | 0.027 a | 14.29 a |
| Treatment | pH | EC | CEC | Ca | Mg | K | Na |
|---|---|---|---|---|---|---|---|
| (Water) | (dS m−1) | (cmol kg−1) | (cmol/kg) | ||||
| 0–15 cm | |||||||
| MCfert | 5.9 a | 0.04 b | 3.0 d | 1.8 d | 0.94 d | 0.068 e | 0.220 f |
| MCbiofert | 6.0 a | 0.05 b | 2.1 b | 1.3 b | 0.48 a | 0.063 d | 0.166 a |
| CCfert | 5.8 a | 0.04 b | 2.9 d | 1.9 e | 0.80 c | 0.067 e | 0.196 b |
| CCbiofert | 5.9 a | 0.05 b | 2.7 c | 1.7 d | 0.70 bc | 0.050 b | 0.199 c |
| MC + CCfert | 5.8 a | 0.06 b | 2.6 c | 1.7 d | 0.65 b | 0.063 d | 0.201 d |
| NPK | 5.6 a | 0.09 c | 1.7 a | 1.1 a | 0.43 a | 0.055 c | 0.207 e |
| Soil Alone | 5.6 a | 0.02 a | 2.2 b | 1.4 c | 0.40 a | 0.047 a | 0.194 b |
| 15–30 cm | |||||||
| MCfert | 5.6 a | 0.037 e | 1.7 e | 1.06 d | 0.44 d | 0.071 e | 0.133 e |
| MCbiofert | 5.6 a | 0.030 cd | 1.2 c | 0.82 c | 0.22 bc | 0.033 b | 0.107 d |
| CCfert | 5.7 a | 0.031 d | 1.2 c | 0.83 c | 0.23 c | 0.032 b | 0.132 e |
| CCbiofert | 5.7 a | 0.026 bc | 0.9 a | 0.59 a | 0.20 b | 0.040 d | 0.067 a |
| MC + CCfert | 5.8 a | 0.024 b | 1.0 b | 0.64 b | 0.21 bc | 0.030 a | 0.075 b |
| NPK | 5.7 a | 0.039 e | 0.9 a | 0.62 ab | 0.14 a | 0.034 b | 0.073 b |
| Soil Alone | 6.0 a | 0.019 a | 1.6 d | 0.61 ab | 0.21 bc | 0.037 c | 0.085 c |
| Topsoil at 0–15 cm (%) | Subsoil at 15–30 cm (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | OC | Sand | Silt | Clay | OC | Sand | Silt | Clay |
| MCfert | 0.29 c | 0.08 bc | 0.04 a | 0.15 c | 0.15 ab | 0.04 a | 0.021 b | 0.09 b |
| MCbiofert | 0.35 d | 0.11 c | 0.05 bc | 0.19 d | 0.16 b | 0.04 a | 0.020 b | 0.09 b |
| CCfert | 0.37 d | 0.10 c | 0.06 c | 0.20 d | 0.15 ab | 0.03 a | 0.020 b | 0.08 b |
| CCbiofert | 0.36 d | 0.10 c | 0.05 bc | 0.20 d | 0.15 ab | 0.04 a | 0.018 b | 0.08 b |
| MC + CCfert | 0.19 b | 0.06 ab | 0.02 a | 0.10 b | 0.15 ab | 0.03 a | 0.016 ab | 0.10 b |
| NPK | 0.20 b | 0.05 a | 0.02 a | 0.12 b | 0.12 ab | 0.04 a | 0.019 b | 0.06 ab |
| Soil Alone | 0.15 a | 0.04 a | 0.02 a | 0.07 a | 0.09 a | 0.03 a | 0.007 a | 0.04 a |
| Topsoil at 0–15 cm (%) | Subsoil at 15–30 cm (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | KMnO4-C | Sand LF | Silt LF | Clay LF | KMnO4-C | Sand LF | Silt LF | Clay LF |
| MCfert | 0.018 b | 0.008 a | 0.0014 b | 0.0023 a | 0.021 e | 0.010 c | 0.0008 a | 0.0032 b |
| MCbiofert | 0.019 c | 0.015 c | 0.0014 b | 0.0026 a | 0.014 b | 0.007 a | 0.0007 a | 0.0033 b |
| CCfert | 0.023 d | 0.018 d | 0.0019 cd | 0.0027 a | 0.019 d | 0.010 c | 0.0009 a | 0.0034 b |
| CCbiofert | 0.019 c | 0.014 c | 0.0016 bc | 0.0030 a | 0.018 c | 0.011 d | 0.0008 a | 0.0029 b |
| MC + CCfert | 0.019 c | 0.010 b | 0.0016 bc | 0.0023 a | 0.015 b | 0.010 c | 0.0008 a | 0.0034 b |
| NPK | 0.018 b | 0.008 a | 0.0014 b | 0.0030 a | 0.018 c | 0.009 b | 0.0007 a | 0.0034 b |
| Soil Alone | 0.017 a | 0.008 a | 0.0008 a | 0.0026 a | 0.012 a | 0.007 a | 0.0008 a | 0.0022 a |
| RF | 0.040 e | 0.033 e | 0.0022 d | 0.0053 b | 0.028 f | 0.019 e | 0.0017 b | 0.0046 c |
| Topsoil at 0–15 cm | Subsoil at 15–30 cm | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | TOC (%) | LI | CPI | CMI | TOC (%) | LI | CPI | CMI |
| MCfert | 0.29 c | 2.5 b | 0.18 c | 46 a | 0.15 ab | 8.10 de | 0.10 ab | 83 e |
| MCbiofert | 0.35 d | 2.2 b | 0.22 d | 49 b | 0.16 b | 5.00 b | 0.11 b | 52 b |
| CCfert | 0.37 d | 2.5 b | 0.24 d | 58 d | 0.15 ab | 7.58 de | 0.10 ab | 74 d |
| CCbiofert | 0.36 d | 2.1 b | 0.23 d | 48 b | 0.15 ab | 6.95 cd | 0.10 ab | 69 c |
| MC + CCfert | 0.19 b | 4.4 d | 0.12 b | 51 c | 0.15 ab | 5.50 bc | 0.10 ab | 57 b |
| NPK | 0.20 b | 3.7 c | 0.13 b | 47 ab | 0.12 ab | 8.89 e | 0.08 ab | 71 cd |
| Soil Alone | 0.15 a | 5.0 e | 0.09 a | 46 a | 0.09 a | 8.07 de | 0.06 a | 47 a |
| RF | 1.58 e | 1.0 a | 1.00 e | 100 d | 1.48 c | 1.00 a | 1.00 c | 100 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fosu-Mensah, B.Y.; Mutaru, D.; MacCarthy, D.S.; Mensah, M. Assessing the Effect of Organic and Inorganic Resources on Carbon Fractions in Soggy Sodic Soil at Sege in Ada West District, Ghana. Soil Syst. 2025, 9, 62. https://doi.org/10.3390/soilsystems9020062
Fosu-Mensah BY, Mutaru D, MacCarthy DS, Mensah M. Assessing the Effect of Organic and Inorganic Resources on Carbon Fractions in Soggy Sodic Soil at Sege in Ada West District, Ghana. Soil Systems. 2025; 9(2):62. https://doi.org/10.3390/soilsystems9020062
Chicago/Turabian StyleFosu-Mensah, Benedicta Yayra, Diawudeen Mutaru, Dilys Sefakor MacCarthy, and Michael Mensah. 2025. "Assessing the Effect of Organic and Inorganic Resources on Carbon Fractions in Soggy Sodic Soil at Sege in Ada West District, Ghana" Soil Systems 9, no. 2: 62. https://doi.org/10.3390/soilsystems9020062
APA StyleFosu-Mensah, B. Y., Mutaru, D., MacCarthy, D. S., & Mensah, M. (2025). Assessing the Effect of Organic and Inorganic Resources on Carbon Fractions in Soggy Sodic Soil at Sege in Ada West District, Ghana. Soil Systems, 9(2), 62. https://doi.org/10.3390/soilsystems9020062








