Exploring the Roles of Plant Growth-Promoting Rhizobacteria (PGPR) and Alternate Wetting and Drying (AWD) in Sustainable Rice Cultivation
Abstract
1. Introduction
2. Alternate Wetting and Drying
2.1. History
2.2. Principles
2.3. Research Achievements and Basis
3. The Role of Alternate Wetting and Drying
3.1. Physiological and Structural Changes in Roots Under AWD
3.1.1. Structural Changes
3.1.2. Physiological Changes
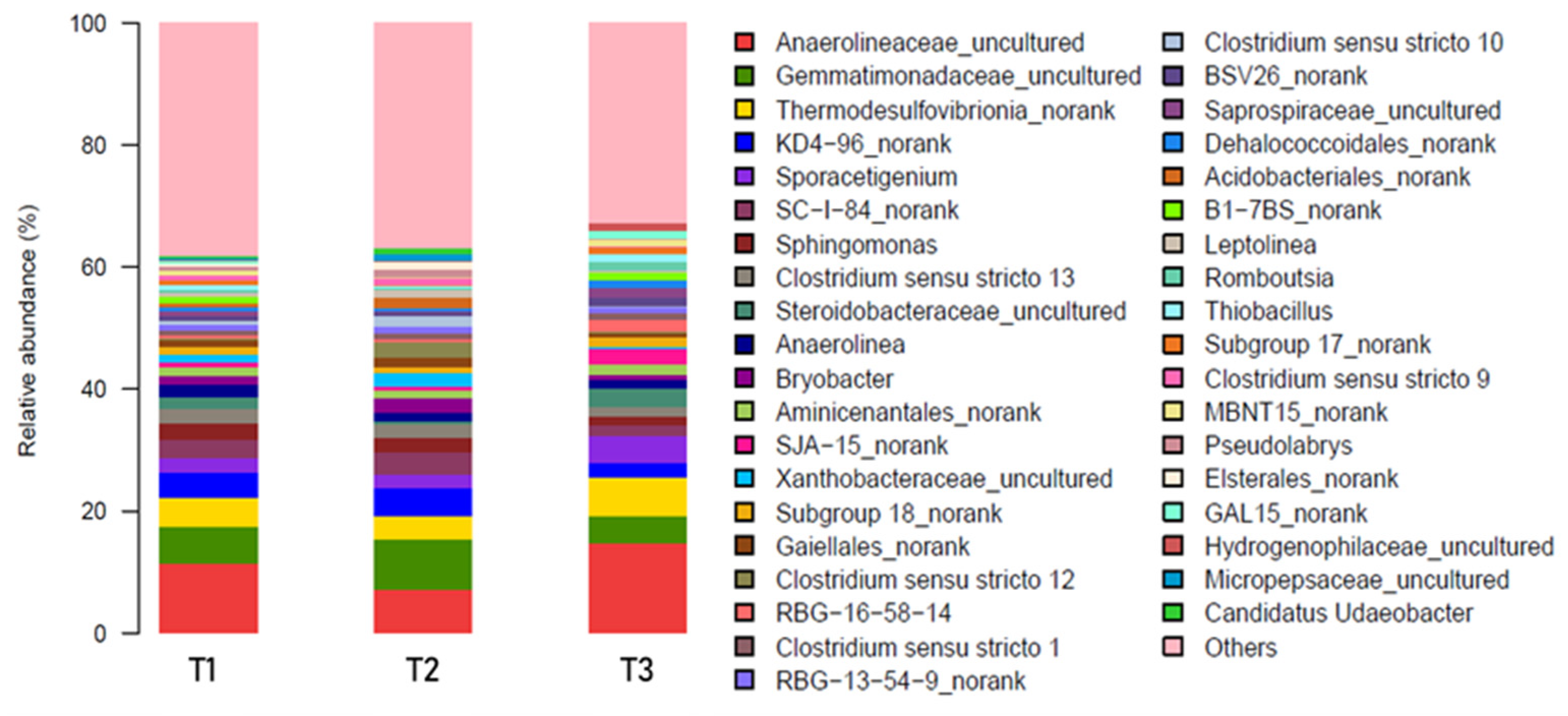
3.2. Microbial Interactions in the Rhizosphere Under AWD
3.2.1. Microbial Interactions
3.2.2. Microbial Density and Contributions
4. The Role of Plant Growth-Promoting Rhizobacteria
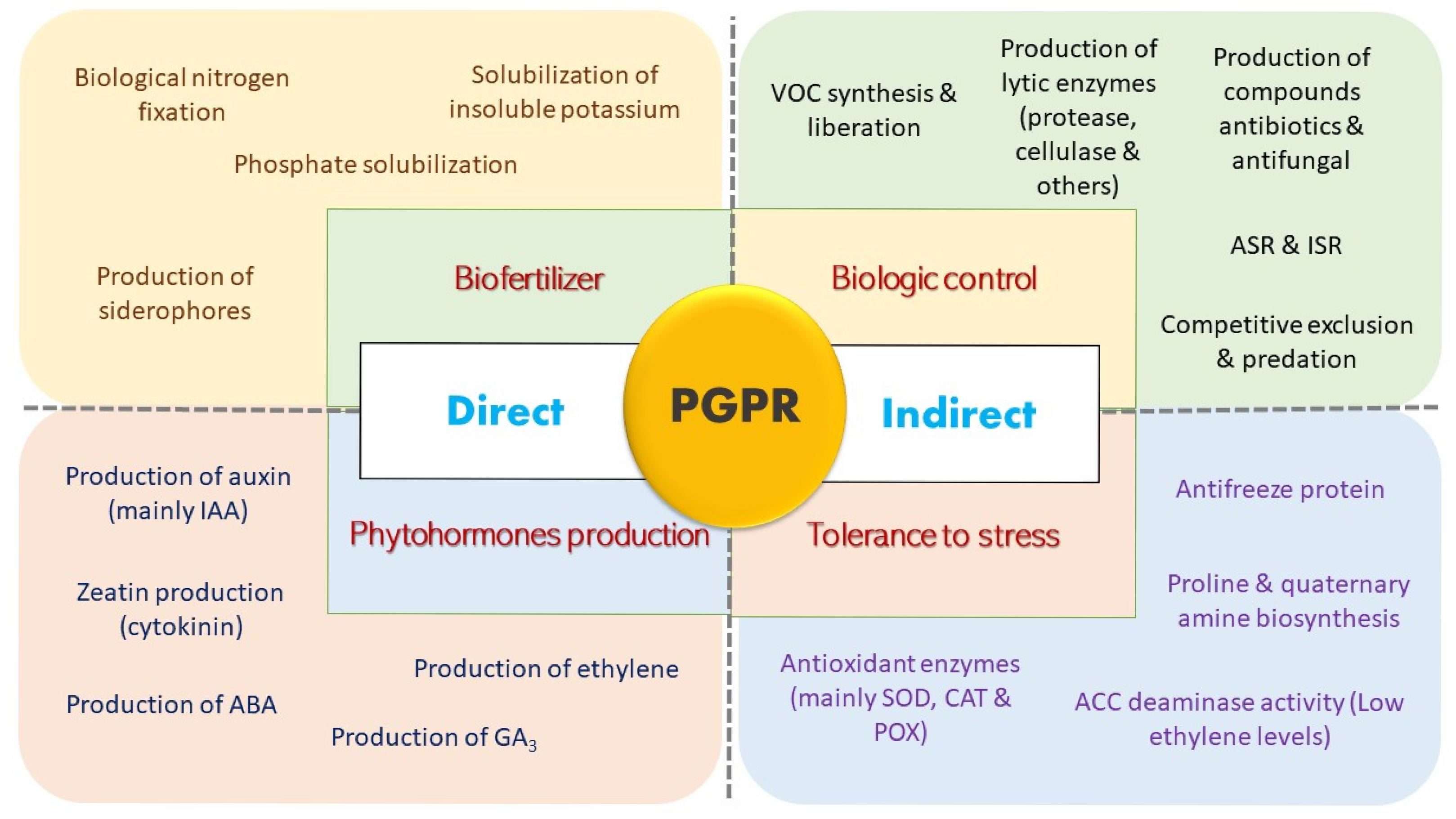
4.1. Plant Growth-Promoting Rhizobacteria
4.2. Mechanism of PGPR
4.3. Benefits of PGPR in Rice Cultivation
4.4. Limitations of PGPR
5. Challenges of PGPR and AWD Synergy
6. Alignment with United Nations Sustainable Development Goals
7. Future Directions
7.1. Information Gaps
7.2. System of Probiotics in Rice Intensification
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahbandeh, M. Leading Countries Based on the Production of Milled Rice in 2022/2023. Available online: https://www.statista.com/statistics/255945/top-countries-of-destination-for-us-rice-exports-2011 (accessed on 2 December 2024).
- United Nations. United Nations Population Division. Available online: https://www.un.org/en/global-issues/population?hl=en-GB (accessed on 2 December 2024).
- Edmond, C. Rice Is Both a Victim and a Villain in Terms of the Climate Crisis. Here’s Why. Available online: https://theprint.in/environment/rice-is-both-a-victim-and-villain-of-climate-crisis-heres-why/1620411/ (accessed on 2 December 2024).
- Ghosh, B.; Ali Md, N. Response of Rice under Salinity Stress: A Review Update. Rice Res. Open Access 2016, 4, 2–9. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Hua, Z.; Xue, H.; Mei, S.; Wang, P.; Wang, S. Comparison of Nitrogen Loss Weight in Ammonia Volatilization, Runoff, and Leaching Between Common and Slow-Release Fertilizer in Paddy Field. Water Air Soil Pollut. 2021, 232, 132. [Google Scholar] [CrossRef]
- Tayefeh, M.; Sadeghi, S.M.; Noorhosseini, S.A.; Bacenetti, J.; Damalas, C.A. Environmental Impact of Rice Production Based on Nitrogen Fertilizer Use. Environ. Sci. Pollut. Res. 2018, 25, 15885–15895. [Google Scholar] [CrossRef]
- External Affairs and Communications. Carbon Emissions from Fertilizers Could Be Reduced by as Much as 80% by 2050. Available online: https://www.cam.ac.uk/research/news/carbon-emissions-from-fertilisers-could-be-reduced-by-as-much-as-80-by-2050 (accessed on 25 November 2024).
- Mboyerwa, P.A.; Kibret, K.; Mtakwa, P.; Aschalew, A. Greenhouse Gas Emissions in Irrigated Paddy Rice as Influenced by Crop Management Practices and Nitrogen Fertilization Rates in Eastern Tanzania. Front. Sustain. Food Syst. 2022, 6, 868479. [Google Scholar] [CrossRef]
- Gao, Y.; Serrenho, A.C. Greenhouse Gas Emissions from Nitrogen Fertilizers Could Be Reduced by up to One-Fifth of Current Levels by 2050 with Combined Interventions. Nat. Food 2023, 4, 170–178. [Google Scholar] [CrossRef]
- Scholz, V.V.; Meckenstock, R.U.; Nielsen, L.P.; Risgaard-Petersen, N. Cable Bacteria Reduce Methane Emissions from Rice-Vegetated Soils. Nat. Commun. 2020, 11, 2020–2021. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant Growth-Promoting Root-Colonizing Bacterial Endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, X.F.; Chaparro, J.M.; Badri, D.V.; Manter, D.K.; Vivanco, J.M.; Guo, J. Root and Bacterial Secretions Regulate the Interaction between Plants and PGPR Leading to Distinct Plant Growth Promotion Effects. Plant Soil 2016, 401, 259–272. [Google Scholar] [CrossRef]
- de Laulanié, H. System of Rice Intensification. Tropicultura 1993, 13, 110–114. [Google Scholar]
- Vuciterna, R.; Ruggeri, G.; Corsi, S.; Facchi, A.; Gharsallah, O. A Bibliometric Analysis of Scientific Literature on Alternate Wetting and Drying (AWD). Paddy Water Environ. 2024, 22, 415–430. [Google Scholar] [CrossRef]
- Ishfaq, M.; Farooq, M.; Zulfiqar, U.; Hussain, S.; Akbar, N.; Nawaz, A.; Anjum, S.A. Alternate Wetting and Drying: A Water-Saving and Ecofriendly Rice Production System. Agric. Water Manag. 2020, 241, 106363. [Google Scholar] [CrossRef]
- Sriphirom, P.; Chidthaisong, A.; Towprayoon, S. Effect of Alternate Wetting and Drying Water Management on Rice Cultivation with Low Emissions and Low Water Used during Wet and Dry Season. J. Clean. Prod. 2019, 223, 980–988. [Google Scholar] [CrossRef]
- Kumar, K.A.; Rajitha, G. Alternate Wetting and Drying (AWD) Irrigation—A Smart Water Saving Technology for Rice: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2561–2571. [Google Scholar] [CrossRef]
- Uphoff, N. Increasing Water Savings While Raising Rice Yields with The System of Rice Intensification (SRI). 1993, pp. 1–17. Available online: http://sri.cals.cornell.edu/conferences/2irc1006/2IRCntu.pdf (accessed on 2 December 2024).
- Mote, K.; Rao, V.P.; Ramulu, V.; Kumar, K.A.; Devi, M.U. Performance of Rice (Oryza sativa (L.)) under AWD Irrigation Practice—A Brief Review. Paddy Water Environ. 2022, 20, 1–21. [Google Scholar] [CrossRef]
- Soliman, E.; Azam, R.; Hammad, S.A.; Mosa, A.A.; Mansour, M.M. Impacts of Alternate Wetting and Drying Technology on Water Use and Soil Nitrogen Transformations for Sustainable Rice Production: A Review. J. Soil Sci. Agric. Eng. 2024, 15, 151–163. [Google Scholar] [CrossRef]
- Fawzi, N.A.; Sadeq, A.; Jalal, A. Design and Implementation of Smart Irrigation System Using Wireless Sensor Network Based on Internet of Things. J. Sci. Eng. Res. 2017, 8, 109–113. [Google Scholar]
- Liu, L.-W.; Ismail, M.H.; Wang, Y.-M.; Lin, W.-S. Internet of Things Based Smart Irrigation Control System for Paddy Field. AGRIVITA 2021, 43, 378–389. [Google Scholar] [CrossRef]
- Liu, L.W.; Lu, C.T.; Wang, Y.M.; Lin, K.H.; Ma, X.; Lin, W.S. Rice (Oryza sativa L.) Growth Modeling Based on Growth Degree Day (GDD) and Artificial Intelligence Algorithms. Agriculture 2022, 12, 59. [Google Scholar] [CrossRef]
- Kima, A.S.; Chung, W.G.; Wang, Y.M.; Traoré, S. Evaluating Water Depths for High Water Productivity in Irrigated Lowland Rice Field by Employing Alternate Wetting and Drying Technique under Tropical Climate Conditions, Southern Taiwan. Paddy Water Environ. 2015, 13, 379–389. [Google Scholar] [CrossRef]
- Pascual, V.J.; Wang, Y. Utilizing Rainfall and Alternate Wetting and Drying Irrigation for High Water Productivity in Irrigated Lowland Paddy Rice in Southern Taiwan. Plant Prod. Sci. 2016, 20, 24–35. [Google Scholar] [CrossRef]
- Zoundou, S.J.P.; Chen, S.; Wang, Y. Comparison of Yields Attributes and Water Productivity under the System of Rice Intensification (SRI) in Southern Taiwan. In Proceedings of the 3rd World Irrigation Forum (WIF3), Bali, Indonesia, 1–7 September 2019; International Commission on Irrigation and Drainage (ICID): Bali, Indonesia, 2019; pp. 1–11. [Google Scholar]
- Schneider, H.M.; Lor, V.S.; Zhang, X.; Saengwilai, P.; Hanlon, M.T.; Klein, S.P.; Davis, J.L.; Borkar, A.N.; Depew, C.L.; Bennett, M.J.; et al. Transcription Factor BHLH121 Regulates Root Cortical Aerenchyma Formation in Maize. Proc. Natl. Acad. Sci. USA 2023, 120, e2219668120. [Google Scholar] [CrossRef] [PubMed]
- Barison, J.; Uphoff, N. Rice Yield and Its Relation to Root Growth and Nutrient-Use Efficiency under SRI and Conventional Cultivation: An Evaluation in Madagascar. Paddy Water Environ. 2011, 9, 65–78. [Google Scholar] [CrossRef]
- Ches, S.; Yamaji, E.; Tsurui, J. Comparison of System of Rice Intensification (SRI) Practices in Irrigated and Rainfed Areas of Cambodia. Int. J. Environ. Rural Dev. 2012, 3, 207–212. [Google Scholar]
- Uren, N.C. Types, Amounts, and Possible Functions of Compounds Released into the Rhizosphere by Soil-Grown Plants. In The Rhizosphere; CRC Press: Boca Raton, FL, USA, 2000; pp. 35–56. [Google Scholar]
- Raaijmakers, J.M.; Weller, D.M. Exploiting Genotypic Diversity of 2,4-Diacetylphloroglucinol-Producing Pseudomonas spp.: Characterization of Superior Root-Colonizing P. fluorescensStrain Q8r1-96. Appl. Environ. Microbiol. 2001, 67, 2545. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J.S. Bioformulation of Biochar as a Potential Inoculant Carrier for Sustainable Agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Reed, L.; Glick, B.R. The Recent Use of Plant-Growth-Promoting Bacteria to Promote the Growth of Agricultural Food Crops. Agriculture 2023, 13, 1089. [Google Scholar] [CrossRef]
- Berkhout, E.; Glover, D.; Kuyvenhoven, A. On-Farm Impact of the System of Rice Intensification (SRI): Evidence and Knowledge Gaps. Agric. Syst. 2015, 132, 157–166. [Google Scholar] [CrossRef]
- Raman, N.; Selvaraj, T.; Rai, M. Tripartite Relationship of Rhizobium, AMF, and Host in Growth Promotion. In Handbook of Microbial Biofertilizers; Rai, M.K., Ed.; The Haworth Press: New York, NY, USA, 2006; p. 51. [Google Scholar]
- Fang, X.; Lee, X.; Twagirayezu, G.; Cheng, H.; Lu, H.; Huang, S.; Deng, L.; Ji, B. A Critical Review of the Effectiveness of Biochar Coupled with Arbuscular Mycorrhizal Fungi in Soil Cadmium Immobilization. J. Fungi 2024, 10, 182. [Google Scholar] [CrossRef]
- Ortega, H.E.; Torres-Mendoza, D.; Cubilla-Rios, L. Patents on Endophytic Fungi for Agriculture and Bio-and Phytoremediation Applications. Microorganisms 2020, 8, 1237. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Abril-Urías, P.; Velasco, P. Endophytic Fungi as Direct Plant Growth Promoters for Sustainable Agricultural Production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Bharti, L.; Yadav, K.; Kumar Chaubey, A. Trichoderma Spp.: Approach for Bio-Control Agent. In Challenges in Plant Disease Detection and Recent Advancements; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A Novel Role for Trichoderma Secondary Metabolites in the Interactions with Plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Korobushkin, D.I.; Butenko, K.O.; Gongalsky, K.B.; Saifutdinov, R.A.; Zaitsev, A.S. Soil Nematode Communities in Temperate Rice-Growing Systems. Eur. J. Soil Biol. 2019, 93, 103099. [Google Scholar] [CrossRef]
- Khan, M.R.; Haque, Z.; Ahamad, F.; Shah, M.H. Nematode Problems in Rice and Their Sustainable Management. In Nematode Diseases of Crops and Their Sustainable Management; Academic Press: Cambridge, MA, USA, 2023; pp. 133–166. [Google Scholar]
- Clarholm, M. Interactions of Bacteria, Protozoa and Plants Leading to Mineralization of Soil Nitrogen. Soil Biol. Biochem. 1985, 17, 181–187. [Google Scholar] [CrossRef]
- Hahn, M.W.; Höfle, M.G. Grazing of Protozoa and Its Effect on Populations of Aquatic Bacteria. FEMS Microbiol. Ecol. 2001, 35, 113–121. [Google Scholar] [CrossRef]
- Panteleit, J.; Horgan, F.G.; Türke, M.; Schmidt, A.; Schädler, M.; Bacht, M.; Brandl, R.; Hotes, S. Effects of Detritivorous Invertebrates on the Decomposition of Rice Straw: Evidence from a Microcosm Experiment. Paddy Water Environ. 2018, 16, 279–286. [Google Scholar] [CrossRef]
- Jernigan, A.; Kao-Kniffin, J.; Pethybridge, S.; Wickings, K. Soil Microarthropod Effects on Plant Growth and Development. Plant Soil 2023, 483, 27–45. [Google Scholar] [CrossRef]
- Reilly, K.; Cavigelli, M.; Szlavecz, K. Agricultural Management Practices Impact Soil Properties More than Soil Microarthropods. Eur. J. Soil Biol. 2023, 117, 103516. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Piwowar, A.; Harasym, J. The Importance and Prospects of the Use of Algae in Agribusiness. Sustainability 2020, 12, 5669. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Paulsen, B.S.; Petersen, D.; Klaveness, D. Extracellular Carbohydrate Polymers from Five Desert Soil Algae with Different Cohesion in the Stabilization of Fine Sand Grain. Carbohydr. Polym. 2003, 54, 33–42. [Google Scholar] [CrossRef]
- He, Z.; Liang, J.; Lu, Y.; Yang, Q.; Lu, C.; Wu, D. Enhanced Soil Moisture Management Using Waste Green Algae-Derived Polymers: Optimization of Application Rate and Mixing Depth. Agronomy 2023, 13, 2335. [Google Scholar] [CrossRef]
- Zhang, X.; Koehler, H. Soil Algae for Combating Soil Degradation—Greenhouse Experiment with Different Soil Amendments. Soil Res. 2022, 61, 70–82. [Google Scholar] [CrossRef]
- Asadian, M.; Fakheri, B.A.; Mahdinezhad, N.; Gharanjik, S.; Beardal, J.; Talebi, A.F. Algal Communities: An Answer to Global Climate Change. CLEAN–Soil Air Water 2018, 48, 1800032. [Google Scholar] [CrossRef]
- Gallois, J.L.; Moury, B.; German-Retana, S. Role of the Genetic Background in Resistance to Plant Viruses. Int. J. Mol. Sci. 2018, 19, 2856. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant Viruses of Agricultural Importance: Current and Future Perspectives of Virus Disease Management Strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Tong, D.; Xu, J. Element Cycling by Environmental Viruses. Natl. Sci. Rev. 2024, 11, nwae459. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Mason-Jones, K. Viruses in Soil: Nano-Scale Undead Drivers of Microbial Life, Biogeochemical Turnover and Ecosystem Functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]
- Rai, M. Potential and Possible Uses of Bacterial and Fungal Biofertilizers. In Handbook of Microbial Biofertilizers; CRC Press: New York, NY, USA, 2006; pp. 26–56. [Google Scholar]
- Kumar, M.; Sharma, N.; Saxena, R.; Tomar, R.S. Uncultivable Soil Microbes Contributing to Sustainable Agriculture. In Rhizosphere Microbes: Biotic Stress Management; Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Sharma, S.K., Eds.; Springer Nature: Singapore, 2021; pp. 267–281. [Google Scholar] [CrossRef]
- Reddy, P.P. Plant Growth Promoting Rhizobacteria for Horticultural Crop Protection; Springer: New Delhi, India, 2014. [Google Scholar] [CrossRef]
- Lee, S.K.; Chiang, M.S.; Hseu, Z.Y.; Kuo, C.H.; Liu, C.T. A Photosynthetic Bacterial Inoculant Exerts Beneficial Effects on the Yield and Quality of Tomato and Affects Bacterial Community Structure in an Organic Field. Front. Microbiol. 2022, 13, 959080. [Google Scholar] [CrossRef]
- Budi Utama, S.P.; Sulistyowati, L.; Chang, P.P.C. Characterization of Plant Growth-Promoting Rhizobacteria (PGPR) from Saline Soil in Taiwan. IOP Conf. Ser. Earth Environ. Sci. 2021, 709, 012079. [Google Scholar] [CrossRef]
- Gou, Z.; Zheng, H.; He, Z.; Su, Y.; Chen, S.; Chen, H.; Chen, G.; Ma, N.L.; Sun, Y. The Combined Action of Biochar and Nitrogen-Fixing Bacteria on Microbial and Enzymatic Activities of Soil N Cycling. Environ. Pollut. 2023, 317, 120790. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Singh, N.; Behl, H.M.; Srivastava, S. Influence of Plant Growth Promoting Bacteria and Its Mutant on Heavy Metal Toxicity in Brassica Juncea Grown in Fly Ash Amended Soil. Chemosphere 2008, 72, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; Tringe, S.G. Interactions between Plants and Soil Shaping the Root Microbiome under Abiotic Stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef]
- Article, R. The Impact of Floods on the Structure and Functional Processes of Floodplain Ecosystems. J. Soil Plant Biol. 2019, 2019, 28–44. [Google Scholar] [CrossRef]
- Jjagwe, J.; Chelimo, K.; Karungi, J.; Komakech, A.J.; Lederer, J. Comparative Performance of Organic Fertilizers in Maize (Zea mays L.) Growth, Yield, and Economic Results. Agronomy 2020, 10, 69. [Google Scholar] [CrossRef]
- Manogaran, M.D.; Shamsuddin, R.; Mohd Yusoff, M.H.; Lay, M.; Siyal, A.A. A Review on Treatment Processes of Chicken Manure. Clean. Circ. Bioeconomy 2022, 2, 100013. [Google Scholar] [CrossRef]
- Anitha, S.; Chellappan, M. Comparison of the System of Rice Intensification (SRI), Recommended Practices, and Farmers’ Methods of Rice (Oryza sativa L.) Production in the Humid Tropics of Kerala, India. J. Trop. Agric. 2011, 49, 64–71. [Google Scholar]
- Kunnathadi, M.; Abraham, C.T.; George Thomas, C.; Girija, T. Comparative Evaluation of SRI with Conventional System in the Irrigated Rice Tracts of Kerala. J. Trop. Agric. 2015, 53, 8–16. [Google Scholar]
- Wayayok, A.; Soom, M.A.M.; Abdan, K.; Mohammed, U. Impact of Mulch on Weed Infestation in System of Rice Intensification (SRI) Farming. Agric. Agric. Sci. Procedia 2014, 2, 353–360. [Google Scholar] [CrossRef]
- Nakhro, N.; Dkhar, M.S. Impact of Organic and Inorganic Fertilizers on Microbial Populations and Biomass Carbon in Paddy Field Soil. J. Agron. 2010, 9, 102–110. [Google Scholar] [CrossRef]
- Xiong, L.; Drosos, M.; Wang, P.; Zhang, W.; Jin, W.; Wang, S.; Scopa, A.; Liu, Z.; Shao, C.; Sun, G.; et al. The Divergent Accumulation Mechanisms of Microbial Necromass C in Paddy Soil under Different Long-Term Fertilization Regimes. Geoderma 2023, 439, 116688. [Google Scholar] [CrossRef]
- Jou, Y.; Tarigan, E.J.; Prayogo, C.; Kobua, C.K.; Weng, Y.; Wang, Y. Effects of Sphingobium Yanoikuyae SJTF8 on Rice (Oryza Sativa) Seed Germination and Root Development. Agriculture 2022, 12, 1890. [Google Scholar] [CrossRef]
- Doni, F.; Suhaimi, N.S.M.; Irawan, B.; Mohamed, Z.; Mispan, M.S. Associations of Pantoea with Rice Plants: As Friends or Foes? Agriculture 2021, 11, 1278. [Google Scholar] [CrossRef]
- Morgenstern, E.; Okon, Y. The Effect of Azospirillum Brasilense and Auxin on Root Morphology in Seedlings of Sorghum Bicolor× Sorghum Sudanense. Arid. Soil Res. Rehabil. 1987, 1, 115–127. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and Molecular Responses of Arabidopsis Thaliana Roots as a Result of Inoculation with the Auxin-Producing Bacterium Azospirillum Brasilense. New Phytol. 2013, 201, 850–861. [Google Scholar] [CrossRef]
- Flores Olivas, A.; Cabello, A.; Olivas, F.; Portugal, O.; Valdés, A.; Alcalá, L. Evaluation of Bacillus Subtilis as Promoters of Plant Growth Evaluación de Cepas de Bacillus Subtilis Como Promotoras de Crecimiento Vegetal. Rev. Bio Ciencias 2019, 6, 418. [Google Scholar]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus Subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Han, Y.; Liu, E.; Liu, L.; Zhang, B.; Wang, Y.; Gui, M.; Wu, R.; Li, P. Rheological, Emulsifying and Thermostability Properties of Two Exopolysaccharides Produced by Bacillus Amyloliquefaciens LPL061. Carbohydr. Polym. 2015, 115, 230–237. [Google Scholar] [CrossRef]
- Kasotia, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. Amelioration of Soybean Plant from Saline-Induced Condition by Exopolysaccharide Producing Pseudomonas-Mediated Expression of High Affinity K+-Transporter (HKT1) Gene. Curr. Sci. 2016, 111, 1961–1967. [Google Scholar] [CrossRef]
- Shin, W.; Siddikee, M.A.; Joe, M.M.; Benson, A.; Kim, K.; Selvakumar, G.; Kang, Y.; Jeon, S.; Samaddar, S.; Chatterjee, P.; et al. Halotolerant Plant Growth Promoting Bacteria Mediated Salinity Stress Amelioration in Plants. Korean J. Soil Sci. Fertil. 2016, 49, 355–367. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schroth, M.N. Plant Growth-Promoting Rhizobacteria on Radishes. In Proceedings of the 4th International Conference on Plant Pathogenic Bactera; Station de Pathologie Vegetale et Phytobacteriologie. INRA: Angers, France, 1978; pp. 879–882. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Sheirdil, R.A.; Hayat, R.; Zhang, X.-X.; Abbasi, N.A.; Ali, S.; Ahmed, M.; Khattak, J.Z.K.; Ahmad, S. Exploring Potential Soil Bacteria for Sustainable Wheat (Triticum aestivum L.) Production. Sustainability 2019, 11, 3361. [Google Scholar] [CrossRef]
- Paliwoda, D.; Mikiciuk, G.; Mikiciuk, M.; Miller, T.; Kisiel, A.; Sas-Paszt, L.; Kozioł, A.; Brysiewicz, A. The Use of Plant Growth Promoting Rhizobacteria to Reduce Greenhouse Gases in Strawberry Cultivation under Different Soil Moisture Conditions. Agronomy 2023, 13, 754. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Mahmood, S.; Ahmad, M.; Ahmad, Z.; Javaid, A.; Ashraf, M. The Role of Mycorrhizae and Plant Growth Promoting Rhizobacteria (PGPR) in Improving Crop Productivity under Stressful Environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.A.; Song, J.; Choe, S.; Jang, G.; Kim, Y. Plant Growth-Promoting Rhizobacterium Bacillus Megaterium Modulates the Expression of Antioxidant-Related and Drought-Responsive Genes to Protect Rice (Oryza sativa L.) from Drought. Front. Microbiol. 2024, 15, 1430546. [Google Scholar] [CrossRef]
- Figueiredo, M.D.V.B.; Bonifacio, A.; Rodrigues, A.C.; de Araujo, F.F. Plant Growth-Promoting Rhizobacteria: Key Mechanisms of Action. In Microbial-Mediated Induced Systemic Resistance in Plants; Springer Nature Singapore: Singapore, 2016; pp. 22–37. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, V.K.; Gautam, H.; Rathod, L.; Chundawat, R.S.; Singh, G.; Verma, R.K.; Sahoo, D.K.; Patel, A. The Role of Plant Growth Promoting Rhizobacteria in Strengthening Plant Resistance to Fluoride Toxicity: A Review. Front. Microbiol. 2023, 14, 1271034. [Google Scholar] [CrossRef]
- Kaleh, A.M.; Singh, P.; Ooi Chua, K.; Harikrishna, J.A. Modulation of Plant Transcription Factors and Priming of Stress Tolerance by Plant Growth-Promoting Bacteria: A Systematic Review. Ann. Bot. 2024, 135, 387–402. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Gallego-Giraldo, C.; Forner-Giner, M.Á.; Ortells-Fabra, R.; Urbaneja, A. Plant Volatile-Triggered Defense in Citrus against Biotic Stressors. Front. Plant Sci. 2024, 15, 1425364. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery upon PGPR Augmentation in Plants under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef]
- Banayo, N.P.M.; Cruz, P.C.S.; Aguilar, E.A.; Badayos, R.B.; Haefele, S.M. Evaluation of Biofertilizers in Irrigated Rice: Effects on Grain Yield at Different Fertilizer Rates. Agriculture 2012, 2, 73–86. [Google Scholar] [CrossRef]
- Midrarullah, M.; Bashir Ahmed, B.A.; Mirza, M.S. Response of Rice to Inoculation with Plant Growth Promoting Rhizobacteria in Control Lab Environment and Field Experiment. Pak. J. Bot. 2014, 46, 1121–1124. [Google Scholar]
- Environ, P.S.; Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Enhancing Grain Iron Content of Rice by the Application of Plant Growth Promoting Rhizobacteria. Plant Soil Environ. 2013, 59, 89–94. [Google Scholar]
- Cavite, H.J.M.; Mactal, A.G.; Evangelista, E.V.; Cruz, J.A. Growth and Yield Response of Upland Rice to Application of Plant Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2021, 40, 494–508. [Google Scholar] [CrossRef]
- Daneshian, J.; Pirdashti, H. Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Leaf Area Duration (LAD) Dynamics of Rice (Oryza sativa L.) Plants under Nitrogen and Water Limited Conditions. Res. Crops 2013, 14, 345–349. [Google Scholar]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of Salt-Tolerant Plant Growth-Promoting Rhizobacteria and the Effect on Growth and Yield of Saline-Affected Rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef]
- Hafez, E.M.; Alsohim, A.S.; Farig, M.; Omara, A.E.D.; Rashwan, E.; Kamara, M.M. Synergistic Effect of Biochar and Plant Growth Promoting Rhizobacteria on Alleviation of Water Deficit in Rice Plants under Salt-Affected Soil. Agronomy 2019, 9, 847. [Google Scholar] [CrossRef]
- Devi Priya, A.; Kalaiselvi, T. Evaluating the Effect of Sphingobium Yanoikuyae MH394206 and Mixed Consortia on Growth of Rice CO 51 in Moisture Deficit Condition. J. Pharmacogn. Phytochem. 2021, 9, 2016–2021. [Google Scholar] [CrossRef]
- Elita, N.; Erlinda, R.; Agustamar, A. The Effect of Bioorganic Dosage with N, P Fertilizer on Rice Production of Sri Methods and Increased Nutrient Content of Paddy Soil Intensification. J. Appl. Agric. Sci. Technol. 2020, 4, 155–169. [Google Scholar] [CrossRef]
- Tarigan, E.J.; Prayogo, C.; Weng, Y.-T.; Kobua, C.K.; Jou, Y.-T.; Wang, Y.-M. Influence of Rhizobacteria on Soil Ion Concentration under Paddy Cultivation. AGRIVITA J. Agric. Sci. 2021, 43, 430–439. [Google Scholar] [CrossRef]
- Kobua, C.K.; Jou, Y.T.; Wang, Y.M. Advantages of Amending Chemical Fertilizer with Plant-Growth-Promoting Rhizobacteria under Alternate Wetting Drying Rice Cultivation. Agriculture 2021, 11, 605. [Google Scholar] [CrossRef]
- Mohamad, H.R.; Zulkarami, B.; Halimi, M.S. Effects of Inoculation of Plant Growth Promoting Rhizobacteria to Minimize Panicle Grain Shattering Habit for Increased Yield of Rice (Oryza sativa L.). Afr. J. Microbiol. Res. 2019, 13, 256–263. [Google Scholar] [CrossRef]
- Shen, F.T.; Yen, J.H.; Liao, C.S.; Chen, W.C.; Chao, Y.T. Screening of Rice Endophytic Biofertilizers with Fungicide Tolerance and Plant Growth-Promoting Characteristics. Sustainability 2019, 11, 1133. [Google Scholar] [CrossRef]
- Kannan, V.R.; Bastas, K.K. Sustainable Approaches to Controlling Plant Pathogenic Bacteria; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Jha, C.K.; Aeron, A.; Patel, B.V.; Maheshwari, D.K.; Saraf, M. Enterobacter: Role in Plant Growth Promotion. In Bacteria in Agrobiology: Plant Growth Responses; Maheshwari, D.K., Ed.; Springer: London, UK, 2011; pp. 159–182. [Google Scholar] [CrossRef]
- Malacrinò, A.; Sadowski, V.A.; Martin, T.K.; De Oliveira, N.C.; Brackett, I.J.; Feller, J.D.; Harris, K.J.; Heredia, O.C.; Vescio, R.; Bennett, A.E. Biological Invasions Alter Environmental Microbiomes: A Meta-Analysis. PLoS ONE 2020, 15, e0240996. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; Enshasy, H.E. Plant Growth Promoting Rhizobacteria (Pgpr) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Colmer, T.D.; Pierik, R.; Millenaar, F.F.; Peeters, A.J.M. How Plants Cope with Complete Submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef]
- Tamang, B.G.; Fukao, T. Plant Adaptation to Multiple Stresses during Submergence and Following Desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef]
- Barkovskii, A.; Bouillant, M.L.; Monrozier, L.J.; Balandreau, J. Azospirillum Strains Use Phenolic Compounds as Intermediates for Electron Transfer under Oxygen-Limiting Conditions. Microb. Ecol. 1995, 29, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kima, A.S.; Chung, W.G.; Wang, Y.M. Improving Irrigated Lowland Rice Water Use Efficiency under Saturated Soil Culture for Adoption in Tropical Climate Conditions. Water 2014, 6, 2830–2846. [Google Scholar] [CrossRef]
- Pascual, V.J.; Wang, Y. Impact of Water Management on Rice Varieties, Yield, and Water Productivity under the System of Rice Intensification in Southern Taiwan. Water 2017, 9, 3. [Google Scholar] [CrossRef]
- Liu, L.-W.; Hsieh, S.-H.; Lin, S.-J.; Wang, Y.-M.; Lin, W.-S. Rice Blast (Magnaporthe oryzae) Occurrence Prediction and the Key Factor Sensitivity Analysis by Machine Learning. Agronomy 2021, 11, 771. [Google Scholar] [CrossRef]
- Zhi, W.; Ge, Z.; He, Z.; Zhang, H. Methods for Understanding Microbial Community Structures and Functions in Microbial Fuel Cells: A Review. Bioresour. Technol. 2014, 171, 461–468. [Google Scholar] [CrossRef]
- Ojala, T.; Häkkinen, A.E.; Kankuri, E.; Kankainen, M. Current Concepts, Advances, and Challenges in Deciphering the Human Microbiota with Metatranscriptomics. Trends Genet. 2023, 39, 686–702. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiong, X.; Danska, J.; Parkinson, J. Metatranscriptomic Analysis of Diverse Microbial Communities Reveals Core Metabolic Pathways and Microbiomespecific Functionality. Microbiome 2016, 4, 2. [Google Scholar] [CrossRef]
- Singer, G.A.C.; Fahner, N.A.; Barnes, J.G.; McCarthy, A.; Hajibabaei, M. Comprehensive Biodiversity Analysis via Ultra-Deep Patterned Flow Cell Technology: A Case Study of EDNA Metabarcoding Seawater. Sci. Rep. 2019, 9, 5991. [Google Scholar] [CrossRef]
- Klymus, K.E.; Marshall, N.T.; Stepien, C.A. Environmental DNA (EDNA) Metabarcoding Assays to Detect Invasive Invertebrate Species in the Great Lakes. PLoS ONE 2017, 12, e0177643. [Google Scholar] [CrossRef]
- Schneider, J.; Valentini, A.; Dejean, T.; Montarsi, F.; Taberlet, P.; Glaizot, O.; Fumagalli, L. Detection of Invasive Mosquito Vectors Using Environmental DNA (EDNA) from Water Samples. PLoS ONE 2016, 11, e0162493. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobua, C.K.; Wang, Y.-M.; Jou, Y.-T. Exploring the Roles of Plant Growth-Promoting Rhizobacteria (PGPR) and Alternate Wetting and Drying (AWD) in Sustainable Rice Cultivation. Soil Syst. 2025, 9, 61. https://doi.org/10.3390/soilsystems9020061
Kobua CK, Wang Y-M, Jou Y-T. Exploring the Roles of Plant Growth-Promoting Rhizobacteria (PGPR) and Alternate Wetting and Drying (AWD) in Sustainable Rice Cultivation. Soil Systems. 2025; 9(2):61. https://doi.org/10.3390/soilsystems9020061
Chicago/Turabian StyleKobua, Chesly Kit, Yu-Min Wang, and Ying-Tzy Jou. 2025. "Exploring the Roles of Plant Growth-Promoting Rhizobacteria (PGPR) and Alternate Wetting and Drying (AWD) in Sustainable Rice Cultivation" Soil Systems 9, no. 2: 61. https://doi.org/10.3390/soilsystems9020061
APA StyleKobua, C. K., Wang, Y.-M., & Jou, Y.-T. (2025). Exploring the Roles of Plant Growth-Promoting Rhizobacteria (PGPR) and Alternate Wetting and Drying (AWD) in Sustainable Rice Cultivation. Soil Systems, 9(2), 61. https://doi.org/10.3390/soilsystems9020061






