Biochar and Kitchen Stove Ash for Improving Nutrient Availability and Microbial Functions of Tropical Acidic Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling, Lime Requirement, and CaCO3 Determination
2.2. Incubation Experiment and Basal Respiration
2.3. Microbial Biomass Carbon and Nitrogen
2.4. Determination of Extracellular Enzyme Activities
2.5. Soil pH, Mineral N, Plant Available P, and Total Soil Nutrients
2.6. Statistical Analysis
3. Results
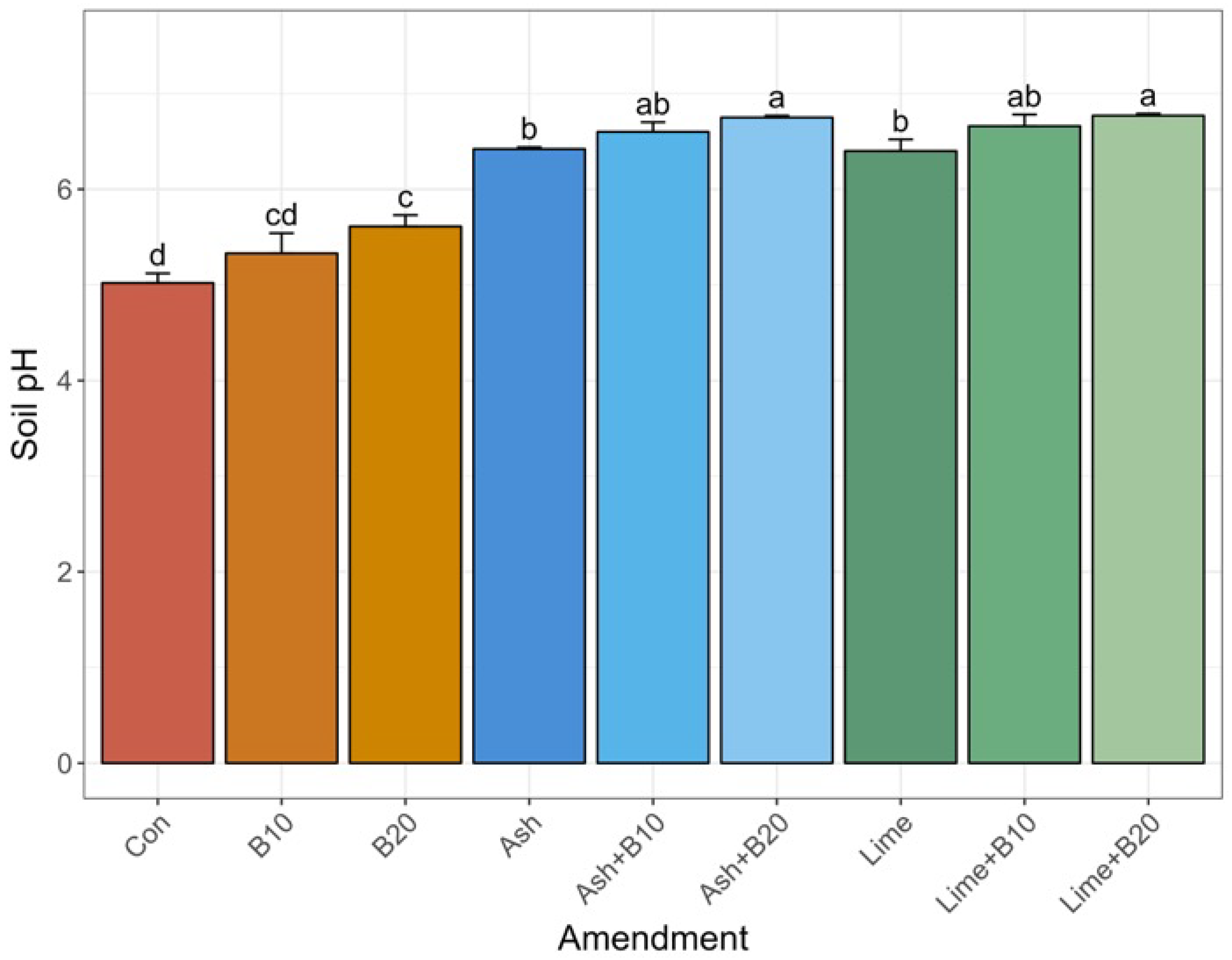
3.1. Treatment Effect on Soil pH
3.2. Mineral Nitrogen and Phosphorus Concentrations
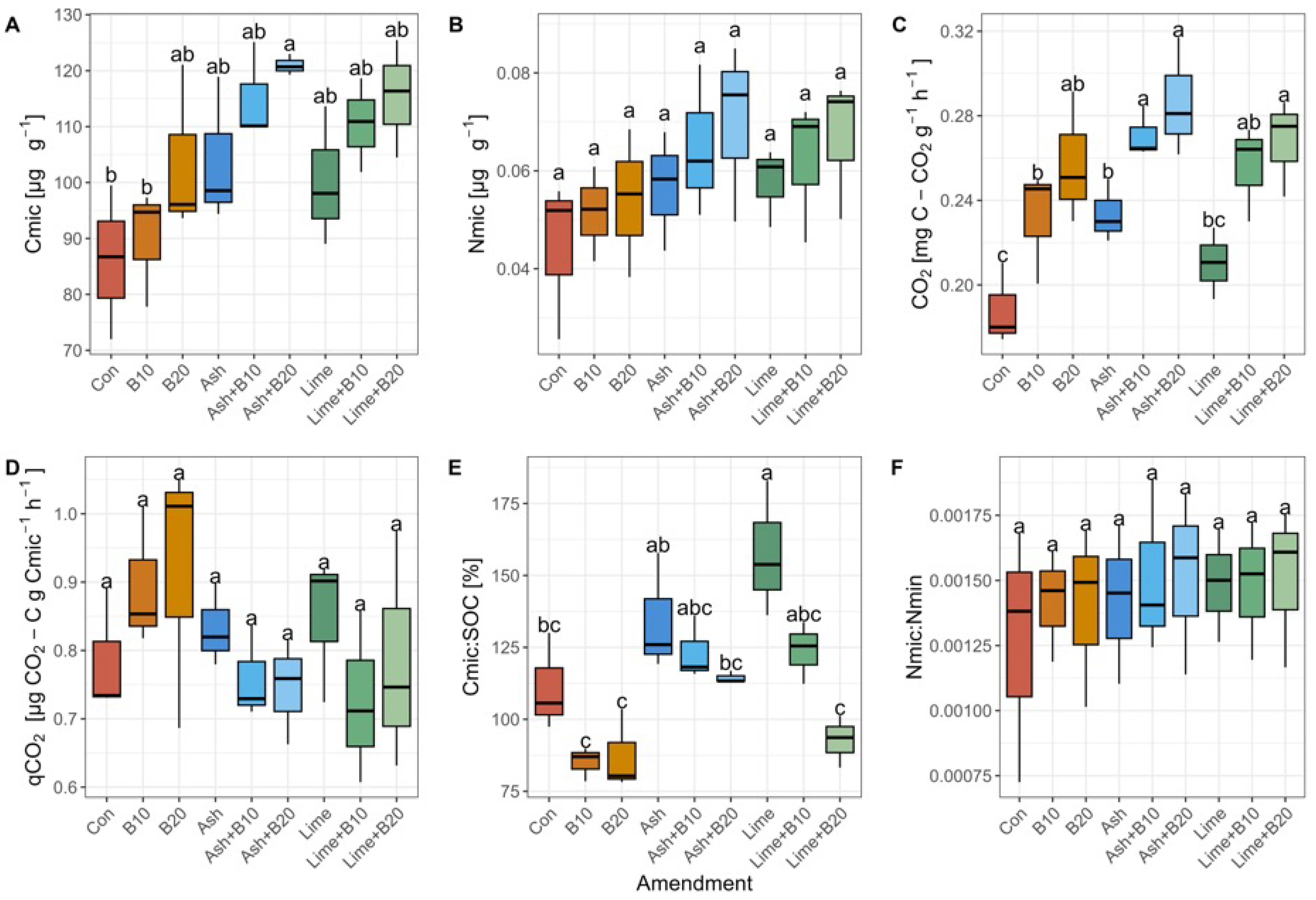
3.3. Microbial Biomass Carbon and Nitrogen, CO2 Emissions, and Metabolic Quotient
3.4. Activities of Soil’s Extracellular Enzymes
3.5. Soil Carbon and Nutrient Composition
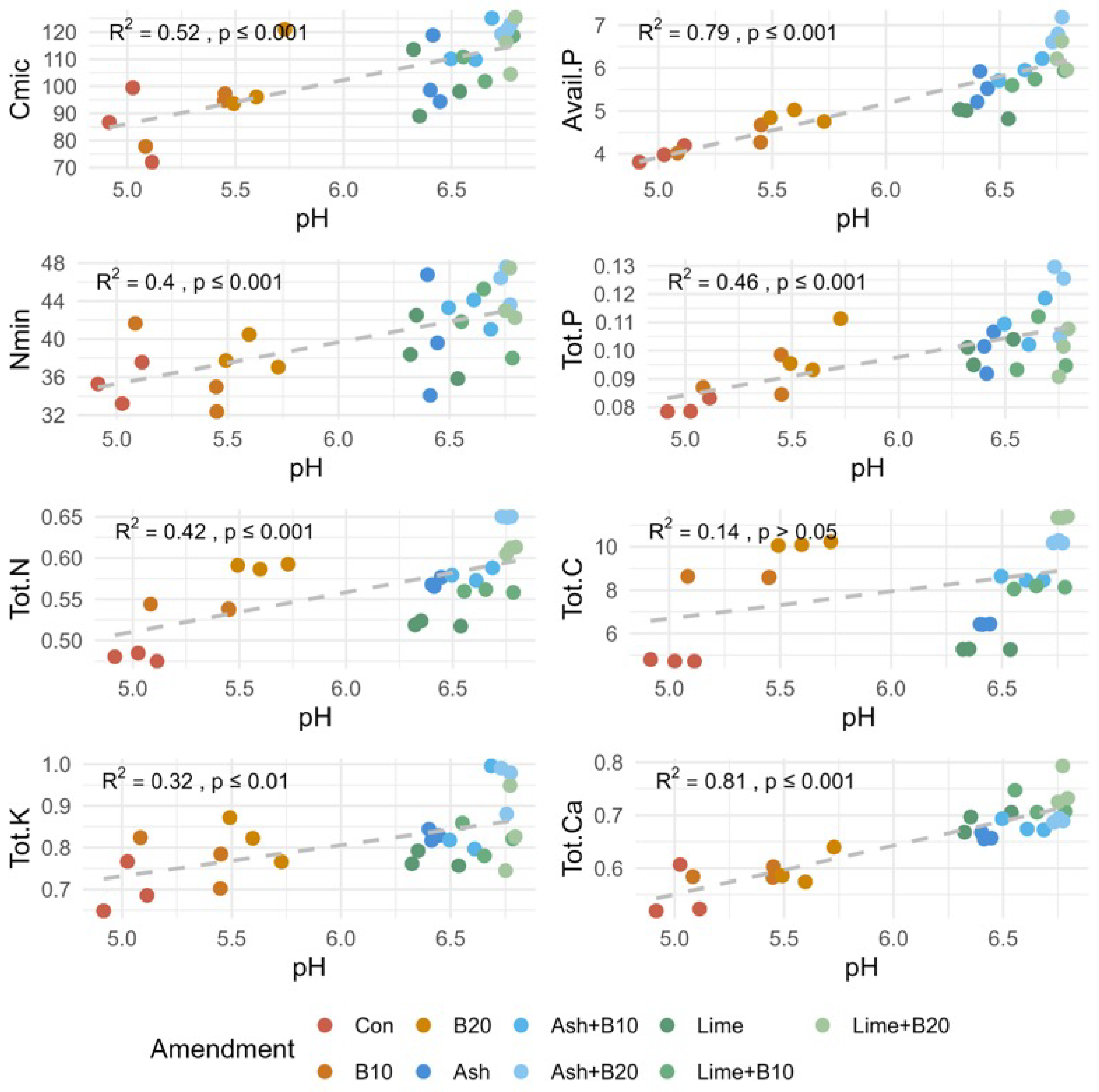
3.6. Principal Component (PCA) and Correlation Analysis
4. Discussion
4.1. Amelioration of Soil Acidity
4.2. Bioavailability of Phosphorus and Nitrogen
4.3. Microbial Biomass Carbon and CO2 Emissions
4.4. Extracellular Enzyme Activities
4.5. Treatment Effect on Total Carbon and Nutrient Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takow, J.A.; Doumbia, M.D.; Hossner, L.R. Acid soil profiles of the semiarid and subhumid tropics in Central and West Africa. In Plant-Soil Interactions at Low pH; Wright, R.J., Baligar, V.C., Murrmann, R.P., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 313–320. ISBN 978-94-010-5520-8. [Google Scholar]
- Sanchez, P.A. Properties and Management of Soils in the Tropics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2019; ISBN 9781316809785. [Google Scholar]
- Sanchez, P.A. Soil fertility and hunger in Africa. Science 2002, 295, 2019–2020. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F. Ameliorating soil acidity in Ghana: A concise review of approaches. ARPN J. Sci. Technol. 2012, 2, 1–9. [Google Scholar]
- Tully, K.; Sullivan, C.; Weil, R.; Sanchez, P. The State of Soil Degradation in Sub-Saharan Africa: Baselines, Trajectories, and Solutions. Sustainability 2015, 7, 6523–6552. [Google Scholar] [CrossRef]
- Buri, M.M.; Issaka, R.N.; Wakatsuki, T. Extent and Management of Low pH Soils in Ghana. J. Soil Sci. Plant Nutr. 2005, 51, 755–759. [Google Scholar] [CrossRef]
- Gupta, A.K.; Maheshwari, A.; Khanam, R. Assessment of phosphorus fixing capacity in different soil orders of India. J. Plant Nutr. 2020, 43, 2395–2401. [Google Scholar] [CrossRef]
- Fageria, N.K.; Nascente, A.S. Management of Soil Acidity of South American Soils for Sustainable Crop Production. In Management of Soil Acidity of South American Soils for Sustainable Crop Production; Fageria, N.K., Nascente, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 221–275. ISBN 9780128021392. [Google Scholar]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manage. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Holland, J.E.; White, P.J.; Glendining, M.J.; Goulding, K.W.T.; McGrath, S.P. Yield responses of arable crops to liming—An evaluation of relationships between yields and soil pH from a long-term liming experiment. Eur. J. Agron. 2019, 105, 176–188. [Google Scholar] [CrossRef]
- Buni, A. Effects of Liming Acidic Soils on Improving Soil Properties and Yield of Haricot Bean. J. Environ. Anal. Toxicol 2014, 5, 1. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Cerutti, P.O.; Sola, P.; Chenevoy, A.; Iiyama, M.; Yila, J.; Zhou, W.; Djoudi, H.; Atyi, R.E.; Gautier, D.J.; Gumbo, D.; et al. The socioeconomic and environmental impacts of wood energy value chains in Sub-Saharan Africa: A systematic map protocol. Environ. Evid. 2015, 4, 12. [Google Scholar] [CrossRef]
- Neina, D.; Faust, S.; Joergensen, R.G. Characterization of charcoal and firewood ash for use in African peri-urban agriculture. Chem. Biol. Technol. Agric. 2020, 7, 5. [Google Scholar] [CrossRef]
- Symanowicz, B.; Becher, M.; Jaremko, D.; Skwarek, K. Possibilities for the Use of Wood Ashes in Agriculture. J. Ecol. Eng. 2018, 19, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Onwuka, M.I.; Osodeke, V.E. Effects of agricultural wastes ash on nutrients concentration and yield of maize (Zea mays L.) in Haplic Acrisol of Southeastern Nigeria. Agro-Sci. 2015, 11, 1–11. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Mair, J.; Etoa, F.X.; Insam, H. Composts with wood ash addition: A risk or a chance for ameliorating acid tropical soils? Geoderma 2009, 153, 402–407. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management; Routledge: Oxfordshire, UK, 2015; ISBN 9780203762264. [Google Scholar]
- Joseph, S.; Cowie, A.L.; van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Amoakwah, E.; Rahman, M.A.; Islam, K.R.; Frimpong, K.A.; Phares, C.A.; Sackey, L.; Asirifi, I.; Arthur, E. Increased humic materials explain aggregate-protected carbon and nitrogen accumulation in biochar-amended tropical soils. Pedosphere 2024, 34, 1086–1099. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 53001. [Google Scholar] [CrossRef]
- Yeboah, E.; Asamoah, G.; Kofi, B.; Abunyewa, A.A. Effect of Biochar Type and Rate of Application on Maize Yield Indices and Water Use Efficiency on an Ultisol in Ghana. Energy Procedia 2016, 93, 14–18. [Google Scholar] [CrossRef]
- Steiner, C.; Bellwood-Howard, I.; Häring, V.; Tonkudor, K.; Addai, F.; Atiah, K.; Abubakari, A.H.; Kranjac-Berisavljevic, G.; Marschner, B.; Buerkert, A. Participatory trials of on-farm biochar production and use in Tamale, Ghana. Agron. Sustain. Dev. 2018, 38, 12. [Google Scholar] [CrossRef]
- Frimpong, K.A.; Phares, C.A.; Boateng, I.; Abban-Baidoo, E.; Apuri, L. One-time application of biochar influenced crop yield across three cropping cycles on tropical sandy loam soil in Ghana. Heliyon 2021, 7, e06267. [Google Scholar] [CrossRef]
- Galinato, S.P.; Yoder, J.K.; Granatstein, D. The economic value of biochar in crop production and carbon sequestration. Energy Policy 2011, 39, 6344–6350. [Google Scholar] [CrossRef]
- WRB (World reference base for soil resources 2014). International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Shoemaker, H.E.; McLean, E.O.; Pratt, P.F. Buffer Methods for Determining Lime Requirement of Soils With Appreciable Amounts of Extractable Aluminum. Soil Sci. Soc. Am. J. 1961, 25, 274–277. [Google Scholar] [CrossRef]
- Ziadi, N.; Sen Tran, T. Lime requirement. In Soil Sampling and Methods of Analysis Carter, 2nd ed.; Martin, R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: London, UK, 2008; ISBN 9780849335860. [Google Scholar]
- Erich, M.S.; Ohno, T. Titrimetric determination of calcium carbonate equivalence of wood ash. Analyst 1992, 117, 993. [Google Scholar] [CrossRef]
- Horváth, B.; Opara-Nadi, O.; Beese, F. A Simple Method for Measuring the Carbonate Content of Soils. Soil Sci. Soc. Am. J. 2005, 69, 1066–1068. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Marx, M.-C.; Wood, M.; Jarvis, S. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- OriginLab Corporation. OriginPro, Version 2022b; OriginLab Corporation: Northampton, MA, USA, 2022. [Google Scholar]
- Blume, H.-P.; Brümmer, G.W.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Schad, P.; Stahr, K.; Wilke, B.-M. Scheffer/Schachtschabel Soil Science; Springer: Berlin, Heidelberg, 2016; ISBN 9783642309410. [Google Scholar]
- Briedis, C.; Sá, J.C.d.M.; Caires, E.F.; Navarro, J.d.F.; Inagaki, T.M.; Boer, A.; Ferreira, A.d.O.; Neto, C.Q.; Canalli, L.B.; dos Santos, J.B. Changes in Organic Matter Pools and Increases in Carbon Sequestration in Response to Surface Liming in an Oxisol under Long-Term No-Till. Soil Sci. Soc. Am. J. 2012, 76, 151–160. [Google Scholar] [CrossRef]
- Akanji, M.A.; Ahmad, M.; Al-Wabel, M.I.; Al-Farraj, A.S.F. Soil Phosphorus Fractionation and Bio-Availability in a Calcareous Soil as Affected by Conocarpus Waste Biochar and Its Acidified Derivative. Agriculture 2022, 12, 2157. [Google Scholar] [CrossRef]
- Antoniadis, V.; Hatzis, F.; Bachtsevanidis, D.; Koutroubas, S.D. Phosphorus Availability in Low-P and Acidic Soils as Affected by Liming and P Addition. Commun. Soil Sci. Plant Anal. 2015, 46, 1288–1298. [Google Scholar] [CrossRef]
- Mkhonza, N.P.; Buthelezi-Dube, N.N.; Muchaonyerwa, P. Effects of lime application on nitrogen and phosphorus availability in humic soils. Sci. Rep. 2020, 10, 8634. [Google Scholar] [CrossRef] [PubMed]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Rønn, R.; Kjøller, R.; Hansen, H.C.B.; Jacobsen, C.S. Wood Ash Induced pH Changes Strongly Affect Soil Bacterial Numbers and Community Composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef]
- Asirifi, I.; Werner, S.; Heinze, S.; Saba, C.K.; Lawson, I.Y.; Marschner, B. Short-term effect of biochar on microbial biomass, respiration and enzymatic activities in wastewater irrigated soils in urban agroecosystems of the West African savannah. Agronomy 2021, 11, 271. [Google Scholar] [CrossRef]
- Grover, S.P.; Butterly, C.R.; Wang, X.; Gleeson, D.B.; Macdonald, L.M.; Tang, C. Liming and priming: The long-term impact of pH amelioration on mineralisation may negate carbon sequestration gains. Soil Secur. 2021, 3, 100007. [Google Scholar] [CrossRef]
- Shah, T.; Khan, S.; Shah, Z. Soil respiration, pH and EC as influenced by biochar. Soil Environ. 2017, 36, 77–83. [Google Scholar] [CrossRef]
- Oladele, S.; Adeyemo, A.; Adegaiye, A.; Awodun, M. Effects of biochar amendment and nitrogen fertilization on soil microbial biomass pools in an Alfisol under rain-fed rice cultivation. Biochar 2019, 1, 163–176. [Google Scholar] [CrossRef]
- Inamdar, A.; Sangawe, V.; Adhapure, N. Enzymes in rhizosphere engineering. In Rhizosphere Engineering; Elsevier Academic Press: Amsterdam, The Netherlands, 2022; ISBN 978-0-323-89973-4. [Google Scholar]
- Kaurin, A.; Cernilogar, Z.; Lestan, D. Revitalisation of metal-contaminated, EDTA-washed soil by addition of unpolluted soil, compost and biochar: Effects on soil enzyme activity, microbial community composition and abundance. Chemosphere 2018, 193, 726–736. [Google Scholar] [CrossRef]
- Lasota, J.; Babiak, T.; Błońska, E. C:N:P stoichiometry associated with biochar in forest soils at historical charcoal production sites in Poland. Geoderma Reg. 2022, 28, e00482. [Google Scholar] [CrossRef]
- Wade, J.; Li, C.; Vollbracht, K.; Hooper, D.G.; Wills, S.A.; Margenot, A.J. Prescribed pH for soil β-glucosidase and phosphomonoesterase do not reflect pH optima. Geoderma 2021, 401, 115161. [Google Scholar] [CrossRef]
- Wang, H.; Ren, T.; Müller, K.; van Zwieten, L.; Wang, H.; Feng, H.; Xu, C.; Yun, F.; Ji, X.; Yin, Q.; et al. Soil type regulates carbon and nitrogen stoichiometry and mineralization following biochar or nitrogen addition. Sci. Total. Environ. 2021, 753, 141645. [Google Scholar] [CrossRef] [PubMed]
- Ndabankulu, K.; Egbewale, S.O.; Tsvuura, Z.; Magadlela, A. Soil microbes and associated extracellular enzymes largely impact nutrient bioavailability in acidic and nutrient poor grassland ecosystem soils. Sci. Rep. 2022, 12, 12601. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Shar, A.G.; Liu, J.; Chen, Y.; Chu, S.; Tian, X. Enhancing organic and inorganic carbon sequestration in calcareous soil by the combination of wheat straw and wood ash and/or lime. PLoS ONE 2018, 13, e0205361. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Camps-Arbestain, M.; Hedley, M. The fate of phosphorus of ash-rich biochars in a soil-plant system. Plant Soil 2014, 375, 61–74. [Google Scholar] [CrossRef]
- Asirifi, I.; Kaetzl, K.; Werner, S.; Heinze, S.; Abagale, F.K.; Wichern, M.; Marschner, B. Biochar for wastewater treatment and soil improvement in irrigated urban agriculture: Single and combined effects on crop yields and soil fertility. J. Soil Sci. Plant Nutr. 2023, 23, 1408–1420. [Google Scholar] [CrossRef]
- Paramisparam, P.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Johan, P.D.; Hamidi, N.H. Co-Application of Charcoal and Wood Ash to Improve Potassium Availability in Tropical Mineral Acid Soils. Agronomy 2021, 11, 2081. [Google Scholar] [CrossRef]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’Ng, H.Y. Combined Use of Charcoal, Sago Bark Ash, and Urea Mitigate Soil Acidity and Aluminium Toxicity. Agronomy 2021, 11, 1799. [Google Scholar] [CrossRef]



| Parameter | Unit | Ash | Biochar |
|---|---|---|---|
| pH | 11.01 ± 1.32 | 7.37 ± 1.10 | |
| TC | % | 14.86 ± 1.55 | 84.93 ± 3.76 |
| TIC | % | 6.46 ± 0.02 | 0.03 ± 0.00 |
| SOC | % | 0.42 ± 0.01 | 76.65 ± 2.67 |
| N | % | 0.51 ± 0.02 | 0.73 ± 0.06 |
| Al | g kg−1 | 12.18 ± 1.93 | 2.60 ± 0.87 |
| Ca | g kg−1 | 217.57 ± 12.42 | 9.32 ± 1.43 |
| P | g kg−1 | 9.28 ± 1.35 | 1.63 ± 0.21 |
| K | g kg−1 | 97.21 ± 14.20 | 9.56 ± 1.01 |
| Mg | g kg−1 | 24.87 ± 3.26 | 5.60 ± 1.02 |
| Fe | g kg−1 | 6.21 ± 1.65 | 1.61 ± 0.58 |
| Na | g kg−1 | 2.25 ± 0.64 | 0.97 ± 0.04 |
| Mn | g kg−1 | 0.61 ± 0.01 | 0.53 ± 0.01 |
| Zn | g kg−1 | 0.10 ± 0.04 | 0.14 ± 0.06 |
| Cu | g kg−1 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| C | N | P | K | Ca | Al | Fe | |
|---|---|---|---|---|---|---|---|
| Amendment | [g kg−1] | ||||||
| Con | 4.75 ± 0.44 d | 0.48 ± 0.05 a | 0.08 ± 0.01 b | 0.70 ± 0.06 c | 0.55 ± 0.05 d | 4.85 ± 0.37 ab | 14.15 ± 0.19 a |
| B10 | 8.61 ± 0.96 b | 0.54 ± 0.02 a | 0.09 ± 0.01 ab | 0.77 ± 0.04 bc | 0.59 ± 0.04 d | 4.56 ± 0.32 abc | 13.86 ± 0.24 a |
| B20 | 10.13 ± 1.10 a | 0.59 ± 0.08 a | 0.10 ± 00 ab | 0.82 ± 0.0 bb | 0.60 ± 0.02 cd | 4.34 ± 0.14 bc | 14.92 ± 0.36 a |
| Akw | 6.43 ± 0.70 cd | 0.57 ± 0.04 a | 0.10 ± 0.01 ab | 0.83 ± 0.06 ab | 0.66 ± 0.02 bc | 5.28 ± 0.08 a | 14.71 ± 1.15 a |
| Lime | 5.28 ± 0.29 d | 0.52 ± 0.05 a | 0.10 ± 0.01 ab | 0.77 ± 0.10 bc | 0.69 ± 0.01 ab | 4.46 ± 0.38 bc | 14.81 ± 0.97 a |
| Ash + B10 | 8.52 ± 0.28 b | 0.58 ± 0.01 a | 0.11 ± 0.01 ab | 0.87 ± 0.11 ab | 0.68 ± 0.03 bc | 3.89 ± 0.33 c | 14.10 ± 0.54 a |
| Lime + B10 | 8.13 ± 0.10 bc | 0.56 ± 0.03 a | 0.10 ± 0.01 ab | 0.82 ± 0.02 ab | 0.72 ± 0.01 ab | 4.49 ± 0.17 bc | 15.48 ± 0.73 a |
| Ash + B20 | 10.22 ± 0.66 a | 0.65 ± 0.06 a | 0.12 ± 0.01 a | 0.95 ± 0.01 a | 0.69 ± 0.01 ab | 4.89 ± 0.14 c | 14.12 ± 0.55 a |
| Lime + B20 | 11.37 ± 0.10 a | 0.61 ± 0.03 a | 0.10 ± 0.01 ab | 0.84 ± 0.06 ab | 0.75 ± 0.01 a | 4.60 ± 0.16 abc | 14.76 ± 0.87 a |
| p-value | p < 0.05 | p > 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asirifi, I.; Makarowsky, L.; Heinze, S.; Herre, M.; Werner, S.; Frimpong, K.A.; Pierburg, R.; Marschner, B. Biochar and Kitchen Stove Ash for Improving Nutrient Availability and Microbial Functions of Tropical Acidic Soil. Soil Syst. 2025, 9, 49. https://doi.org/10.3390/soilsystems9020049
Asirifi I, Makarowsky L, Heinze S, Herre M, Werner S, Frimpong KA, Pierburg R, Marschner B. Biochar and Kitchen Stove Ash for Improving Nutrient Availability and Microbial Functions of Tropical Acidic Soil. Soil Systems. 2025; 9(2):49. https://doi.org/10.3390/soilsystems9020049
Chicago/Turabian StyleAsirifi, Isaac, Lars Makarowsky, Stefanie Heinze, Michael Herre, Steffen Werner, Kwame Agyei Frimpong, Robin Pierburg, and Bernd Marschner. 2025. "Biochar and Kitchen Stove Ash for Improving Nutrient Availability and Microbial Functions of Tropical Acidic Soil" Soil Systems 9, no. 2: 49. https://doi.org/10.3390/soilsystems9020049
APA StyleAsirifi, I., Makarowsky, L., Heinze, S., Herre, M., Werner, S., Frimpong, K. A., Pierburg, R., & Marschner, B. (2025). Biochar and Kitchen Stove Ash for Improving Nutrient Availability and Microbial Functions of Tropical Acidic Soil. Soil Systems, 9(2), 49. https://doi.org/10.3390/soilsystems9020049









