Aridification Inhibits the Release of Dissolved Organic Carbon from Alpine Soils in Southwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Analysis Methods
3. Results
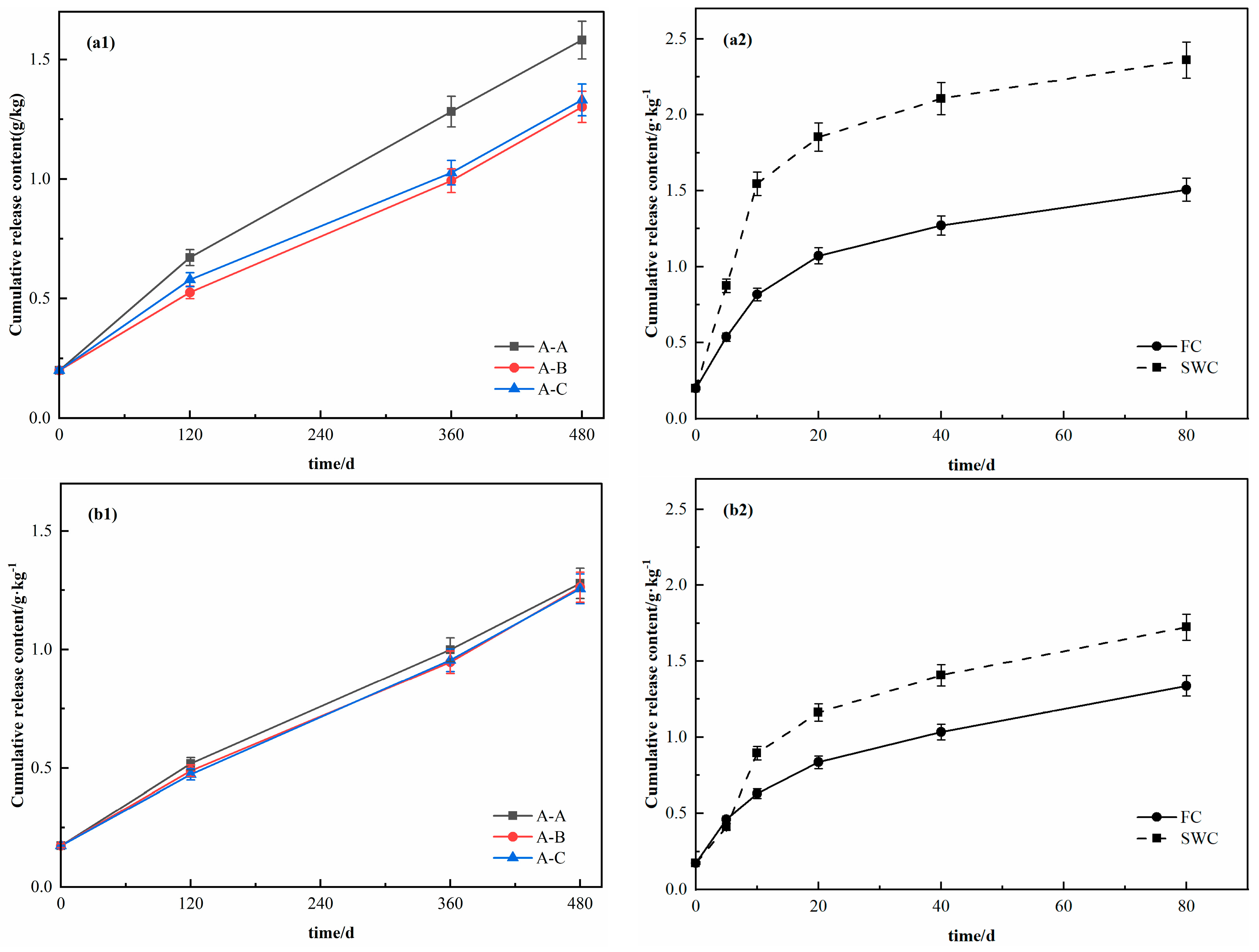
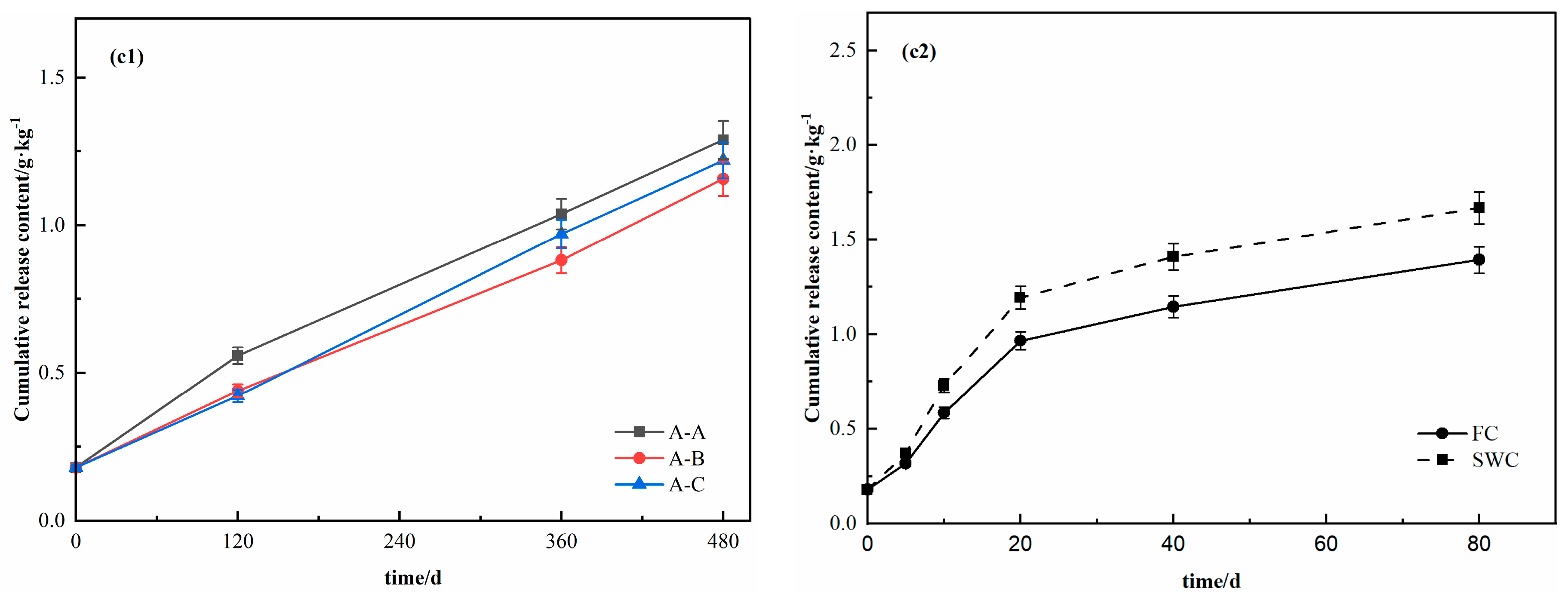
3.1. Release Characteristics of DOC in Response to Moisture Changes at Different Soil Depths
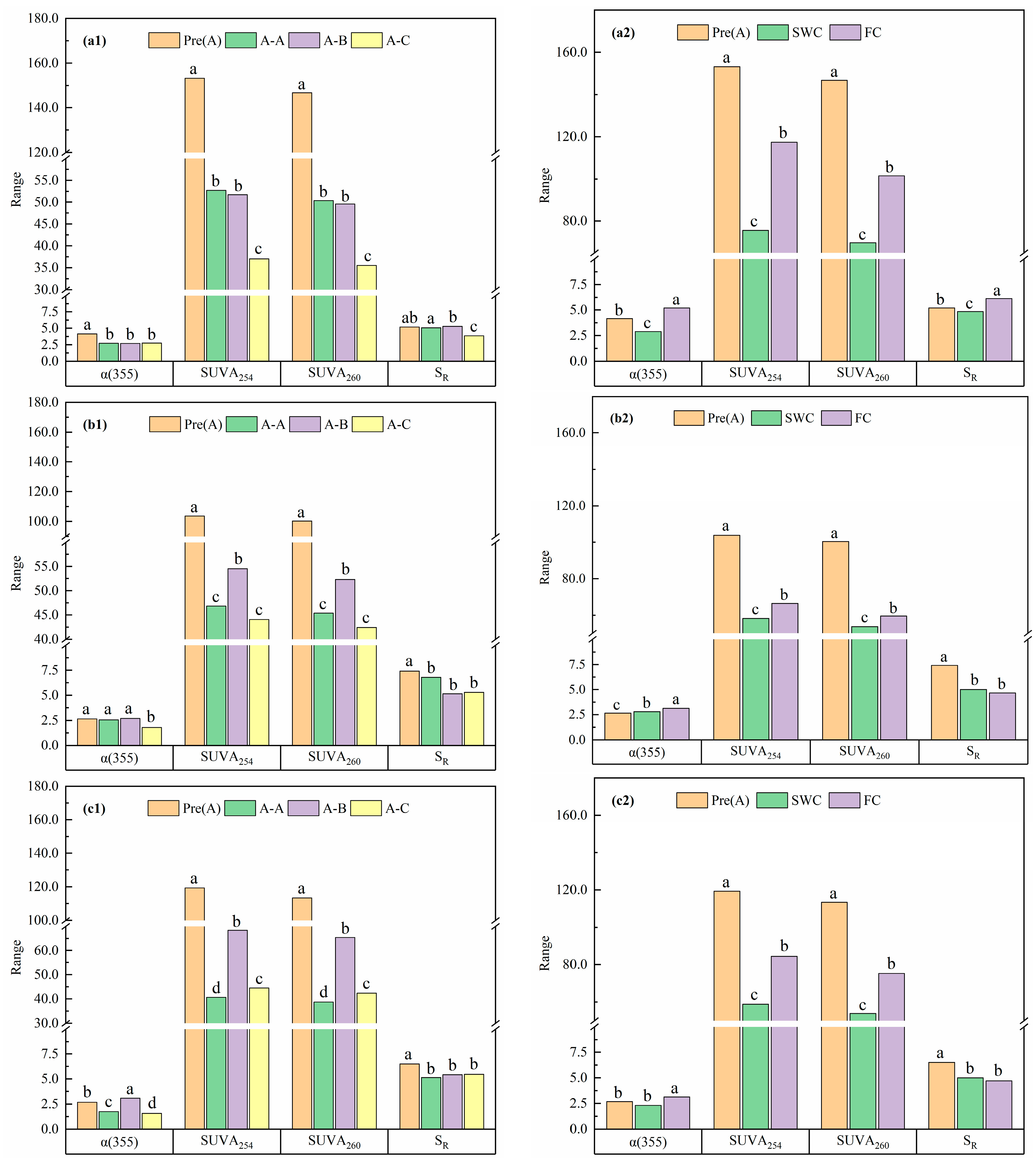
3.2. Absorption Spectral Characteristics of Soil DOC Under Different Water Contents
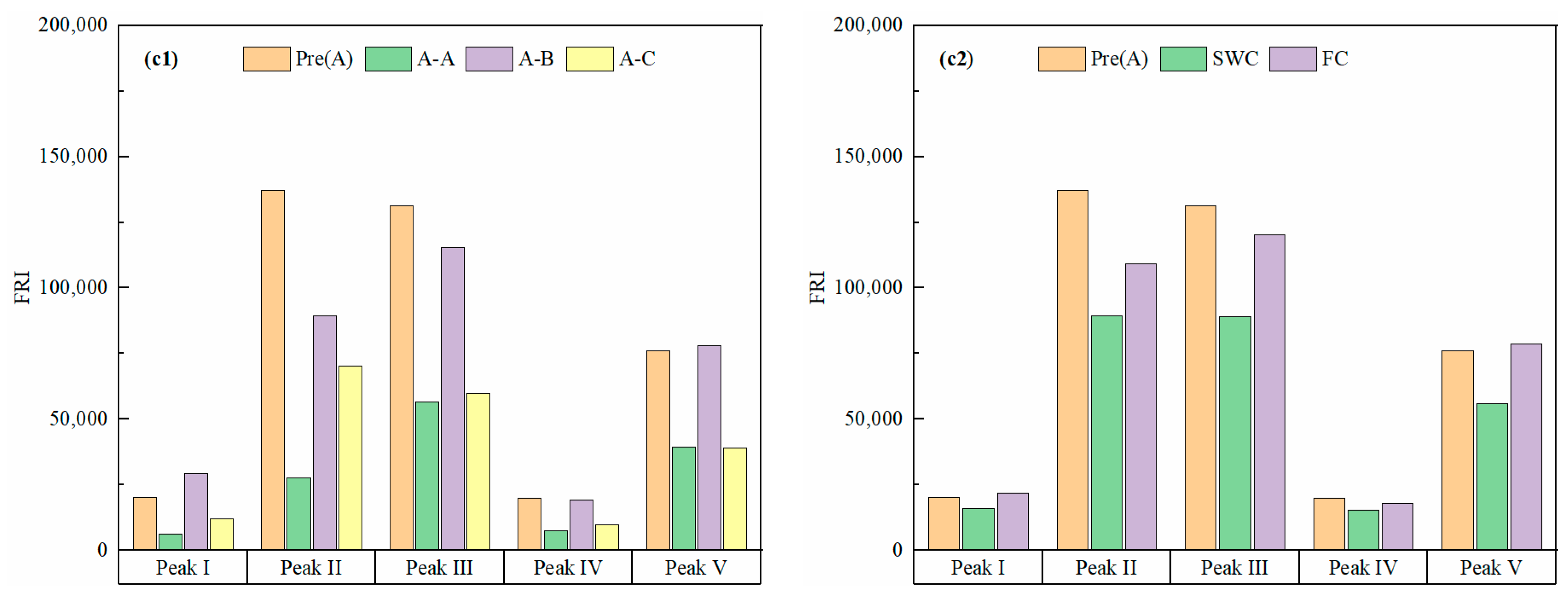
3.3. Three-Dimensional Fluorescence Regional Integration (FRI) of DOC Under Different Water Contents
4. Discussion
4.1. Response of Alpine DOC to Environmental Changes
4.2. Influence Mechanism of Moisture on DOC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, J.; Yuan, X.; Zhu, B. Effects of warming on carbon and nitrogen cycling in alpine grassland ecosystems on the tibetan plateau: A meta-analysis. Geoderma 2020, 370, 114363. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Hou, J.; Wang, M.; Fan, B.; Yan, J.; Huang, L.; He, Y. Spatio-temporal patterns of centennial-scale climate change over the Tibetan Plateau during the past two millennia and their possible mechanisms. Quat. Sci. Rev. 2022, 292, 107664. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Liu, S.; Li, J.; Zhou, H.; Wu, G.; Haregeweyn, N. Editorial: Patterns, functions, and processes of alpine grassland ecosystems under global change. Front. Plant Sci. 2022, 13, 1048031. [Google Scholar] [CrossRef]
- Piao, S.L.; Zhang, X.Z.; Wang, T.; Liang, E.Y.; Wang, S.P.; Zhu, J.T.; Niu, B.J.C.S.B. Responses and feedback of the Tibetan Plateau’s alpine ecosystem to climate change. Chin. Sci. Bull. 2019, 64, 2842–2855. [Google Scholar] [CrossRef]
- Song, C.; Pei, T.; Zhou, C.H. Progress in the study of temperature changes in the Qinghai-Tibet Plateau since 1960. Prog. Geogr. 2012, 31, 1503–1509. [Google Scholar]
- Gao, Q.-Z.; Li, Y.; Xu, H.-M.; Wan, Y.-F.; Jiangcun, W.-Z. Adaptation strategies of climate variability impacts on alpine grassland ecosystems in tibetan plateau. Mitig. Adapt. Strateg. Glob. Change 2014, 19, 199–209. [Google Scholar] [CrossRef]
- Li, Y.; Song, B.; Kang, X. Vegetation, ecosystem processing and carbon budget of wetlands under global change. Front. Plant Sci. 2023, 14, 1160319. [Google Scholar] [CrossRef]
- Qiang, H.F.; Jin, X.Y.; Zhao, L.; Lu, A.H. Dry and wet changes in Zoige wetland in recent 56 years based on relative humidity index. Res. Soil Water Conserv. 2018, 25, 172–177+182. [Google Scholar] [CrossRef]
- Yang, A.; Kang, X.; Li, Y.; Zhang, X.; Zhang, K.; Kang, E.; Yan, Z.; Li, M.; Wang, X.; Niu, Y.; et al. Alpine wetland degradation reduces carbon sequestration in the Zoige Plateau, China. Front. Ecol. Evol. 2022, 10, 980441. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhang, Y.; Qian, H.; Xie, Z.; Hu, Y.; Huang, Y.; Zhao, C.; Cheng, W.; Feng, X.; et al. Soil Organic Carbon Dynamics and Influencing Factors in the Zoige Alpine Wetland from the 1980s to 2020 Based on a Random Forest Model. Land 2023, 12, 1923. [Google Scholar] [CrossRef]
- Li, P.; Wu, M.; Li, T.; Dumbrell, A.J.; Saleem, M.; Kuang, L.; Luan, L.; Wang, S.; Li, Z.; Jiang, J. Molecular weight of dissolved organic matter determines its interactions with microbes and its assembly processes in soils. Soil Biol. Biochem. 2023, 184, 109117. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Gmach, M.R.; Cherubin, M.R.; Kaiser, K.; Cerri, C.E.P. Processes that influence dissolved organic matter in the soil: A review. Sci. Agric. 2019, 77, e20180164. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Surey, R.; Schimpf, C.M.; Sauheitl, L.; Mueller, C.W.; Rummel, P.S.; Dittert, K.; Kaiser, K.; Böttcher, J.; Mikutta, R. Potential denitrification stimulated by water-soluble organic carbon from plant residues during initial decomposition. Soil Biol. Biochem. 2020, 147, 107841. [Google Scholar] [CrossRef]
- Preston, M.D.; Eimers, M.C.; Watmough, S.A. Effect of moisture and temperature variation on DOC release from a peatland: Conflicting results from laboratory, field and historical data analysis. Sci. Total Environ. 2011, 409, 1235–1242. [Google Scholar] [CrossRef]
- Chen, Q.; Niu, B.; Hu, Y.; Luo, T.; Zhang, G. Warming and increased precipitation indirectly affect the composition and turnover of labile-fraction soil organic matter by directly affecting vegetation and microorganisms. Sci. Total Environ. 2020, 714, 136787. [Google Scholar] [CrossRef]
- Naden, P.S.; Allott, N.; Arvola, L.; Järvinen, M.; Jennings, E.; Moore, K.; Aonghusa, C.N.; Pierson, D. Modelling the impacts of climate change on dissolved organic carbon. In The Impact of Climate Change on European Lakes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 221–252. [Google Scholar] [CrossRef]
- Nie, X.; Wang, D.; Ren, L.; Du, Y.; Zhou, G. Storage and controlling factors of soil organic carbon in alpine wetlands and meadow across the Tibetan Plateau. Eur. J. Soil Sci. 2023, 74, e13383. [Google Scholar] [CrossRef]
- Bai, W.; Chen, X.; Tang, Y.; He, Y.; Zheng, Y. Temporal and spatial changes of soil moisture and its response to temperature and precipitation over the Tibetan Plateau. Hydrol. Sci. J. 2019, 64, 1370–1384. [Google Scholar] [CrossRef]
- Evans, C.D.; Freeman, C.; Monteith, D.T.; Reynolds, B.; Fenner, N. Climate change (Communication arising): Terrestrial export of organic carbon. Nature 2002, 415, 862–863. [Google Scholar] [CrossRef]
- Freeman, C.; Evans, C.D.; Monteith, D.T.; Reynolds, B.; Fenner, N. Export of organic carbon from peat soils. Nature 2001, 412, 785. [Google Scholar] [CrossRef] [PubMed]
- Tiefenbacher, A.; Weigelhofer, G.; Klik, A.; Mabit, L.; Santner, J.; Wenzel, W.; Strauss, P. Antecedent soil moisture and rain intensity control pathways and quality of organic carbon exports from arable land. Catena 2021, 202, 105297. [Google Scholar] [CrossRef]
- Fan, S.; Qin, J.; Sun, H.; Dan, Z.; Li, Z.; Yang, J. Different Responses of Soil Nutrient Dynamics and Microbial Activities to Soil Moisture Changes in Alpine Wetlands and Meadows on the Tibetan Plateau. Land Degrad. Dev. 2025; early review. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, T.; Li, R.; Wei, H.; Li, X.; Du, M.; Tang, Y. Precipitation alters temperature effects on ecosystem respiration in tibetan alpine meadows. Agric. For. Meteorol. 2018, 252, 121–129. [Google Scholar] [CrossRef]
- Borken, W.; Xu, Y.-J.; Brumme, R.; Lamersdorf, N. A climate change scenario for carbon dioxide and dissolved organic carbon fluxes from a temperate forest soil drought and rewetting effects. Soil Sci. Soc. Am. J. 1999, 63, 1848–1855. [Google Scholar] [CrossRef]
- Gao, J.; Feng, J.; Zhang, X.; Yu, F.-H.; Xu, X.; Kuzyakov, Y. Drying-rewetting cycles alter carbon and nitrogen mineralization in litter-amended alpine wetland soil. Catena 2016, 145, 285–290. [Google Scholar] [CrossRef]
- Jiang, P.; He, S.; Xiao, L.; Li, L.; Liu, M. Effects of Drought on Dissolved Organic Carbon Content in Grassland and Forest Soils. Russ. J. Ecol. 2023, 54, 516–525. [Google Scholar] [CrossRef]
- Xiang, S.; Guo, R.; Wu, N.; Sun, S. Current status and future prospects of Zoige Marsh in Eastern Qinghai-Tibet Plateau. Ecol. Eng. 2009, 35, 553–562. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ma, J. Water-holding capacity of ground covers and soils in alpine and sub-alpine shrubs in western Sichuan, China. Acta Ecol. Sin. 2006, 26, 2775–2781. [Google Scholar] [CrossRef]
- Yi, X.S.; Li, G.S.; Yin, Y.Y. Comparison of three methods to develop pedotransfer functions for the saturated water content and field water capacity in permafrost region. Cold Reg. Sci. Technol. 2013, 88, 10–16. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Wang, S.; Qin, J.; Sun, H.; Chen, W.; Xie, B. Photodegradation characteristics of dissolved organic matter (DOM) in alpine soils of the eastern margin of Tibetan Plateau. Pol. J. Environ. Stud. 2021, 30, 2815–2826. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Sun, H.; Liu, C.; Wang, X.Q. Characteristics of chromophoric dissolved organic matter (CDOM) in natural rivers of Western Sichuan Plateau. Environ. Sci. 2019, 40, 5318–5329. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation? Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Gaitán, J.J.; Maestre, F.T.; Bran, D.E.; Buono, G.G.; Dougill, A.J.; Martínez, G.G.; Ferrante, D.; Guuroh, R.T.; Linstädter, A.; Massara, V.; et al. Biotic and abiotic drivers of topsoil organic carbon concentration in drylands have similar effects at regional and global scales. Ecosystems 2019, 22, 1445–1456. [Google Scholar] [CrossRef]
- Gao, J.; Lei, G.; Zhang, X.; Wang, G. Can delta C-13 abundance, water-soluble carbon, and light fraction carbon be potential indicators of soil organic carbon dynamics in Zoige wetland? Catena 2014, 119, 21–27. [Google Scholar] [CrossRef]
- Puissant, J.; Mills, R.T.E.; Robroek, B.J.M.; Gavazov, K.; Perrette, Y.; De Danieli, S.; Spiegelberger, T.; Buttler, A.; Brun, J.-J.; Cécillon, L. Climate change effects on the stability and chemistry of soil organic carbon pools in a subalpine grassland. Biogeochemistry 2017, 132, 123–139. [Google Scholar] [CrossRef]
- Sowerby, A.; Emmett, B.A.; Tietema, A.; Beier, C. Contrasting effects of repeated summer drought on soil carbon efflux in hydric and mesic heathland soils. Glob. Change Biol. 2008, 14, 2388–2404. [Google Scholar] [CrossRef]
- Sowerby, A.; Emmett, B.A.; Williams, D.; Beier, C.; Evans, C.D. The response of dissolved organic carbon (DOC) and the ecosystem carbon balance to experimental drought in a temperate shrubland. Eur. J. Soil Sci. 2010, 61, 697–709. [Google Scholar] [CrossRef]
- Ding, J.; Chen, L.; Ji, C.; Hugelius, G.; Li, Y.; Liu, L.; Qin, S.; Zhang, B.; Yang, G.; Li, F.; et al. Decadal soil carbon accumulation across Tibetan permafrost regions. Nat. Geosci. 2017, 10, 420–424. [Google Scholar] [CrossRef]
- Li, N.; Wang, G.X.; Gao, Y.H.; Ji, C.Z. Research progress on soil organic carbon in Qinghai-Tibet Plateau ecosystem. Soil 2009, 41, 512–519. [Google Scholar]
- Oechel, W.C.; Vourlitis, G.L.; Hastings, S.J.; Zulueta, R.C.; Hinzman, L.; Kane, D. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 2000, 406, 978–981. [Google Scholar] [CrossRef]
- Sawicka, K.; Clark, J.M.; Vanguelova, E.; Monteith, D.T.; Wade, A.J. Spatial properties affecting the sensitivity of soil water dissolved organic carbon long-term median concentrations and trends. Sci. Total Environ. 2021, 780, 146670. [Google Scholar] [CrossRef] [PubMed]
- Sanderman, J.; Baldock, J.A.; Amundson, R. Dissolved organic carbon chem-istry and dynamics in contrasting forest and grassland soils. Biogeochemistry 2008, 89, 181–198. [Google Scholar] [CrossRef]
- Guo, L.L.; Li, A.D.; Shang, H.L.; Sun, S.Q. Total and Labile Organic Carbon in Soils of Three Subalpine Forest Types in Gongga Mountain, Western Sichuan. Chin. J. Agrometeorol. 2018, 39, 636. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, Y.; Tang, S.; Liu, J. Spatial distribution of soil organic carbon in the Zoige alpine wetland, northeastern Qinghai–Tibet Plateau. Catena 2016, 144, 102–108. [Google Scholar] [CrossRef]
- Schrumpf, M.; Kaiser, K.; Guggenberger, G.; Persson, T.; Kögel-Knabner, I.; Schulze, E.-D. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 2013, 10, 31675–31691. [Google Scholar] [CrossRef]
- Garcia-Franco, N.; Wiesmeier, M.; Buness, V.; Berauer, B.J.; Schuchardt, M.A.; Jentsch, A.; Schlingmann, M.; Andrade-Linares, D.; Wolf, B.; Kiese, R.; et al. Rapid loss of organic carbon and soil structure in mountainous grassland topsoils induced by simulated climate change. Geoderma 2024, 442, 116807. [Google Scholar] [CrossRef]
- Han, C.; Wang, Z.; Si, G.; Lei, T.; Yuan, Y.; Zhang, G. Increased precipitation accelerates soil organic matter turnover associated with microbial community composition in topsoil of alpine grassland on the eastern Tibetan Plateau. Can. J. Microbiol. 2017, 63, 811–821. [Google Scholar] [CrossRef]
- Davenport, R.; Bowen, B.P.; Lynch, L.M.; Kosina, S.M.; Shabtai, I.; Northen, T.R.; Lehmann, J. Decomposition decreases molecular diversity and ecosystem similarity of soil organic matter. Proc. Natl. Acad. Sci. USA 2023, 120, e2303335120. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Sun, H. Temporal dynamics of light-fraction organic carbon of subalpine-alpine soil along an altitudinal gradient in the Southwestern China. Res. Soil Water Conserv. 2015, 22, 51–55. [Google Scholar] [CrossRef]
- Jia, J.; Feng, X.; He, J.-S.; He, H.; Lin, L.; Liu, Z. Comparing microbial carbon sequestration and priming in the subsoil versus topsoil of a Qinghai-Tibetan alpine grassland. Soil Biol. Biochem. 2017, 104, 141–151. [Google Scholar] [CrossRef]
- Wang, S.; Qin, J.; Xie, B.; Liu, C.; Chen, Y.; Tang, X.; Sun, H. Spectroscopic characteristics of dissolved organic matter (DOM)in Zoige alpine peatland soils along a soil moisture gradient. Ecol. Environ. Sci. 2020, 29, 676–685. [Google Scholar] [CrossRef]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef]
- Tipping, E.; Woof, C.; Rigg, E.; Harrison, A.; Ineson, P.; Taylor, K.; Benham, D.; Poskitt, J.; Rowland, A.; Bol, R.; et al. Climatic influences on the leaching of dissolved organic matter from upland UK moorland soils, investigated by a field manipulation experiment. Environ. Int. 1999, 25, 83–95. [Google Scholar] [CrossRef]
- Xu, G.; Kang, X.; Wang, F.; Zhuang, W.; Yan, W.; Zhang, K. Alpine wetlands degradation leads to soil nutrient imbalances that affect plant growth and microbial diversity. Commun. Earth Environ. 2024, 5, 397. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.; Wang, M.; Yang, C.; Miao, L. Analysis of Soil Carbon, Nitrogen and Phosphorus Storage and Sequestration Effects in Alpine Grassland with Different Degradation Levels in Maqu County, China. Eurasian Soil Sci. 2024, 57, 794–805. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.-P.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Zhu, M.; Kong, F.; Li, Y.; Li, M.; Zhang, J.; Xi, M. Effects of moisture and salinity on soil dissolved organic matter and ecological risk of coastal wetland. Environ. Res. 2020, 187, 109659. [Google Scholar] [CrossRef]
- Pu, Y.; Lang, S.; Wang, A.; Zhang, S.; Li, T.; Qian, H.; Wang, G.; Jia, Y.; Xu, X.; Yuan, D.; et al. Distribution and functional groups of soil aggregate-associated organic carbon along a marsh degradation gradient on the Zoige plateau, China. Catena 2022, 209, 105811. [Google Scholar] [CrossRef]
- Hu, M.; Yao, Z.; Shi, L.; Wu, Q.; Tang, S.; Shao, X. Patchy degradation-induced changes in soil aggregates and organic carbon in an alpine swamp meadow on the Qinghai-Tibetan Plateau. Land Degrad. Dev. 2024, 35, 4713–4725. [Google Scholar] [CrossRef]
- Huang, X.Y.; Chen, K.L.; Wu, C.Y. Diurnal variation and seasonal dynamics of soil respiration in the alpine meadow in the Qinghai Tibet Plateau. Yunnan Geogr. Environ. Res. 2016, 28, 66–71. [Google Scholar]
- Liu, L.; Chen, H.; Liu, X.; Yang, Z.; Zhu, D.; He, Y.; Liu, J. Contemporary, modern and ancient carbon fluxes in the Zoige peatlands on the Qinghai-Tibetan plateau. Geoderma 2019, 352, 138–149. [Google Scholar] [CrossRef]
- Sierra, C.A.; Malghani, S.; Loescher, H.W. Interactions among temperature, moisture, and oxygen concentrations in controlling decomposition rates in a boreal forest soil. Biogeoences 2017, 14, 703–710. [Google Scholar] [CrossRef]
- Yu, H.; Zha, T.; Zhang, X.; Ma, L. Vertical distribution and influencing factors of soc in the Loess Plateau China. Sci. Total Environ. 2019, 693, 133632. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Jiang, H.; Hu, Y.; Lu, X. Influences of litter diversity and soil moisture on soil microbial communities in decomposing mixed litter of alpine steppe species. Geoderma 2020, 377, 114577. [Google Scholar] [CrossRef]
- Fan, S.; Qin, J.; Sun, H.; Jia, Z.; Chen, Y. Alpine soil microbial community structure and diversity are largely influenced by moisture content in the zoige wetland. Int. J. Environ. Sci. Technol. 2021, 19, 4369–4378. [Google Scholar] [CrossRef]
- McLatchey, G.P.; Reddy, K.R. Regulation of organic matter decomposition and nutrient release in a wetland soil. J. Environ. Qual. 1998, 27, 1268–1274. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Wang, Q.; Wang, S.; Zhang, Z.; Zhang, Z.; Zhao, Y. Climate change affects soil labile organic carbon fractions in a tibetan alpine meadow. J. Soils Sediments 2017, 17, 326–339. [Google Scholar] [CrossRef]
- Larionova, A.A.; Maltseva, A.N.; de Gerenyu, V.O.L.; Kvitkina, A.K.; Bykhovets, S.S.; Zolotareva, B.N.; Kudeyarov, V.N. Effect of temperature and moisture on the mineralization and humification of leaf litter in a model incubation experiment. Eurasian Soil Sci. 2017, 50, 422–431. [Google Scholar] [CrossRef]
- Han, Y.; Qu, C.; Hu, X.; Wang, P.; Wan, D.; Cai, P.; Rong, X.; Chen, W.; Huang, Q. Warming and humidification mediated changes of DOM composition in an Alfisol. Sci. Total Environ. 2022, 805, 150198. [Google Scholar] [CrossRef] [PubMed]
- Moche, M.; Gutknecht, J.; Schulz, E.; Langer, U.; Rinklebe, J. Monthly dynamics of microbial community structure and their controlling factors in three floodplain soils. Soil Biol. Biochem. 2015, 90, 169–178. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J. Soil organic matter accumulation and carbon fractions along a moisture gradient of forest soils. Forests 2017, 8, 448. [Google Scholar] [CrossRef]
- Li, C.; Gao, S.; Zhang, J.; Zhao, L.; Wang, L. Moisture Effect on Soil Humus Characteristics in a Laboratory Incubation Experiment. Soil Water Res. 2016, 11, 37–43. [Google Scholar] [CrossRef]


| Position | Bulk Density (g/cm3) | pH | ORP (mV) | DOC (g/kg) | TOC (g/kg) | TN (g/kg) | GWC (%) | |
|---|---|---|---|---|---|---|---|---|
| A | 0–5 cm | 0.80 ± 0.12 | 5.25 ± 0.02 | 76 ± 7 | 0.20 ± 0.00 | 90.27 ± 10.91 | 7.82 ± 0.85 | 89.71 ± 10.92 |
| 5–10 cm | 0.78 ± 0.04 | 5.14 ± 0.04 | 113 ± 5 | 0.17 ± 0.00 | 65.67 ± 0.59 | 6.01 ± 0.14 | 78.04 ± 4.52 | |
| 10–15 cm | 1.00 ± 0.15 | 5.25 ± 0.05 | 147 ± 9 | 0.18 ± 0.00 | 53.94 ± 1.86 | 4.72 ± 0.22 | 69.15 ± 4.44 | |
| B | 0–5 cm | 0.90 ± 0.01 | 5.37 ± 0.01 | 251 ± 13 | 0.25 ± 0.00 | 75.33 ± 5.28 | 6.99 ± 0.70 | 81.87 ± 13.36 |
| 5–10 cm | 0.73 ± 0.05 | 5.49 ± 0.01 | 265 ± 12 | 0.28 ± 0.00 | 57.54 ± 3.51 | 5.22 ± 0.28 | 72.43 ± 3.59 | |
| 10–15 cm | 0.93 ± 0.04 | 5.00 ± 0.04 | 297 ± 9 | 0.23 ± 0.00 | 70.40 ± 3.63 | 6.61 ± 0.31 | 66.88 ± 1.05 | |
| C | 0–5 cm | 0.94 ± 0.01 | 5.61 ± 0.02 | 333 ± 3 | 0.27 ± 0.00 | 97.39 ± 10.08 | 9.06 ± 1.04 | 76.78 ± 13.36 |
| 5–10 cm | 0.83 ± 0.02 | 5.41 ± 0.03 | 316 ± 14 | 0.19 ± 0.00 | 59.36 ± 1.49 | 5.56 ± 0.18 | 69.85 ± 6.09 | |
| 10–15 cm | 0.75 ± 0.02 | 5.17 ± 0.03 | 311 ± 13 | 0.20 ± 0.00 | 64.50 ± 0.85 | 5.94 ± 0.22 | 59.09 ± 5.15 | |
| Spectral Parameters | Computational Formula | Formula Parameters |
|---|---|---|
| a(355) | a(λ) is the absorption coefficient (m−1), D(λ) is the absorbance at a wavelength of λ, l is the optical path (m) | |
| SUVA254 | DOC is dissolved organic carbon (mg/L) | |
| SUVA260 | / | |
| SR | and are the slope of the absorption spectra in the 275~295 nm and 350~400 nm bands, respectively. |
| Fluorescence Peak | Fluorescence Peak Excitation and Emission Wavelength Range |
|---|---|
| Peak I | = 200–250 nm, = 280–330 nm |
| Peak II | = 200–250 nm, = 330–380 nm |
| Peak III | = 200–250 nm, = 380–550 nm |
| Peak IV | = 250–450 nm, = 280–330 nm |
| Peak V | = 250–450 nm, = 380–550 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qin, J.; Chen, Y.; Sun, H.; Hu, X. Aridification Inhibits the Release of Dissolved Organic Carbon from Alpine Soils in Southwest China. Soil Syst. 2025, 9, 24. https://doi.org/10.3390/soilsystems9010024
Li Y, Qin J, Chen Y, Sun H, Hu X. Aridification Inhibits the Release of Dissolved Organic Carbon from Alpine Soils in Southwest China. Soil Systems. 2025; 9(1):24. https://doi.org/10.3390/soilsystems9010024
Chicago/Turabian StyleLi, Yanmei, Jihong Qin, Yuwen Chen, Hui Sun, and Xinyue Hu. 2025. "Aridification Inhibits the Release of Dissolved Organic Carbon from Alpine Soils in Southwest China" Soil Systems 9, no. 1: 24. https://doi.org/10.3390/soilsystems9010024
APA StyleLi, Y., Qin, J., Chen, Y., Sun, H., & Hu, X. (2025). Aridification Inhibits the Release of Dissolved Organic Carbon from Alpine Soils in Southwest China. Soil Systems, 9(1), 24. https://doi.org/10.3390/soilsystems9010024






