Impact of Abiotic Stressors on Soil Microbial Communities: A Focus on Antibiotics and Their Interactions with Emerging Pollutants
Abstract
1. Introduction
2. Survey Methodology
3. Soil Microbiome and Its Importance
4. Abiotic Factors Shaping the Soil Microbiome
5. Abiotic Stressors Influencing the Soil Microbiome
6. The Impact of Various Materials on Soil Microorganisms
6.1. Nanomaterials
6.2. Plastics
6.3. Biocides
6.4. Heavy Metals
6.5. Antibiotic Storm: Emerging Trends
7. Distribution of Antibiotics in the Soil
7.1. Factors Affecting the Distribution of Antibiotics
7.2. Fate and Degradation of Antibiotics
7.2.1. Half-Lives, Rate of Degradation, and Mobility of Antibiotics
7.2.2. Degradation Pathways of Antibiotics
7.2.3. Degradation by Microorganisms
7.3. Antibiotic Resistance
7.3.1. Antibiotic Resistance Genes (ARGs)
7.3.2. Factors Driving the ARG Pattern
7.4. Impact of Antibiotics on Soil Health
7.5. Soil Resistance and Human Health
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Brussaard, L. Ecosystem services provided by the soil biota. In Soil Ecology and Ecosystem Services; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Vasu, D.; Tiwary, P.; Chandran, P.; Singh, S.K. Soil quality for sustainable agriculture. In Nutrient Dynamics for Sustainable Crop Production; Meena, R.S., Ed.; Springer: Singapore, 2020; pp. 41–66. [Google Scholar]
- Banerjee, S.; Van Der Heijden, M.G. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Yang, X.; Liu, G.; Cui, Q.; Indree, T.; Ye, X.; Huang, Z. The Relationship and Influencing Factors between Endangered Plant Tetraena mongolica and Soil Microorganisms in West Ordos Desert Ecosystem, Northern China. Plants 2023, 12, 1048. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil health and sustainable agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Janzen, H.H.; Janzen, D.W.; Gregorich, E.G. The ‘soil health’ metaphor: Illuminating or illusory? Soil Biol. Biochem. 2021, 159, 108167. [Google Scholar] [CrossRef]
- Rinot, O.; Levy, G.J.; Steinberger, Y.; Svoray, T.; Eshel, G. Soil health assessment: A critical review of current methodologies and a proposed new approach. Sci. Total Environ. 2019, 648, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2019, 147, 103356. [Google Scholar] [CrossRef]
- Curtis, T.P.; Sloan, W.T.; Scannell, J.W. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad. Sci. USA 2002, 99, 10494–10499. [Google Scholar] [CrossRef] [PubMed]
- Gans, J.; Wolinsky, M.; Dunbar, J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, X.; Qian, X.; Gu, H.; Li, J.; Chen, X.; Shen, G. Soil health assessment in the Yangtze River Delta of China: Method de-velopment and application in orchards. Agric. Ecosyst. Environ. 2023, 341, 108190. [Google Scholar] [CrossRef]
- Biswas, B.; Nirola, R.; Biswas, J.K.; Pereg, L.; Willett, I.R.; Naidu, R. Environmental microbial health under changing climates: State, implication and initiatives for high-performance soils. In Sustainable Agriculture Reviews 29: Sustainable Soil Management: Preventive and Ameliorative Strategies; Lal, R., Francaviglia, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–3. [Google Scholar]
- Bini, D.; dos Santos, C.A.; Carmo, K.B.D.; Kishino, N.; Andrade, G.; Zangaro, W.; Nogueira, M.A. Effects of land use on soil organic carbon and microbial processes associated with soil health in southern Brazil. Eur. J. Soil Biol. 2013, 55, 117–123. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Rahim, H.U.; Ali, W.; Uddin, M.; Ahmad, S.; Khan, K.; Bibi, H.; Alatalo, J.M. Abiotic stresses in soils, their effects on plants, and mitigation strategies: A literature review. Chem. Ecol. 2024, 12, 1–34. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Gahan, J.; Schmalenberger, A. The role of bacteria and mycorrhiza in plant sulfur supply. Front. Plant Sci. 2014, 5, 723. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Abdul Rahman, N.S.N.; Abdul Hamid, N.W.; Nadarajah, K. Effects of abiotic stress on soil microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef] [PubMed]
- Dassen, S.; Cortois, R.; Martens, H.; de Hollander, M.; Kowalchuk, G.A.; van der Putten, W.H.; De Deyn, G.B. Differential responses of soil bacteria, fungi, archaea and protists to plant species richness and plant functional group identity. Mol. Ecol. 2017, 26, 4085–4098. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, X.; Hale, L.; Yuan, M.; Ning, D.; Feng, J.; Shi, Z.; Li, Z.; Feng, B.; Gao, Q.; et al. Climate warming accelerates temporal scaling of grassland soil microbial biodiversity. Nat. Ecol. Evol. 2019, 3, 612–619. [Google Scholar] [CrossRef]
- Oliverio, A.M.; Bradford, M.A.; Fierer, N. Identifying the microbial taxa that consistently respond to soil warming across time and space. Glob. Chang. Biol. 2017, 23, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Sheik, C.S.; Beasley, W.H.; Elshahed, M.S.; Zhou, X.; Luo, Y.; Krumholz, L.R. Effect of warming and drought on grassland microbial communities. ISME J. 2011, 5, 1692–1700. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Jiang, Y.; Li, J.; Fu, Q.; Qiu, Y.; Fu, X.; Yao, Z.; Dai, Z.; Qiu, Y.; et al. Effects of simulated warming on soil microbial community diversity and composition across diverse ecosystems. Sci. Total Environ. 2024, 911, 168793. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Averill, C.; Cates, L.L.; Dietze, M.C.; Bhatnagar, J.M. Spatial vs. temporal controls over soil fungal community similarity at continental and global scales. ISME J. 2019, 13, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Prober, S.M.; Leff, J.W.; Bates, S.T.; Borer, E.T.; Firn, J.; Harpole, W.S.; Lind, E.M.; Seabloom, E.W.; Adler, P.B.; Bakker, J.D.; et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015, 18, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhou, R.; Liu, S.; Xing, W.; Yuan, Y. Seasonal Changes in the Soil Microbial Community Structure in Urban Forests. Biology 2024, 13, 31. [Google Scholar] [CrossRef]
- Xu, T.; Shen, Y.; Ding, Z.; Zhu, B. Seasonal dynamics of microbial communities in rhizosphere and bulk soils of two temperate forests. Rhizosphere 2023, 25, 100673. [Google Scholar] [CrossRef]
- Koch, M.S.; Snedaker, S.C. Factors influencing Rhizophora mangle L. seedling development in Everglades carbonate soils. Aquat. Bot. 1997, 59, 87–98. [Google Scholar] [CrossRef]
- Xiao, D.-S.; Xu, C.-M.; Wang, D.-Y.; Chen, S.; Chu, G.; Liu, Y.-H. Effects of Aeration Methods on Microbial Diversity and Community Structure in Rice Rhizosphere. Huan Jing Ke Xue = Huanjing Kexue 2023, 44, 6362–6376. [Google Scholar] [CrossRef] [PubMed]
- Frindte, K.; Pape, R.; Werner, K.; Löffler, J.; Knief, C. Temperature and soil moisture control microbial community composition in an arctic–alpine ecosystem along elevational and micro-topographic gradients. ISME J. 2019, 13, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Mayor, J.R.; Sanders, N.J.; Classen, A.T.; Bardgett, R.D.; Clément, J.-C.; Fajardo, A.; Lavorel, S.; Sundqvist, M.K.; Bahn, M.; Chisholm, C.; et al. Elevation alters ecosystem properties across temperate treelines globally. Nature 2017, 542, 91–95. [Google Scholar] [CrossRef]
- Shen, C.; Shi, Y.; Fan, K.; He, J.S.; Adams, J.M.; Ge, Y.; Chu, H. Soil pH dominates elevational diversity pattern for bacteria in high elevation alkaline soils on the Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, fiz003. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Wang, S.; Xu, D.; Yu, H.; Wu, L.; Lin, Q.; Hu, Y.; Li, X.; He, Z.; et al. The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J. 2014, 8, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, B.; Pump, J.; Dumont, M.G. Microbial community structure in the rhizosphere of rice plants. Front. Microbiol. 2016, 6, 1537. [Google Scholar] [CrossRef] [PubMed]
- Buyer, J.S.; Roberts, D.P.; Russek-Cohen, E. Soil and plant effects on microbial community structure. Can. J. Microbiol. 2002, 48, 955–964. [Google Scholar] [CrossRef]
- Wang, L.; Li, X. Soil Microbial Community and Their Relationship with Soil Properties across Various Landscapes in the Mu Us Desert. Forests 2023, 14, 2152. [Google Scholar] [CrossRef]
- Bai, Z.; Jia, A.; Li, H.; Wang, M.; Qu, S. Explore the soil factors driving soil microbial community and structure in Songnen alkaline salt degraded grassland. Front. Plant Sci. 2023, 14, 1110685. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Tian, L.; Nasir, F.; Bahadur, A.; Batool, A.; Luo, S.; Yang, F.; Wang, Z.; Tian, C. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Burns, J.H.; Anacker, B.L.; Strauss, S.Y.; Burke, D.J. Soil microbial community variation correlates most strongly with plant species identity, followed by soil chemistry, spatial location and plant genus. AoB Plants 2015, 7, plv030. [Google Scholar] [CrossRef]
- Davies, L.O.; Schäfer, H.; Marshall, S.; Bramke, I.; Oliver, R.G.; Bending, G.D. Light structures phototroph, bacterial and fungal communities at the soil surface. PLoS ONE 2013, 8, e69048. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Bao, J.; Wu, T.; Chai, B. Effect of Light Heterogeneity Caused by Photovoltaic Panels on the Plant–Soil–Microbial System in Solar Park. Land 2023, 12, 367. [Google Scholar] [CrossRef]
- Huličová, P.; Fazekašová, D.; Fazekaš, J. Impact of flooding on soil enzyme activity in environmentally sensitive areas. Carpathian J. Earth Environ. Sci. 2018, 13, 567–574. [Google Scholar] [CrossRef]
- Ren, Q.; Li, C.; Yang, W.; Song, H.; Ma, P.; Wang, C.; Schneider, R.L.; Morreale, S.J. Revegetation of the riparian zone of the Three Gorges Dam Reservoir leads to increased soil bacterial diversity. Environ. Sci. Pollut. Res. 2018, 25, 23748–23763. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Bai, M.; Peng, T.; Li, H.; Xu, H.-J.; Guo, G.; Bai, H.; Rong, N.; Sahu, S.K.; et al. Soil conditions and the plant microbiome boost the accumulation of monoterpenes in the fruit of Citrus reticulata ‘Chachi’. Microbiome 2023, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, G.; Ye, C.; Liu, W. Bacterial community and climate change implication affected the diversity and abundance of antibiotic resistance genes in wetlands on the Qinghai-Tibetan Plateau. J. Hazard. Mater. 2019, 361, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, D.; Wu, J.; Chen, Q.; Long, C.; Li, Y.; Cheng, X. Anti-seasonal submergence dominates the structure and composition of prokaryotic communities in the riparian zone of the Three Gorges Reservoir, China. Sci. Total Environ. 2019, 663, 662–672. [Google Scholar] [CrossRef]
- Zhuang, J.; Tian, Y. Effects of Precipitation on Forestry Soil Microorganisms. Pol. J. Environ. Stud. 2023, 32, 5923–5931. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X.; Yu, L. Salinity controls soil microbial community structure and function in coastal estuarine wetlands. Environ. Microbiol. 2021, 23, 1020–1037. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Wu, Y.; Yu, J.; Shen, G.; Jiang, M.; Wang, X.; Zhang, X. Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 2019, 338, 118–127. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Garkoti, S.C. Influence of vegetation types on soil physical and chemical properties, microbial biomass and stoichiometry in the central Himalaya. Catena 2023, 222, 06835. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Q.; Chang, D.; Ndayisenga, F.; Yu, Z. Assessing the effect of physicochemical properties of saline and sodic soil on soil microbial communities. Agriculture 2022, 12, 782. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Yin, M.; Ma, X.; Yu, X.; Guo, X.; Du, N.; Eller, F.; Guo, W. Soil salinity, not plant genotype or geographical distance, shapes soil microbial community of a reed wetland at a fine scale in the Yellow River Delta. Sci. Total Environ. 2023, 856, 159136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H. Salinity is a key determinant for soil microbial communi-ties in a desert ecosystem. Msystems 2019, 4, 10-1128. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Wang, W.; Song, X.; Yu, S. Soil depth determines the composition and diversity of bacterial and archaeal communities in a poplar plantation. Forests 2019, 10, 550. [Google Scholar] [CrossRef]
- Spitzer, C.M.; Wardle, D.A.; Lindahl, B.D.; Sundqvist, M.K.; Gundale, M.J.; Fanin, N.; Kardol, P. Root traits and soil micro-organisms as drivers of plant–soil feedbacks within the sub-arctic tundra meadow. J. Ecol. 2022, 110, 466–478. [Google Scholar] [CrossRef]
- Frey, B.; Walthert, L.; Perez-Mon, C.; Stierli, B.; Köchli, R.; Dharmarajah, A.; Brunner, I. Deep soil layers of drought-exposed forests harbor poorly known bacterial and fungal communities. Front. Microbiol. 2021, 12, 674160. [Google Scholar] [CrossRef] [PubMed]
- Leewis, M.-C.; Lawrence, C.R.; Schulz, M.S.; Tfaily, M.M.; Ayala-Ortiz, C.O.; Flores, G.E.; Mackelprang, R.; McFarland, J.W. The influence of soil development on the depth distribution and structure of soil microbial communities. Soil Biol. Biochem. 2022, 174, 108808. [Google Scholar] [CrossRef]
- Xin, Y.; Wu, Y.; Zhang, H.; Li, X.; Qu, X. Soil depth exerts a stronger impact on microbial communities and the sulfur biological cycle than salinity in salinized soils. Sci. Total Environ. 2023, 894, 164898. [Google Scholar] [CrossRef]
- Hao, J.; Xu, W.; Song, J.; Gao, G.; Bai, J.; Yu, Q.; Ren, G.; Feng, Y.; Wang, X. Adaptability of agricultural soil microbial nutrient utilization regulates community assembly under mulching measures on the Loess Plateau. Agric. Ecosyst. Environ. 2023, 357, 108702. [Google Scholar] [CrossRef]
- He, L.; Sun, X.; Li, S.; Zhou, W.; Chen, Z.; Bai, X. The vertical distribution and control factor of microbial biomass and bacterial community at macroecological scales. Sci. Total Environ. 2023, 869, 161754. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cao, X.; Zhou, Y.; Li, Z.; Yang, Y.; Zhao, D.; Li, Y.; Xu, Z.; Zhang, C.-S. Effect of planting salt-tolerant legumes on coastal saline soil nutrient availability and microbial communities. J. Environ. Manag. 2023, 345, 118574. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, B.N.; Dwivedi, P.; Rajawat, M.V.S. Interference of climate change on plant-microbe interaction: Present and future prospects. Front. Agron. 2022, 3, 725804. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Shen, C.; Wang, J.; Singh, B.K.; Ge, Y. Plant diversity improves resistance of plant biomass and soil microbial communities to drought. J. Ecol. 2022, 110, 1656–1672. [Google Scholar] [CrossRef]
- Manivel, T.; Sandhiya, T.; Deepika, S.; Selvakumar, S.V.; Karnan, T.M.; Adeyemi, D.E.; Thanapaul, R.J. Chemical communication between plant roots and microbes within the rhizosphere. Plant-Microbe Interact.-Recent Adv. Mol. Bio-Chem. Approaches 2023, 1, 141–164. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.; Liu, B.; Zhang, J.; Hua, J.; Liu, D.; Bhople, P.; Zhang, Y.; Zhang, H.; Zhang, C.; et al. Exploration of Soil Microbial Diversity and Community Structure along Mid-Subtropical Elevation Gradients in Southeast China. Forests 2023, 14, 769. [Google Scholar] [CrossRef]
- Luo, H.; Wang, C.; Zhang, K.; Ming, L.; Chu, H.; Wang, H. Elevational changes in soil properties shaping fungal community assemblages in terrestrial forest. Sci. Total Environ. 2023, 900, 165840. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, Y.; Li, J.; Li, X.; Ruan, H.; Bhople, P.; Keiblinger, K.; Mao, L.; Liu, D. Interaction among soil nutrients, plant diversity and hypogeal fungal trophic guild modifies root-associated fungal diversity in coniferous forests of Chinese Southern Himalayas. Plant Soil 2022, 481, 395–408. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.-J.; Banerjee, S.; Zhou, N.; Zhao, Z.-Y.; Zhang, K.; Tian, C.-Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef]
- Fang, J.; Wei, S.; Gao, Y.; Zhang, X.; Cheng, Y.; Wang, J.; Ma, J.; Shi, G.; Bai, L.; Xie, R.; et al. Character variation of root space microbial community composition in the response of drought-tolerant spring wheat to drought stress. Front. Microbiol. 2023, 14, 1235708. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J.; Hou, J.; Yang, Y.; Yao, T.; Knight, R.; Chu, H. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol. 2012, 14, 2457–2466. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Janssens, I.A.; Deng, Y.; He, X.; Liu, L.; Yi, Y.; Xiao, N.; Wang, X.; Li, C.; et al. Divergent rhizosphere and non-rhizosphere soil microbial structure and function in long-term warmed steppe due to altered root exudation. Glob. Chang. Biol. 2023, 30, e17111. [Google Scholar] [CrossRef]
- Dennis, P.G.; Newsham, K.K.; Rushton, S.P.; O’Donnell, A.G.; Hopkins, D.W. Soil bacterial diversity is positively associated with air temperature in the maritime Antarctic. Sci. Rep. 2019, 9, 2686. [Google Scholar] [CrossRef]
- Feng, J.; Ma, H.; Wang, C.; Gao, J.; Zhai, C.; Jiang, L.; Wan, S. Water rather than nitrogen availability predominantly modulates soil microbial beta-diversity and co-occurrence networks in a secondary forest. Sci. Total Environ. 2024, 907, 167996. [Google Scholar] [CrossRef] [PubMed]
- Stamou, G.P.; Monokrousos, N.; Papapostolou, A.; Papatheodorou, E.M. Recurring heavy rainfall resulting in degraded-upgraded phases in soil microbial networks that are reflected in soil functioning. Soil Ecol. Lett. 2023, 5, 220161. [Google Scholar] [CrossRef]
- Su, W.; Han, Q.; Yang, J.; Yu, Q.; Wang, S.; Wang, X.; Qu, J.; Li, H. Heavy rainfall accelerates the temporal turnover but decreases the deterministic processes of buried gravesoil bacterial communities. Sci. Total Environ. 2022, 836, 155732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, B.; Wu, L.; Gao, Q.; Wang, F.; Wen, C.; Wang, M.; Liang, Y.; Hale, L.; Zhou, J.; et al. Zonal soil type determines soil microbial responses to maize cropping and fertilization. mSystems 2016, 1, e00075-16. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Chomel, M.; Segura, N.A.; de Castro, F.; Goodall, T.; Magilton, M.; Rhymes, J.M.; Delgado-Baquerizo, M.; Griffiths, R.I.; Baggs, E.M.; et al. Land management shapes drought responses of dominant soil microbial taxa across grasslands. Nat. Commun. 2024, 15, 29. [Google Scholar] [CrossRef]
- Xie, J.; Lou, X.; Lu, Y.; Huang, H.; Yang, Q.; Zhang, Z.; Zhao, W.; Li, Z.; Liu, H.; Du, S.; et al. Suitable light combinations enhance cadmium accumulation in Bidens pilosa L. by regulating the soil microbial communities. Environ. Exp. Bot. 2023, 205, 105128. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, H.; Shi, J.; Wang, Z.; Lv, J.; Li, L.; Wang, X. Soil microbial composition, diversity, and network stability in intercropping versus monoculture responded differently to drought. Agric. Ecosyst. Environ. 2024, 365, 108915. [Google Scholar] [CrossRef]
- Al-Hadidi, S.H.; Abumaali, D.A.; Ahmed, T.; Al-Khis, A.F.; Al-Malki, S.A.; Yaish, M.; Rahim, H.U.; Khalid, M.F.; Hassan, H.; Alatalo, J.M. The effect of type and combination of fertilizers on eukaryotic microbiome of date palm rhizosphere. Plant Growth Regul. 2024, 103, 439–451. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Fu, Y.; Jiang, Z.; Jia, S.; Song, B.; Liu, D.; Zhou, X. Drought-induced changes in rare microbial community promoted contribution of microbial necromass C to SOC in a subtropical forest. Soil Biol. Biochem. 2024, 189, 109252. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Ma, T.; Lv, W.; Zhang, Z.; Shen, Y.; Yang, Q.; Wang, X.; Li, J.; Xiang, Q.; et al. Effects of short-term drought, nitrogen application and their interactions on the composition and functional genes of soil microbial communities in alfalfa grassland on the Loess Plateau. Front. Sustain. Food Syst. 2023, 7, 1332683. [Google Scholar] [CrossRef]
- Ares, Á.; Brisbin, M.M.; Sato, K.N.; Martín, J.P.; Iinuma, Y.; Mitarai, S. Extreme storms cause rapid but short-lived shifts in nearshore subtropical bacterial communities. Environ. Microbiol. 2020, 22, 4571–4588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, L.; Xiang, J.; Liao, Q.; Zhang, D.; Liu, J. Response of the microbial community structure to the environmental factors during the extreme flood season in Poyang Lake, the largest freshwater lake in China. Front. Microbiol. 2024, 15, 1362968. [Google Scholar] [CrossRef]
- Shah, A.; Shah, S.; Shah, V. Impact of flooding on the soil microbiota. Environ. Chall. 2021, 4, 100134. [Google Scholar] [CrossRef]
- Idbella, M.; Stinca, A.; El-Gawad, A.M.A.; Motti, R.; Mazzoleni, S.; Bonanomi, G. Windstorm disturbance sets off plant species invasion, microbiota shift, and soilborne pathogens spread in an urban Mediterranean forest. For. Ecol. Manag. 2023, 540, 121058. [Google Scholar] [CrossRef]
- Kan, J. Storm events restructured bacterial community and their biogeochemical potentials. J. Geophys. Res. Biogeosciences 2018, 123, 2257–2269. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Dathe, J.; Davinic, M.; Perry, G.; Wester, D.; Schwilk, D.W.; Rideout-Hanzak, S. Forest wildfire and grassland prescribed fire effects on soil biogeochemical processes and microbial communities: Two case studies in the semi-arid Southwest. Appl. Soil Ecol. 2016, 99, 118–128. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Erickson, T.E.; Martini, D.; Dixon, K.W.; Merritt, D.J. Soil physicochemical and microbiological indicators of short, medium and long term post-fire recovery in semi-arid ecosystems. Ecol. Indic. 2016, 63, 14–22. [Google Scholar] [CrossRef]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Stein dorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; de Menezes, A.B.; Macdonald, L.M.; Toscas, P.; Bissett, A.; Baker, G.; Farrell, M.; Richardson, A.E.; Wark, T.; Thrall, P.H. Wildfire impact: Natural experiment reveals differential short-term changes in soil microbial communities. Soil Biol. Biochem. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Sietiö, O.-M.; Pumpanen, J.; Heinonsalo, J.; Köster, K.; Berninger, F. Wildfire effects on soil bacterial community and its potential functions in a permafrost region of Canada. Appl. Soil Ecol. 2020, 156, 103713. [Google Scholar] [CrossRef]
- Silva, D.E.; Costa, R.M.; Campos, J.R.; Rocha, S.M.; de Araujo Pereira, A.P.; Melo, V.M.; Oliveira, F.A.; de Alcantara Neto, F.; Mendes, L.W.; Araujo, A.S. Short-term restoration practices change the bacterial community in degraded soil from the Brazilian semiarid. Sci. Rep. 2024, 14, 6845. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Y.; Sun, H.; Zhou, J.; Li, R. Reforestation regulated soil bacterial community structure along vertical profiles in the Loess Plateau. Front. Microbiol. 2023, 14, 1324052. [Google Scholar] [CrossRef]
- Zheng, B.; Su, L.; Hui, N.; Jumpponen, A.; Kotze, D.J.; Lu, C.; Pouyat, R.; Szlavecz, K.; Wardle, D.A.; Yesilonis, I.; et al. Urbanisation shapes microbial community composition and functional attributes more so than vegetation type in urban greenspaces across climatic zones. Soil Biol. Biochem. 2024, 191, 109352. [Google Scholar] [CrossRef]
- Dong, D.; Guo, Z.; Wu, F.; Yang, X.; Li, J. Plastic residues alter soil microbial community compositions and metabolite profiles under realistic conditions. Sci. Total Environ. 2024, 906, 167352. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; de Medeiros, E.V.; da Costa, D.P.; Pereira, A.P.d.A.; Mendes, L.W. From desertification to restoration in the Brazilian semiarid region: Unveiling the potential of land restoration on soil microbial properties. J. Environ. Manag. 2024, 351, 119746. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.C.; Hartmann, M.; Conz, R.F.; Six, J.; Solly, E.F. Drought-induced tree mortality in Scots pine mesocosms promotes changes in soil microbial communities and trophic groups. Appl. Soil Ecol. 2024, 194, 105198. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Lal, R. Soil Erosion and Gaseous Emissions. Appl. Sci. 2020, 10, 2784. [Google Scholar] [CrossRef]
- Li, Z.; Fang, H. Impacts of climate change on water erosion: A review. Earth-Sci. Rev. 2016, 163, 94–117. [Google Scholar] [CrossRef]
- Mo, S.-H.; Zheng, F.-L.; Feng, Z.-Z.; Yi, Y. Effects of soil erosion and deposition on the spatial distribution of soil microbial quantity in Mollisol area of Northeast China. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2022, 33, 685–693. [Google Scholar] [CrossRef]
- Fernández-González, A.J.; Lasa, A.V.; Cobo-Díaz, J.F.; Villadas, P.J.; Pérez-Luque, A.J.; García-Rodríguez, F.M.; Tringe, S.G.; Fernández-López, M. Long-Term Persistence of Three Microbial Wildfire Biomarkers in Forest Soils. Forests 2023, 14, 1383. [Google Scholar] [CrossRef]
- Ramm, E.; Ambus, P.L.; Gschwendtner, S.; Liu, C.; Schloter, M.; Dannenmann, M. Fire intensity regulates the short-term postfire response of the microbiome in Arctic tundra soil. Geoderma 2023, 438, 116627. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, A.; Singh, V.K.; Minkina, T.M.; Sushkova, S. Impact of nanoparticles on soil resource. In Nanomaterials for Soil Remediation; Amrane, A., Mo-han, D., Nguyen, T.A., Assadi, A.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 65–85. [Google Scholar]
- Han, L.; Chen, L.; Feng, Y.; Kuzyakov, Y.; Chen, Q.; Zhang, S.; Chao, L.; Cai, Y.; Ma, C.; Sun, K.; et al. Microplastics alter soil structure and microbial community composition. Environ. Int. 2024, 185, 108508. [Google Scholar] [CrossRef]
- Ko, K.; Chung, H.; Kim, W.; Kim, M.-J. Effects of different sizes of polystyrene micro(nano)plastics on soil microbial communities. NanoImpact 2023, 30, 100460. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Y.; Du, S.; Li, Y.; Wang, J. Nanoplastics promote the dissemination of antibiotic resistance genes and diversify their bacterial hosts in soil. Eco-Environ. Health 2024, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Yan, Y.; Doyle, E.; Zhu, C.; Jin, X.; Chen, Z.; Wang, C.; He, H.; Zhou, D.; Gu, C. Microplastics altered soil microbiome and nitrogen cycling: The role of phthalate plasticizer. J. Hazard. Mater. 2022, 427, 127944. [Google Scholar] [CrossRef]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021, 93, 24–32. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, A.; Chen, S.; He, X.; Jin, L.; Yu, X.; Yang, S.; Li, B.; Fan, L.; Ji, L.; et al. Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J. Environ. Manag. 2020, 257, 109980. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xu, J.; Xiang, G.; Cao, Z.; Yan, Q.; Mi, J.; Ma, B.; Zou, Y.; Zhang, N.; Liao, X.; et al. Multiple driving factors contribute to the variations of typical antibiotic resistance genes in different parts of soil-lettuce system. Ecotoxicol. Environ. Safety 2021, 225, 112815. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, X.; Li, Q.; Liu, J.; Ding, J.; Wu, H.; Zhao, Z.; Ba, Y.; Cheng, X.; Cui, L.; et al. Bacterial community structure and abundances of antibiotic resistance genes in heavy metals contaminated agricultural soil. Environ. Sci. Pollut. Res. 2018, 25, 9547–9555. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, Y.; Zheng, L.; Ji, L.; Kong, X.; Du, J.; Wang, H.; Duan, L.; Niu, T.; Liu, J.; et al. Identification and Distribution of Antibiotic Resistance Genes and Antibiotic Resistance Bacteria in the Feces Treatment Process: A Case Study in a Dairy Farm, China. Water 2024, 16, 1575. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, Y.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of heavy metals and antibiotics on antibiotic resistance genes and microbial communities in soil. Process Saf. Environ. Prot. 2023, 169, 418–427. [Google Scholar] [CrossRef]
- Gupta, S.; Graham, D.W.; Sreekrishnan, T.R.; Ahammad, S.Z. Heavy metal and antibiotic resistance in four Indian and UK rivers with different levels and types of water pollution. Sci. Total Environ. 2023, 857, 159059. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhou, J.; Zheng, S.; Yang, Z.; Lu, Z.; Jiang, X.; Zhao, L.; Yan, B.; Yang, X.; Chen, T. Geochemical properties, heavy metals and soil microbial community during revegetation process in a production Pb-Zn tailings. J. Hazard. Mater. 2024, 463, 132809. [Google Scholar] [CrossRef]
- Yang, Z.; Sui, H.; Zhang, T.; Wang, Y.; Song, Y. Response of surface soil microbial communities to heavy metals and soil properties for five different land-use types of Yellow River Delta. Environ. Earth Sci. 2023, 82, 599. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Z.-H.; Wang, Z.-H. Effects of heavy metals on soil microbial community of different land use types. J. Mt. Sci. 2023, 20, 3582–3595. [Google Scholar] [CrossRef]
- Du, P.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Effects of trifluralin on the soil microbial community and functional groups involved in nitrogen cycling. J. Hazard. Mater. 2018, 353, 204–213. [Google Scholar] [CrossRef]
- Feld, L.; Hjelmsø, M.H.; Nielsen, M.S.; Jacobsen, A.D.; Rønn, R.; Ekelund, F.; Krogh, P.H.; Strobel, B.W.; Jacobsen, C.S. Pesticide Side Effects in an Agricultural Soil Ecosystem as Measured by amoA Expression Quantification and Bacterial Diversity Changes. PLoS ONE 2015, 10, e0126080. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Jia, Z.; Conrad, R.; Seeger, M. Simazine application inhibits nitrification and changes the ammonia-oxidiing bacterial communities in a fertilized agricultural soil. FEMS Microbiol. Ecol. 2011, 78, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Reiß, F.; Kiefer, N.; Purahong, W.; Borken, W.; Kalkhof, S.; Noll, M. Active soil microbial composition and proliferation are directly affected by the presence of biocides from building materials. Sci. Total Environ. 2024, 912, 168689. [Google Scholar] [CrossRef]
- Tan, H.; Xu, M.; Li, X.; Zhang, H.; Zhang, C. Effects of chlorimuron-ethyl application with or without urea fertilization on soil ammonia-oxidizing bacteria and archaea. J. Hazard. Mater. 2013, 260, 368–374. [Google Scholar] [CrossRef]
- Ma, C.; Han, L.; Shang, H.; Hao, Y.; Xu, X.; White, J.C.; Wang, Z.; Xing, B. Nanomaterials in agricultural soils: Ecotoxicity and application. Curr. Opin. Environ. Sci. Health 2023, 31, 100432. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Ruraż, K.; Kosewska, O.; Oćwieja, M.; Gorczyca, A. The impact of various forms of silver nanoparticles on the rhizosphere of wheat (Triticum aestivum L.)–Shifts in microbiome structure and predicted microbial metabolic functions. Sci. Total Environ. 2024, 914, 169824. [Google Scholar] [CrossRef]
- da Silva Júnior, A.H.; Mulinari, J.; de Oliveira, P.V.; de Oliveira, C.R.; Júnior, F.W. Impacts of metallic nanoparticles application on the agricultural soils microbiota. J. Hazard. Mater. Adv. 2022, 7, 100103. [Google Scholar] [CrossRef]
- Khalid, M.F.; Khan, R.I.; Jawaid, M.Z.; Shafqat, W.; Hussain, S.; Ahmed, T.; Rizwan, M.; Ercisli, S.; Pop, O.L.; Marc, R.A. Nanoparticles: The plant saviour under abiotic stresses. Nanomaterials 2022, 12, 3915. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Q.; Lu, Y.; Zhang, L.; Bian, X. Insight into the short-term effects of TiO2 nanoparticles on the cultivation of medicinal plants: Comprehensive analysis of Panax ginseng physiological indicators, soil physicochemical properties and microbiome. Sci. Total Environ. 2024, 951, 175581. [Google Scholar] [CrossRef]
- Sim, J.X.; Doolette, C.L.; Vasileiadis, S.; Drigo, B.; Wyrsch, E.R.; Djordjevic, S.P.; Donner, E.; Karpouzas, D.G.; Lombi, E. Pesticide effects on nitrogen cycle related microbial functions and community composition. Sci. Total Environ. 2022, 807, 150734. [Google Scholar] [CrossRef]
- Liu, X.; Wei, H.; Ahmad, S.; Wang, R.; Gao, P.; Chen, J.; Song, Y.; Liu, C.; Ding, N.; Tang, J. Effects and mechanism of microplastics on abundance and transfer of antibiotic resistance genes in the environment—A critical review. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1852–1874. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.; Wu, X.; Zhao, L.; Wang, N.; Mao, D.; Ren, H.; Luo, Y. Antibiotic contamination amplifies the impact of foreign antibiotic-resistant bacteria on soil bacterial community. Sci. Total Environ. 2021, 758, 143693. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhu, L.; Wang, J.; Xing, B. Antibiotic resistance in agricultural soils: Source, fate, mechanism and attenuation strategy. Crit. Rev. Environ. Sci. Technol. 2022, 52, 847–889. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; Li, L.; Liu, J.; Yan, X. Distribution, sources, and potential risks of antibiotic resistance genes in wastewater treatment plant: A review. Environ. Pollut. 2022, 310, 119870. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-F.; Ying, G.-G.; Liu, S.; Zhou, L.-J.; Zhao, J.-L.; Tao, R.; Peng, P.-A. Biological degradation and microbial function effect of norfloxacin in a soil under different conditions. J. Environ. Sci. Health Part B 2012, 47, 288–295. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K. Dissipation of sulfamethoxazole in pasture soils as affected by soil and environmental factors. Sci. Total Environ. 2014, 479, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.; Mezcua, M.; Fernandezalba, A.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, J.; Li, C.; Zhu, Q.; Zhang, Y.; Lichtfouse, E.; Marmier, N. The effect review of various biological, physical and chemical methods on the removal of antibiotics. Water 2022, 14, 3138. [Google Scholar] [CrossRef]
- Sabljić, A.; Güsten, H.; Verhaar, H.; Hermens, J. QSAR modelling of soil sorption. Improvements and systematics of log KOC vs. log KOW correlations. Chemosphere 1995, 31, 4489–4514. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. Hydrolysis of amphenicol and macrolide antibiotics: Chloramphenicol, florfenicol, spiramycin, and tylosin. Chemosphere 2015, 134, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S.; Seibicke, T.; Schulten, H.; Leinweber, P. TECHNICAL REPORTS-Organic Compounds in the Environment-Sorption of Sulfonamide Pharmaceutical Antibiotics on Whole Soils and Particle-Size Fractions. J. Environ. Qual. 2004, 33, 1331–1342. [Google Scholar] [CrossRef]
- Martínez-Hernández, V.; Meffe, R.; López, S.H.; de Bustamante, I. The role of sorption and biodegradation in the removal of acetaminophen, carbamazepine, caffeine, naproxen and sulfamethoxazole during soil contact: A kinetics study. Sci. Total Environ. 2016, 559, 232–241. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci. Total Environ. 2017, 579, 466–473. [Google Scholar] [CrossRef]
- Dalkmann, P.; Willaschek, E.; Schiedung, H.; Bornemann, L.; Siebe, C.; Siemens, J. Long-term Wastewater Irrigation Reduces Sulfamethoxazole Sorption, but Not Ciprofloxacin Binding, in Mexican Soils. J. Environ. Qual. 2014, 43, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, A.; Siemens, J.; Welp, G.; Xue, Q.; Lin, X.; Liu, X.; Amelung, W. Leaching of veterinary antibiotics in calcareous Chinese croplands. Chemosphere 2013, 91, 928–934. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Wang, J. Zn-Fe-CNTs catalytic in situ generation of H2O2 for Fenton-like degradation of sulfamethoxazole. J. Hazard. Mater. 2018, 342, 166–176. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Y.; Jing, M.; Chen, J. Sorption and transport of sulfonamides in soils amended with wheat straw-derived biochar: Effects of water pH, coexistence copper ion, and dissolved organic matter. J. Soils Sediments 2017, 17, 771–779. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Wen, Y.-Y.; Niu, Z.-L.; Yin, K.; Xu, D.-X.; Chen, L.-X. Isolation and characterization of sulfonamide-degrading bacteria Escherichia sp. HS21 and Acinetobacter sp. HS51. World J. Microbiol. Biotechnol. 2012, 28, 447–452. [Google Scholar] [CrossRef]
- Erickson, B.D.; Elkins, C.A.; Mullis, L.B.; Heinze, T.M.; Wagner, R.D.; Cerniglia, C.E. A metallo-β-lactamase is responsible for the degradation of ceftiofur by the bovine intestinal bacterium Bacillus cereus P41. Vet. Microbiol. 2014, 172, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dick, W.A. Growth of soil bacteria, on penicillin and neomycin, not previously exposed to these antibiotics. Sci. Total Environ. 2014, 493, 445–453. [Google Scholar] [CrossRef]
- Xin, Z.; Fengwei, T.; Gang, W.; Xiaoming, L.; Qiuxiang, Z.; Hao, Z.; Wei, C. Isolation, identification and characterization of human intestinal bacteria with the ability to utilize chloramphenicol as the sole source of carbon and energy. FEMS Microbiol. Ecol. 2012, 82, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Nghiem, L.D.; Oh, S. Aerobic biotransformation of the antibiotic ciprofloxacin by Bradyrhizobium sp. isolated from activated sludge. Chemosphere 2018, 211, 600–607. [Google Scholar] [CrossRef]
- Leng, Y.; Bao, J.; Chang, G.; Zheng, H.; Li, X.; Du, J.; Snow, D.; Li, X. Biotransformation of tetracycline by a novel bacterial strain Stenotrophomonas maltophilia DT1. J. Hazard. Mater. 2016, 318, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.A.S.; Lalung, J.; Noman, E.A.; Bala, J.D.; Norli, I. Removal of heavy metals and antibiotics from treated sewage effluent by bacteria. Clean Technol. Environ. Policy 2015, 17, 2101–2123. [Google Scholar] [CrossRef]
- Hong, X.; Zhao, Y.; Zhuang, R.; Liu, J.; Guo, G.; Chen, J.; Yao, Y. Bioremediation of tetracycline antibiotics-contaminated soil by bioaugmentation. RSC Adv. 2020, 10, 33086–33102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, H.; Pei, J.; Liu, F.; Li, D. Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO. Desalination Water Treat. 2016, 57, 19981–19987. [Google Scholar] [CrossRef]
- Cheng, X.; Delanka-Pedige, H.M.; Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.; Smith, G.B.; Nirmalakhandan, N.; Zhang, Y. Removal of antibiotic resistance genes in an algal-based wastewater treatment system employing Galdieria sulphuraria: A comparative study. Sci. Total Environ. 2020, 711, 134435. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; An, W.; Lu, J.; Hu, J.; Yang, M. Antibiotic resistomes in drinking water sources across a large geographical scale: Multiple drivers and co-occurrence with opportunistic bacterial pathogens. Water Res. 2020, 183, 116088. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Yasseen, B.T. Microbial ecology of Qatar, the arabian gulf: Possible roles of microorganisms. Front. Mar. Sci. 2021, 8, 697269. [Google Scholar] [CrossRef]
- Kayal, A.; Mandal, S. Microbial degradation of antibiotic: Future possibility of mitigating antibiotic pollution. Environ. Monit. Assess. 2022, 194, 639. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Suh, S.; Hamon, M.; Hong, J.W. Determination of antibiotic EC50using a zero-flow microfluidic chip based growth phenotype assay. Biotechnol. J. 2015, 10, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Kotwani, A.; Joshi, J.; Kaloni, D. Pharmaceutical effluent: A critical link in the interconnected ecosystem promoting antimicrobial resistance. Environ. Sci. Pollut. Res. 2021, 28, 32111–32124. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 April 2024).
- Lozano-Huntelman, N.A.; Singh, N.; Valencia, A.; Mira, P.; Sakayan, M.; Boucher, I.; Tang, S.; Brennan, K.; Gianvecchio, C.; Fitz-Gibbon, S.; et al. Evolution of antibiotic cross-resistance and collateral sensitivity in Staphylococcus epidermidis using the mutant prevention concentration and the mutant selection window. Evol. Appl. 2020, 13, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Virulence Mech. Bact. Pathog. 2016, 22, 481–511. [Google Scholar] [CrossRef]
- Han, B.; Ma, L.; Yu, Q.; Yang, J.; Su, W.; Hilal, M.G.; Li, X.; Zhang, S.; Li, H. The source, fate and prospect of antibiotic resistance genes in soil: A review. Front. Microbiol. 2022, 13, 976657. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Song, D.; Jiang, L.; Zhang, D.; Sun, Y.; Chen, G.; Xu, H.; Mei, W.; Li, Y.; Luo, C.; et al. Large-scale biogeographical patterns of antibiotic resistome in the forest soils across China. J. Hazard. Mater. 2021, 403, 123990. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, L.; Ashbolt, N.; Wang, X.; Cui, Y.; Zhu, X.; Xu, Y.; Yang, Y.; Mao, D.; Luo, Y. Arctic antibiotic resistance gene contamination, a result of anthropogenic activities and natural origin. Sci. Total Environ. 2018, 621, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ye, M.; Wu, J.; Feng, Y.; Shen, F.; Tian, D.; Liu, K.; Hu, F.; Li, H.; Jiang, X.; et al. Impact of bioaccessible pyrene on the abundance of antibiotic resistance genes during Sphingobium sp.- and sophorolipid-enhanced bioremediation in soil. J. Hazard. Mater. 2015, 300, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, K.; Athiappan, M.; Devarajan, N.; Samykannu, G.; Parray, J.A.; Aruljothi, K.; Shameem, N.; Alqarawi, A.A.; Hashem, A.; Abd_Allah, E.F. Pesticide degrading natural multidrug resistance bacterial flora. Microb. Pathog. 2018, 114, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, M.; Baek, S.; Shin, J.; Shin, J.; Shin, S.G.; Kim, Y.M.; Cho, K.H. Hydrometeorological Influence on Antibiotic-Resistance Genes (ARGs) and Bacterial Community at a Recreational Beach in Korea. J. Hazard. Mater. 2021, 403, 123599. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Z.; Wei, Y.; Chen, T.; Feng, W.; Chen, H. High-throughput profiling of seasonal variations of antibiotic resistance gene transport in a peri-urban river. Environ. Int. 2018, 114, 87–94. [Google Scholar] [CrossRef]
- Molaei, A.; Lakzian, A.; Haghnia, G.; Astaraei, A.; Rasouli-Sadaghiani, M.; Ceccherini, M.T.; Datta, R. Assessment of some cultural experimental methods to study the effects of antibiotics on microbial activities in a soil: An incubation study. PLoS ONE 2017, 12, e0180663. [Google Scholar] [CrossRef] [PubMed]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Aga, D.S.; Lenczewski, M.; Snow, D.; Muurinen, J.; Sallach, J.B.; Wallace, J.S. Challenges in the Measurement of Antibiotics and in Evaluating Their Impacts in Agroecosystems: A Critical Review. J. Environ. Qual. 2016, 45, 407–419. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Wang, J.; Zhou, H.; Jiang, C. The combined effect of sulfadiazine and copper on soil microbial activity and community structure. Ecotoxicol. Environ. Saf. 2016, 134, 43–52. [Google Scholar] [CrossRef]
- Lewe, N.; Hermans, S.; Lear, G.; Kelly, L.T.; Thomson-Laing, G.; Weisbrod, B.; Wood, S.A.; Keyzers, R.A.; Deslippe, J.R. Phospholipid fatty acid (PLFA) analysis as a tool to estimate absolute abundances from compositional 16S rRNA bacterial metabarcoding data. J. Microbiol. Methods 2021, 188, 106271. [Google Scholar] [CrossRef] [PubMed]
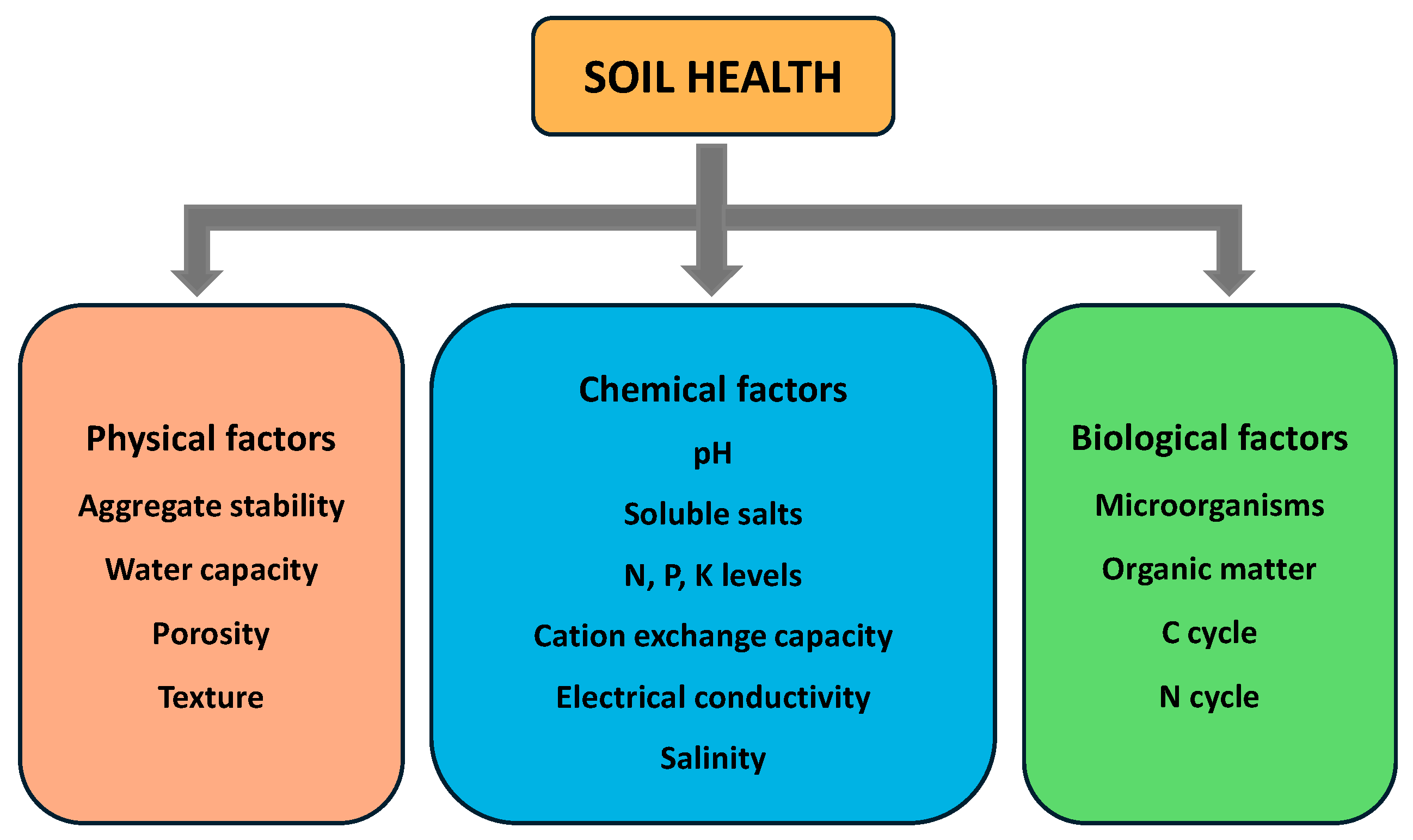
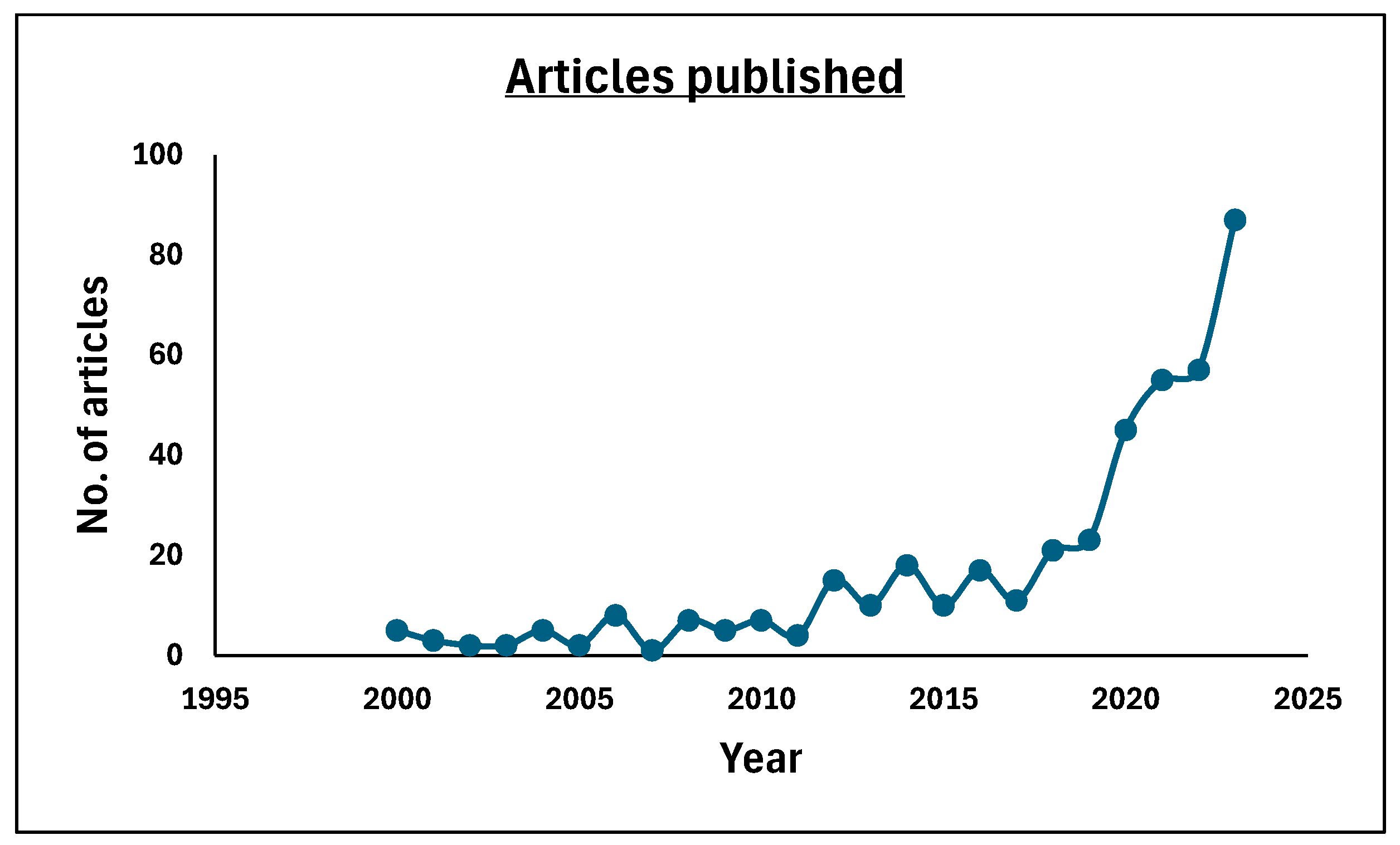


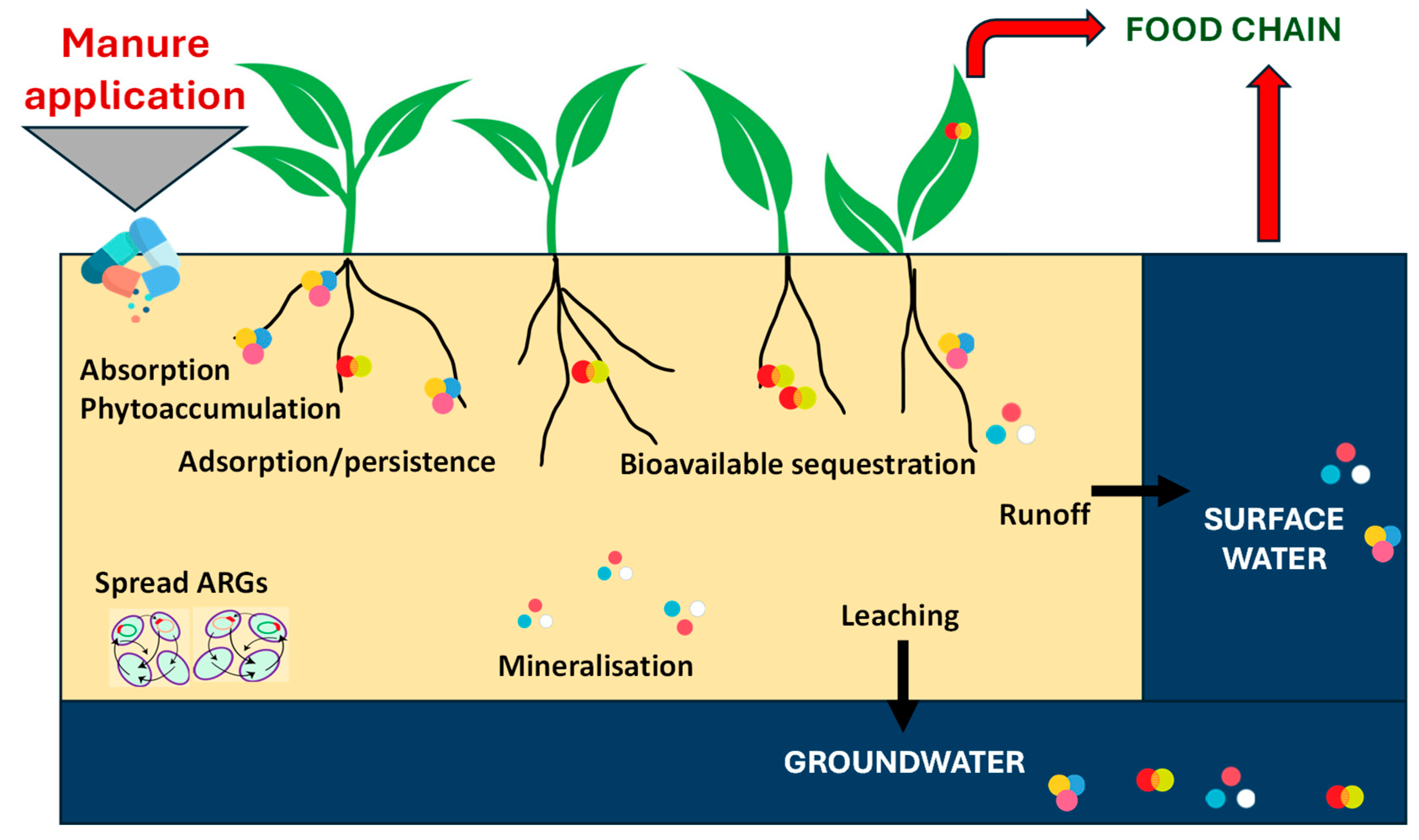
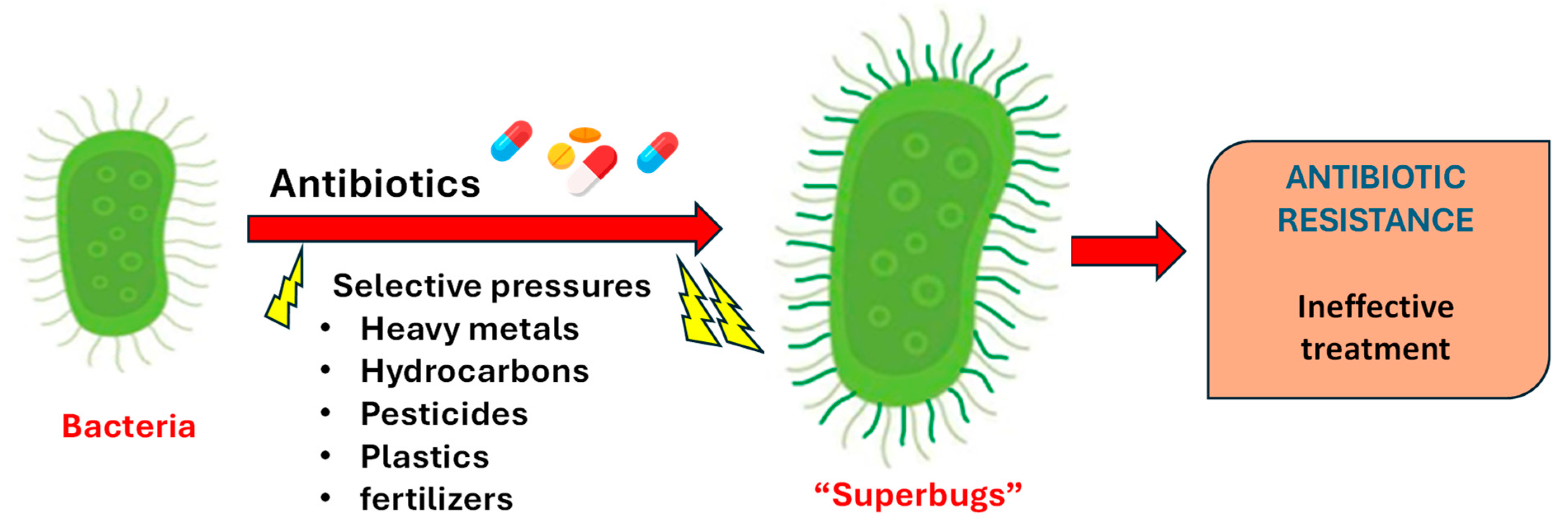
| Factors Affecting Microbial Community | Comments | References |
|---|---|---|
| Temperature | Directly or indirectly affect microbial community, complexity, community composition, stability of their interaction, and nutrient cycling (C, P, K, N). | [25,26,27,28,29] |
| Seasonal dynamics | Microbial diversity, composition, and function display variations based on the seasons. | [30,31,32,33,34] |
| Aeration | Varied according to the soil depth and properties; oxygenation of soil rhizosphere enhanced soil health and optimized soil microbiome. | [35,36] |
| Elevation | Soil properties, climatic conditions, etc., play a dominant role in structuring bacterial communities along the elevational gradient. | [37,38,39,40] |
| Soil physical properties (soil type, texture, clay content, grain content) | Soil type resulting from land use management practices influence the structure and composition of microbial communities. | [41,42,43] |
| Saline alkali degradation/restoration | Saline and alkali degradation has a negative effect on bacterial and fungal community structure; soil amendments increased the abundance of bacteria. | [44,45] |
| Light | Important effects on the microbial community structure and interaction; light structures the phototrophic, bacterial, and fungal communities at the soil surface. | [46,47,48] |
| Soil water content (rainfall, precipitation, moisture etc.) | Modulates microbial abundance and biodiversity. | [49,50,51,52,53,54] |
| Soil pH | Universal factor directly affecting soil microbial diversity, activity, and community composition. | [55,56,57,58] |
| Soil chemical properties (pH, NO3−-N, available phosphorus, C:N:P) | Governs the bacterial and fungal community distribution and composition. | [50,59,60,61] |
| Soil salinity (assessed as electrical conductivity (EC)) | Salinity stress in different saline habitats induces various responses in the soil microbial community and microbial functional genes due to the alterations in soil properties (i.e., low water availability and ionic toxicity). | [62,63,64,65] |
| Soil depth | Soil microbial diversity decreased with depth in soils. | [66,67,68,69,70,71] |
| Soil nutrient availability | Enriched various microbial taxa and communities and their interconnections across soil depth. | [67,72,73] |
| Abiotic Stressors Influencing Microbial Community | Remarks | References |
|---|---|---|
| Drought/water stress | Significantly altered microbial community composition and diversity, enriched the genes controlling biogeochemical cycles and metabolism. | [91,92,93,94] |
| Flood/submergence | Dynamic distribution of nutrients and microorganisms. | [94,95,96] |
| Storm/hurricane | Physico-chemical and community changes after storm; other run-off components during storm impacted the soil microbiome. | [97,98,99] |
| Wildfire | The impact of fire and recovery may vary from days to months to year. Microbial communities vary in burned and unburned areas. | [100,101,102,103,104] |
| Habitat degradation (deforestation, land conversion, urbanization) | Degradation of natural habitats, reducing biodiversity and altering ecosystem structure and function. | [105,106,107] |
| Restoration of degraded areas | Enhanced the soil fertility and the soil microbial properties; increased generalist microbes. | [105,108] |
| Soil Contaminants | Remarks | References |
|---|---|---|
| Plastic (microplastics (MPs)/nanoplastics) | Influence on the bacterial community depends on the characteristics (size, type, composition, concentration) of the MPs. | [117,118,119,120,121,122] |
| Antibiotics | Abundance of the bacterial community composition and functions altered. | [93,123,124,125,126] |
| Heavy metals | Spatial distribution of the microbiome varies in accordance with the land use types and revegetation. Heavy metals, nutrients, and antibiotics directly/indirectly affect the ARGs and variations in bacteria. | [127,128,129,130,131] |
| Biocides | Shifts in the bacterial–fungal community and functionalities. | [132,133,134,135,136] |
| Manufactured nano materials (MNOs) (nanosensors, nanopesticides, nanofertilizers) | Various MNOs may have negative, positive, or neutral effects on the soil microbiota. | [137,138,139,140] |
| Documents by Year | Antibiotics and Heavy Metals | Antibiotics and Plastics | Antibiotics and Biocides |
|---|---|---|---|
| 2019 | 341 | 252 | 111 |
| 2020 | 386 | 290 | 110 |
| 2021 | 524 | 414 | 148 |
| 2022 | 610 | 439 | 163 |
| 2023 | 616 | 597 | 152 |
| Microorganism | Degraded Antibiotics | % of Degradation | References |
|---|---|---|---|
| Acenitobacter sp. | Sulfathiazole (Sulfonamide) | 45–67% | [161] |
| Bacillus sp. | Ceftiofur (Cephalosporin) | >50% | [162] |
| Escherichia sp. | Sulfapyridine (sulfonamide) | 66–72% | [161] |
| Klebsiella sp. Escherischia sp. | p-Nitroaromatic antibiotic chloramphenicol (CAP) | 10.5%– 45% 95% | [164] |
| Bradyrhizobium sp. | Ciprofloxacin | 70% | [165] |
| Strenotrophomonas sp. | Tetracycline | 11.11% | [166] |
| Bacillus sp. | Penicillin Cefalexin Ampicillin Amoxicillin | 68% 10.62% 22.59% 25.03% | [167] |
| Burkholderia sp. | Tetracycline Methoprim, ciprofloxacin | 82.31 ± 0.62% 79.2 ± 0.32% | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheela, A.R.P.; Khalid, M.F.; Abumaali, D.A.; Alatalo, J.M.; Ahmed, T. Impact of Abiotic Stressors on Soil Microbial Communities: A Focus on Antibiotics and Their Interactions with Emerging Pollutants. Soil Syst. 2025, 9, 2. https://doi.org/10.3390/soilsystems9010002
Rasheela ARP, Khalid MF, Abumaali DA, Alatalo JM, Ahmed T. Impact of Abiotic Stressors on Soil Microbial Communities: A Focus on Antibiotics and Their Interactions with Emerging Pollutants. Soil Systems. 2025; 9(1):2. https://doi.org/10.3390/soilsystems9010002
Chicago/Turabian StyleRasheela, Abdul Rashid P., Muhammad Fasih Khalid, Dana A. Abumaali, Juha M. Alatalo, and Talaat Ahmed. 2025. "Impact of Abiotic Stressors on Soil Microbial Communities: A Focus on Antibiotics and Their Interactions with Emerging Pollutants" Soil Systems 9, no. 1: 2. https://doi.org/10.3390/soilsystems9010002
APA StyleRasheela, A. R. P., Khalid, M. F., Abumaali, D. A., Alatalo, J. M., & Ahmed, T. (2025). Impact of Abiotic Stressors on Soil Microbial Communities: A Focus on Antibiotics and Their Interactions with Emerging Pollutants. Soil Systems, 9(1), 2. https://doi.org/10.3390/soilsystems9010002









