Evaluation of Industrial Hemp Cultivar and Biochar Rate to Remediate Heavy-Metal-Contaminated Soil from the Tar Creek Superfund Site, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection, Processing, and Analyses
2.2. Treatments Evaluated and Experimental Design
2.3. Biochar Characterization
2.4. Pot Preparation
2.5. Initial Seeding, Plant Establishment, and Pot Management
2.6. Plant Watering Scheme
2.7. Plant Measurements and Sample Collection, Processing, and Analyses
2.8. Biochar Collection, Processing, and Analyses
2.9. Statistical Analyses
3. Results and Discussion
3.1. Initial Soil Properties
3.2. Aboveground and Belowground Plant Response
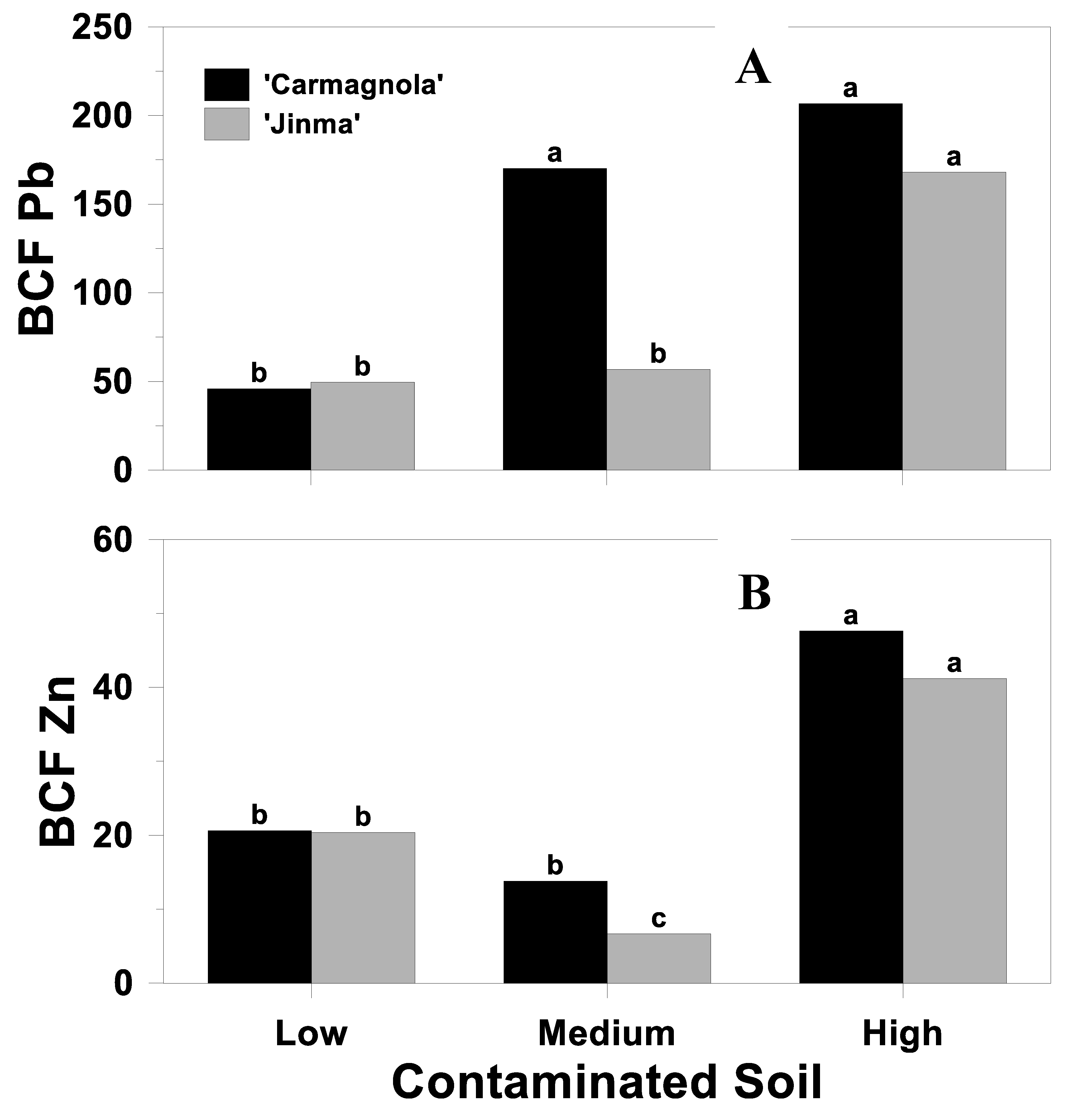
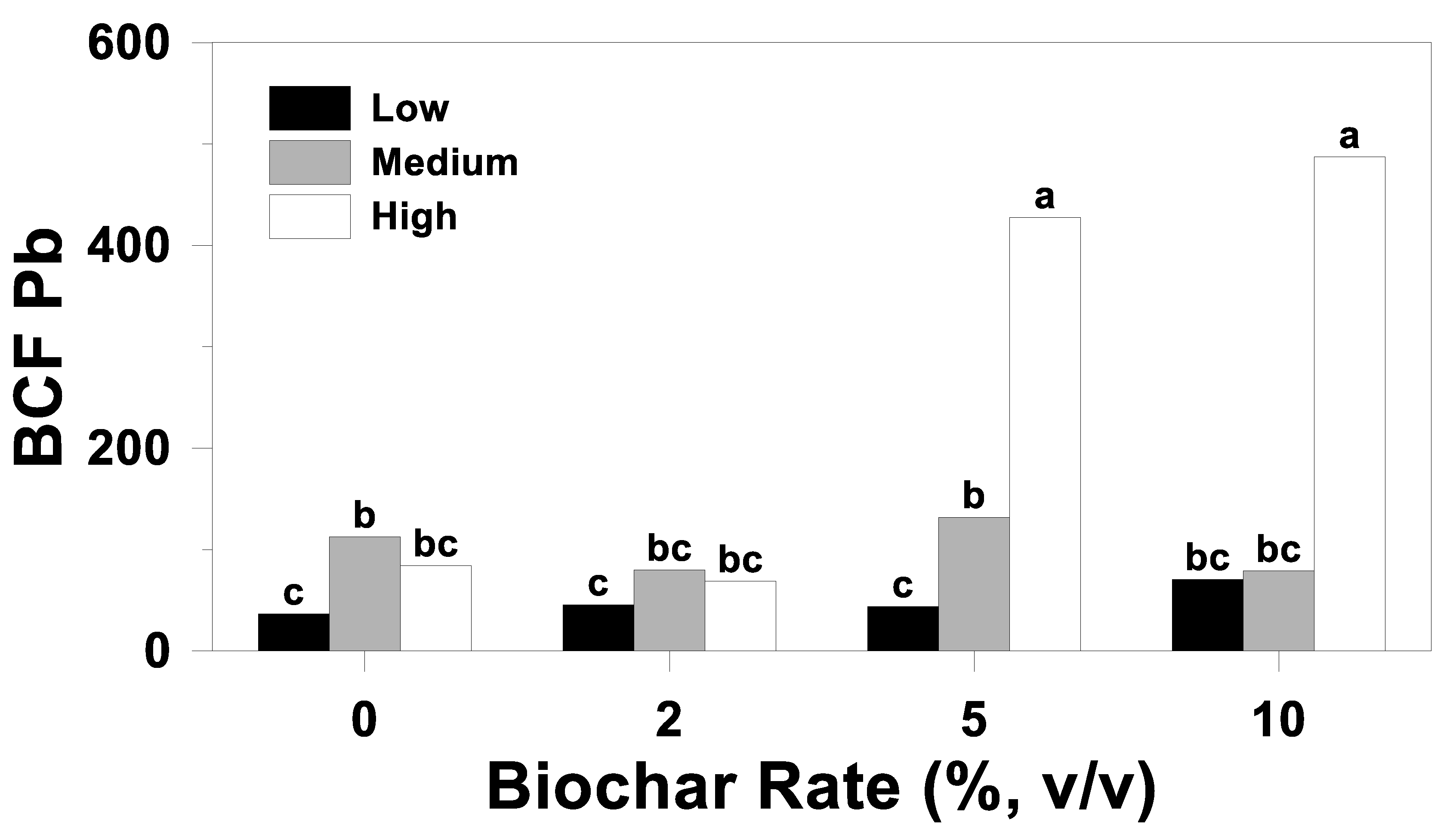
3.3. Bioconcentration Factor
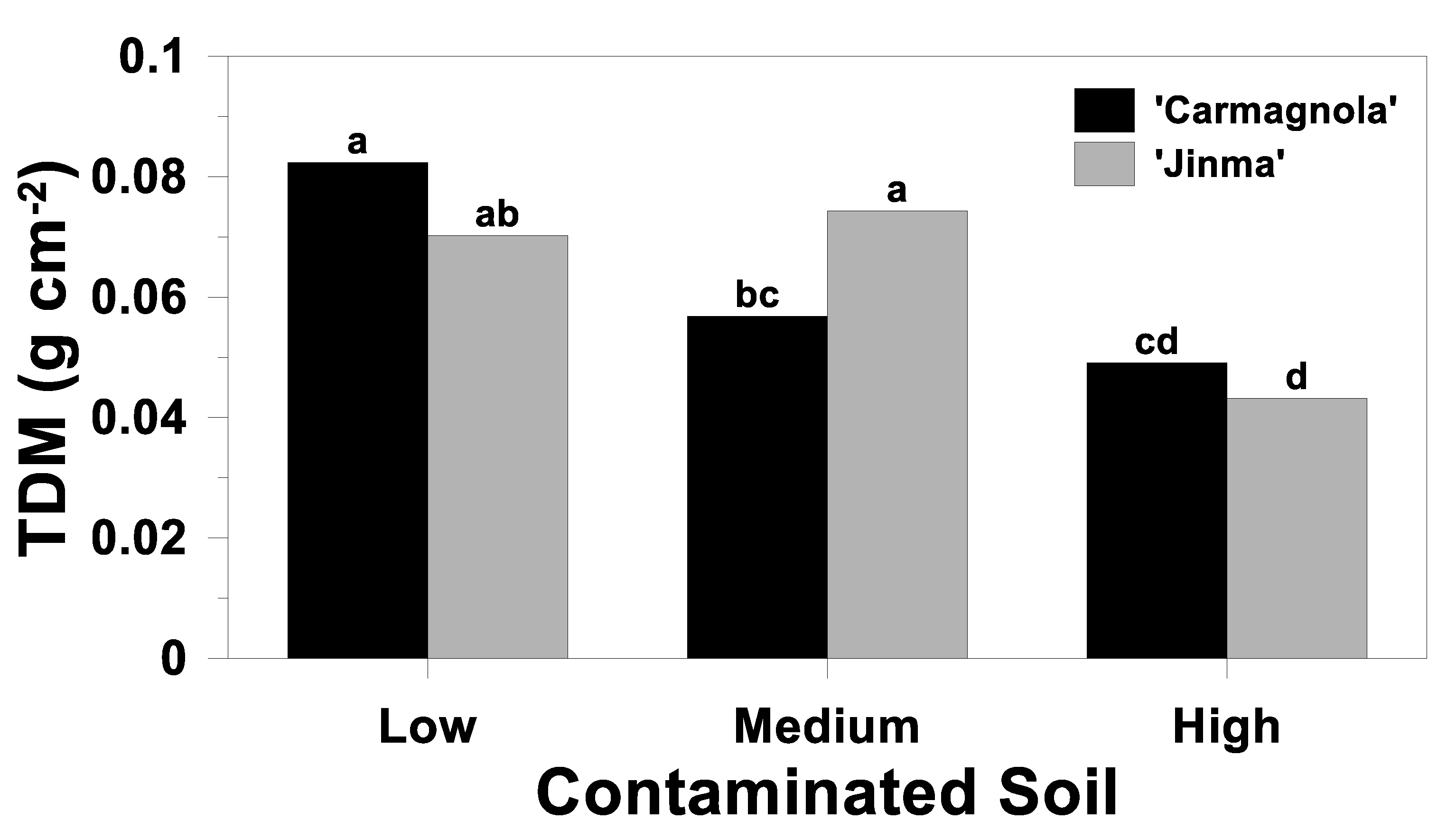
3.4. Whole-Plant Response
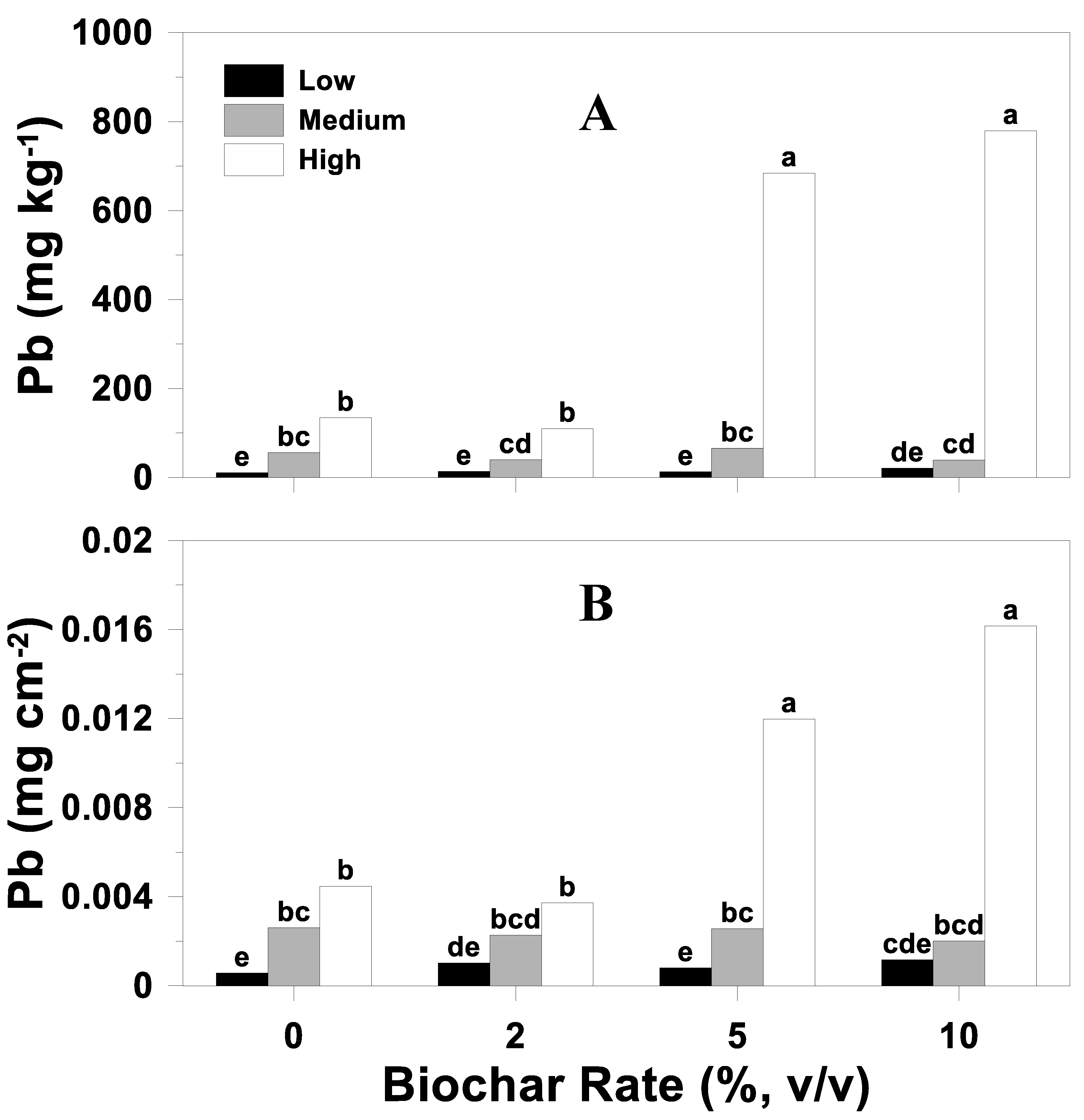
3.5. Biochar Heavy-Metal Response
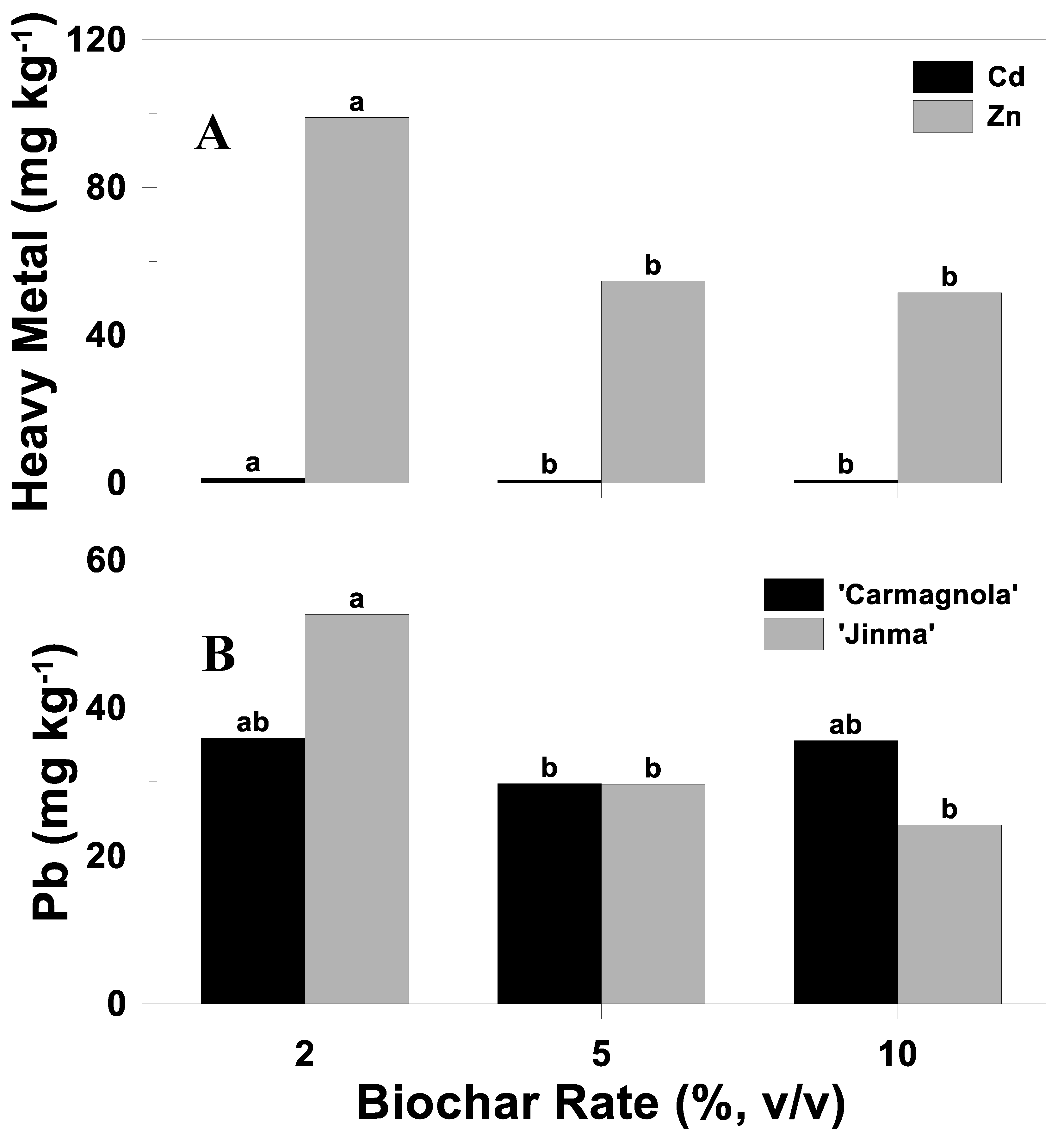
3.5.1. Concentration
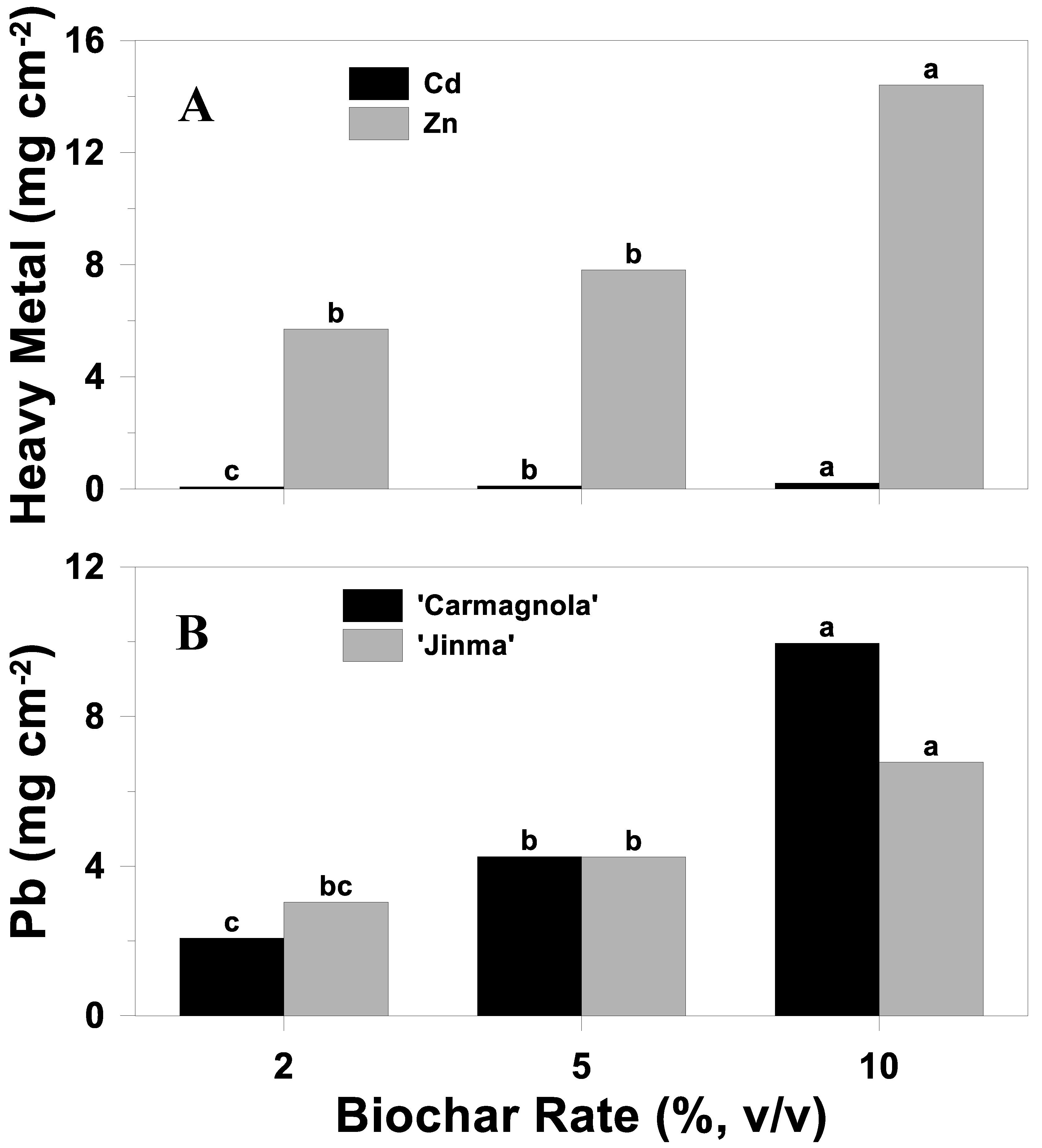
3.5.2. Uptake
4. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKnight, T.; Fischer, R. Geology and Ore Deposits of the Oklahoma and Kansas Picher. Available online: https://pubs.usgs.gov/pp/0588/report.pdf (accessed on 29 September 2024).
- United States Environmental Protection Agency. Tar Creek Superfund Site Strategic Plan, Cleanup Progress, and Plans for the Future. Available online: https://semspub.epa.gov/work/06/100017221.pdf (accessed on 29 September 2024).
- United States Environmental Protection Agency. Review of Peer-Reviewed Documents on Treatment Technologies Used at Mining Waste Sites. Available online: https://semspub.epa.gov/work/HQ/100002899.pdf (accessed on 29 September 2024).
- United States Environmental Protection Agency. Tar Creek (Ottowa County). Available online: https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.Cleanup&id=0601269#bkground (accessed on 29 September 2024).
- Oklahoma Department of Environmental Quality. Tar Creek Superfund Site. Available online: https://www.deq.ok.gov/land-protection-division/cleanup-redevelopment/superfund/tar-creek-superfund-site/ (accessed on 29 September 2024).
- Beattie, R.E.; Henke, W.; Davis, C.; Mottaleb, M.A.; Campbell, J.H.; McAliley, L.R. Quantitative analysis of the extent of heavy-metal contamination in soils near Picher, Oklahoma, within the Tar Creek superfund site. Chemosphere 2017, 172, 89–95. [Google Scholar] [CrossRef]
- Neuberger, J.S.; Hu, S.C.; Drake, K.D.; Jim, R. Potential health impacts of heavy-metal exposure at the Tar Creek Superfund site, Ottowa County, Oklahoma. Environ. Geochem. Health 2009, 31, 47–59. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Superfund: National Priorities List (NPL). Available online: https://www.epa.gov/superfund/superfund-national-priorities-list-npl (accessed on 29 September 2024).
- United States Geological Survey. Acid Mine Drainage. Available online: https://www.usgs.gov/mission-areas/water-resources/science/mine-drainage (accessed on 29 September 2024).
- Schaider, L.A.; Senn, D.B.; Brabander, D.J.; McCarthy, K.D.; Shine, J.P. Characterization of zinc, lead, and cadmium in mine waste: Implications for transport, exposure, and bioavailability. Environ. Sci. Technol. 2007, 41, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E.L.; Rice, P.J.; Rice, P.J.; Anderson, T.A.; Baladi, S.M.; Henderson, K.L.D.; Coats, J.R. Phytoremediation—An overview. Crit. Rev. Plant Sci. 2007, 24, 109–122. [Google Scholar] [CrossRef]
- Salt, D.E.; Blaylock, M.; Kumar, N.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 1995, 13, 468–474. [Google Scholar] [CrossRef]
- Nanda-Kumar, N.A.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef]
- Picchi, C.; Giorgetti, L.; Morelli, E.; Landi, M.; Rosellini, I.; Grifoni, M.; Franchi, E.; Petruzzelli, G.; Barbafieri, M. Cannabis sativa L. and Brassica juncea L. grown on arsenic-contaminated industrial soil: Potentiality and limitation for phytoremediation. Environ. Sci. Pollut. Res. 2021, 11, 15983–15998. [Google Scholar] [CrossRef]
- Stonehouse, G.C.; McCarron, B.J.; Guignardi, A.F.M.; Lima, L.W.; Sirine, C.F.; Pilon-Smits, E.A.H. Selenium metabolism in hemp (Cannabis sativa L.)—Potential for phytoremediation and biofortification. Environ. Sci. Technol. 2020, 54, 4221–4230. [Google Scholar] [CrossRef]
- Ahmad, R.; Tehsin, Z.; Malik, S.T.; Asad, S.A.; Shahzad, M.; Bilal, M.; Shah, M.M.; Khan, A.A. Phytoremediation potential of hemp (Cannabis sativa L.): Identification and characterization of heavy metals responsive genes. CLEAN Soil Air Water 2016, 44, 195–201. [Google Scholar] [CrossRef]
- Nason, S.L.; Stanley, C.J.; Peterpaul, C.E.; Blumenthal, M.F.; Zuverza-Mena, N.; Silliboy, R.J. A community based PFAS phytoremediation project at the former Loring Air Force Base. iScience 2021, 24, 102777. [Google Scholar] [CrossRef]
- Linger, P.; Ostwald, A.; Haensler, J. Cannabis sativa L. growing on heavy metal contaminated soil: Growth, cadmium uptake and photosynthesis. Biol. Plant. 2005, 49, 567–576. [Google Scholar] [CrossRef]
- Awwad, E.; Mabsout, M.; Hamad, B.; Farran, M.T.; Khatib, H. Studies on fiber-reinforced concrete using industrial hemp fibers. Construct. Build. Mat. 2012, 35, 710–717. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Lee, H.S.; Shin, H.S. Competitive adsorption of heavy metals onto modified biochars: Comparison of biochar properties and modification methods. Environ. Manag. 2021, 299, 113651. [Google Scholar] [CrossRef]
- Niu, L.Q.; Jia, P.; Li, S.; Kuang, J.-L.; He, X.; Zhou, W.; Liao, B.; Shu, W.; Li, J. Slash-and-char: An ancient agricultural technique holds new promise for management of soils contaminated by Cd, Pb, and Zn. Environ. Pollut. 2015, 205, 333–339. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.; Lee, S.J.; Bolan, N.; Chung, J.W.; Edraki, M. Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water Air Soil Pollut. 2013, 224, 1711. [Google Scholar] [CrossRef]
- Lao, E.J.; Mbega, E.R. Biochar as a feed additive for improving the performance of farm animals. Malaysian J. Sustain. Agric. 2020, 4, 86–93. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Biochar Technical Report. Available online: https://www.ams.usda.gov/sites/default/files/media/NOPBiocharTechnicalReport.pdf (accessed on 29 September 2024).
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils; Pearson, Pearson, Edinburgh Gate: Harlow, Essex, UK, 2017. [Google Scholar]
- Antonangelo, J.A.; Zhang, H. Heavy metal phytoavailability in a contaminated soil of northeastern Oklahoma as affected by biochar amendment. Environ. Sci. Pollut. Res. 2019, 26, 33582–33593. [Google Scholar] [CrossRef]
- Brye, K.R.; Slaton, N.A. Carbon and nitrogen storage in a typic albaqualf as affected by assessment method. Commun. Soil Sci. Plant Anal. 2003, 34, 1637–1655. [Google Scholar] [CrossRef]
- Gee, W.G.; Or, D. Particle size analysis. In Methods of Soil Analysis, Part 4, Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Sikora, F.J.; Kissel, D.E. Soil pH. In Soil Test Methods from the Southeastern United States; Sikora, F.J., Moore, K.P., Eds.; University of Georgia: Athens, GA, USA, 2014; pp. 48–53. [Google Scholar]
- Wang, J.J.; Provin, T.; Zhang, H. Measurement of soil salinity and sodicity. In Soil Test Methods from the Southeastern United States; Sikora, F.J., Moore, K.P., Eds.; University of Georgia: Athens, GA, USA, 2014; pp. 185–193. [Google Scholar]
- Zhang, H.; Wang, J. Loss on ignition. In Soil Test Methods from the Southeastern United States; Sikora, F.J., Moore, K.P., Eds.; University of Georgia: Athens, GA, USA, 2014; pp. 155–157. [Google Scholar]
- Zhang, H.; Hardy, D.H.; Mylavarapu, R.; Wang, J. Mehlich-3. In Soil Test Methods from the Southeastern United States; Sikora, F.J., Moore, K.P., Eds.; University of Georgia: Athens, GA, USA, 2014; pp. 101–110. [Google Scholar]
- United States Environmental Protection Agency. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. Available online: https://www.epa.gov/esam/epa-method-3050b-acid-digestion-sediments-sludges-and-soils (accessed on 29 September 2024).
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fert. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- McLaughlin, H.; Shields, F.; Jagiello, J.; Thiele, G. Analytical Options for Biochar Adsorption and Surface Area. Available online: https://mcl.mse.utah.edu/wp-content/uploads/sites/84/2024/03/Analytical-Options-for-BioChar-full-paper-2012.pdf (accessed on 29 September 2024).
- ASTM-D1762-84; American Society for Testing and Materials. Standard Test Method for Chemical Analysis of Wood Charcoal. Available online: www.astm.org/catalogsearch/result/?q=d1762 (accessed on 29 September 2024).
- Williams, D.W.; Mundell, R. Agronomic recommendations for Industrial Hemp Production in Kentucky. Available online: https://www.kyagr.com/marketing/documents/HEMP_APP_UK-agronomic-recommendations.pdf (accessed on 29 September 2024).
- University of Arkansas Extension. Wheat Production in Arkansas. Available online: https://www.uaex.uada.edu/farm-ranch/crops-commercial-horticulture/wheat/ (accessed on 29 September 2024).
- Roseberg, R.J.; Jeliazkov, V.D.; Angima, S.D. Soil, Seedbed Preparation and Seeding for Hemp. Available online: https://www.researchgate.net/publication/336232127_Soil_Seedbed_Preparation_and_Seeding_for_Hemp (accessed on 29 September 2024).
- Geneve, R.L.; Janes, E.W.; Kester, S.T.; Hildebrand, D.F.; Davis, D. Temperature limits for seed germination in Industrial Hemp (Cannabis sativa L.). Crops 2022, 2, 415–427. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Soil-Plant-Atmosphere-Water Field, and Pond Hydrology. Available online: https://www.nrcs.usda.gov/resources/tech-tools/spaw-version-602 (accessed on 29 September 2024).
- Huang, Y.L.; Schulte, E.E. Digestion of plant tissue for analysis by ICP emission spectroscopy. Commun. Soil Sci. Plant Anal. 1985, 16, 943–958. [Google Scholar] [CrossRef]
- Zarcinas, B.A.; Cartwright, B.; Spouncer, L. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 1987, 18, 131–146. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, O.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Dalvi, A.A.; Bhalerao, S.A. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Annals Plant Sci. 2013, 2, 362–368. [Google Scholar]
- Farmtiva. Jinma Hemp Fiber Seeds. Available online: www.farmtiva.com/2022-seed-catalog/inma-fiber-seeds (accessed on 29 September 2024).
- Coolong, T.; Bagby, T. Fiber Hemp Production in Georgia. Available online: https://secure.caes.uga.edu/extension/publications/files/pdf/C%201236_1.PDF (accessed on 29 September 2024).
- Candilo, M.D.; Ranalli, P.; Re, L.D. Heavy metal tolerance and uptake of Cd, Pb, and Tl by hemp. Adv. Hort. Sci. 2004, 18, 138–144. [Google Scholar]
- Juraszek, A.M.; Rivier, P.A.; Rasse, D.; Joner, E.J. Biochar affects heavy metal uptake in plants through interactions in the rhizosphere. J. Appl. Sci. 2020, 10, 5105. [Google Scholar] [CrossRef]
- Cui, L.; Pan, G.; Li, L.; Yan, J.; Zhang, A.; Bian, R.; Chang, A. The reduction of wheat Cd uptake in contaminated soil via biochar amendment: A two-year field experiment. BioResources 2012, 7, 5666–5676. [Google Scholar] [CrossRef]
- Fellet, G.; Marchiol, L.; Vedove, G.D.; Peressotti, A. Application of biochar on mine tailings: Effects and perspectives for land reclamation. Chemosphere 2011, 83, 1262–1267. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.-K.; Ye, X.-Z.; Xiao, W.-D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotox. Environ. Safety 2016, 132, 94–100. [Google Scholar] [CrossRef]
- Linger, P.; Mussig, J.; Fischer, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre and phytoremediation potential. Indust. Crops Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468–469, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of biochar on availability and plant uptake of heavy metals—A meta-analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead, and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Ferreiro, J.P.; Lu, H.; Fu, S.; Mendez, A.; Gasco, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Liao, W.; Thomas, S.C. Biochar particle size and post-pyrolysis mechanical processing affect soil pH, water retention capacity, and plant performance. Soil Syst. 2014, 3, 14. [Google Scholar] [CrossRef]
- Sarfraz, R.; Yang, W.; Wang, S.; Zhou, B.; Xing, S. Short term effects of biochar with different particle sizes on phosphorus availability and microbial communities. Chemosphere 2020, 256, 12582. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; Cristina de Melo, I.; Melo, L.C.A.; Magriotis, Z.M.; Sanchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of hemp (Cannabis sativa L.) for paired phytoremediation and bioenergy production. Global Change Biol. Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Sauve, S.; McBride, M.; Hendershot, W. Soil solution speciation of lead (II): Effects of organic matter and pH. Soil Sci. Soc. Am. J. 1998, 62, 618–621. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 14, 752708. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Kim, H.J.; Yoon, J.H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.H. Effect of biochar on heavy metals immobilization and uptake by lettuce (Lactuca sativa L.). Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]



| Soil Property | p-Value | Soil Contamination Level | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Sand (g g−1) | <0.01 | 0.23 c† | 0.44 a | 0.35 b |
| Silt (g g−1) | <0.01 | 0.48 b | 0.45 b | 0.56 a |
| Clay (g g−1) | <0.01 | 0.28 a | 0.11 b | 0.09 b |
| pH | 0.01 | 6.27 b | 6.23 b | 6.53 a |
| Electrical conductivity (dS m−1) | 0.01 | 1.95 a | 1.35 b | 1.32 b |
| Soil organic matter (%) | <0.01 | 3.1 b | 2.1 c | 4.6 a |
| Total C (%) | <0.01 | 0.88 c | 1.12 b | 3.69 a |
| Total N (%) | <0.01 | 0.10 b | 0.07 c | 0.17 a |
| C:N ratio | <0.01 | 9.1 c | 15.9 b | 22.3 a |
| Water-soluble elements (mg kg−1) | ||||
| P | 0.03 | 0.04 b | 0.04 b | 0.07 a |
| K | <0.01 | 20.0 c | 35.0 b | 43.7 a |
| Na | <0.01 | 573 a | 25.0 b | 20.6 c |
| Pb | <0.01 | 0.3 b | 0.5 b | 1.6 a |
| Zn | <0.01 | 5.1 c | 45.1 a | 36.0 b |
| Cd | <0.01 | 0.2 b | 1.3 a | 1.1 a |
| Mehlich-3 extractable elements (mg kg−1) | ||||
| P | <0.01 | 1.0 c | 7.3 a | 5.7 b |
| K | <0.01 | 138 a | 119 b | 88.7 c |
| Na | <0.01 | 595 a | 31.6 b | 24.7 c |
| Pb | <0.01 | 275 c | 587 b | 4197 a |
| Zn | <0.01 | 554 c | 4470 b | 10,092 a |
| Cd | <0.01 | 3.8 c | 24.4 b | 38.8 a |
| Total recoverable elements (mg kg−1) | ||||
| Pb | <0.01 | 308 c | 978 b | 10,251 a |
| Zn | <0.01 | 763 c | 5353 b | 21,179 a |
| Cd | <0.01 | 4.6 c | 34.4 b | 107 a |
| Biochar Property | Mean (+/− Standard Error) |
|---|---|
| Cadmium (mg kg−1) | 0.15 (0.04) |
| Lead (mg kg−1) | 2.5 (0.4) |
| Zinc (mg kg−1) | 15.8 (3.4) |
| pH † | 8.93 |
| Surface area (m2 g−1) ‡ | 308 |
| Total ash (%) †† | 2.1 |
| Source of Variation | Plant Height | AGDM | BGDM | TDM | AG Concentration | AG Uptake | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd | Pb | Zn | |||||
| Soil (S) | <0.01 | <0.01 | 0.41 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cultivar (C) | <0.01 | 0.77 | 0.61 | 0.93 | 0.32 | 0.02 | 0.02 | 0.55 | 0.09 | 0.06 |
| S*C | 0.37 | 0.06 | 0.10 | 0.03 | 0.72 | 0.02 | 0.04 | 0.18 | 0.22 | 0.38 |
| Biochar rate (B) | 0.67 | 0.77 | 0.63 | 0.71 | 0.62 | <0.01 | 0.28 | 0.92 | 0.05 | 0.36 |
| S*B | 0.60 | 0.71 | 0.62 | 0.80 | 0.04 | <0.01 | 0.08 | 0.18 | 0.03 | 0.48 |
| C*B | 0.13 | 0.13 | 0.93 | 0.20 | 0.72 | 0.31 | 0.36 | 0.46 | 0.74 | 0.77 |
| S*C*B | 0.49 | 0.14 | 0.75 | 0.21 | 0.01 | 0.19 | 0.23 | 0.09 | 0.78 | 0.95 |
| Plant Property | Soil | ||
|---|---|---|---|
| Low | Medium | High | |
| Plant height (cm) | 87.6 a† | 78.9 a | 65.5 b |
| Aboveground dry matter (g cm−2) | 0.06 a | 0.05 a | 0.03 b |
| Aboveground Cd (µg cm−2) | 0.084 b | 0.694 a | 0.791 a |
| Aboveground Zn (mg cm−2) | 0.006 c | 0.021 b | 0.044 a |
| Soil | Cultivar | Biochar Rate (%, v/v) | Cd (mg kg−1) | BCF Cd |
|---|---|---|---|---|
| Low | Carmagnola | 0 | 1.7 i† | 8.3 fghi |
| 2 | 1.3 i | 6.6 ghi | ||
| 5 | 1.1 i | 5.5 i | ||
| 10 | 1.7 i | 8.3 fghi | ||
| Jinma | 0 | 1.1 i | 5.3 i | |
| 2 | 1.3 i | 6.3 hi | ||
| 5 | 1.8 i | 8.8 fghi | ||
| 10 | 1.3 i | 6.7 ghi | ||
| Medium | Carmagnola | 0 | 22.8 bcde | 17.5 bcde |
| 2 | 12.2 fgh | 9.4 fghi | ||
| 5 | 13.6 efgh | 10.5 efgh | ||
| 10 | 13.8 efgh | 10.6 efgh | ||
| Jinma | 0 | 15.0 defgh | 11.6 defg | |
| 2 | 15.5 defgh | 11.9 defg | ||
| 5 | 11.9 gh | 9.2 fghi | ||
| 10 | 8.9 h | 6.8 ghi | ||
| High | Carmagnola | 0 | 15.5 defgh | 14.1 cdef |
| 2 | 20.9 cdefg | 19.0 bcde | ||
| 5 | 47.1 a | 42.9 a | ||
| 10 | 33.7 abc | 30.6 ab | ||
| Jinma | 0 | 27.8 abcd | 25.2 abc | |
| 2 | 21.8 cdef | 19.9 bcd | ||
| 5 | 19.6 cdefg | 17.8 bcde | ||
| 10 | 40.6 ab | 36.9 a |
| Source of Variation | BCF | ||
|---|---|---|---|
| Cd | Pb | Zn | |
| Soil (S) | <0.01 | <0.01 | <0.01 |
| Cultivar (C) | 0.32 | 0.02 | 0.02 |
| S*C | 0.72 | 0.02 | 0.04 |
| Biochar rate (B) | 0.62 | <0.01 | 0.28 |
| S*B | 0.04 | <0.01 | 0.08 |
| C*B | 0.72 | 0.31 | 0.36 |
| S*C*B | 0.01 | 0.19 | 0.23 |
| Source of Variation | Biochar Concentration | Biochar Uptake | ||||
|---|---|---|---|---|---|---|
| Cd | Pb | Zn | Cd | Pb | Zn | |
| Soil (S) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cultivar (C) | 0.83 | 0.99 | 0.55 | 0.81 | 0.99 | 0.55 |
| S*C | 0.14 | 0.35 | 0.40 | 0.14 | 0.35 | 0.40 |
| Biochar rate (B) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| S*B | 0.16 | 0.13 | 0.10 | 0.15 | 0.12 | 0.10 |
| C*B | 0.73 | 0.04 | 0.07 | 0.74 | 0.04 | 0.07 |
| S*C*B | 0.26 | 0.54 | 0.47 | 0.28 | 0.54 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurston, D.V.; Brye, K.R.; Miller, D.M.; Moore, P.A., Jr.; Johnson, D.M.; Richardson, M. Evaluation of Industrial Hemp Cultivar and Biochar Rate to Remediate Heavy-Metal-Contaminated Soil from the Tar Creek Superfund Site, USA. Soil Syst. 2024, 8, 114. https://doi.org/10.3390/soilsystems8040114
Thurston DV, Brye KR, Miller DM, Moore PA Jr., Johnson DM, Richardson M. Evaluation of Industrial Hemp Cultivar and Biochar Rate to Remediate Heavy-Metal-Contaminated Soil from the Tar Creek Superfund Site, USA. Soil Systems. 2024; 8(4):114. https://doi.org/10.3390/soilsystems8040114
Chicago/Turabian StyleThurston, Dietrich V., Kristofor R. Brye, David M. Miller, Philip A. Moore, Jr., Donald M. Johnson, and Mike Richardson. 2024. "Evaluation of Industrial Hemp Cultivar and Biochar Rate to Remediate Heavy-Metal-Contaminated Soil from the Tar Creek Superfund Site, USA" Soil Systems 8, no. 4: 114. https://doi.org/10.3390/soilsystems8040114
APA StyleThurston, D. V., Brye, K. R., Miller, D. M., Moore, P. A., Jr., Johnson, D. M., & Richardson, M. (2024). Evaluation of Industrial Hemp Cultivar and Biochar Rate to Remediate Heavy-Metal-Contaminated Soil from the Tar Creek Superfund Site, USA. Soil Systems, 8(4), 114. https://doi.org/10.3390/soilsystems8040114





