Growth Responses of Holcus lanatus L. (Velvet Grass) in Soils Contaminated with Cesium or Strontium
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed
2.2. Soil
2.3. Experimental Design and Treatment Combinations for Hydroponic Experiment
2.4. Data Recording of Seedling Growth
2.5. Experimental Design, Treatment Combinations, and Data Recording for Soil Experiment
2.6. Analysis of Plant Samples
2.7. Statistical Analysis
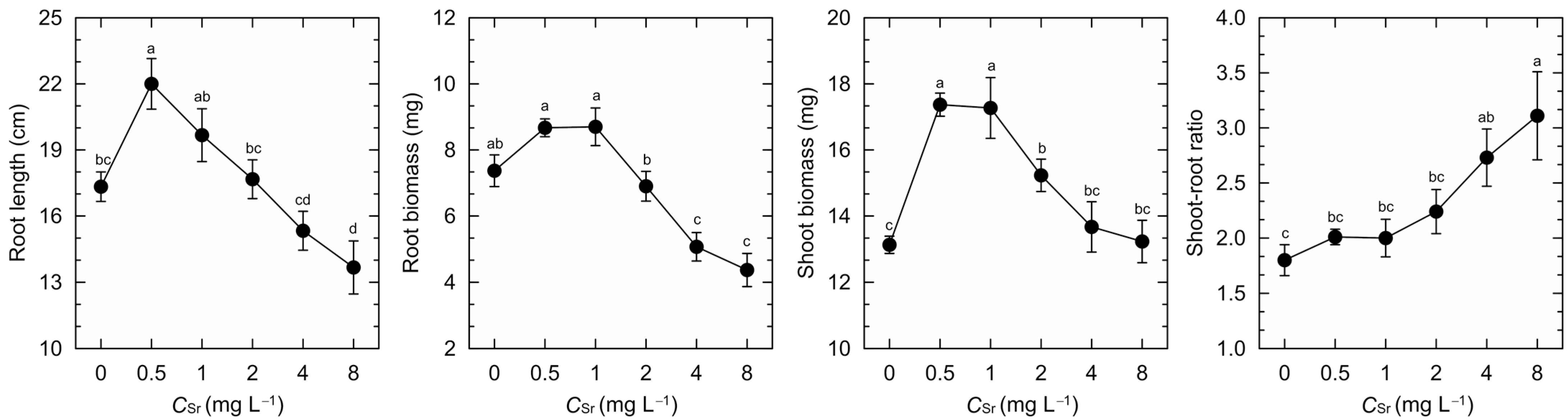
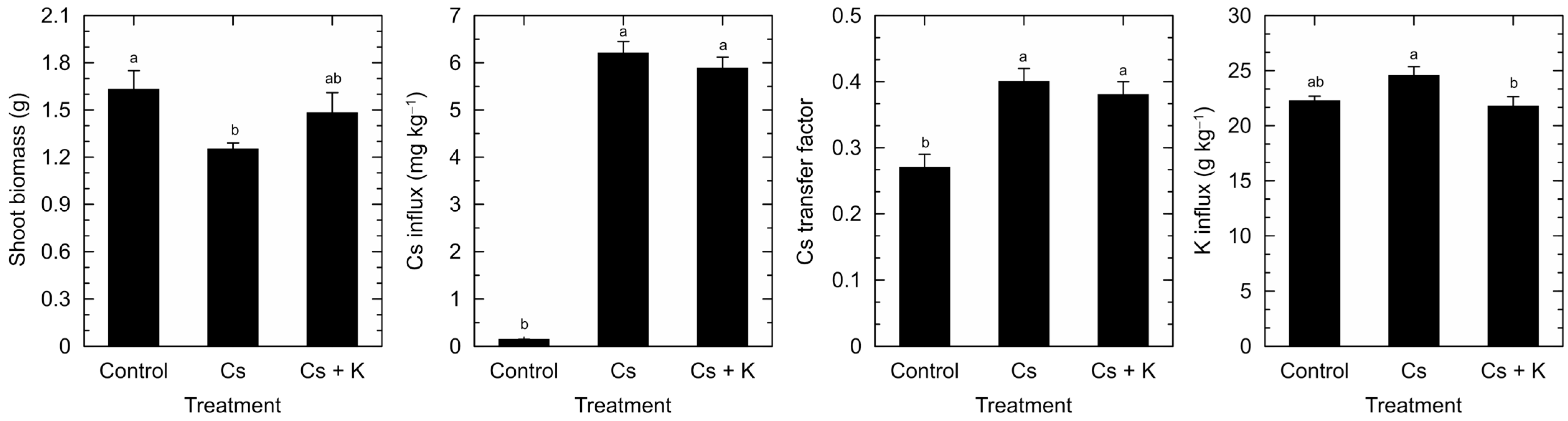
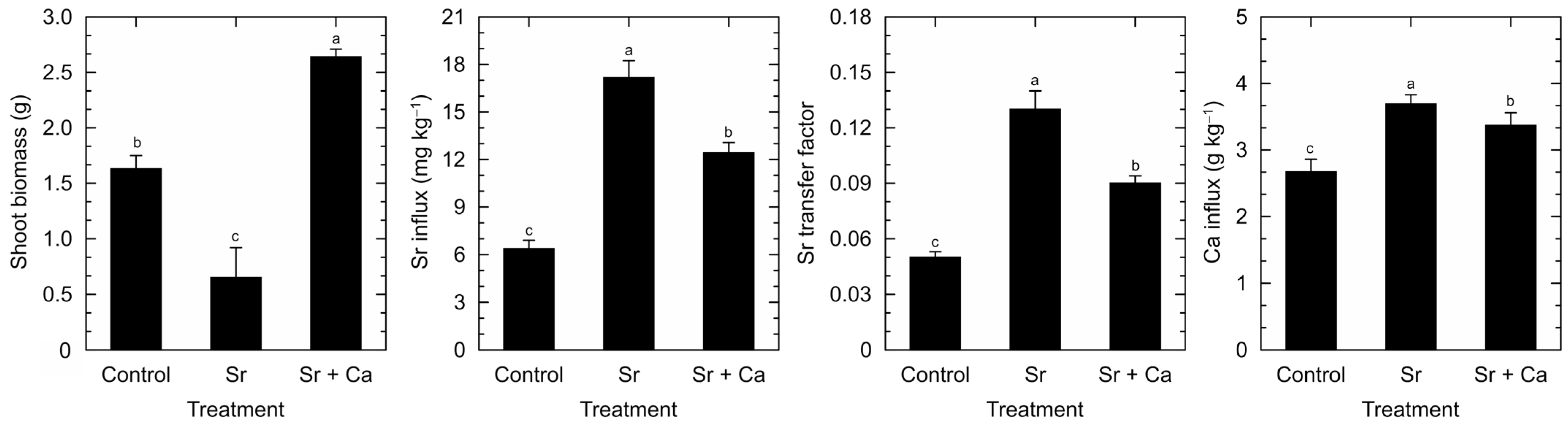
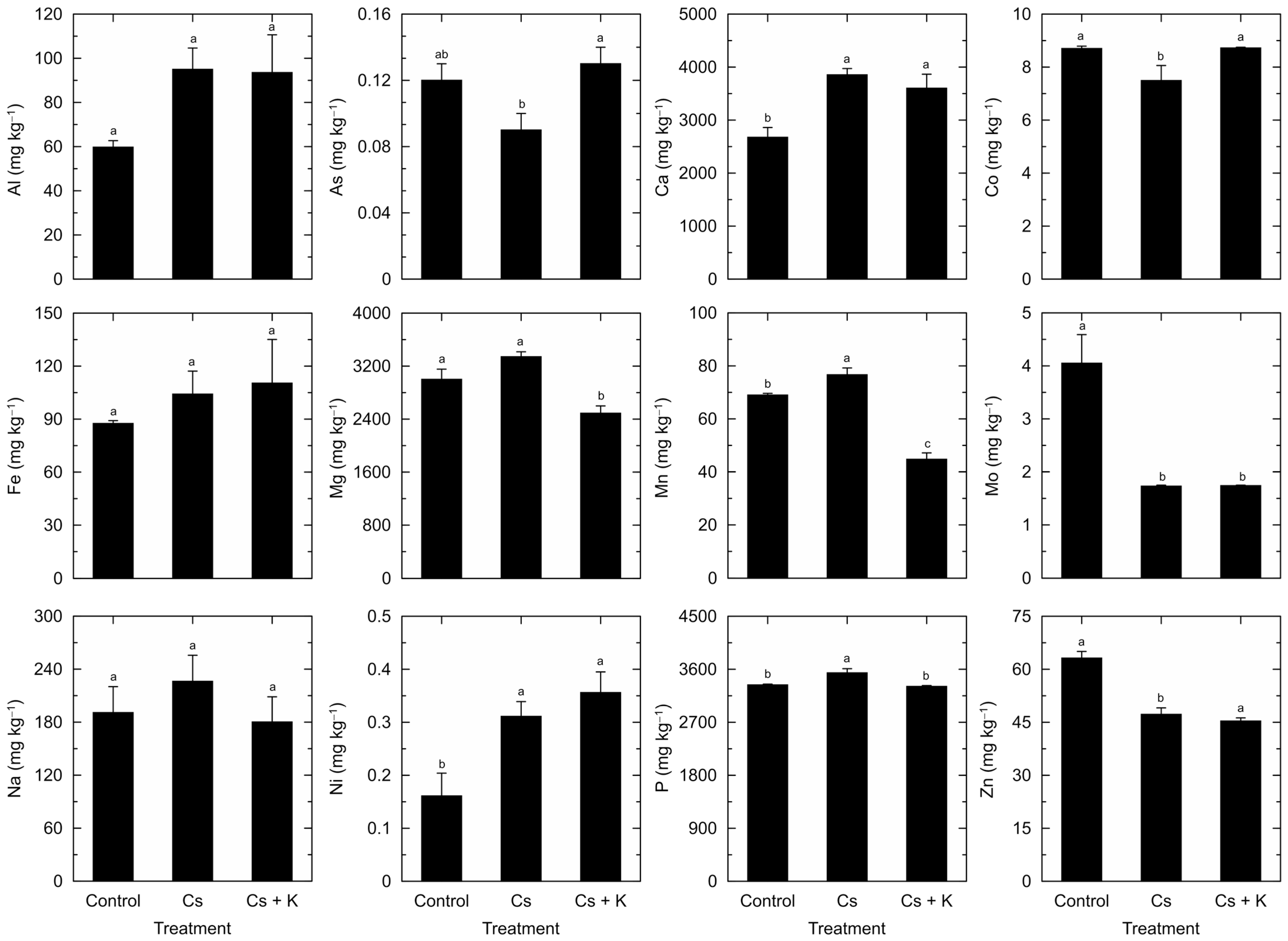
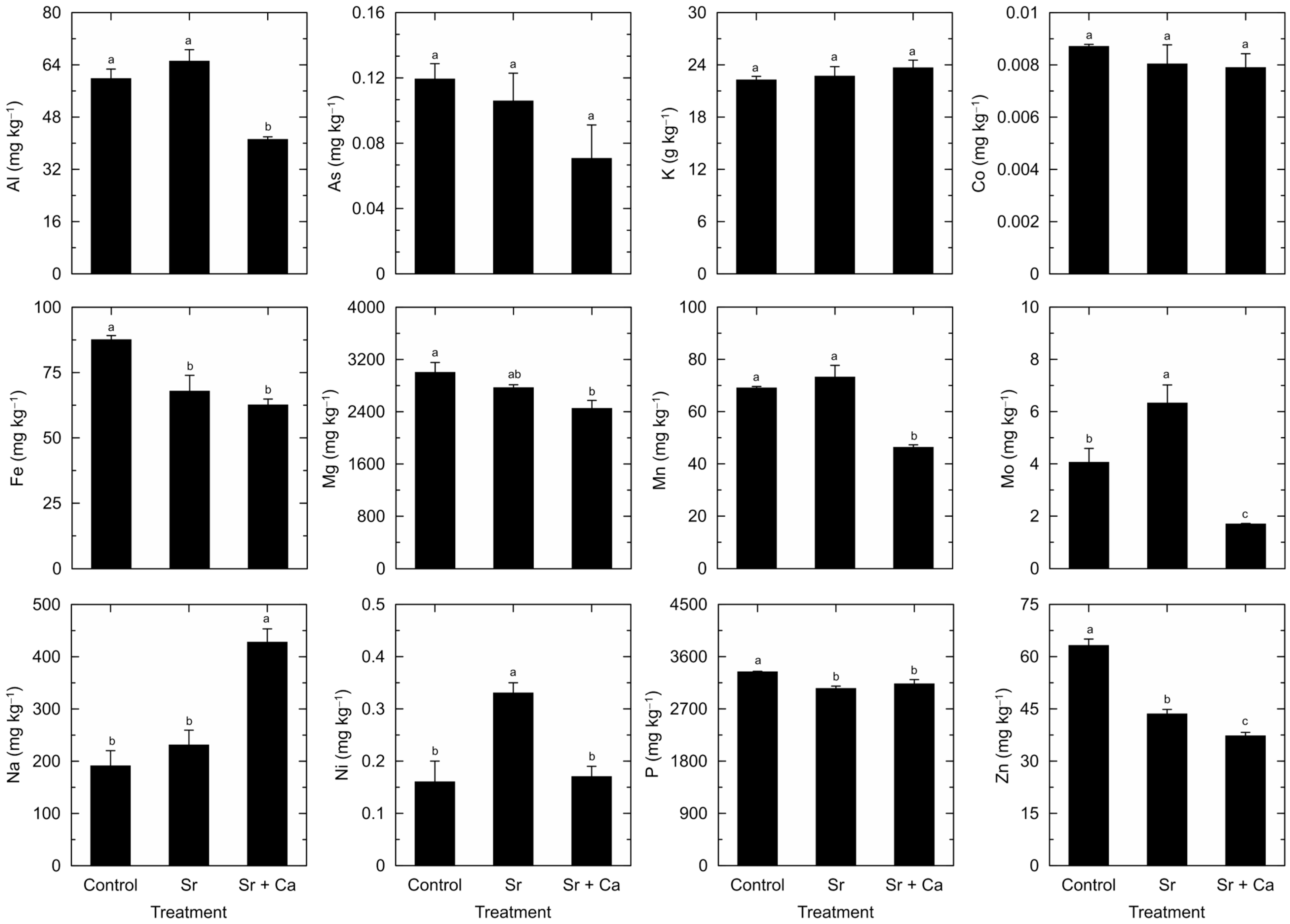
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, D.K.; Schulz, W.; Steinhauser, G.; Walther, C. Radiostrontium transport in plants and phytoremediation. Environ. Sci. Pollut. R. 2018, 25, 29996–30008. [Google Scholar] [CrossRef] [PubMed]
- Topping, C.E.W.; Abella, M.K.I.L.; Berkowitz, M.E.; Molina, M.R.; Nikolić-Hughes, I.; Hughes, E.W.; Ruderman, M.A. In situ measurement of cesium-137 contamination in fruits from the northern Marshall Islands. Proc. Natl. Acad. Sci. USA 2019, 116, 15414–15419. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Matsuzaki, S.-i.S.; Hayashi, S. Different factors determine 137Cs concentration factors of freshwater fish and aquatic organisms in lake and river ecosystems. J. Environ. Radioact. 2020, 213, 106102. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Hayashi, S.; Abe, T.; Igura, M.; Kuramata, M.; Tanikawa, H.; Iino, M.; Saito, T.; Ono, Y.; Ishikawa, T.; et al. Low-cesium rice: Mutation in OsSOS2 reduces radiocesium in rice grains. Sci. Rep. 2017, 7, 2432. [Google Scholar] [CrossRef] [PubMed]
- Mikhailovskaya, L.N.; Modorov, M.V.; Pozolotina, V.N.; Antonova, E.V. Heterogeneity of soil contamination by 90Sr and its absorption by herbaceous plants in the East Ural Radioactive Trace area. Sci. Total Environ. 2019, 651, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Mechanisms of caesium uptake by plants. New Phytol. 2000, 147, 241–256. [Google Scholar] [CrossRef]
- Hampton, J.G.; Tolentino, M.S.; Hill, M.J. Effect of plant growth regulators on seed yield in velvet grass (Holcus lanatus L.) cv. Massey basyn. N. Z. J. Agric. Res. 1992, 35, 35–40. [Google Scholar] [CrossRef]
- Komínková, D.; Berchová-Bímová, K.; Součková, L. Influence of potassium concentration gradient on stable caesium uptake by Calla palustris. Ecotoxicol. Environ. Saf. 2018, 165, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Luo, X.; Liu, B.; Zhou, J.; Feng, J.; Zhu, W.; Wang, S.; Zhang, Y.; Lin, X.; Chen, P. Bayberry tannin immobilized bovine serum albumin nanospheres: Characterization, irradiation stability and selective removal of uranyl ions from radioactive wastewater. J. Mater. Chem. A 2018, 6, 15359–15370. [Google Scholar] [CrossRef]
- Rosén, K.; Vinichuk, M.; Nikolova, I.; Johanson, K. Long-term effects of single potassium fertilization on 137Cs levels in plants and fungi in a boreal forest ecosystem. J. Environ. Radioact. 2011, 102, 178–184. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.-l.; Tao, Z.-y.; Han, N.; Wu, G. Characterizations of bio-accumulations, subcellular distribution and chemical forms of cesium in Brassica juncea, and Vicia faba. J. Environ. Radioact. 2016, 154, 52–59. [Google Scholar] [CrossRef]
- Genies, L.; Orjollet, D.; Carasco, L.; Camilleri, V.; Frelon, S.; Vavasseur, A.; Leonhardt, N.; Henner, P. Uptake and translocation of cesium by Arabidopsis thaliana in hydroponics conditions: Links between kinetics and molecular mechanisms. Environ. Exp. Bot. 2017, 138, 164–172. [Google Scholar] [CrossRef]
- Rinaldi, F.; Komínková, D.; Berchová, K.; Daguenet, J.; Pecharová, E. Stable cesium (133Cs) uptake by Calla palustris from different substrates. Ecotoxicol. Environ. Saf. 2017, 139, 301–307. [Google Scholar] [CrossRef]
- Lai, J.-l.; Luo, X.-g. Comparative transcriptomics analysis of potassium uptake pathways mediated cesium accumulation differences and related molecular mechanisms in Brassica juncea and Vicia faba. Ecotoxicol. Environ. Saf. 2019, 179, 31–39. [Google Scholar] [CrossRef]
- Cline, J.F.; Hungate, F.P. Accumulation of potassium, cesium, and rubidium in bean plants grown in nutrient solutions. Plant Physiol. 1960, 35, 826–829. [Google Scholar] [CrossRef]
- Soudek, P.; Tykva, R.; Vaněk, T. Laboratory analyses of 137Cs uptake by sunflower, reed and poplar. Chemosphere 2004, 55, 1081–1087. [Google Scholar] [CrossRef]
- Marčiulionienė, D.; Lukšienė, B.; Jefanova, O. Accumulation and translocation peculiarities of 137Cs and 40K in the soil—Plant system. J. Environ. Radioact. 2015, 150, 86–92. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Bunkowski, M.; Puschenreiter, M.; Horak, O. Rhizosphere characteristics of indigenously growing nickel hyperaccumulator and excluder plants on serpentine soil. Environ. Pollut. 2003, 123, 131–138. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, Q.; Wang, X. Identification of weed plants excluding the uptake of heavy metals. Environ. Int. 2005, 31, 829–834. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Rahman, I.M.M.; Khan, B.M. Physiological responses of wild grass Holcus lanatus L. to potentially toxic elements in soils: A review. Environ. Sci. Pollut. R. 2023, 30, 54470–54482. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, Q.; Wang, X. Characteristics of 18 species of weed hyperaccumulating heavy metals in contaminated soils. J. Basic Sci. Eng. 2003, 11, 152–159. [Google Scholar]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 01143. [Google Scholar] [CrossRef]
- Jackson, S.A.; Iwata, A.; Lee, S.-H.; Schmutz, J.; Shoemaker, R. Sequencing crop genomes: Approaches and applications. New Phytol. 2011, 191, 915–925. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Robin Buell, C. Advances in plant genome sequencing. Plant J. 2012, 70, 177–190. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yokosho, K.; Kashino, M.; Zhao, F.-J.; Yamaji, N.; Ma, J.F. Adaptation to acidic soil is achieved by increased numbers of cis-acting elements regulating ALMT1 expression in Holcus lanatus. Plant J. 2013, 76, 10–23. [Google Scholar] [CrossRef]
- Meharg, C.; Khan, B.; Norton, G.; Deacon, C.; Johnson, D.; Reinhardt, R.; Huettel, B.; Meharg, A.A. Trait-directed de novo population transcriptome dissects genetic regulation of a balanced polymorphism in phosphorus nutrition/arsenate tolerance in a wild grass, Holcus lanatus. New Phytol. 2014, 201, 144–154. [Google Scholar] [CrossRef]
- Young, E.; Carey, M.; Meharg, A.A.; Meharg, C. Microbiome and ecotypic adaption of Holcus lanatus (L.) to extremes of its soil pH range, investigated through transcriptome sequencing. Microbiome 2018, 6, 48. [Google Scholar] [CrossRef]
- US EPA. Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices (Method 3052); United States Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- Kingston, H.M.; Walter, P.J. The art and science of microwave sample preparation for trace and ultra-trace elemental analysis. In Inductively Coupled Plasma Mass Spectrometry; Montaser, A., Ed.; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Burger, A.; Lichtscheidl, I. Stable and radioactive cesium: A review about distribution in the environment, uptake and translocation in plants, plant reactions and plants’ potential for bioremediation. Sci. Total Environ. 2018, 618, 1459–1485. [Google Scholar] [CrossRef]
- Adams, E.; Miyazaki, T.; Saito, S.; Uozumi, N.; Shin, R. Cesium inhibits plant growth primarily through reduction of potassium influx and accumulation in Arabidopsis. Plant Cell Physiol. 2018, 60, 63–76. [Google Scholar] [CrossRef]
- Mohamed, S.; Sentenac, H.; Guiderdoni, E.; Véry, A.-A.; Nieves-Cordones, M. Internal Cs+ inhibits root elongation in rice. Plant Signal. Behav. 2018, 13, e1428516. [Google Scholar] [CrossRef]
- Hill, J.O.; Simpson, R.J.; Moore, A.D.; Chapman, D.F. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 2006, 286, 7–19. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V.; Horst, W.J.; Martin, P. Root-induced changes in the rhizosphere: Importance for the mineral nutrition of plants. Z. Pflanz. Bodenkunde 1986, 149, 441–456. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Sarath, G.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Gilroy, S.; Jones, D.L. Through form to function: Root hair development and nutrient uptake. Trends Plant Sci. 2000, 5, 56–60. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Gojon, A.; Nacry, P.; Davidian, J.-C. Root uptake regulation: A central process for NPS homeostasis in plants. Curr. Opin. Plant Biol. 2009, 12, 328–338. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol. 2011, 156, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Bolsunovsky, A.; Muratova, E.; Sukovaty, A.; Kornilova, M. The effect of radionuclide and heavy metal contamination of the Yenisei River on cytogenetics of aquatic plant Elodea canadensis. Radioprotection 2009, 44, 83–88. [Google Scholar] [CrossRef]
- Davies, H.S.; Rosas-Moreno, J.; Cox, F.; Lythgoe, P.; Bewsher, A.; Livens, F.R.; Robinson, C.H.; Pittman, J.K. Multiple environmental factors influence 238U, 232Th and 226Ra bioaccumulation in arbuscular mycorrhizal-associated plants. Sci. Total Environ. 2018, 640–641, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Rodkin, O.I.; Ivanukovič, V.A.; Pronko, S.K.; Kresova, E.V. Willow wood production on radionuclide polluted areas. Zb. Matice Srp. Za Prir. Nauk. 2010, 119, 105–113. [Google Scholar] [CrossRef]
- Fokin, A.D.; Torshin, S.P.; Bebneva, Y.M.; Gadzhiagaeva, R.A.; Taldykina, L.G. Role of plants in the spatial differentiation of 137Cs and 90Sr statuses on the aggregate level. Eurasian Soil Sci. 2016, 49, 412–421. [Google Scholar] [CrossRef]
- Prorok, V.V.; Dacenko, O.I.; Bulavin, L.A.; Poperenko, L.V.; White, P.J. Mechanistic interpretation of the varying selectivity of cesium-137 and potassium uptake by radish (Raphanus sativus L.) under field conditions near Chernobyl. J. Environ. Radioact. 2016, 152, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Schulze, E.-D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Carbonell, A.A.; Aarabi, M.A.; DeLaune, R.D.; Gambrell, R.P.; Patrick, W.H., Jr. Arsenic in wetland vegetation: Availability, phytotoxicity, uptake and effects on plant growth and nutrition. Sci. Total Environ. 1998, 217, 189–199. [Google Scholar] [CrossRef]
- Fowler, B.A.; Chou, C.H.S.J.; Jones, R.L.; Chen, C.J. Arsenic. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Academic Press: Burlington, MA, USA, 2007; pp. 367–406. [Google Scholar] [CrossRef]
- Barua, A.; Gupta, S.D.; Mridha, M.A.U.; Bhuiyan, M.K. Effect of arbuscular mycorrhizal fungi on growth of Gmelina arborea in arsenic-contaminated soil. J. For. Res. 2010, 21, 423–432. [Google Scholar] [CrossRef]
- Smith, S.E.; Christophersen, H.M.; Pope, S.; Smith, F.A. Arsenic uptake and toxicity in plants: Integrating mycorrhizal influences. Plant Soil 2010, 327, 1–21. [Google Scholar] [CrossRef]
- Mench, M.; Martin, E. Mobilization of cadmium and other metals from two soils by root exudates of Zea mays L., Nicotiana tabacum L. and Nicotiana rustica L. Plant Soil 1991, 132, 187–196. [Google Scholar] [CrossRef]
- Delvaux, B.; Kruyts, N.; Cremers, A. Rhizospheric mobilization of radiocesium in soils. Environ. Sci. Technol. 2000, 34, 1489–1493. [Google Scholar] [CrossRef]
- Pulhani, V.A.; Dafauti, S.; Hegde, A.G.; Sharma, R.M.; Mishra, U.C. Uptake and distribution of natural radioactivity in wheat plants from soil. J. Environ. Radioact. 2005, 79, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, D.; Thorne, M.C. An investigation into the upward transport of uranium-series radionuclides in soils and uptake by plants. J. Radiol. Prot. 2014, 34, 545. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Lanzirotti, A. 241Am, 137Cs, Sr and Pb uptake by tobacco as influenced by application of Fe chelators to soil. J. Environ. Radioact. 2005, 82, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, H.; Nakamura, Y. Transfer factors of 31 elements in several agricultural plants collected from 150 farm fields in Aomori, Japan. J. Radioanal. Nucl. Chem. 1998, 236, 123–131. [Google Scholar] [CrossRef]
- Connolly, E.L.; Guerinot, M.L. Iron stress in plants. Genome Biol. 2002, 3, reviews1024.1. [Google Scholar] [CrossRef]
- Vigani, G.; Maffi, D.; Zocchi, G. Iron availability affects the function of mitochondria in cucumber roots. New Phytol. 2009, 182, 127–136. [Google Scholar] [CrossRef]
- Jeong, J.; Guerinot, M.L. Homing in on iron homeostasis in plants. Trends Plant Sci. 2009, 14, 280–285. [Google Scholar] [CrossRef]
- Conte, S.S.; Walker, E.L. Transporters contributing to iron trafficking in plants. Mol. Plant 2011, 4, 464–476. [Google Scholar] [CrossRef]
- Khan, B.M.; Deacon, C.; Meharg, C.; Norton, G.; Johnson, D.; Meharg, A.A. A balanced polymorphism in biomass resource allocation controlled by phosphate in grasses screened through arsenate tolerance. Environ. Exp. Bot. 2013, 96, 43–51. [Google Scholar] [CrossRef]
- Afzal, M.; Hindawi, S.E.S.; Alghamdi, S.S.; Migdadi, H.H.; Khan, M.A.; Hasnain, M.U.; Arslan, M.; Habib ur Rahman, M.; Sohaib, M. Potential breeding strategies for improving salt tolerance in crop plants. J. Plant Growth Regul. 2023, 42, 3365–3387. [Google Scholar] [CrossRef]
- Melino, V.; Tester, M. Salt-tolerant crops: Time to deliver. Annu. Rev. Plant Biol. 2023, 74, 671–696. [Google Scholar] [CrossRef]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.-H.; Siddique, K.H.M.; Zhuang, W.; et al. Developing drought-smart, ready-to-grow future crops. Plant Genome 2023, 16, e20279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qin, F. The battle of crops against drought: Genetic dissection and improvement. J. Integr. Plant Biol. 2023, 65, 496–525. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Chaudhry, U.K.; Raza, A.; Charagh, S.; Bakhsh, A.; Bohra, A.; Ali, S.; Chitikineni, A.; Saeed, Y.; Visser, R.G.F.; et al. Developing future heat-resilient vegetable crops. Funct. Integr. Genom. 2023, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.K.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Asati, R.; Chauhan, S.; Tiwari, P.N.; Payasi, D.K. Genome editing and improvement of abiotic stress tolerance in crop plants. Life 2023, 13, 1456. [Google Scholar] [CrossRef] [PubMed]
- Sugita, R.; Kobayashi, N.I.; Hirose, A.; Saito, T.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Visualization of uptake of mineral elements and the dynamics of photosynthates in arabidopsis by a newly developed real-time radioisotope imaging system (RRIS). Plant Cell Physiol. 2016, 57, 743–753. [Google Scholar] [CrossRef]
- Kanno, S.; Yamawaki, M.; Ishibashi, H.; Kobayashi, N.I.; Hirose, A.; Tanoi, K.; Nussaume, L.; Nakanishi, T.M. Development of real-time radioisotope imaging systems for plant nutrient uptake studies. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1501–1508. [Google Scholar] [CrossRef]
- Suzui, N.; Tanoi, K.; Furukawa, J.; Kawachi, N. Recent advances in radioisotope imaging technology for plant science research in Japan. Quantum Beam Sci. 2019, 3, 18. [Google Scholar] [CrossRef]
- Branco, S.; Schauster, A.; Liao, H.-L.; Ruytinx, J. Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol. 2022, 235, 2158–2175. [Google Scholar] [CrossRef] [PubMed]
- Boege, K.; Dirzo, R.; Siemens, D.; Brown, P. Ontogenetic switches from plant resistance to tolerance: Minimizing costs with age? Ecol. Lett. 2007, 10, 177–187. [Google Scholar] [CrossRef] [PubMed]

| Properties | Mean | SD | |
|---|---|---|---|
| Texture | Sand (%) | 64.4 | 0.34 |
| Silt (%) | 33.4 | 0.31 | |
| Clay (%) | 2.2 | 0.12 | |
| pH | 7.15 | 0.02 | |
| Organic matter (%) | 30.69 | 0.33 | |
| Element content (mg kg−1) | Al | 53945 | 3502 |
| As | 3.618 | 0.074 | |
| Ca | 20670 | 1189 | |
| Cd | 0.228 | 0.013 | |
| Co | 1.58 | 0.017 | |
| Cr | 7.37 | 1.304 | |
| Cs | 0.52 | 0.033 | |
| Cu | 79.5 | 4.716 | |
| Fe | 11089 | 491.8 | |
| K | 9232 | 136.8 | |
| Mg | 5318 | 363.5 | |
| Mn | 538.2 | 32.03 | |
| Mo | 1.04 | 0.521 | |
| Na | 3620 | 88.53 | |
| Ni | 6.55 | 0.457 | |
| P | 4035 | 46.56 | |
| Pb | 9.514 | 0.642 | |
| Sr | 119.5 | 6.062 | |
| Zn | 244.8 | 8.758 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, B.M.; Alam, M.F.; Begum, Z.A.; Rahman, I.M.M. Growth Responses of Holcus lanatus L. (Velvet Grass) in Soils Contaminated with Cesium or Strontium. Soil Syst. 2024, 8, 57. https://doi.org/10.3390/soilsystems8020057
Khan BM, Alam MF, Begum ZA, Rahman IMM. Growth Responses of Holcus lanatus L. (Velvet Grass) in Soils Contaminated with Cesium or Strontium. Soil Systems. 2024; 8(2):57. https://doi.org/10.3390/soilsystems8020057
Chicago/Turabian StyleKhan, Bayezid M., M. Ferdous Alam, Zinnat A. Begum, and Ismail M. M. Rahman. 2024. "Growth Responses of Holcus lanatus L. (Velvet Grass) in Soils Contaminated with Cesium or Strontium" Soil Systems 8, no. 2: 57. https://doi.org/10.3390/soilsystems8020057
APA StyleKhan, B. M., Alam, M. F., Begum, Z. A., & Rahman, I. M. M. (2024). Growth Responses of Holcus lanatus L. (Velvet Grass) in Soils Contaminated with Cesium or Strontium. Soil Systems, 8(2), 57. https://doi.org/10.3390/soilsystems8020057







