Biofertilization with Liquid Vermicompost-Activated Biochar Enhances Microbial Activity and Soil Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar and LVE Characteristics
2.2. Formulation of LVE-Activated Biochar and Microbiological Analysis
2.2.1. Biochar Sterilization and Contamination Check
2.2.2. Preparation of Water- and LVE-Activated Biochars
2.2.3. Bacterial Quantification
2.2.4. Bacterial Isolation and Molecular Identification
2.3. pH and EC Determination in LVE-Activated Biochar
2.4. Validation of the Biochar-Activation Protocol on Two Different Soils
2.4.1. Soil Experimental Setup
2.4.2. Soil Recovering
2.4.3. Soil Analyses
2.5. Statistical Analyses
3. Results
3.1. Biochar Sterilization Test
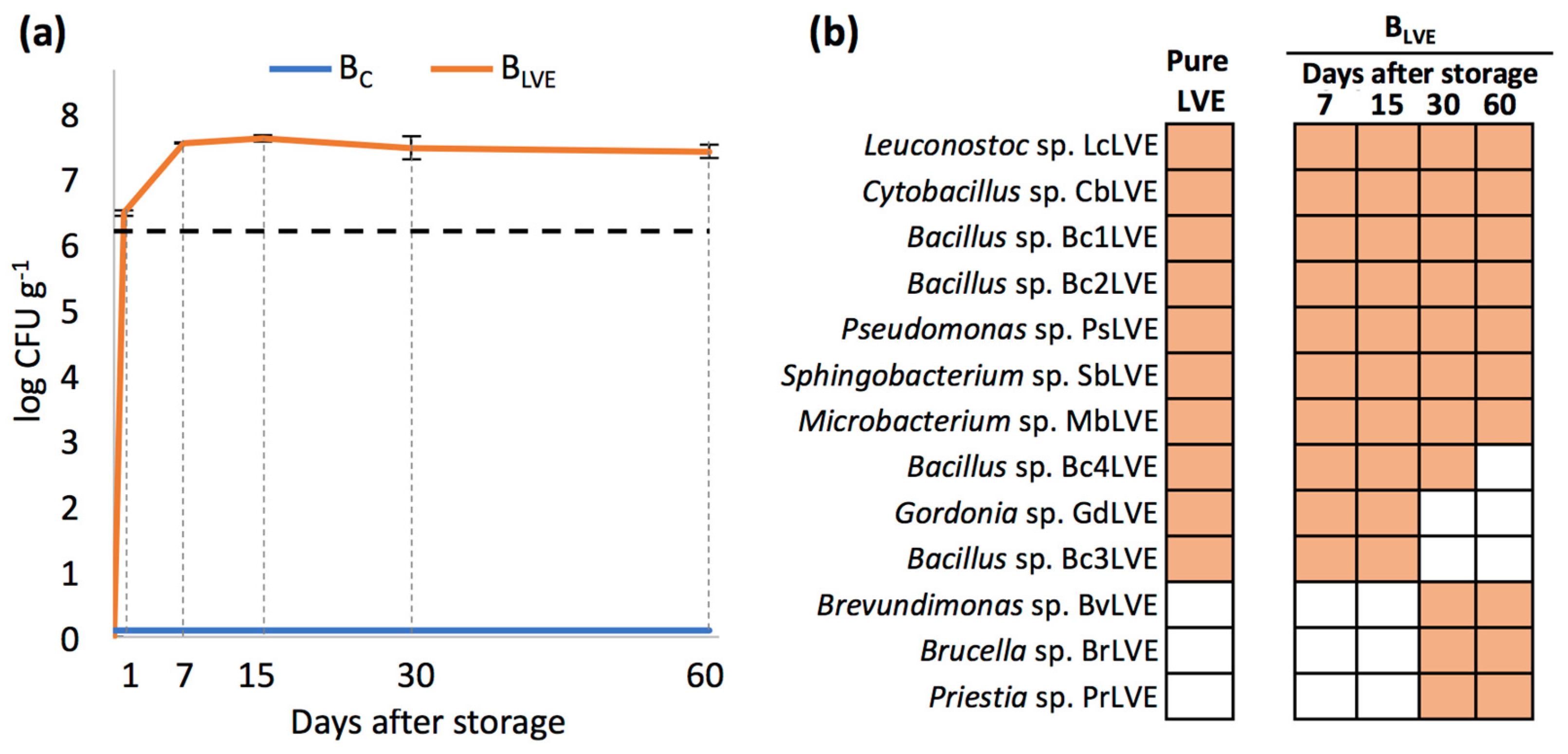
3.2. Quantitative and Qualitative Analyses of Bacteria across BC and BLVE Storage
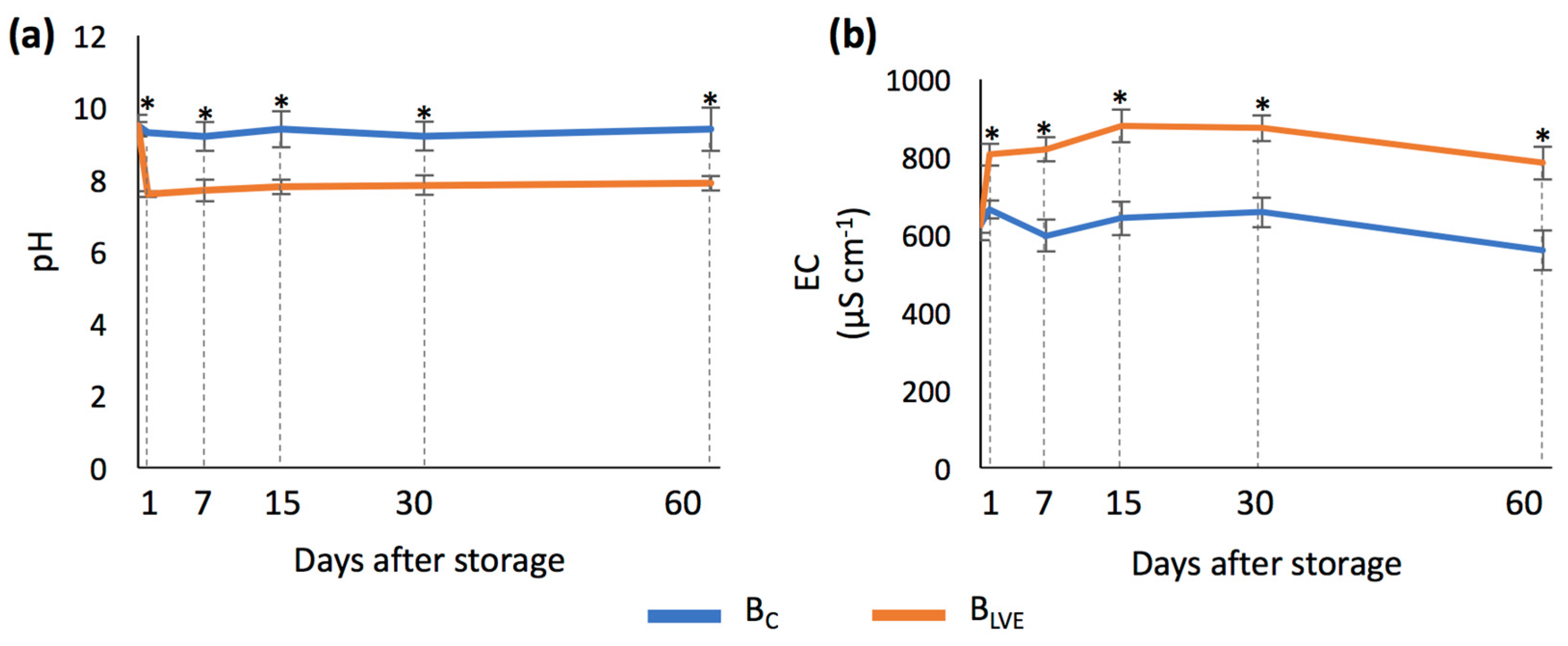
3.3. pH and EC Analyses of BC and BLVE
3.4. Testing the Effect of Increasing Doses on Soil Quality
3.4.1. Carbon Fractions and BFI
3.4.2. Enzyme Activities and SAI3
4. Discussion
4.1. Potential Properties of LVE-Activated Biochar
4.2. Validation of LVE-Activated Biochar on Soil
4.2.1. Effects on Linked C Parameters and Biological Fertility of Soil
4.2.2. Effects on Enzyme Activities and Quality of Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez-Hernandez, J.C.; Ro, K.S.; Díaz, F.J. Biochar and earthworms working in tandem: Research opportunities for soil bioremediation. Sci. Total Environ. 2019, 688, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Nyambo, P.; Zhou, L.; Chuma, T.; Sokombela, A.; Malobane, M.E.; Musokwa, M. Prospects of Vermicompost and Biochar in Climate Smart Agriculture. In Vermicomposting for Sustainable Food Systems in Africa; Springer Nature: Singapore, 2023; pp. 145–159. [Google Scholar]
- Álvarez Bernal, D.; Lastiri Hernández, M.A.; Buelna Osben, H.R.; Contreras Ramos, S.M.; Mora, M. Vermicompost as an alternative of management for water hyacinth. Rev. Int. Contam. 2016, 32, 425–433. [Google Scholar] [CrossRef]
- Koskey, G.; Avio, L.; Turrini, A.; Sbrana, C.; Bàrberi, P. Biostimulatory effect of vermicompost extract enhances soil mycorrhizal activity and selectively improves crop productivity. Plant Soil 2023, 484, 183–199. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Bairwa, H.L.; Kumar, U.; Javed, T.; Asad, M.; Lal, K.; Abdelsalam, N.R. Influence of organic manures on soil nutrient content, microbial population, yield and quality parameters of pomegranate (Punica granatum L.) cv. Bhagwa. PLoS ONE 2022, 17, e0266675. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jha, G. Utilization of microbiome to develop disease resistance in crop plants against phytopathogens. Front. Plant Sci. 2023, 14, 1204896. [Google Scholar] [CrossRef] [PubMed]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Haider, G.; Iqbal, M.; Hameed, S.; Ahmad, N.; ur Rehman, M.Z.; Ali, S. Effect of alkaline and chemically engineered biochar on soil properties and phosphorus bioavailability in maize. Chemosphere 2021, 266, 128980. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Bolan, N. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, G.; Liao, S.; Shan, S.; Wang, G.; Guo, Z. Immobilization of cadmium by immobilized Alishewanella sp. WH16-1 with alginate-lotus seed pods in pot experiments of Cd-contaminated paddy soil. J. Hazard. Mater. 2018, 357, 431–439. [Google Scholar] [CrossRef]
- Quaik, S.; Embrandiri, A.; Rupani, P.F.; Ibrahim, M.H. Potential of vermicomposting leachate as organic foliar fertilizer and nutrient solution in hydroponic culture: A review. In Proceedings of the 2nd International Conference on Environment and BioScience IPCBEE, Phnom Penh, Cambodia, 28–29 September 2012; IACSIT Press: Singapore, 2012; Volume 44, pp. 43–47. [Google Scholar]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Pecina, V. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.D.; Chaves, L.H.; Mendes, J.S.; Chaves, I.B.; Tito, G.A. Alterations in soil salinity with the use of different biochar doses. Rev. Ciênc. 2019, 42, 89–98. [Google Scholar]
- Jahan, S.; Ahmad, F.; Rasul, F.; Amir, R.; Shahzad, S. Physicochemical Analysis of Vermicompost-Perlite Based Activated Biochar and its Influence on Wheat (Triticum aestivum L.) Growth Under Water Stress. J. Soil Sci. Plant Nutr. 2023, 23, 3034–3050. [Google Scholar] [CrossRef]
- Karki, R.; Solberg, S.Ø. Activated BIOCHAR with VERMICOMPOST as an alternative PEAT substitute. Int. J. Biosci. Healthc. Agric. Technol. 2018, 9, 64. [Google Scholar]
- Yuvaraj, A.; Thangaraj, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; Awasthi, M.K.; Kannan, S. Activation of biochar through exoenzymes prompted by earthworms for vermibiochar production: A viable resource recovery option for heavy metal contaminated soils and water. Chemosphere 2021, 278, 130458. [Google Scholar] [CrossRef] [PubMed]
- Becagli, M.; Guglielminetti, L.; Cardelli, R. Effects of Combined Biochar and Vermicompost Solution on Leachate Characterization and Nitrogen Balance from a Greenhouse Tomato (Solanum lycopersicum) Cultivation Soil. Commun. Soil Sci. Plant Anal. 2021, 52, 1879–1893. [Google Scholar] [CrossRef]
- Becagli, M.; Arduini, I.; Cardelli, R. Using Biochar and Vermiwash to Improve Biological Activities of Soil. Agriculture 2022, 12, 178. [Google Scholar] [CrossRef]
- Abbey, L.; Cai, J.; Gunupuru, L.R.; Ijenyo, M.; Esan, E.O.; Lin, S. Nutrient Release Pattern and Greenhouse-Grown Swiss Chard Response to Biochar Inoculated with Vermicast. Int. J. Agron. 2020, 2020, 7852187. [Google Scholar] [CrossRef]
- Dura, O.; Dura, S.; Kepenekci, H. Nematicidal potential of vermicompost tea against Meloidogyne incognita on tomato. In Proceedings of the IV. Balkan Agricultural Congress, Edirne, Turkey, 31 August–2 September 2022; pp. 742–748. [Google Scholar]
- Edenborn, S.L.; Johnson, L.M.; Edenborn, H.M.; Albarran-Jack, M.R.; Demetrion, L.D. Amendment of a hardwood biochar with compost tea: Effects on plant growth, insect damage and the functional diversity of soil microbial communities. Biol. Agric. Hortic. 2018, 34, 88–106. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Mukherjee, A. Application of biochar and vermicompost against the rice root-knot nematode (Meloidogyne graminicola): An eco-friendly approach in nematode management. J. Plant Dis. Prot. 2011, 128, 819–829. [Google Scholar] [CrossRef]
- Brtnicky, M.; Mustafa, A.; Hammerschmiedt, T.; Kintl, A.; Trakal, L.; Beesley, L.; Holatko, J. Pre-activated biochar by fertilizers mitigates nutrient leaching and stimulates soil microbial activity. Chem. Biol. Technol. Agric. 2023, 10, 57. [Google Scholar] [CrossRef]
- Schommer, V.A.; Nazari, M.T.; Melara, F.; Braun, J.C.A.; Rempel, A.; Dos Santos, L.F.; Piccin, J.S. Techniques and mechanisms of bacteria immobilization on biochar for further environmental and agricultural applications. Microbiol. Res. 2023, 278, 127534. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.I.; Franke-Whittle, I.H.; Haindl, S.; Insam, H.; Braun, R. Microbiological community analysis of vermicompost tea and its influence on the growth of vegetables and cereals. Can. J. Microbiol. 2012, 58, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Si, S.; Zhang, Z.; Geng, P.; Shen, Y.; Wang, J.; Zhu, X. Synergistic passivation performance of cadmium pollution by biochar combined with sulfate reducing bacteria. Environ. Technol. Innov. 2023, 32, 103356. [Google Scholar] [CrossRef]
- Ouyang, P.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Luo, Y.; Ma, Y. Integrating biochar and bacteria for sustainable remediation of metal-contaminated soils. Biochar 2023, 5, 63. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, L.; Wang, S.; Dong, M.; Gao, A.; Han, Z.; Zhang, H. Bioremediation of metribuzin-contaminated soil by corn straw biochar-immobilized Bacillus cereus N1. Process Biochem. 2023, 130, 520–533. [Google Scholar] [CrossRef]
- International Biochar Initiative. 2024. Available online: http://www.european-biochar.org/en/ebc-ibi (accessed on 11 January 2024).
- Hristov, A.N.; Callaway, T.R.; Lee, C.; Dowd, S.E. Rumen bacterial, archaeal, and fungal diversity of dairy cows in response to ingestion of lauric or myristic acid. J. Anim. Sci. 2012, 90, 4449–4457. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Wiley: Hoboken, NJ, USA, 1991; pp. 115–175. [Google Scholar]
- Celletti, S.; Bergamo, A.; Benedetti, V.; Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Phytotoxicity of hydrochars obtained by hydrothermal carbonization of manure-based digestate. J. Environ. Manag. 2021, 280, 111635. [Google Scholar] [CrossRef]
- Colombo, C.; Miano, T. Metodi di Analisi Chimica del Suolo; Pubblicità & Stampa: Modugno, BA, USA, 2015. [Google Scholar]
- Levi-Minzi, R.; Riffaldi, R.; Saviozzi, A. Carbon mineralization in soil amended with different organic materials. Agric. Ecosyst. Environ. 1990, 31, 325–335. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass carbon. Soil Biol. Biochem. 1987, 19, 403–707. [Google Scholar] [CrossRef]
- Renzi, G.; Canfora, L.; Salvati, L.; Benedetti, A. Validation of the soil Biological Fertility Index (BFI) using a multidimensional statistical approach: A country-scale exercise. Catena 2017, 149, 294–299. [Google Scholar] [CrossRef]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Renella, G.; Moreno, J.L.; Falchini, L.; Nannipieri, P. Influence of cadmium on the metabolic quotient, L-: D-glutamic acid respiration ratio and enzyme activity: Microbial biomass ratio under laboratory conditions. Biol. Fertil. Soils 2000, 32, 8–16. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Sequi, P.; Benedetti, A.; Dell’Abate, M.T.; ATLAS. Atlante di Indicatori della Qualità del Suolo; Franco Angeli: Milano, Italy, 2006. [Google Scholar]
- Brookes, P.C. The use of microbial parameters inmonitoring soil pollution by heavy metals. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Bloem, J.; Benedetti, A.; Hopkins, D. Microbial Methods Assessing Soil Quality; CABI: London, UK, 2006. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soil. Soil Biol. Biochem. 1997, 9, 167–172. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Puglisi, E.; Del Re, A.A.M.; Rao, M.A.; Gianfreda, L. Development and validation of numerical indexes integrating enzyme activities of soils. Soil Biol. Biochem. 2006, 38, 1673–1681. [Google Scholar] [CrossRef]
- Maron, P.A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Ranjard, L. High microbial diversity promotes soil ecosystem functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, M.; Sun, X.; Li, Z.; Xu, L.; Li, L. Characterization of Nematicidal Activity and Nematode-Toxic Metabolites of a Soilborne Brevundimonas bullata Isolate. Pathogens 2022, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Castelo Sousa, H.; Gomes de Sousa, G.; de Araújo Viana, T.V.; Prudêncio de Araújo Pereira, A.; Nojosa Lessa, C.I.; Pires de Souza, M.V.; Barbosa da Silva, F.D. Bacillus aryabhattai Mitigates the Effects of Salt and Water Stress on the Agronomic Performance of Maize under an Agroecological System. Agriculture 2023, 13, 1150. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Portieles, R.; Du, L.; Gao, X.; Borras-Hidalgo, O. Endophytic bacterium Bacillus aryabhattai induces novel transcriptomic changes to stimulate plant growth. PLoS ONE 2022, 17, e0272500. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, N.; Liang, X.; Huang, T.; Li, B. Bacillus aryabhattai LAD impacts rhizosphere bacterial community structure and promotes maize plant growth. J. Sci. Food Agric. 2022, 102, 6650–6657. [Google Scholar] [CrossRef] [PubMed]
- Dergham, Y.; Sanchez-Vizuete, P.; Le Coq, D.; Deschamps, J.; Bridier, A.; Hamze, K.; Briandet, R. Comparison of the genetic features involved in Bacillus subtilis biofilm formation using multi-culturing approaches. Microorganisms 2021, 9, 633. [Google Scholar] [CrossRef]
- Leathers, T.D.; Bischoff, K.M. Biofilm formation by strains of Leuconostoc citreum and L. mesenteroides. Biotechnol. Lett. 2011, 33, 517–523. [Google Scholar] [CrossRef]
- Majed, R.; Faille, C.; Kallassy, M.; Gohar, M. Bacillus cereus biofilms—Same, only different. Front. Microbiol. 2016, 7, 1054. [Google Scholar] [CrossRef]
- Blanchet, M.; Pringault, O.; Bouvy, M.; Catala, P.; Oriol, L.; Caparros, J.; Joux, F. Changes in bacterial community metabolism and composition during the degradation of dissolved organic matter from the jellyfish Aurelia aurita in a Mediterranean coastal lagoon. Environ. Sci. Pollut. Res. 2015, 22, 13638–13653. [Google Scholar] [CrossRef]
- Gowda, K.; Ping, D.; Mani, M.; Kuehn, S. Genomic structure predicts metabolite dynamics in microbial communities. Cell 2022, 185, 530–546. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Fernández de Córdova, P.; Cebolla-Cornejo, J. Assessment of biochar and hydrochar as minor to major constituents of growing media for containerized tomato production. J. Sci. Food Agric. 2017, 97, 3675–3684. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Plaza, C.; Giannetta, B.; Fernández, J.M.; López-de-Sá, E.G.; Polo, A.; Gascó, G.; Méndez, A.; Zaccone, C. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 150–159. [Google Scholar] [CrossRef]
- Gross, A.; Bromm, T.; Glaser, B. Soil organic carbon sequestration after biochar application: A global meta-analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Becagli, M.; Lauria, G.; Cantini, V.; Ceccanti, C.; Cardelli, R.; Massai, R.; Remorini, D.; Guidi, L.; Landi, M. Biochar as a soil amendment in the tree establishment phase: What are the consequences for tree physiology, soil quality and carbon sequestration? Sci. Total Environ. 2022, 844, 157175. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Wang, P.; Liu, X.; Cheng, K.; Li, L.; Zheng, J.; Zhang, X.; Zheng, J.; Crowley, D.; et al. Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A Meta-analysis. Agric. Ecosyst. Environ. 2017, 239, 80–89. [Google Scholar] [CrossRef]
- Moridi, A.; Zarei, M.; Moosavi, A.A.; Ronaghi, A. Effect of liquid organic fertilizers and soil moisture status on some biological and physical properties of soil. Polish J. Soil Sci. 2021, 54, 41–58. [Google Scholar] [CrossRef]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Liu, X.; Chang, T.; Wang, Q.; Shaghaleh, H.; Hamoud, Y.A. Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front. Environ. Sci. 2023, 10, 1060277. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Y.; Zhou, B.; Wang, X.; Yang, Z.; Li, M.H. Biochar addition increases subsurface soil microbial biomass but has limited effects on soil CO2 emissions in subtropical moso bamboo plantations. Appl. Soil Ecol. 2019, 142, 155–165. [Google Scholar] [CrossRef]
- Weng, Z.H.; Van Zwieten, L.; Singh, B.P.; Kimber, S.; Morris, S.; Cowie, A.; Macdonald, L.M. Plant-biochar interactions drive the negative priming of soil organic carbon in an annual ryegrass field system. Soil Biol. Biochem. 2015, 90, 111–121. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Miyasaka, S.C. Pak Choi (Brassica rapa, Chinensis group) yield, phytonutrient content, and soil biological properties as affected by vermicompost-to-water ratio used for extraction. HortScience 2012, 47, 395–402. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Insam, H.; Haselwandter, K. Metabolic quotient of the soil microflora in relation to plant succession. Oecologia 1989, 79, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.H.; Domsch, K.H. Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol. Fertil. Soils 1985, 1, 81–89. [Google Scholar] [CrossRef]
- Powlson, D.S.; Jenkinson, D.S. A comparison of the organic matter, biomass, adenosine triphosphate, and mineralizable nitrogen contents of ploughed and direct drilled soils. J. Agric. Sci. 1981, 97, 713–721. [Google Scholar] [CrossRef]
- Trivedi, P.; Singh, K.; Pankaj, U.; Verma, S.K.; Verma, R.K.; Patra, D.D. Effect of organic amendments and microbial application on sodic soil properties and growth of an aromatic crop. Ecol. Eng. 2017, 102, 127–136. [Google Scholar] [CrossRef]
- Yilmaz, F.I.; Kurt, S. The effects of biochar and vermicompost applications on some enzyme activities in rhizosphere root zone of corn (Zea mays L.) plant. Comptes Rendus L’académie Bulg. Sci. 2020, 73, 58–65. [Google Scholar]
- Moscatelli, M.C.; Lagomarsino, A.; Marinari, S.; De Angelis, P.; Grego, S. Soil microbial indices as bioindicators of environmental changes in a poplar plantation. Ecol. Indic. 2005, 5, 171–179. [Google Scholar] [CrossRef]
- Luis Moreno, J.; Bastida, F.; Díaz-López, M.; Li, Y.; Zhou, Y.; López-Mondéjar, R.; Benavente-Ferraces, I.; Rojas, R.; Rey, A.; Carlos García-Gil, J.; et al. Response of soil chemical properties, enzyme activities and microbial communities to biochar application and climate change in a Mediterranean agroecosystem. Geoderma 2022, 407, 115536. [Google Scholar] [CrossRef]
- Das, S.K. Soil carbon footprint, budgeting, and dynamics in a biomass conversion-based long-term organic production system. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Lehmann, J. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Gcb Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhanitabar, A.; Maghsoodi, M.R.; Lajayer, B.A.; Chang, S.X. Biochar affects the fate of phosphorus in soil and water: A critical review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef]
- Günal, E.; Erdem, H.; Demirbaş, A. Effects of three biochar types on activity of β-glucosidase enzyme in two agricultural soils of different textures. Arch. Agron. Soil Sci. 2018, 64, 1963–1974. [Google Scholar] [CrossRef]
- Foster, E.J.; Fogle, E.J.; Cotrufo, M.F. Sorption to biochar impacts β-glucosidase and phosphatase enzyme activities. Agriculture 2018, 8, 158. [Google Scholar] [CrossRef]
- Jayaswal, K.; Christian, J.; Singh, N.K.; Padhiyar, H.; Yadav, M.; Sanghvi, G. Effect of vermicompost on soil quality parameters for different land use patterns. IOP Conf. Ser. Earth Environ. Sci. 2023, 1280, 012054. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, J.; Zhao, R.; Dai, H.; Zhang, Z. Application of vermicompost improves strawberry growth and quality through increased photosynthesis rate, free radical scavenging and soil enzymatic activity. Sci. Hortic. 2018, 233, 132–140. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Nogales, R.; Insam, H.; Romero, E.; Goberna, M. Continuous-feeding vermicomposting as a recycling management method to revalue tomato-fruit wastes from greenhouse crops. Waste Manag. 2010, 30, 2461–2468. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Taušnerová, H.; Hanč, A.; Tlustoš, P. Stabilization of different starting materials through vermicomposting in a continuous-feeding system: Changes in chemical and biological parameters. Waste Manag. 2017, 62, 33–42. [Google Scholar] [CrossRef]
- Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Koçak, B. Importance of urease activity in soil. In Proceedings of the International Scientific and Vocational Studies Congress—Science and Health (BILMES SH 2020), Ankara, Turkey, 12–15 December 2020. [Google Scholar]
- Aruna, S.; Manikandavelu, D.; Uma, A.; Antony, C.; Jayakumar, N. Assessment of phosphorus mobilizing capacity of Bacillus spp. isolated from mangrove rhizospheric sediment and its potential application in aquaculture. Indian J. Anim. Res. 2023, 57, 1506–1511. [Google Scholar] [CrossRef]
- Fitriatin, B.N.; Arief, D.H.; Simarmata, T.; Santosa, D.A.; Joy, B. Phosphatase-producing bacteria isolated from Sanggabuana forest and their capability to hydrolyze organic phosphate. J. Soil Sci. Environ. 2011, 2, 299–303. [Google Scholar]
- Hwang, T.K.; Park, Y.J.; Kim, M.J.; Park, M.K.; Kim, M.C.; Jung, M.; Shin, J.H. Isolation of Bacillus aryabhattai GW320 from the Cucumber Rhizospheric Soil and Evaluation of Plant Growth Promoting Activity. Korean J. Soil. Sci. Fert. 2021, 54, 126–139. [Google Scholar] [CrossRef]
- Parhamfar, M.; Badoei-Dalfard, A.; Parhamfar, M.; Fahimi Rad, S. Purification and Characterization of an Extracellular Phosphatase Enzyme From Bacillus spp. J. Cell Mol. Res. 2016, 8, 90–97. [Google Scholar]
- Ramesh, A.; Sharma, S.K.; Joshi, O.P.; Khan, I.R. Phytase, phosphatase activity and P-nutrition of soybean as influenced by inoculation of Bacillus. Indian J. Microbiol. 2011, 51, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.R.; Estrela, A.B.; Nikitin, D.I.; Smit, J.; Vancanneyt, M. Brevundimonas halotolerans sp. nov., Brevundimonas poindexterae sp. nov. and Brevundimonas staleyi sp. nov., prosthecate bacteria from aquatic habitats. Int. J. Syst. Evol. Microbiol. 2010, 60, 1837–1843. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Yaraguppi, D.A.; Mulla, S.I.; Deshpande, S.H. Response surface methodology based optimization, partial purification and characterization of alkaline phosphatase isolated from Pseudomonas asiatica strain ZKB1 and its application in plant growth promotion. Mol. Biotechnol. 2022, 64, 984–1002. [Google Scholar] [CrossRef]
- Paul, D.; Sinha, S.N. Phosphate solubilization potential and phosphatase activity of some bacterial strains isolated from thermal power plant effluent exposed water of river Ganga. CIBTech J. Microbiol. 2013, 2, 1–7. [Google Scholar]
- Riedel, R.; Commichau, F.M.; Benndorf, D.; Hertel, R.; Holzer, K.; Hoelzle, L.E.; Martienssen, M. Biodegradation of selected aminophosphonates by the bacterial isolate Ochrobactrum sp. BTU1. Microbiol. Res. 2024, 280, 127600. [Google Scholar] [CrossRef] [PubMed]
- Baldi, P.C.; Giambartolomei, G.H. Brucella. In Molecular Medical Microbiology; Academic Press: Cambridge, MA, USA, 2024; pp. 1657–1679. [Google Scholar]
- Cheng, W.M.; Liu, J.D.; Feng, Y.; Hu, X.M.; Zhao, Y.Y.; Liu, Y. Study on the cooperation mechanism of urea-hydrolysis bacteria and biosurfactant bacteria for dust suppression. Chem. Eng. J. 2024, 480, 148008. [Google Scholar] [CrossRef]

| Parameter | Unit | W-S | M-S |
|---|---|---|---|
| Sand | % | 86 | 74 |
| Silt | % | 8 | 16 |
| Clay | % | 6 | 10 |
| WHC | % | 21 | 25 |
| pH | - | 8.2 ± 0.1 | 7.6 ± 0.1 |
| TC | % | 0.7 ± 0.1 | 1.6 ± 0.1 |
| Total CaCO3 | % | 0.9 ± 0.1 | 2.5 ± 0.2 |
| TOC | % | 0.6 ± 0.1 | 1.3 ± 0.1 |
| SOM | % | 1.0 ± 0.1 | 2.2 ± 0.1 |
| CEC | cmol(+) kg−1 | 4.93 ± 0.05 | 8.12 ± 0.03 |
| Biochar | Storage Time (h) | |||
|---|---|---|---|---|
| 24 | 48 | 72 | 96 | |
| Non-autoclaved | 2.5 ± 0.5 | 9.3 ± 0.8 | 20.0 ± 2.0 | 20.0 ± 1.0 |
| Autoclaved | 0 | 0 | 0 | 0 |
| M-S | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Res_Bas | Res_Cum | MB-C | SOM | qCO2 | qM | BFI | BFI lv. |
| Cnt | 21.6 ± 0.4 d | 1302 ± 14 cd | 849 ± 33 e | 2.2 ± 0.1 d | 1.06 ± 0.04 a | 10.0 ± 0.1 a | 14.0 ± <0.1 e | III |
| L-BC | 21.2 ± 0.7 d | 1285 ± 49 cd | 963 ± 23 bc | 3.1 ± 0.1 c | 0.92 ± 0.02 cd | 7.1 ± 0.3 d | 17.0 ± <0.1 cd | III |
| M-BC | 21.3 ± 0.4 d | 1299 ± 2 d | 1025 ± 85 b | 3.4 ± 0.2 b | 0.87 ± 0.07 d | 6.4 ± <0.1 ef | 18.3 ± 0.6 c | III |
| H-BC | 23.6 ± 0.8 bcd | 1405 ± 49 bc | 1025 ± 10 b | 4.8 ± 0.2 a | 0.92 ± 0.03 cd | 5.1 ± 0.2 g | 20.7 ± 0.6 b | IV |
| L-LVE | 23.9 ± 1.0 abcd | 1388 ± 67 bcd | 886 ± 22 de | 2.4 ± 0.2 d | 1.12 ± 0.03 a | 9.8 ± 0.5 ab | 14.7 ± 1.2 e | III |
| M-LVE | 22.5 ± 1.1 d | 1360 ± 7 bcd | 939 ± 19 cd | 2.4 ± 0.2 d | 1.00 ± 0.02 b | 9.8 ± 0.1 a | 14.3 ± 0.6 e | III |
| H-LVE | 22.8 ± 0.4 cd | 1315 ± 26 cd | 967 ± 16 bc | 2.4 ± 0.2 d | 0.98 ± 0.02 b | 9.2 ± 0.2 b | 15.3 ± 0.6 de | III |
| L-BLVE | 26.6 ± 2.1 a | 1406 ± 13 bc | 1110 ± 29 a | 3.1 ± 0.2 c | 1.00 ± 0.03 b | 8.0 ± 0.1 c | 18.3 ± 1.5 c | III |
| M-BLVE | 25.5 ± 0.5 abc | 1447 ± 20 b | 1109 ± 35 a | 3.6 ± 0.2 b | 0.96 ± 0.01 bc | 7.0 ± 0.1 de | 20.3 ± 0.6 b | IV |
| H-BLVE | 26.3 ± 1.5 ab | 1753 ± 54 a | 1136 ± 12 a | 4.8 ± 0.2 a | 0.96 ± 0.01 bc | 6.3 ± 0.2 f | 22.7 ± 0.6 a | IV |
| W-S | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Res_Bas | Res_Cum | MB-C | SOM | qCO2 | qM | BFI | BFI lv. |
| Cnt | 13.1 ± 1.0 c | 896 ± 81 b | 807 ± 71 c | 1.0 ± 0.1 d | 0.68 ± 0.06 ab | 14.7 ± 1.3 bc | 10.7 ± 0.6 f | II |
| L-BC | 13.5 ± 2.0 bc | 959 ± 87 b | 840 ± 37 c | 1.5 ± 0.2 c | 0.67 ± 0.03 ab | 10.4 ± 0.9 de | 11.3 ± 0.6 ef | II |
| M-BC | 13.4 ± 1.0 bc | 1032 ± 94 b | 946 ± 33 b | 2.2 ± 0.1 b | 0.64 ± 0.02 bc | 8.1 ± 0.7 ef | 13.0 ± 0.6 cde | III |
| H-BC | 13.4 ± 0.5 bc | 1103 ± 100 ab | 869 ± 43 bc | 3.4 ± 0.1 a | 0.59 ± 0.02 c | 5.6 ± 0.5 f | 16.3 ± 0.6 ab | III |
| L-LVE | 14.4 ± 0.4 bc | 948 ± 86 b | 894 ± 97 bc | 1.0 ± 0.1 d | 0.68 ± 0.08 ab | 15.4 ± 1.4 ab | 11.0 ± 1.0 f | II |
| M-LVE | 14.5 ± 1.0 bc | 992 ± 90 b | 901 ± 34 bc | 1.0 ± 0.1 d | 0.67 ± 0.03 ab | 16.6 ± 1.5 ab | 11.7 ± 1.2 ef | II |
| H-LVE | 14.6 ± 1.0 abc | 1098 ± 100 ab | 957 ± 50 ab | 1.0 ± 0.1 d | 0.64 ± 0.03 bc | 18.2 ± 1.7 a | 12.3 ± 0.6 def | II |
| L-BLVE | 15.4 ± 0.3 abc | 1103 ± 100 ab | 892 ± 26 bc | 1.7 ± 0.2 c | 0.72 ± 0.02 a | 11.5 ± 1.0 cd | 13.7 ± 0.6 cd | III |
| M-BLVE | 15.8 ± 0.4 ab | 1153 ± 105 ab | 902 ± 14 bc | 2.2 ± 0.2 b | 0.73 ± 0.01 a | 8.8 ± 0.8 def | 14.7 ± 0.6 bc | III |
| H-BLVE | 17.2 ± 0.2 a | 1318 ± 120 a | 1052 ± 45 a | 3.4 ± 0.1 a | 0.68 ± 0.03 ab | 6.6 ± 0.6 f | 17.3 ± 0.6 a | III |
| M-S | W-S | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | APA | βGA | UA | SAI3 | APA | βGA | UA | SAI3 |
| Cnt | 80 ± 2 d | 152 ± 6 a | 156 ± 6 e | 1.1 ± 0.2 e | 166 ± 9 f | 60 ± 4 a | 13 ± 4 c | −6.6 ± 0.7 f |
| L-BC | 101 ± 3 b | 139 ± 5 ab | 163 ± 4 e | −1.0 ± 0.1 d | 187 ± 7 de | 56 ± 4 a | 18 ± 5 bc | −8.2 ± 0.1 de |
| M-BC | 105 ± 5 ab | 125 ± 9 cd | 187 ± 5 bcd | −2.4 ± 0.7 c | 197 ± 7 cd | 52 ± 5 ab | 19 ± 4 bc | −9.1 ± 0.6 cd |
| H-BC | 111 ± 3 a | 118 ± 13 cde | 201 ± 7 a | −3.4 ± 0.8 ab | 197 ± 2 cd | 43 ± 7 b | 21 ± 3 b | −9.6 ± 0.4 bc |
| L-LVE | 87 ± 2 c | 121 ± 5 cd | 182 ± 3 cd | −1.5 ± 0.2 d | 178 ± 9 e | 59 ± 3 a | 13 ± 3 c | −7.4 ± 0.6 ef |
| M-LVE | 106 ± 4 ab | 115 ± 6 de | 187 ± 2 bcd | −3.0 ± 0.2 bc | 184 ± 6 e | 57 ± 5 a | 17 ± 5 bc | −8.0 ± 0.2 e |
| H-LVE | 105 ± 6 ab | 119 ± 9 cd | 180 ± 3 d | −2.6 ± 0.4 bc | 185 ± 5 e | 56 ± 4 a | 18 ± 3 bc | −8.1 ± 0.1 e |
| L-BLVE | 101 ± 3 b | 139 ± 5 ab | 190 ± 4 bc | −1.5 ± 0.1 d | 205 ± 2 c | 55 ± 6 a | 29 ± 4 a | −9.5 ± 0.4 bc |
| M-BLVE | 108 ± 4 ab | 130 ± 4 bc | 187 ± 5 bcd | −2.3 ± 0.1 c | 218 ± 6 b | 53 ± 8 ab | 31 ± 3 a | −10.4 ± 0.8 b |
| H-BLVE | 109 ± 4 a | 104 ± 5 c | 195 ± 7 ab | −3.9 ± 0.3 a | 278 ± 4 a | 51 ± 6 ab | 32 ± 3 a | −14.1 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carril, P.; Becagli, M.; Celletti, S.; Fedeli, R.; Loppi, S.; Cardelli, R. Biofertilization with Liquid Vermicompost-Activated Biochar Enhances Microbial Activity and Soil Properties. Soil Syst. 2024, 8, 54. https://doi.org/10.3390/soilsystems8020054
Carril P, Becagli M, Celletti S, Fedeli R, Loppi S, Cardelli R. Biofertilization with Liquid Vermicompost-Activated Biochar Enhances Microbial Activity and Soil Properties. Soil Systems. 2024; 8(2):54. https://doi.org/10.3390/soilsystems8020054
Chicago/Turabian StyleCarril, Pablo, Michelangelo Becagli, Silvia Celletti, Riccardo Fedeli, Stefano Loppi, and Roberto Cardelli. 2024. "Biofertilization with Liquid Vermicompost-Activated Biochar Enhances Microbial Activity and Soil Properties" Soil Systems 8, no. 2: 54. https://doi.org/10.3390/soilsystems8020054
APA StyleCarril, P., Becagli, M., Celletti, S., Fedeli, R., Loppi, S., & Cardelli, R. (2024). Biofertilization with Liquid Vermicompost-Activated Biochar Enhances Microbial Activity and Soil Properties. Soil Systems, 8(2), 54. https://doi.org/10.3390/soilsystems8020054









