Abstract
More exact information on soil nutrient management is crucial due to environmental protection, nature conservation, decreasing sources for mining, general precaution, etc. Soil magnesium (Mg) analytical methods of potassium chloride (KCl), Mehlich 3 (M3), water (WA) and cobalt hexamine (CoHex) extractions are compared with an elemental analysis and X-ray fluorescence (XRF) analysis. The ratio of the available to the total Mg content was calculated and compared on the whole dataset. The results showed that the linear regressions between all the pairs of Mg content measurement methods were significant. The linear relationship between the KCl and CoHex methods has the highest determination coefficient (R2 = 0.96), followed by WA–M3 (R2 = 0.68), M3–CoHex (R2 = 0.66) and M3–KCl (R2 = 0.60). The M3 solution demonstrated a greater capacity for extracting Mg from the soil. The second part is the analysis of the influence of CaCO3, pH, soil texture and clay content on the measurable magnesium content of soils. It was established that the extraction methods, the soil and the classification method of the soil properties affect the evaluation. These results may help through the nutrient replenishment and the melioration of soils. These results can help the examination of mineral nutrients, especially the Mg uptake.
1. Introduction
Magnesium is the eighth most common element in the crust of the Earth and the fourth most abundant cation in the human body, as it is an essential co-factor required for many biochemical reactions and functions [1]. Magnesium plays an important role, for example, in glucose metabolism [2,3], ATP (Adenosine triphosphate) synthesis [4], blood pressure regulation [5] and signal transduction [6], and its deficiency can be associated with different diseases [7]. Magnesium is also an essential nutrient for cultivated plants. Magnesium is directly involved in photosynthesis and many other processes; its deficiency affects yield and crop quality either directly or by adversely affecting the utilization of other plant nutrients [8,9,10,11].
Among many other reasons, growing crops on magnesium-deficient soils is one of the reasons why the daily intake of magnesium is often insufficient, as the reduction in the magnesium content of cultivated crops subsequently affects the entire food chain [12,13]. On the other hand, ensuring an adequate supply of magnesium can improve the quantity and quality of crops grown [8,14,15].
Although soil is an essential resource and a vital part of the natural environment from which most of the global food is produced, due to the increasingly high demands for food and competing land uses caused by population increase, soils are under pressure [16,17].
Approximately 33% of global soils are degraded [18,19]. Developing a strategy to maintain or improve soil fertility is challenging for the farming communities. To harmonize soil fertility preservation with farming objectives, proper soil nutrient management strategies are needed. These strategies should be based on data-driven information on the current fertility status of soil. Soil analysis is also a valuable tool in cost management, as it contributes to optimizing inputs while considering environmental and sustainability concerns.
Most soil analytical methods aim to measure the phytoavailability of nutrients. While measuring soil nutrient content is technically a relatively simple task, it is much more difficult to determine the amount of nutrients that can be taken up by plants based on test results [20]. The main reason for this is that soil test results provide a snapshot of the properties of the soil being tested, but it is difficult to model the nutrient uptake of plants based on this information, and it is much more difficult to conclude the future based on the current state [21].
Numerous methods are used for soil analysis around the world and even in the European Union. Water extraction of soil samples can be used to determine the soluble magnesium content, where soluble magnesium content is defined as the dissolved magnesium in the soil solution and the magnesium present as a water-soluble precipitate. By using saline instead of water, a fraction of the exchangeable magnesium content can be measured, which is important because the exchangeable magnesium content is considered to be the magnesium that can be taken up by the plants [22]. Such methods are the calcium chloride method [23,24] and the potassium chloride method, which has been the standard method in Hungary since the 1980s [25]. The CaCl2 method measures about 50–60% of the exchangeable magnesium content in clay soils, about 60–80% in loamy soils and about 80–90% in sandy soils [26].
The calcium chloride and potassium chloride methods are typically used for the determination of only one nutrient (or at least not used for the determination of P and K), and therefore, there is a lot of research into universal extraction methods that are suitable for the simultaneous determination of several nutrients [27]. The Mehlich 3 (M3) method is used for the determination of the plant-available soil fractions of phosphorous, potassium, calcium, magnesium, sodium, manganese and zinc [28,29]. The A-L (ammonium lactate) method (Egnér et al., 1960) [30] was developed to determine P and K content but can also be used to determine magnesium [27], although the acidic extractant of the A-L method (pH = 3.7) may measure a larger proportion of the magnesium content of the soil, as some of the slowly exchangeable and structurally bound magnesium may also leach into the solution, which does not occur or occurs only slowly under normal field conditions [28]. This effect can also be expected for other acidic extractants.
The calcium chloride extract is used in Poland, Slovenia, Germany and Austria; the potassium chloride method is used in Hungary and in Russia, Belarus, Ukraine and the Balkan countries; and the Mehlich 3 method is used in Czechia, Slovakia and Estonia. In Latvia, the D-L (Egner-Riehm) method [30,31] is used, and in Lithuania and Sweden, the A-L (Egner-Riehm-Domingo) method is used to determine the magnesium in the soil together with phosphorus and potassium [27].
In addition to these methods, there are numerous methods specifically designed to determine the cation exchange capacity (CEC) and exchangeable cations of soils. These methods operate with some buffered or non-buffered saline solution (e.g., NH4OAc, NH4Cl, BaCl2). These methods can give a good estimate of the number of exchangeable cations, but in saline, calcareous or gypsiferous soil, the lack of soluble salt removal or carbonate dissolution inhibition may cause problems. In addition, the extraction step used may affect the usable analytical methods or their analytical performance [32,33,34]. The CoHex method [33], which we used, provides a simple solution for measuring these parameters. The exchange is carried out by simply shaking the test portion in an unbuffered, low-concentration saline solution. The CEC can be determined by a simple spectrophotometric method, and the exchangeable cations can be determined by various analytical methods.
The non-exchangeable fractions of soil magnesium are mineral, acid-soluble and organic complexed magnesium. These are usually the forms in which the majority of magnesium is present in the soil. Acid-soluble magnesium can be considered a reserve pool of Mg [22]. A part of this can be measured by the methods using acidic extractants, as mentioned earlier.
Total element analysis methods are widely used to determine the toxic element content of soils but can also be used to determine the total nutrient content, including total magnesium content. Concentrated inorganic acids and acid mixtures (for example HCl, HNO3, HClO4) are widely used to determine the total element content of soils [35]. However, these methods measure only a semi-total elemental content, as less acid-soluble components such as metal silicates are not fully decomposed by the procedure and thus are not included in the analytical measurement [36]. Total elemental content can be determined after digestion with acid mixtures containing hydrogen fluoride [37], but this is generally not recommended due to its hazardous nature, corrosivity and possible matrix effect [36]. Fusion methods based on alkaline, acidic and peroxide fusion, such as Na2CO3, Na2O2, LiF and LiBO2/Li2B4O7, can also be used to dissolve silicate-based matrices in geological applications [35,38]. However, fusion techniques require large amounts of flux relative to the sample size; therefore, its impurities are a source of contamination, and the high salt content causes problems for atomic spectroscopy and mass spectrometry detection techniques (e.g., instability and high background values, as well as interferences) [38]. XRF technology is a real-time or near-real-time and cost-effective alternative to classical laboratory analysis and is also suitable for on-site measurements, but comparisons with laboratory measurements have variable success (point measurements vs. laboratory homogenized samples, matrix effect, sample heterogeneity), and most digestion techniques extract only part of the material analyzed, while XRF, as a physical technique, analyzes the total elemental content, regardless of chemical bonding. Therefore a positive bias can be expected [39,40,41].
The different methods give non-equivalent test results. The choice between methods can be made based on advantages and disadvantages and technical feasibility, but it is always important to use the limits of the method when evaluating the results.
In addition to the many analytical methods for determining the magnesium content of soils, an additional difficulty in determining magnesium supply is that the amount of magnesium that can be taken up by plants can be influenced by several other factors. Such influencing factors include soil pH, soil texture, soil texture and CaCO3 content [21,22,27,42,43].
Given the ultimate need to collect and compare results obtained by the different methods in a harmonized way, our present work is aimed at summarizing the soil analytical methods used in Hungary and comparing them with the relevant methods used internationally. In a former publication, the comparison of the methods was analyzed [44]. In the recent paper, the main aim is to analyze the goodness of the methods compared to the XRF measurements and, on the other hand, along the influencing parameters, such as CaCO3, pH, soil texture and clay content.
2. Materials and Methods
2.1. Collection of the Soil Samples
Seventy geo-referenced soil samples from the 0–20 cm top layer of arable fields (Figure 1) were taken in Hungary, in the summer of 2017.
Figure 1.
Sampling locations of the soil samples in Hungary.
The locations of the 70 samples were selected according to Minasny-McBratney [45] and Roudier-Hedley [46]. Factors taken into account in this selection were land use, soil type, climate data, accessibility and market value.
2.2. Laboratory Analyses
The soil-test methods implemented in this study included four different extraction methods with the use of the Mehlich 3 (M3) solution, a cobalt hexamine trichloride (CoHex) solution, deionized water (WA) and a potassium chloride solution (KCl) solution as a measure of different pools of the plant-available magnesium content of soils and an energy dispersive X-ray fluorescence (ED-XRF) method as a measure of the total magnesium content.
The Mehlich 3 method [28] was selected as a multielement extraction method, which is applicable for the determination of the bioavailable pool of different nutrients. The Mehlich 3 method was implemented after the recommendations of Recommended Soil Testing Procedures for the Northeastern United States [47]. The soil samples are extracted with the Mehlich-3 solution, which contains 0.2 mol dm−3 acetic acid, 0.015 mol dm−3 ammonium fluoride, 0.013 mol dm−3 nitric acid, 0.25 mol dm−3 ammonium nitrate and 0.001 mol dm−3 ethylenediaminetetraacetic acid and has a pH value of 2.5. The extract was prepared with the application of a soil-to-solvent ratio of 1:10 (m V−1). The suspension was shaken for 5 min then filtered and measured by inductively coupled plasma mass spectrometry (ICP-MS).
The cobalt hexamine trichloride method [33] is relatively simple and allows the determination of multiple exchangeable cations and CEC in one procedure without compromising accuracy. Cations retained by the soil sample are exchanged with the aqueous solution of cobalt-hexamine (Cohex) ions (0.0166 mol dm−3) after shaking for 60 min. The CEC is determined as the difference between the initial quantity of cobalt-hexamine in the solution and the quantity remaining in the extract after the cation exchange reaction. The quantities of exchanged cations (e.g., magnesium) can be measured in the same extract. The measurement of magnesium concentration in the extract is performed by the ICP-MS method.
The aqueous extract is commonly used to determine pH and soil electrical conductivity (EC) but can also be used to determine the fraction of nutrients that can be easily taken up by plants. A total of 12 g of air-dried soil was mixed with deionized water, in the ratio of 1:5 (m V−1), and was subjected to 30 min of shaking. The filtered extract was analyzed by ICP-MS.
The method using the traditionally accepted Hungarian Standard [25] was used to measure the Mg content of the soil samples after extracting them with a 1 mol dm−3 KCl solution (soil-to-solvent ratio of 1:2.5, stirring for 1 h). The filtered extracts were analyzed with inductively coupled plasma atomic emission spectroscopy (ICP-AES).
The XRF method was used to measure the total magnesium contents. The XRF is a compromise between information that can be obtained, cost, environmental impact and accuracy. The ED-XRF method was implemented after milling a subsample of 30 g to 1 mm particle size and pelleting. The XRF analysis was done following the International Organization for Standardization (ISO) standard 18227:2014.
2.3. The Role of Soil Properties Affecting the Magnesium Extraction Efficiency
A potentiometric method was used to determine pH value according to the MSZ-08-0206-2:1978 [48]. The pH (KCl) value was measured in a soil suspension, prepared with 1 mol dm−3 KCl solution using a soil-to-solvent ratio of 1:2.5 (m V−1). The suspension was left to stand overnight before measuring.
The gas volumetric method by Scheibler according to MSZ-08-0206-2:1978 [48] was used to determine the CaCO3 content. The carbonates present in the sample were converted into CO2 by adding an HCl solution to the sample. Carbonate content was calculated from the volume, the temperature and the pressure of the generated gas.
Particle size distribution was measured using laser diffractometry (Fritsch Analysette 22 Microtech Plus). For breaking down the aggregates, organic matter and CaCO3 content were removed from the samples using H2O2 and 10% HCl, respectively. For the complete disaggregation, 0.5 mol dm−3 sodium-pyrophosphate addition and ultrasonic treatment were applied during the measurement. To calculate the size distribution, the Mie theory was used, applying a 1.54 refractive index value.
2.4. Analyses of the Influencing Factors
To evaluate the role of soil properties affecting magnesium extraction efficiency, soil samples were grouped according to pH (KCl), CaCO3 content and clay content.
Soils were divided into five groups based on their pH (KCl) value (Table 1), basically following the original categories. The distribution of the data in the entire data set allowed us to have a minimum of 11 samples per group, which was important for the statistical analyses.

Table 1.
pH, CaCO3 and clay content groups with group thresholds and the number of samples classified.
In the Hungarian Integrated Soil Advisory System, CaCO3 content is an influencing factor for the assessment of phosphorous availability in the soil, but not for magnesium, although it has long been known that calcium can reduce the uptake of magnesium in calcareous alkaline soils [22,49]. Most of the samples tested in our study were in the lime-free (<0.1%) or low-lime (0.1–4.9%) categories. To investigate the dependence of magnesium content on lime content in a detailed manner, five groups (Table 1) were created, taking into account having enough samples per group for statistical purposes. Sample numbers for the most optimal statistical analyses were the basis for creating the groups. With the appropriate choice of boundaries, the minimum sample number per group was seven.
According to the measurement results, the clay content (particle size < 0.002 mm) of the soil samples was between 6.82% and 24.89%. The entire data set was divided into 6 groups (Table 1). The grouping was based on the approximately uniform distribution and a statistically adequate number of elements per group.
2.5. Statistical Analysis
For the statistical characterization of the entire data set, we used the following descriptive statistical indicators: arithmetic means, median, coefficient of variation (CV), relative standard deviation (RSD) and maximum (Max) and minimum (Min) values.
Regression analysis was used to determine the relationship between the different Mg determination methods, where R2 presents a measure to match the relationship of the different methods.
Pearson correlation analysis was used to determine the relationship between the extraction methods and the influencing soil parameters (pH, CaCO3 content, clay content).
The normality of the data series of the different analysis methods was tested with the Kolmogorov–Smirnov test. A non-parametric Friedmann ANOVA test was used for not normally distributed data. If the data of the analysis methods showed normal distribution, then a parametric, the repeated measures ANOVA test was used.
The Wilcoxon signed-rank test, a non-parametric statistical hypothesis test, was used to compare the analysis methods (WA, M3, CoHex, KCl, XRF) to assess whether their mean ranks differed.
The pairwise analyses test was used to investigate the pH (KCl), CaCO3-content, Arany-type texture index and clay content dependence of the used soil parameter measurement methods. This is a type of location test that is used to compare measurements of the analysis methods to assess whether their means differed. The proportions of measured Mg compared to the total amount (XRF) were used in the comparisons, and they were classified according to specified pH, CaCO3 content, Arany-type texture index and clay content groups.
The box plot method was used to display the variation in the magnesium determination methods in the specific groups of pH (KCl), CaCO3 content and clay content.
3. Results
Our present work is aimed at comparing the selected soil analytical methods used for the determination of magnesium content in Hungary. The total magnesium content in the soil has been reported to vary greatly from 0.5 to 40 g kg−1 (Yan és Hou, 2018), and this relatively wide range can be even wider if we take into account the different effectiveness levels of different soil testing methods. Therefore, as a first step in our methodological comparison, we examined the basic statistical indicators for the entire data set, for all the different magnesium determination methods.
3.1. Descriptive Statistics of the Mg Analysis Results
The descriptive statistics of the soil sample sets are presented in Table 2.

Table 2.
The basic statistical data of the soils as Mg contents were determined by the different methods (No. of samples (n) = 70).
The highest Mg content was measured by XRF analysis, which determines the total amount of Mg in the soil. The other methods measured much less because these extractants dissolve only a part of the total Mg content of the soils, hence they are applied to represent the available magnesium content in the soil. Comparing the four other methods, WA showed the lowest, whereas M3 had the highest available Mg content in the soil. The mean and median of the Mg content measured by the four methods resulted in the following order: WA < KCl < CoHex < M3 < XRF.
3.2. Comparison of Magnesium Determination Methods
The results of the analyses of the soil Mg content measured by the different soil testing methods (water extract, Mehlich 3, CoHex, KCl and XRF methods) are shown in Table 3.

Table 3.
The linear regression with a significance level of 5% between the Mg contents measured by WA, M3, CoHex, KCl and XRF methods.
The linear regressions between all the pairs of Mg content measurement methods are significant. Remarkably, only four of them explain more than 60% of the total variation (R2 ≥ 0.96). The linear relationship between the KCl and CoHex methods has the highest determination coefficient (R2 = 0.96), followed by WA—M3 (R2 = 0.68), M3—CoHex (R2 = 0.66) and M3—KCl (R2 = 0.60).
3.3. Comparison of Extraction Efficiency of the Four Wet Chemical Methods
The study aims to compare the extraction efficiency of different methods. As the XRF method was applied to determine the total magnesium contents, based on its results, the percentage of the total Mg (XRF) content that could be measured with the other analytical methods was calculated. Table 4 shows the calculated values.

Table 4.
The proportion of measured Mg from the total amount (XRF).
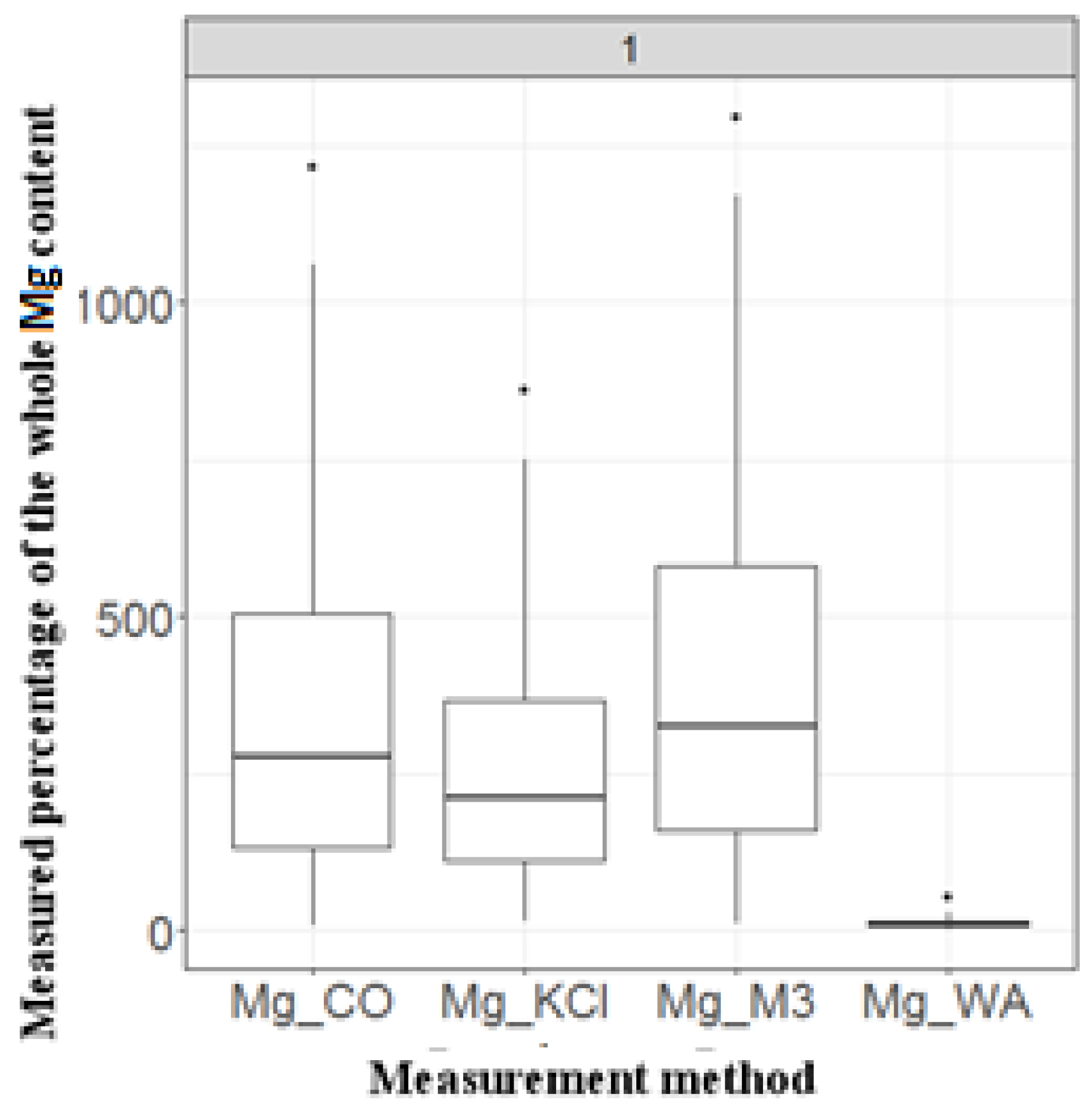
The mean, median and min and max percentage values resulting from all the Mg determination methods showed the following order of measured magnitude: WA < KCl < CoHex < M3.
According to the Kolmogorov–Smirnov test, the distribution of the data in the entire data set did not follow a normal distribution; therefore, the non-parametric Friedman’s two-way analysis of variance by ranks (ANOVA) test was used to evaluate the differences between the methods. The results of the statistical analysis (Figure 2) showed that the applied methods pairwise provide significantly different results (Fr = 181.766, df = 3, p < 0.001), except for the M3 and CoHex methods (p = 0.521).
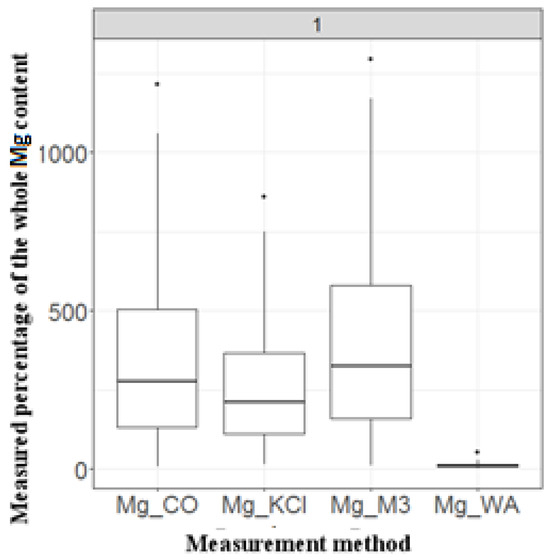
Figure 2.
Results of the box plot analyses of four different Mg-extraction methods based on the percentage that each method could measure from the total amount of Mg, measured by a fifth method (XRF). (WA = water soluble, M3 = Mehlich 3, CO = cobalt hexamine, KCl = potassium chloride.).
3.4. The Effect of Soil Parameters on the Mg Analysis Methods
3.4.1. Pearson Correlation Analysis
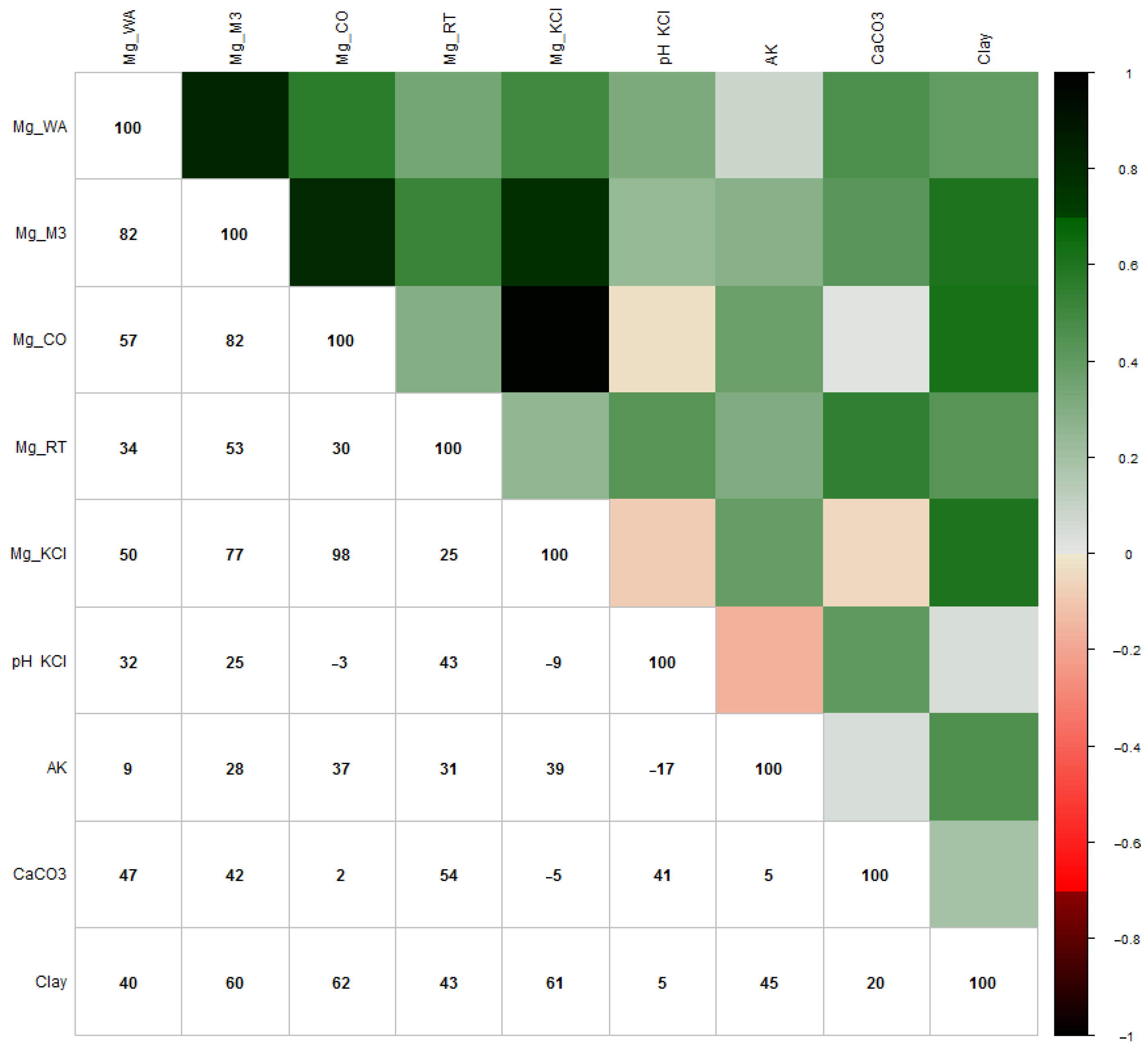
Figure 3 shows the results of the Pearson correlation analysis to get an overview of which soil parameters affect the amount of measured magnesium by different analysis methods.
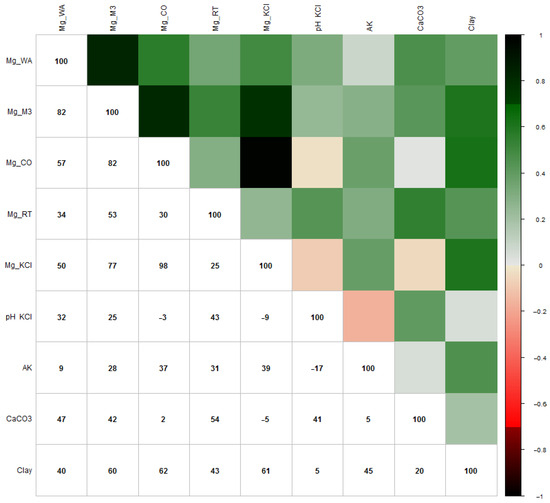
Figure 3.
Pearson correlation analysis (×100) between the soil parameters and Mg analysis methods. (Red: the darker the red, the weaker the correlation; green: the darker the green, the stronger the correlation).
Comparing the Mg analysis methods, there was a very strong correlation between M3 and WA (r = 0.82), CoHex and M3 (r = 0.82) and KCl and CoHex (r = 0.98). A strong correlation was found between KCl and M3 (r = 0.77), and a moderate correlation was found between CoHex and WA (r = 0.57), XRF and M3 (r = 0.53) and KCl and WA (r = 0.50).
Evaluating the effect of influencing soil parameters on the measured magnesium content, the clay content showed a strong correlation with the analysis results of M3 (r = 0.60), CoHex (r = 0.62) and KCl (r = 0.61) methods. The amount of CaCO3 content showed a moderate correlation with the amount of Mg that the WA (r = 0.47), M3 (r = 0.42) and XRF (r = 0.54) methods measured. The pH (KCl) showed a moderate correlation with the Mg measurements of XRF (r = 0.43) and a weak correlation with the analysis results of WA (r = 0.32) and M3 (r = 0.25) analysis methods. The KA values had a weak correlation with the amount of Mg that M3 (r = 0.28), CoHex (r = 0.37), XRF (r = 0.31) and KCl (r = 0.39) methods measured.
3.4.2. Evaluating the Effect of Soil Parameters with Pairwise Analysis
Further investigating the effect of soil parameters, pairwise analysis was used to compare measurements of the four magnesium analysis methods to assess whether their means differed. The proportions of measured Mg to the total amount (XRF) were used in the comparison, and they were grouped according to specified pH, CaCO3 content and clay content groups.
Comparison of the Measured Values in the Classic pH Groups
In the first group (soil pH 3.39–4.35), the results of the CoHex method did not differ significantly from the other methods. There was no significant difference between M3 vs. KCl and M3 vs. CoHex. The WA method did not differ significantly from CoHex but differed significantly from M3 and KCl. The other methods are significantly different from each other. In the second group (pH 4.36–5.47), the WA method measured significantly different Mg levels compared to the other three methods. The results of the M3 and CoHex methods were also significantly different. There was no significant difference between the results of M3 vs. KCl and CoHex vs. KCl. In the case of slightly acidic soils (pH 5.48–6.78), the WA method also measured significantly different Mg levels compared to the other three methods, but there was no significant difference between the following pairs: M3 vs. CoHex, M3 vs. KCl and CoHex vs. KCl. In neutral soils (pH 6.79–7.2), only one pair was not significantly different: the results of M3 and CoHex methods. All the other pairs resulted in significant differences. In the fifth group (pH 7.21–8.14), all the different analysis methods were significantly different from each other (Table 5).

Table 5.
Results of the pairwise analysis of the measured magnesium percentages compared with the measured total magnesium amounts based on the pH (KCl) group.
Based on the pH (KCl) groups, we can conclude that WA vs. M3 and WA vs. KCl methods showed the biggest differences; these were significantly different in all pH groups. In the strongly acidic soils group, the differences between the methods are less significant compared to the direction of neutral and alkaline groups.
It can be concluded that in the strongly acidic soils (pH 3.39–4.35), there were less significant differences compared to the pH ranges of 6.79–7.2 and 7.21–8.14. There was one exception; there was no significant difference between the results of M3 and CoHex methods in the fourth pH group.
The least significant difference was between M3 vs. CoHex and M3 vs. KCl methods. These were the most “similar” methods.
Comparison of the Measured Values Based on the CaCO3-Content Groups
In lime-free or very low lime-containing soils (CaCO3 < 0.1 and 0.11–0.84%), there was no significant difference between the M3 and CoHex methods; all the other methods were significantly different from each other. In the third group (CaCO3 between 0.85% and 3.16%) and the fourth group (CaCO3 between 3.17% and 7.02%), there were no significant differences between the methods. In soils with the highest lime content (CaCO3 between 8.79% and 18.71%), there was no significant difference between the results of M3 vs. KCl and M3 vs. CoHex methods, but the WA was significantly different from M3, CoHex and KCl. The results of magnesium measurements measured by Cohex and KCl were also significantly different (Table 6).

Table 6.
Pairwise analysis of the measured magnesium percentages compared with the measured total magnesium amounts based on the CaCO3 groups.
In the lime-free or low calcareous soils, there are significant differences between all methods except the M3 vs. CoHex methods. In the third carbonate group (CaCO3 0.85–3.16%), there was no significant difference between the methods. The most obvious differences are between the WA and M3, CoHex and KCl methods. The less significant differences were between the results of the M3 and CoHex methods.
Comparison of the Measured Values Based on the Clay-Content Groups
In the first clay group (6.82–9.64%), only WA was different from KCl, and the other pairs did not differ significantly. In the second and third clay groups (10–12.74% and 12.99–15.69%, respectively), all the methods were significantly different except for M3 vs. CoHex. In the fourth and fifth clay groups (15.99–18.59%, 19.16–21.82%), M3 was not different from the KCl and CoHex methods. There was no significant difference between the CoHex and KCl methods. The CoHex method measured significantly different Mg content compared to KCl. WA was significantly different compared to all the other methods. In the sixth clay group (21.83–24.89), M3 did not differ from CoHex, and CoHex did not differ from the KCL method significantly. The results of WA were significantly different compared to all the other methods (Table 7).

Table 7.
Pairwise analysis of the measured magnesium percentages compared with the total magnesium amounts (XRF) based on the clay-content groups.
There was no significant difference between the M3 and CoHex methods; they were “similar” (not only nonsignificant, but p was 1 in all cases except for one case, but even here, the p was 0.942, so close to one) in all clay categories. The M3 and KCl methods did not differ significantly in the lowest clay content group and from 15.70% clay content.
The smallest clay content resulted in the smallest number of differences, while the most significant differences were between 9.65 and 15.69% clay content. The WA method was significantly different from the other methods in all clay groups except for Group 1. Overall, small clay content resulted in the least differences between the methods.
3.4.3. Evaluation of the Effect of Soil Parameters on Magnesium Measurements
Summarizing the effect of the soil parameters on Mg measurements, calculations were made to determine how many percentages of the results of the pairwise analysis was significant, along with the three influencing factors (pH (KCl), CaCO3 and clay content) (Table 8). It helped to evaluate the methods and establish general order.

Table 8.
The average of the number of significant results of all the pairwise analyses of the Mg% measurements along with the three influencing factors (pH (KCl), CaCO3 and clay).
Based on the average of the significance levels of all the pairwise analysis results along with the three influencing factors (pH (KCl), CaCO3 and clay content), the largest number of non-significantly different results belonged to the M3 vs. CoHex method. There were only two significant differences between their results in the pH groups 2 (pH 4.36–5.47) and 5 (pH 7.21–8.14); all the other results of the pairwise comparison was not significantly different.
Even though the results of the statistical analysis of the overall data showed that all the methods are significantly different except for M3 vs. Cohex, the detailed analysis of the categories of pH, CaCO3, KA and clay resulted in different outcomes: more methods were not significantly different.
The smallest difference was between the results of the M3 vs. CoHex methods. The M3 and KCl methods showed the least number of significant differences in the KA and clay classification, while M3 and KCl showed the least significant differences in pH and CaCO3 classification.
The WA method measured a significantly different amount of magnesium compared to the other three methods. Evaluating the differences based on all parameters, the following order can be made (1—smallest difference, 6—biggest difference):
- M3 vs. CoHex
- M3 vs. KCl
- CoHex vs. KCl
- WA vs. CoHex
- WA vs. KCl
- WA vs. M3
By evaluating the differences based on all parameters, it can be concluded that M3 vs. CoHex were not significantly different from each other. The greatest significant difference was between the results of the P-WA vs. M3 methods.
4. Discussion
4.1. The Role of Mg and the Effect of Deficiency and Excess Amount (Toxicity)
Magnesium has particular importance as an essential nutrient for our cultivated plants and farm animals and also plays an important role in the human diet [9,50]. In the case of our cultivated plants, an adequate magnesium supply is necessary for the proper functioning of photosynthesis, transport of photoassimilates, enzyme activation and formation and utilization of ATP, and magnesium also has a positive effect on nitrogen- (N) use efficiency and grain N accumulation [9]. The insufficient soluble Mg in the soil is also hazardous for grazing animals; the disease “grass tetany” is caused by magnesium deficiency. However, an excess of magnesium can also be risky.
Magnesium toxicity is only expected at extremely high concentration levels; applications of up to 2000 mg Mg kg−1 soil did not lead to Mg toxicity [51], but there is a negative correlation between K and Mg content of the soil, leaf, root and stem [51,52], which suggests that Mg can inhibit translocation of K. Magnesium nutrition may increase some diseases such as bacterial spot of tomato and pepper, although the amount, the source, the time of application, the nutrient status of the plant and interactions with other minerals in the plant or pathogen are important considerations in understanding the role of Mg in disease resistance or susceptibility [53].
4.2. Complexity and Difficulties in Measuring Mg Content of the Soil
Accurate knowledge on the magnesium content of arable soils would be extremely important for practice, but according to current soil testing practice, the situation is quite complicated. Mg is a common constituent in many minerals, but most of the soil’s Mg content is incorporated in the crystal lattice structure of minerals and is therefore not directly available for plants. The phytoavailable amount of Mg depends on many factors [54]. There are several soil analysis methods used for predicting the phytoavailability of the nutrients, making a direct comparison of the results often difficult [21].
In this comparative analysis study, difficulties were also found. The data analyses proved that there were significant differences between the methods. A comparison of methods with linear regression showed an unexpectedly strong relationship (R2 = 0.96) between the KCl and CoHex methods based on the percentage of the variability (R2). Martins et al. [55] found a similarly close relationship between NH4OAc and M3 soluble magnesium contents in oven-dry (R2 = 0.945) and field-moist (R2 = 0.978) soil samples, as Rogers et al. [56] also found (r2 = 0.89), but these methods are generally considered to extract comparable or slightly different amounts of Mg from soils. However, the two methods used in our study should be more dissimilar from one another, as the KCl method “only” measures the soluble and the readily exchangeable part of the Mg in the soil, while the CoHex method can also measure the slowly exchangeable part. The KCl and M3 methods were expected to produce similar results with a high determination coefficient, but they showed a weaker relationship (R2 = 0.60). The M3 and CoHex methods had a similar low determination coefficient of 0.66.
4.3. Comparing Multiple Methodologies
The complexity of a soil analysis was proven by Staugaitis and Rutkauskienė [27], who compared six different Mg extraction and determination methods and found that the extraction procedure strongly impacts the outcome, similarly seen in this study. Nevertheless, all mild extraction procedures, including CaCl2, KCl, NH4OAc and the M3 method, showed quite high correlations over all three sampling depths investigated, indicating similar extraction characteristics for the soil Mg. These results do not reflect our findings, where the KCl extraction resulted in much lower amounts, compared to those of the M3 method. A comparison of methods showed expected differences between the KCl and CoHex methods (as the KCl method measures significantly lower amounts) with the Wilcoxon signed-rank test, since KCl does not bring the slowly exchangeable Mg into the solution, so it does not measure it. The basic statistical figures of the mean and median values produced the same order of magnitude for the methods; the WA method measured the smallest, followed by the KCl method in the middle range, which was followed by the CoHex method and then by the M3 method.
The results of a joint institutional project in Czechia, Hungary, Poland and the Netherlands on the calibration of the 0.01 M CaCl2 soil testing procedure for Mg are presented by Loch et al. [57]. With the relatively weak extractant, 0.01 M CaCl2, somewhat lower quantities of Mg were extracted from the soil samples compared to the extraction solutions used in Poland (0.0125 M CaCl2), in Hungary (1 M KCl) and in the Netherlands (0.5 M NaCl). With the relatively strong extractant used in Czechia (Mehlich II), more Mg was extracted. Based on the study of Zbíral and Němec [58], significant correlations were found between the M2 and M3 methods for all the nutrients (the correlation coefficients were in the range of 0.97–0.99.) This result corresponds with our findings, where the M3 method measured more Mg than the KCl method. In general, the amount of Mg extracted increases, i.e., 0.01 M CaCl2 < Schachtschabel < Mehlich-2.
The amount of Mg that can be extracted from the soil depends on the extracting solution and the ratio of the soil-to-extracting solution. The readily soluble Mg-containing solid constituents and Mg bound to the cation exchange complex of the soil are regarded as the plant-available fraction. Extraction of the soil elements with unbuffered soil solutions like 1 M KCl [59] is used to extract this plant-available fraction from the soil. The use of acidic extractants is not advised, because they also extract part of the Mg reserves of the soil. Despite this, Mehlich [28,29] developed a multi-nutrient extractant, buffered at pH = 2.5, for the determination of the plant-available soil fractions of Mg [60]. The Mehlich-2 method also extracts part of the Mg reserves of the soil, especially in soils containing dolomite [60]. The acidified extractants may promote the dissolution of structural forms like Mg containing carbonates and minerals [61]. In our study, it was also visible that the M3 method extracted more Mg compared to the KCl method.
4.4. Extractability and Other Influencing Factors
In most comparative studies, the results of the two extraction procedures are related by using statistical techniques like (multiple) linear regression. To increase the explained variance of the relationships, soil characteristics like the soil type, organic matter, clay and carbonate contents are also arbitrarily included [57,62]. We can also conclude that not only the extraction method but also soil properties like the carbonate content, affect the evaluation of the magnesium measurements. Our results showed that in the case of the CaCO3-free or low-CaCO3-content soils, there was a strong relationship between the three methods, but the increased CaCO3 content showed weaker correlations. Van Erp [63] compared the relationship between 0.01 M CaCl2, BaCl2 and KCl extractants. An analysis of the difference in the Mg extracted among the BaCl2 and KCl methods showed that the difference was related to the clay content of the soils and not to the organic C content or carbonate content. Similarly, Hailes et al. [64] observed that exchangeable Mg was not significantly correlated with organic carbon. Because the structural and exchangeable Mg is related to clay minerals, clayey soils generally contain adequate Mg for plant requirements, whilst magnesium deficiencies are most likely to occur on acidic, sandy soils [22]. Dontsova and Lloyd Darrell [65] reported on the degrading effect of a high Mg content on the soil structure and clay dispersion. Contrarily, Wang et al. [15] found no effect of texture on the Mg availability.
The relationship between the extractable nutrient content of soil and the response of growing plants is often weak. A soil analysis just provides a picture of the current situation at a given site; it is not capable of perfectly simulating the plant characteristics on the Mg uptake. The phytoavailability of Mg in the soil solution depends on the duration and intensity of weathering, soil moisture, soil pH and root–microbial activity in the soil. Furthermore, this available amount is generally small compared with the amounts needed to sustain high crop yield and quality [54]. Choosing an appropriate extraction method has an important role in the correct assessment of Mg availability in the soils. Consequently, a soil analysis only gives information on the potential of soil to provide the respective nutrients [20].
5. Conclusions
In conclusion of the experiments, it can be stated that both physicochemical properties and the chosen classification method influenced the outcome of magnesium measurements. The Mehlich-3 solution demonstrated a greater capacity of extracting Mg from the soil, compared with other extracting solutions. Magnesium content measured by the four methods resulted in the following order: WA < KCl < CoHex < M3 < XRF. The linear regression between all the pairs of Mg content measurement methods is significant, but only four of them explain more than 60% of the total variation. The linear relationship between the KCl and CoHex methods has the highest determination coefficient (R2 = 0.96), followed by WA–M3 (R2 = 0.68), M3–CoHex (R2 = 0.66) and M3–KCl (R2 = 0.60). The CoHex vs. KCl methods showed an unexpectedly strong relationship. However, these two methods should be more dissimilar from one another, as the KCl method “only” measures the soluble and readily exchangeable part of the Mg in the soil, while the CoHex method can also measure the slowly exchangeable part. The KCl and M3 methods were expected to produce similar results with a high determination coefficient, but they showed a weaker relationship (R2 = 0.60). The M3 and CoHex methods had a similar low determination coefficient of 0.66. The results of the pairwise analysis based on the percentage that each method could measure from the total amount of Mg (XRF) proved that all the methods were significantly different, except for the M3 and CoHex methods. The further comparison of the methods based on the influencing factors, such as pH, lime content and clay content showed the differences between the different methods. Linear regression and Pearson correlation analysis showed the strongest correlation between CoHex and KCl. The pairwise analysis showed other aspects. The pairwise analysis showed that the least significant differences were between the results of M3 vs. CoHex and KCl vs. M3 methods. Evaluating the differences based on all parameters, the following order can be made (1—smallest difference, 6—biggest difference):
- M3 vs. CoHex
- M3 vs. KCl
- CoHex vs. KCl
- WA vs. CoHex
- WA vs. KCl
- WA vs. M3
By evaluating the differences based on all parameters, it can be concluded that M3 vs. CoHex were not significantly different from each other. The greatest significant difference was between the results of the WA vs. M3 methods. Concerning the comparison and evaluation of the different Mg determination methods, they should be further investigated to find the most appropriate method for the different varieties of influential soil properties. An advisory system could be formulated based on the main soil types (e.g., following the already existing Hungarian system: forest soils, meadow soils, chernozems, sandy soils, etc.), and the influencing factors that should be soil-type specific (e.g., sandy soils’ Mg-content measurements are influenced by organic carbon content or forests soils are influenced by clay content or pH, etc.). This can be a future research topic to help farmers and other interested businesses with finding the proper amount of Mg needed in certain soils to produce not only a good quantity but also quality crops.
Author Contributions
Conceptualization, V.V., R.K., Z.G., C.C., M.V. and Z.B.; methodology, R.K., V.V., Z.B., I.K. and L.K.; validation, V.V., S.Z., Z.G. and I.K.; formal analysis, V.V., R.K., M.V. and C.C.; investigation, V.V., M.V. and C.C.; resources, V.V. and M.V.; data curation, Z.G., Z.B., V.V. and I.K.; writing—original draft preparation, R.K. and V.V.; writing—review and editing, R.K., V.V. and C.C.; visualization, T.S. and L.K.; supervision, V.V.; project administration, V.V. and M.V.; funding acquisition, V.V. and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, K.R.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research—A review. J. Magn. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Paolisso, G.; Scheen, A.; D’Onofrio, F.; Lefebvre, P. Magnesium and Glucose Homeostasis. Diabetologia 1990, 33, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; Xiao, C.W.; Ratnayake, W.M.N.; Fernandez, L.; Lavergne, C.; Wood, C.; Swist, E. Lower Serum Magnesium Concentration Is Associated with Diabetes, Insulin Resistance, and Obesity in South Asian and White Canadian Women but Not Men. Food Nutr. Res. 2015, 59, 25974. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Hong, S.; Pedersen, P.L. Chemical Mechanism of ATP Synthase. J. Biol. Chem. 1999, 274, 28853–28856. [Google Scholar] [CrossRef] [PubMed]
- Sontia, B.; Touyz, R.M. Role of Magnesium in Hypertension. Arch. Biochem. Biophys. 2007, 458, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, A.; O’Neill, J.S. Signal Transduction: Magnesium Manifests as a Second Messenger. Curr. Biol. 2018, 28, R1403–R1405. [Google Scholar] [CrossRef] [PubMed]
- Bergman, C.; Gray-Scott, D.; Chen, J.-J.; Meacham, S. What Is Next for the Dietary Reference Intakes for Bone Metabolism Related Nutrients Beyond Calcium: Phosphorus, Magnesium, Vitamin D, and Fluoride? Crit. Rev. Food Sci. Nutr. 2009, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The Power of Magnesium: Unlocking the Potential for Increased Yield, Quality, and Stress Tolerance of Horticultural Crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Magnesium in Crop Production, Food Quality and Human Health. Plant Soil 2013, 368, 1–4. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The Significance of Magnesium for Crop Quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium Deficiency in Plants: An Urgent Problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Cazzola, R.; Della Porta, M.; Manoni, M.; Iotti, S.; Pinotti, L.; Maier, J.A. Going to the Roots of Reduced Magnesium Dietary Intake: A Tradeoff between Climate Changes and Sources. Heliyon 2020, 6, e05390. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Yazici, A.M. Magnesium: A Forgotten Element in Crop Production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium Fertilization Improves Crop Yield in Most Production Systems: A Meta-Analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Laws of sustainable soil management. Agron. Sustain. Dev. 2009, 29, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- FAO & ITPS. Status of the World’s Soil Resources Report. Technical Summary; FAO: Rome, Italy, 2015; p. 79. [Google Scholar]
- Fischer, M.; Rounsevell, M.; Torre-Marin Rando, A.; Mader, A.; Church, A.; Elbakidze, M.; Elias, V.; Hahn, T.; Harrison, P.A.; Hauck, J.; et al. Summary for Policymakers of the Regional Assessment Report on Biodiversity and Ecosystem Services for Europe and Central Asia of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2018. [Google Scholar]
- Kopittke, P.M.; Menzies, N.W. A Review of the Use of the Basic Cation Saturation Ratio and the “Ideal” Soil. Soil Sci. Soc. Am. J. 2007, 71, 259–265. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium Mobility in Soils as a Challenge for Soil and Plant Analysis, Magnesium Fertilization and Root Uptake under Adverse Growth Conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Mayland, H.F.; Wilkinson, S.R. Soil Factors Affecting Magnesium Availability in Plant-Animal Systems: A Review. J. Anim. Sci. 1989, 67, 3437. [Google Scholar] [CrossRef]
- Schachtschabel, P. Das pflanzenverfügbare Magnesium des Boden und seine Bestimmung. Z. Pflanzenernähr. Düng. Bodenkd. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Novozamsky, I.; Huybregts, A.W.M.; Van Der Lee, J.J. Comparison of Soil Extractions by 0.01M CaCl2, by EUF and by Some Conventional Extraction Procedures. Plant Soil 1986, 96, 433–437. [Google Scholar] [CrossRef]
- MSZ 20135; Hungarian Standard for Magnesium Determination. Magyar Szabványügyi Testület: Budapest, Hungary, 1999.
- Schachtschabel, P. Der Magnesiumversorgungsgrad nordwestdeutscher Böden und seine Beziehungen zum Auftreten von Mangelsymptomen an Kartoffeln. Z. Pflanzenernähr. Düng. Bodenkd. 1956, 74, 202–219. [Google Scholar] [CrossRef]
- Staugaitis, G.; Rutkauskiene, R. Comparison of Magnesium Determination Methods as Influenced by Soil Properties. Agriculture 2010, 97, 105–116. [Google Scholar]
- Mehlich, A. New Extractant for Soil Test Evaluation of Phosphorus, Potassium, Magnesium, Calcium, Sodium, Manganese and Zinc. Commun. Soil Sci. Plant Anal. 1978, 9, 477–492. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Egnér, H.; Riehm, H.; Domingo, W. Untersuchungen uber Die Chemische Bodenanalyse als Grundlage fur Die Beurteilung des Nährstoffzustandes der Böden. (Using Chemical Soil Analysis as a Basis for Assessing Soil Nutrient Status). K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Vuorinen, J.; Mäkitie, O. The Method of Soil Testing in Use in Finland. Agrogeology 1955, 63, 1–44. [Google Scholar]
- Ciesielski, H.; Sterckeman, T.; Santerne, M.; Willery, J.P. A comparison between three methods for the determination of cation exchange capacity and exchangeable cations in soils. Agronomie 1997, 17, 9–16. [Google Scholar] [CrossRef]
- Ciesielski, H.; Sterckeman, T.; Santerne, M.; Willery, J.P. Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Effects of experimental conditions. Agronomie 1997, 17, 1–7. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods—Australasia; CSIRO Publishing: Melbourne, Australia, 2010. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. (Eds.) Methods of Soil Analysis: Part 3: Chemical Methods; Soil Science Society of America, Inc.; American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar] [CrossRef]
- Caporale, A.G.; Adamo, P.; Capozzi, F.; Langella, G.; Terribile, F.; Vingiani, S. Monitoring metal pollution in soils using portable-XRF and conventional laboratory-based techniques: Evaluation of the performance and limitations according to metal properties and sources. Sci. Total Environ. 2018, 643, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Yawar, W.; Naeem, K.; Akhter, P.; Rehana, I.; Saeed, M. Assessment of three digestion procedures for Zn contents in Pakistani soil by flame atomic absorption spectrometry. J. Saudi Chem. Soc. 2010, 14, 125–129. [Google Scholar] [CrossRef]
- Zimmermann, T.; Von Der Au, M.; Reese, A.; Klein, O.; Hildebrandt, L.; Pröfrock, D. Substituting HF by HBF 4—An optimized digestion method for multi-elemental sediment analysis via ICP-MS/MS. Anal. Methods 2020, 12, 3778–3787. [Google Scholar] [CrossRef] [PubMed]
- Towett, E.K.; Shepherd, K.D.; Tondoh, J.E.; Winowiecki, L.A.; Lulseged, T.; Nyambura, M.; Sila, A.; Vågen, T.-G.; Cadisch, G. Total elemental composition of soils in Sub-Saharan Africa and relationship with soil forming factors. Geoderma Reg. 2015, 5, 157–168. [Google Scholar] [CrossRef]
- Lemière, B. A review of pXRF (field portable X-ray fluorescence) applications for applied geochemistry. J. Geochem. Explor. 2018, 188, 350–363. [Google Scholar] [CrossRef]
- Ravansari, R.; Wilson, S.C.; Tighe, M. Portable X-ray fluorescence for environmental assessment of soils: Not just a point and shoot method. Environ. Intern. 2020, 134, 105250. [Google Scholar] [CrossRef] [PubMed]
- Hariadi, Y.; Shabala, S. Screening Broad Beans (Vicia faba) for Magnesium Deficiency. I. Growth Characteristics, Visual Deficiency Symptoms and Plant Nutritional Status. Funct. Plant Biol. 2004, 31, 529. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current Understandings on Magnesium Deficiency and Future Outlooks for Sustainable Agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Vona, V.; Centeri, C.; Giczi, Z.; Kalocsai, R.; Biró, Z.; Jakab, G.; Milics, G.; J. Kovács, A. Comparison of magnesium determination methods on Hungarian soils. Soil Water Res. 2020, 15, 173–180. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. A Conditioned Latin Hypercube Method for Sampling in the Presence of Ancillary Information. Comp. Geosci. 2006, 32, 1378–1388. [Google Scholar] [CrossRef]
- Roudier, P.; Hedley, C.B. Smart sampling to assist on-farm nutrient management. In Accurate and Efficient Use of Nutrients on Farms; Currie, L.D., Christensen, C.L., Eds.; Occasional Report No. 26; Fertilizer and Lime Research Centre, Massey University: Palmerston North, NZ, USA, 2013. [Google Scholar]
- Wolf, A.; Beegle, D. (Eds.) Recommended soil tests for macro and micronutrients. In Northeastern Regional Publication: Recommended Soil Testing Procedures for the Northeastern United States, 3rd ed.; Northeastern Regional Publication No. 493; Cooperative Extension; University of Delaware: Newark, DE, USA, 2009; pp. 39–48. [Google Scholar]
- MSZ-08-0206-2; Hungarian Standard for pH and CaCO3 Determination. Magyar Szabványügyi Testület: Budapest, Hungary, 1978.
- Taalab, A.; Ageeb, G.; Siam, H.S.; Mahmoud, S.A. Some Characteristics of Calcareous Soils. A Review. Middle East J. Agric. Res. 2019, 8, 96–105. [Google Scholar]
- Soeten, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Venkatesan, S.; Jayaganesh, S. Characterisation of Magnesium Toxicity, its Influence on Amino Acid Synthesis Pathway and Biochemical Parameters of Tea. Res. J. Phytochem. 2010, 4, 67–77. [Google Scholar] [CrossRef]
- Kobayashi, H.; Masaoka, Y.; Sato, S. Effects of Excess Magnesium on the Growth and Mineral Content of Rice and Echinochloa. Plant Prod. Sci. 2005, 8, 38–43. [Google Scholar] [CrossRef]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2013, 368, 73–85. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Martins, P.O.; Slaton, N.A.; Roberts, T.L.; Norman, R.J. Comparison of Field-Moist and Oven-Dry Soil on Mehlich-3 and Ammonium Acetate Extractable Soil Nutrient Concentrations. Soil Sci. Soc. Am. J. 2015, 79, 1792–1803. [Google Scholar] [CrossRef]
- Rogers, C.W.; Dari, B.; Schroeder, K.L. Comparison of Soil-Test Extractants for Potassium, Calcium, Magnesium, Sulfur, and Micronutrients in Idaho Soils. Agrosys. Geosci. Environ. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Loch, J.; Jaszberenyi, I.; Vago, I. Hundredth molar calcium chloride extraction procedure. Part III: Calibration with conventional soil testing methods for magnesium. Comm. Soil Sci. Plant Anal. 1998, 29, 1633–1640. [Google Scholar] [CrossRef]
- Zbíral, J.; Němec, P. Data presentation, interpretation, and communication—Integrating of Mehlich 3 extractant into the Czech soil testing scheme. Comm. Soil Sci. Plant Anal. 2000, 31, 2171–2182. [Google Scholar] [CrossRef]
- Mazaeva, M.M. The critical magnesium content of soils. Agroh. Moszkva 1967, 10, 93–105. (In Russian) [Google Scholar]
- Loch, J. Relation between the Magnesium Content of Soils and the Magnesium Taken Up by Plants. Ph.D. Thesis, Hungarian Academy of Sciences, Budapest, Hungary, 1970. (In Hungarian). [Google Scholar]
- Sposito, G. Chemical Equilibria and Kinetics in Soils; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Mamo, T.; Richter, C.; Heiligtag, B. Comparison of extractants for the determination of available P, K, Ca, Mg, and Na in some Ethiopian and German soils. Comm. Soil Sci. Plant Anal. 1996, 27, 2197–2212. [Google Scholar] [CrossRef]
- Van Erp, P.J. The Potential of Multi-Nutrient Soil Extraction with CaCl2 in Nutrient Management. Master’s Thesis, Department of Sub-Department of Soil Quality, Wageningen University, Wageningen, The Netherlands, 2002. [Google Scholar]
- Hailes, K.J.; Aitken, R.L.; Menzies, N.W. Magnesium in tropical and subtropical soils from north-eastern Australia. I. Magnesium fractions and interrelationships with soil properties. Aust. J. Soil Res. 1997, 35, 615–628. [Google Scholar] [CrossRef]
- Dontsova, K.M.; Norton, L.D. Clay dispersion, infiltration, and erosion as influenced by exchangeable Ca and Mg. Soil Sci. 2002, 167, 184–193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).