Impact of Biosolids-Derived Biochar on the Remediation and Ecotoxicity of Diesel-Impacted Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample and Characterization
2.2. Biochar Production and Characterization
2.3. Soil Contamination and Bioremediation Experiment
2.4. Total Petroleum Hydrocarbon (TPH) Analysis
2.5. Bacterial Community Analysis
2.5.1. DNA Extraction
2.5.2. Quantification of Total and Alkane-Degrading Bacteria
2.5.3. Bacterial Community Analysis via Next-Generation Sequencing
2.6. Ecotoxicity Testing
2.7. Data Analysis
3. Results and Discussion
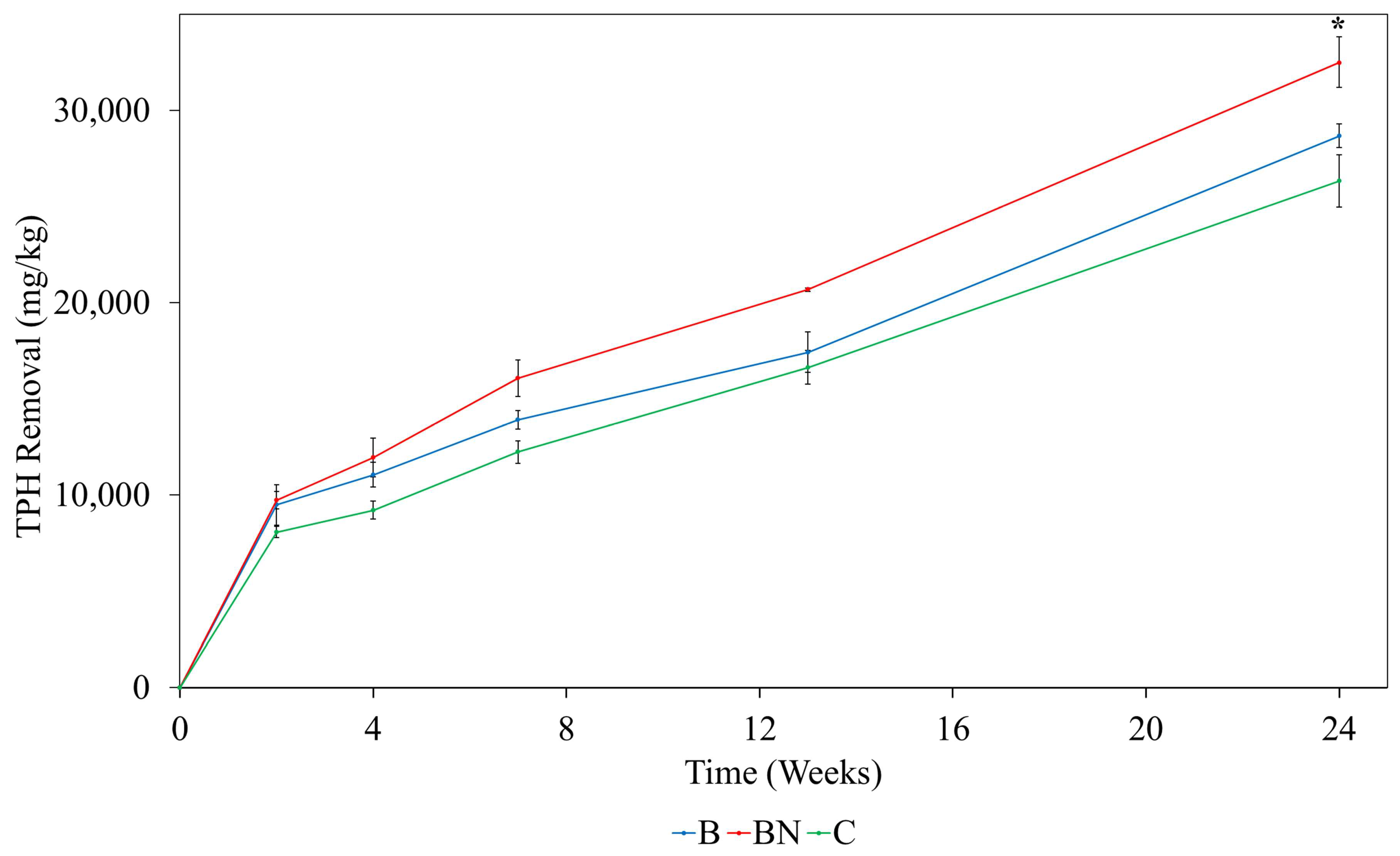
3.1. Effects of Biochar on Hydrocarbon Removal from Contaminated Soil
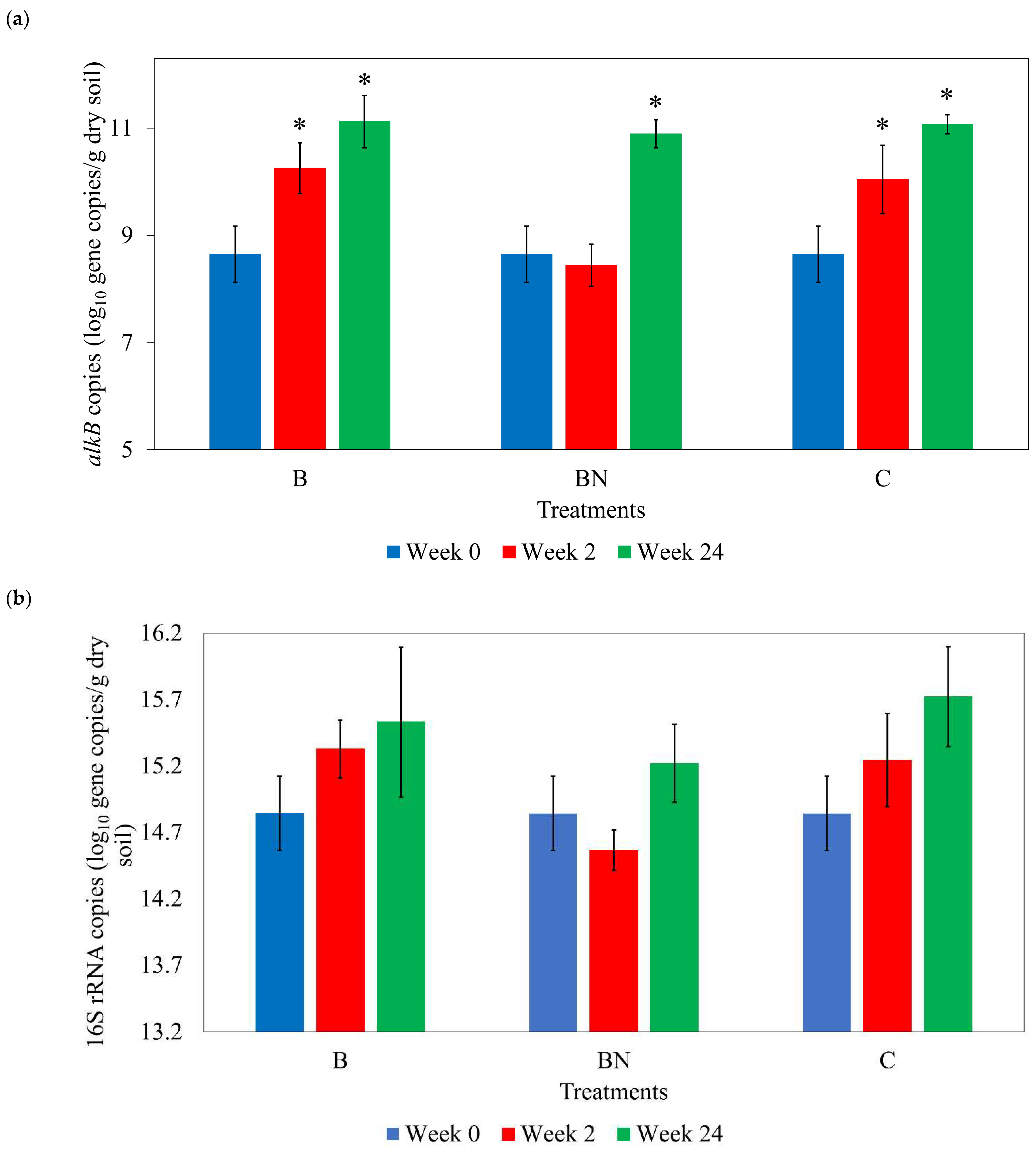
3.2. Bacterial Gene Copies
3.3. Bacterial Diversity
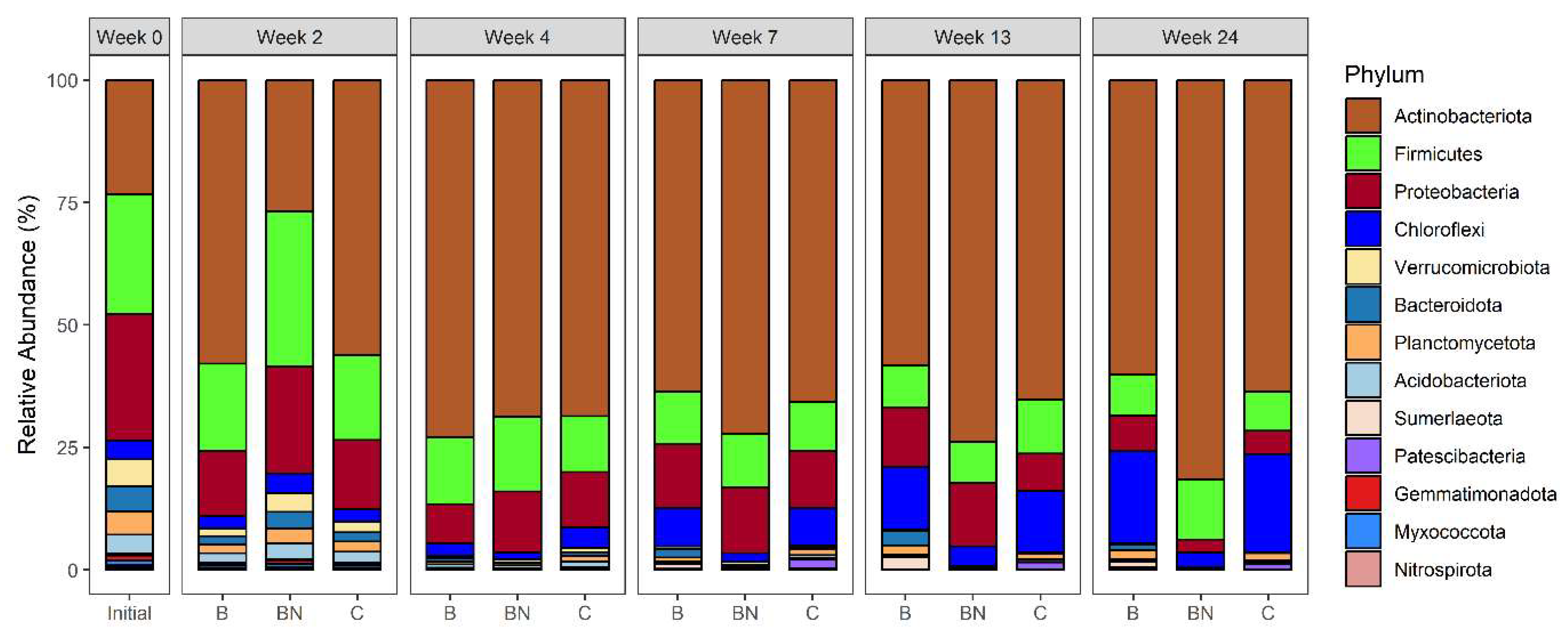
3.4. Bacterial Community Structure and Abundance
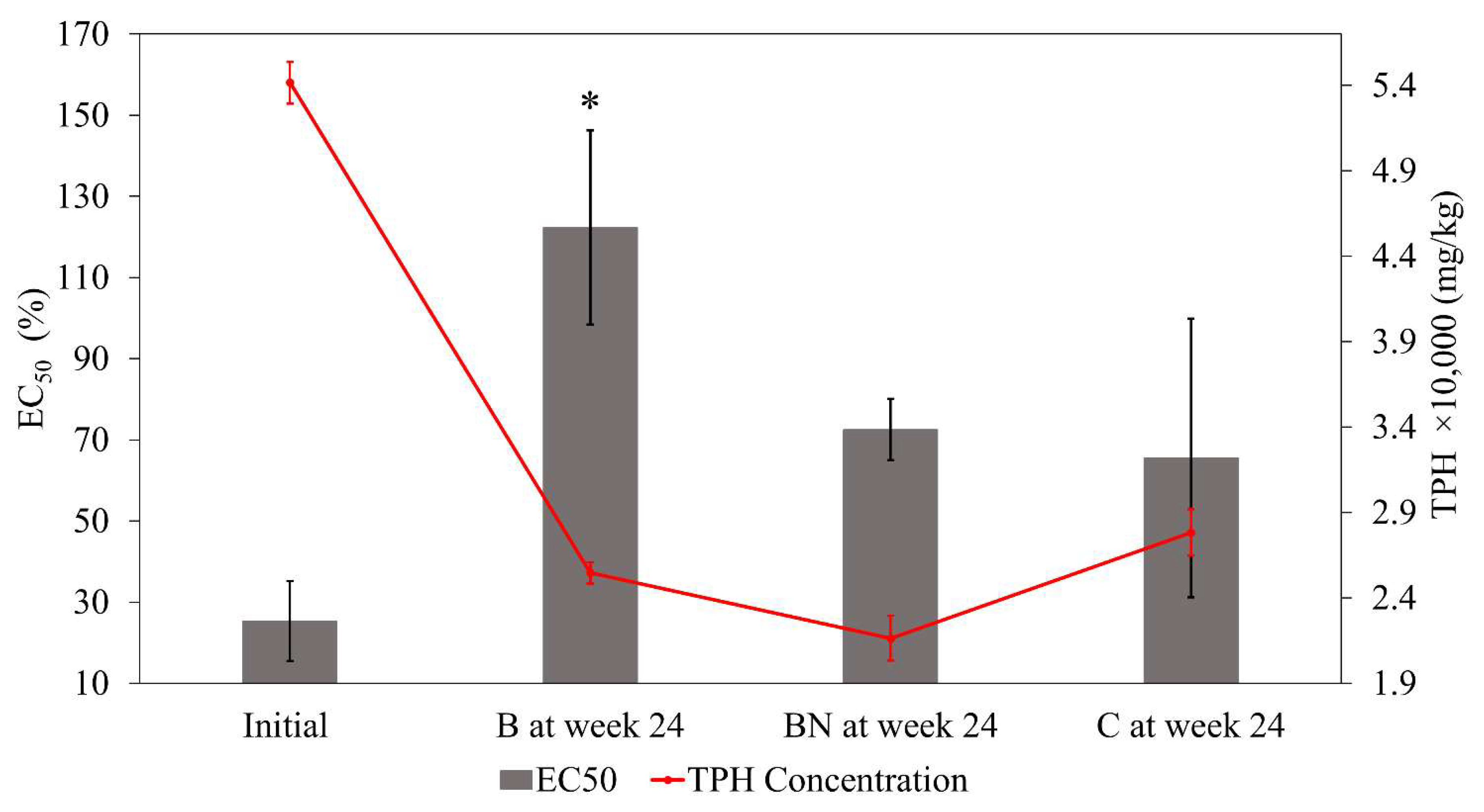
3.5. Ecotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Yang, C.; Yang, Z.; Hollebone, B.; Brown, C.E.; Landriault, M.; Sun, J.; Mudge, S.M.; Kelly-Hooper, F.; Dixon, D.G. Fingerprinting of petroleum hydrocarbons (PHC) and other biogenic organic compounds (BOC) in oil-contaminated and background soil samples. J. Environ. Monit. 2012, 14, 2367–2381. [Google Scholar] [CrossRef] [PubMed]
- Asquith, E.A.; Geary, P.M.; Nolan, A.L.; Evans, C.A. Comparative bioremediation of petroleum hydrocarbon-contaminated soil by biostimulation, bioaugmentation and surfactant addition. J. Environ. Sci. Eng. A 2012, 1, 637–665. [Google Scholar]
- National Oil Spill Detection and Response Agency. Nigerian Oil Spill Monitor; National Oil Spill Detection and Response Agency: Abuja, Nigeria, 2022.
- Ince, M.; Ince, O.K. Introductory Chapter: Sources, Health Impact, and Environment Effect of Hydrocarbons. In Hydrocarbon Pollution and its Effect on the Environment; Ince, M., Ince, O.K., Eds.; IntechOpen: London, UK, 2019; p. 1. [Google Scholar]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Lim, M.W.; Von Lau, E.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil—Present works and future directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef] [PubMed]
- Shackley, S.; Sohi, S.; Brownsort, P.; Carter, S.; Cook, J.; Cunningham, C.; Gaunt, J.; Hammond, J.; Ibarrola, R.; Mašek, O. An Assessment of the Benefits and Issues Associated with The Application of Biochar to Soil; Department for Environment, Food and Rural Affairs, UK Government: London, UK, 2010.
- Chaudhary, D.K.; Bajagain, R.; Jeong, S.-W.; Kim, J. Insights into the biodegradation of diesel oil and changes in bacterial communities in diesel-contaminated soil as a consequence of various soil amendments. Chemosphere 2021, 285, 131416. [Google Scholar] [CrossRef] [PubMed]
- Dike, C.C.; Khudur, L.S.; Hakeem, I.G.; Rani, A.; Shahsavari, E.; Surapaneni, A.; Shah, K.; Ball, A.S. Biosolids-derived biochar enhances the bioremediation of diesel-contaminated soil. J. Environ. Chem. Eng. 2022, 10, 108633. [Google Scholar] [CrossRef]
- Aziz, S.; Ali, M.I.; Farooq, U.; Jamal, A.; Liu, F.-J.; He, H.; Guo, H.; Urynowicz, M.; Huang, Z. Enhanced bioremediation of diesel range hydrocarbons in soil using biochar made from organic wastes. Environ. Monit. Assess. 2020, 192, 56. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Ilyas, N.; Bibi, F.; Shabir, S.; Jayachandran, K.; Sayyed, R.; Shati, A.A.; Alfaifi, M.Y.; Show, P.L.; Rizvi, Z.F. Development of novel kinetic model based on microbiome and biochar for in-situ remediation of total petroleum hydrocarbons (TPHs) contaminated soil. Chemosphere 2023, 324, 138311. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Niu, X.; Zhang, N.; Li, T. Effect of biochar-immobilized Sphingomonas sp. PJ2 on bioremediation of PAHs and bacterial community composition in saline soil. Chemosphere 2021, 279, 130427. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, X.; Tang, J. Enhanced degradation of petroleum hydrocarbons by immobilizing multiple bacteria on wheat bran biochar and its effect on greenhouse gas emission in saline-alkali soil. Chemosphere 2022, 286, 131663. [Google Scholar] [CrossRef]
- Yousaf, U.; Khan, A.H.A.; Farooqi, A.; Muhammad, Y.S.; Barros, R.; Tamayo-Ramos, J.A.; Iqbal, M.; Yousaf, S. Interactive effect of biochar and compost with Poaceae and Fabaceae plants on remediation of total petroleum hydrocarbons in crude oil contaminated soil. Chemosphere 2022, 286, 131782. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Y.; Zhou, Q.; Zhao, X.; Sun, Z. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard. Mater. 2018, 343, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Rong, X.; Song, H.; Chen, J. Remediation of petroleum-contaminated soil using bulrush straw powder, biochar and nutrients. Bull. Environ. Contam. Toxicol. 2017, 98, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Agarry, S.E.; Oghenejoboh, K.M.; Solomon, B.O. Kinetic modelling and half life study of adsorptive bioremediation of soil artificially contaminated with bonny light crude oil. J. Ecol. Eng. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Spinosa, L.; Molinari, L. Standardization: A Necessary Support for the Utilization of Sludge/Biosolids in Agriculture. Standards 2023, 3, 385–399. [Google Scholar] [CrossRef]
- Mohajerani, A.; Lound, S.; Liassos, G.; Kurmus, H.; Ukwatta, A.; Nazari, M. Physical, mechanical and chemical properties of biosolids and raw brown coal fly ash, and their combination for road structural fill applications. J. Clean. Prod. 2017, 166, 1–11. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Walkiewicz, A.; Neugschwandtner, R.W.; Kopecký, M.; Zamanian, K.; Chen, W.-H.; Bucur, D. Feasibility of biochar derived from sewage sludge to promote sustainable agriculture and mitigate GHG emissions—A review. Int. J. Environ. Res. Public Health 2022, 19, 12983. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Buelow, M.C.; Shang, J.; Peng, J.; Rodriguez, M.; Mackay, D.M.; Pignatello, J.J.; Sihota, N.; Hoelen, T.P.; Parikh, S.J. Biochar amendment as a remediation strategy for surface soils impacted by crude oil. Environ. Pollut. 2020, 265, 115006. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Gaston, L.A.; Li, J.; Fultz, L.M.; DeLaune, R.D.; Dodla, S.K. Remediation of crude oil-contaminated coastal marsh soil: Integrated effect of biochar, rhamnolipid biosurfactant and nitrogen application. J. Hazard. Mater. 2020, 396, 122595. [Google Scholar] [CrossRef]
- AL-Khafaji, J.K.T. Preparation of modified selective medium for isolation of Enterococcus faecalis in pure culture from heavy sources (rapid diagnosis). Med. J. Babylon 2021, 18, 340–342. [Google Scholar]
- Aguirre-Garrido, J.F.; Martínez-Abarca, F.; Montiel-Lugo, D.; Hernández-Soto, L.M.; Ramírez-Saad, H. Metagenomic analyses uncover the differential effect of azide treatment on bacterial community structure by enriching a specific Cyanobacteria present in a saline-alkaline environmental sample. Int. Microbiol. 2020, 23, 467–474. [Google Scholar] [CrossRef]
- Maila, M.P.; Cloete, T.E. The use of biological activities to monitor the removal of fuel contaminants—Perspective for monitoring hydrocarbon contamination: A review. Int. Biodeterior. Biodegrad. 2005, 55, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, M. Bioremediation of crude oil-contaminated soil: Comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 2010, 183, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Khudur, L.S.; Shahsavari, E.; Miranda, A.F.; Morrison, P.D.; Nugegoda, D.; Ball, A.S. Evaluating the efficacy of bioremediating a diesel-contaminated soil using ecotoxicological and bacterial community indices. Environ. Sci. Pollut. Res. 2015, 22, 14809–14819. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Agronomic and remedial benefits and risks of applying biochar to soil: Current knowledge and future research directions. Environ. Int. 2016, 87, 1–12. [Google Scholar] [CrossRef]
- Gad, S.C. Diesel Fuel. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 115–118. [Google Scholar]
- Zhang, B.; Zhang, L.; Zhang, X. Bioremediation of petroleum hydrocarbon-contaminated soil by petroleum-degrading bacteria immobilized on biochar. RSC Adv. 2019, 9, 35304–35311. [Google Scholar] [CrossRef] [PubMed]
- Khudur, L.S.; Ball, A.S. RemScan: A tool for monitoring the bioremediation of Total Petroleum Hydrocarbons in contaminated soil. MethodsX 2018, 5, 705–709. [Google Scholar] [CrossRef]
- Shahsavari, E.; Aburto-Medina, A.; Taha, M.; Ball, A.S. A quantitative PCR approach for quantification of functional genes involved in the degradation of polycyclic aromatic hydrocarbons in contaminated soils. MethodsX 2016, 3, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Beker, S.A.; Khudur, L.S.; Krohn, C.; Cole, I.; Ball, A.S. Remediation of groundwater contaminated with dye using carbon dots technology: Ecotoxicological and microbial community responses. J. Environ. Manag. 2022, 319, 115634. [Google Scholar] [CrossRef]
- Amplicon, P.; Clean-Up, P.; Index, P. 16s Metagenomic Sequencing Library Preparation; Illumina: San Diego, CA, USA, 2013. [Google Scholar]
- Krohn, C.; Jin, J.; Wood, J.L.; Hayden, H.L.; Kitching, M.; Ryan, J.; Fabijański, P.; Franks, A.E.; Tang, C. Highly decomposed organic carbon mediates the assembly of soil communities with traits for the biodegradation of chlorinated pollutants. J. Hazard. Mater. 2021, 404, 124077. [Google Scholar] [CrossRef]
- James, R.; Dindal, A.; Willenberg, Z.; Riggs, K. Environmental Technology Verification Report: Strategic Diagnostics Microtox Rapid Toxicity Testing System; Batelle and U.S. Environmental Protection Agency: Columbus, OH, USA, 2003.
- Koshlaf, E.; Shahsavari, E.; Haleyur, N.; Osborn, A.M.; Ball, A.S. Impact of necrophytoremediation on petroleum hydrocarbon degradation, ecotoxicity and soil bacterial community composition in diesel-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 31171–31183. [Google Scholar] [CrossRef]
- Dike, C.C.; Shahsavari, E.; Surapaneni, A.; Shah, K.; Ball, A.S. Can biochar be an effective and reliable biostimulating agent for the remediation of hydrocarbon-contaminated soils? Environ. Int. 2021, 154, 106553. [Google Scholar] [CrossRef]
- Skipper, H.D.; Westermann, D.T. Comparative effects of propylene oxide, sodium azide, and autoclaving on selected soil properties. Soil Biol. Biochem. 1973, 5, 409–414. [Google Scholar] [CrossRef]
- Kebede, G.; Tafese, T.; Abda, E.M.; Kamaraj, M.; Assefa, F. Factors influencing the bacterial bioremediation of hydrocarbon contaminants in the soil: Mechanisms and impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Kong, L.; Song, B.; Zhang, T.; Gao, K.; Liu, J. Effects of soil organic matter on biochar application in developing the biodegradation potentials of polycyclic aromatic hydrocarbons (PAHs). Appl. Soil Ecol. 2021, 167, 104046. [Google Scholar] [CrossRef]
- EPA Victoria. Waste Disposal Categories—Characteristics and Thresholds. Available online: https://www.epa.vic.gov.au/about-epa/publications/1828-2 (accessed on 17 March 2024).
- Tazangi, M.H.; Ebrahimi, S.; Nasrabadi, R.G.; Naeeni, S.A.M. Kinetic Monitoring of Bioremediators for Biodegradation of Gasoil-Polluted Soil. Water Air Soil Pollut. 2020, 231, 418. [Google Scholar] [CrossRef]
- Agarry, S.E.; Aremu, M.O.; Aworanti, O.A. Kinetic modelling and half-life study on enhanced soil bioremediation of bonny light crude oil amended with crop and animal-derived organic wastes. J. Pet. Environ. Biotechnol. 2013, 4, 137. [Google Scholar] [CrossRef]
- Kim, S.H.; Woo, H.; An, S.; Chung, J.; Lee, S.; Lee, S. What determines the efficacy of landfarming for petroleum-contaminated soils: Significance of contaminant characteristics. Chemosphere 2022, 290, 133392. [Google Scholar] [CrossRef]
- Schloter, M. Effects of different compost amendments on the abundance and composition of alkB harboring bacterial communities in a soil under industrial use contaminated with hydrocarbons. Front. Microbiol. 2014, 5, 67345. [Google Scholar]
- Dasgupta, D.; Brahmaprakash, G. Soil microbes are shaped by soil physico-chemical properties: A brief review of existing literature. Int. J. Plant Soil Sci. 2021, 33, 59–71. [Google Scholar] [CrossRef]
- Galitskaya, P.; Akhmetzyanova, L.; Selivanovskaya, S. Biochar-carrying hydrocarbon decomposers promote degradation during the early stage of bioremediation. Biogeosciences 2016, 13, 5739–5752. [Google Scholar] [CrossRef]
- Noonin, C.; Peerapen, P.; Thongboonkerd, V. Contamination of bacterial extracellular vesicles (bEVs) in human urinary extracellular vesicles (uEVs) samples and their effects on uEVs study. J. Extracell. Biol. 2022, 1, e69. [Google Scholar] [CrossRef]
- Thongboonkerd, V.; Saetun, P. Bacterial overgrowth affects urinary proteome analysis: Recommendation for centrifugation, temperature, duration, and the use of preservatives during sample collection. J. Proteome Res. 2007, 6, 4173–4181. [Google Scholar]
- Lichstein, H.C.; Soule, M.H. Studies of the effect of sodium azide on microbic growth and respiration: I. The action of sodium azide on microbic growth. J. Bacteriol. 1944, 47, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bruckberger, M.C.; Bastow, T.P.; Morgan, M.J.; Gleeson, D.; Banning, N.; Davis, G.; Puzon, G.J. Biodegradability of polar compounds formed from weathered diesel. Biodegradation 2018, 29, 443–461. [Google Scholar] [CrossRef]
- Bruckberger, M.C.; Morgan, M.J.; Walsh, T.; Bastow, T.P.; Prommer, H.; Mukhopadhyay, A.; Kaksonen, A.H.; Davis, G.; Puzon, G.J. Biodegradability of legacy crude oil contamination in Gulf War damaged groundwater wells in Northern Kuwait. Biodegradation 2019, 30, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P. Actinobacteria. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1819–1838. [Google Scholar]
- Tischler, D.; Van Berkel, W.J.; Fraaije, M.W. Actinobacteria, a source of biocatalytic tools. Front. Microbiol. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.C.; Amande, T.J.; McGenity, T.J. Prokaryotic hydrocarbon degraders. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; McGenity, T., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Greer, C.; Whyte, L.; Niederberger, T. Microbial communities in hydrocarbon-contaminated temperate, tropical, alpine, and polar soils. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Brust, G.E. Management strategies for organic vegetable fertility. In Safety and Practice for Organic Food; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–212. [Google Scholar]
- Bao, H.; Wang, J.; Zhang, H.; Li, J.; Li, H.; Wu, F. Effects of biochar and organic substrates on biodegradation of polycyclic aromatic hydrocarbons and microbial community structure in PAHs-contaminated soils. J. Hazard. Mater. 2020, 385, 121595. [Google Scholar] [CrossRef]
- Qin, G.; Gong, D.; Fan, M.-Y. Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegrad. 2013, 85, 150–155. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Zhu, Q.; Zhang, Z.; Lin, X. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci. Rep. 2017, 7, 40093. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Bao, J.; Li, S.; Liu, D.; Dai, D.; Qv, M.; Zhu, L. Biochar amendment of aerobic composting for the effective biodegradation of heavy oil and succession of bacterial community. Bioresour. Technol. 2022, 362, 127820. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhan, J.; Li, L.; Zhu, Y.; Liu, J.; Guo, X. Total petroleum hydrocarbons and influencing factors in co-composting of rural sewage sludge and organic solid wastes. Environ. Pollut. 2022, 319, 120911. [Google Scholar] [CrossRef] [PubMed]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Cébron, A.; Riom, J.-M.; Guibaud, G.; Esposito, G.; van Hullebusch, E.D. Effect of digestate application on microbial respiration and bacterial communities’ diversity during bioremediation of weathered petroleum hydrocarbons contaminated soils. Sci. Total Environ. 2019, 670, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Sowani, H.; Kulkarni, M.; Zinjarde, S. Harnessing the catabolic versatility of Gordonia species for detoxifying pollutants. Biotechnol. Adv. 2019, 37, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, R.; Song, J.; Ren, Y.; Luo, X.; Li, Y.; Li, X.; Li, T.; Wang, X.; Zhou, Q. Combined phyto-microbial-electrochemical system enhanced the removal of petroleum hydrocarbons from soil: A profundity remediation strategy. J. Hazard. Mater. 2021, 420, 126592. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, X.; Zhu, Q.; Ma, J.; Hou, H.; Zhang, S. Bioremediation of petroleum-contaminated soil enhanced by aged refuse. Chemosphere 2019, 222, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Al Masud, M.A.; Shin, W.S.; Sarker, A.; Septian, A.; Das, K.; Deepo, D.M.; Iqbal, M.A.; Islam, A.R.M.T.; Malafaia, G. A critical review of sustainable application of biochar for green remediation: Research uncertainty and future directions. Sci. Total Environ. 2023, 904, 166813. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Scharf, R.; Stierstorfer, J.; Unger, C.C. Toxicity assessment of energetic materials by using the luminescent bacteria inhibition test. Propellants Explos. Pyrotech. 2021, 46, 114–123. [Google Scholar] [CrossRef]
- Schafer, H.; Muyzer, G. Denaturing gradient gel electrophoresis in marine microbial ecology. Methods Microbiol. 2001, 30, 425–468. [Google Scholar]
- Pérez-de-Mora, A.; Engel, M.; Schloter, M. Abundance and diversity of n-alkane-degrading bacteria in a forest soil co-contaminated with hydrocarbons and metals: A molecular study on alkB homologous genes. Microb. Ecol. 2011, 62, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Khudur, L.S.; Gleeson, D.B.; Ryan, M.H.; Shahsavari, E.; Haleyur, N.; Nugegoda, D.; Ball, A.S. Implications of co-contamination with aged heavy metals and total petroleum hydrocarbons on natural attenuation and ecotoxicity in Australian soils. Environ. Pollut. 2018, 243, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Adetutu, E.M.; Anderson, P.A.; Ball, A.S. Plant residues—a low cost, effective bioremediation treatment for petrogenic hydrocarbon-contaminated soil. Sci. Total Environ. 2013, 443, 766–774. [Google Scholar] [CrossRef]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y.; Zhang, Q. Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning. J. Hazard. Mater. 2020, 398, 122941. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dike, C.C.; Krohn, C.; Khudur, L.S.; Batra, A.R.; Nnorom, M.-A.; Surapaneni, A.; Shah, K.; Ball, A.S. Impact of Biosolids-Derived Biochar on the Remediation and Ecotoxicity of Diesel-Impacted Soil. Soil Syst. 2024, 8, 40. https://doi.org/10.3390/soilsystems8020040
Dike CC, Krohn C, Khudur LS, Batra AR, Nnorom M-A, Surapaneni A, Shah K, Ball AS. Impact of Biosolids-Derived Biochar on the Remediation and Ecotoxicity of Diesel-Impacted Soil. Soil Systems. 2024; 8(2):40. https://doi.org/10.3390/soilsystems8020040
Chicago/Turabian StyleDike, Charles Chinyere, Christian Krohn, Leadin S. Khudur, Alka Rani Batra, Mac-Anthony Nnorom, Aravind Surapaneni, Kalpit Shah, and Andrew S. Ball. 2024. "Impact of Biosolids-Derived Biochar on the Remediation and Ecotoxicity of Diesel-Impacted Soil" Soil Systems 8, no. 2: 40. https://doi.org/10.3390/soilsystems8020040
APA StyleDike, C. C., Krohn, C., Khudur, L. S., Batra, A. R., Nnorom, M.-A., Surapaneni, A., Shah, K., & Ball, A. S. (2024). Impact of Biosolids-Derived Biochar on the Remediation and Ecotoxicity of Diesel-Impacted Soil. Soil Systems, 8(2), 40. https://doi.org/10.3390/soilsystems8020040






