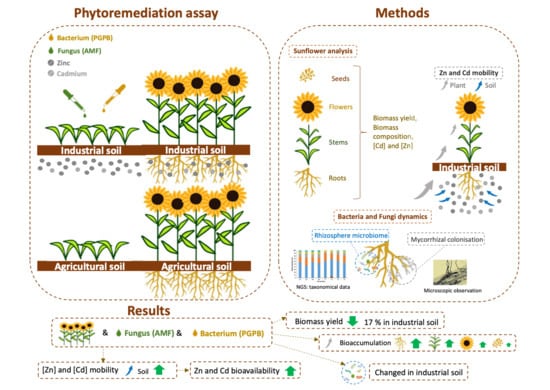
Phytomanagement of Zn- and Cd-Contaminated Soil: Helianthus annuus Biomass Production and Metal Remediation Abilities with Plant-Growth-Promoting Microbiota Assistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Growth
2.2. Plant Biomass and Metal Accumulation Monitoring
2.3. Bioaccumulation Factor and Remediation Ratio
2.4. Cd and Zn Mobilization in Soil
2.5. Monitoring of Bacterial Community in Soils
2.6. Root Colonization by AMF
2.7. Statistical Analysis
3. Results
3.1. H. annuus Biomass Production Yields
3.2. Metal Accumulation in H. annuus Plants
3.3. Bioaccumulation and Translocation Factors and Remediation Ratios
3.4. Cd and Zn Mobilization in Soils
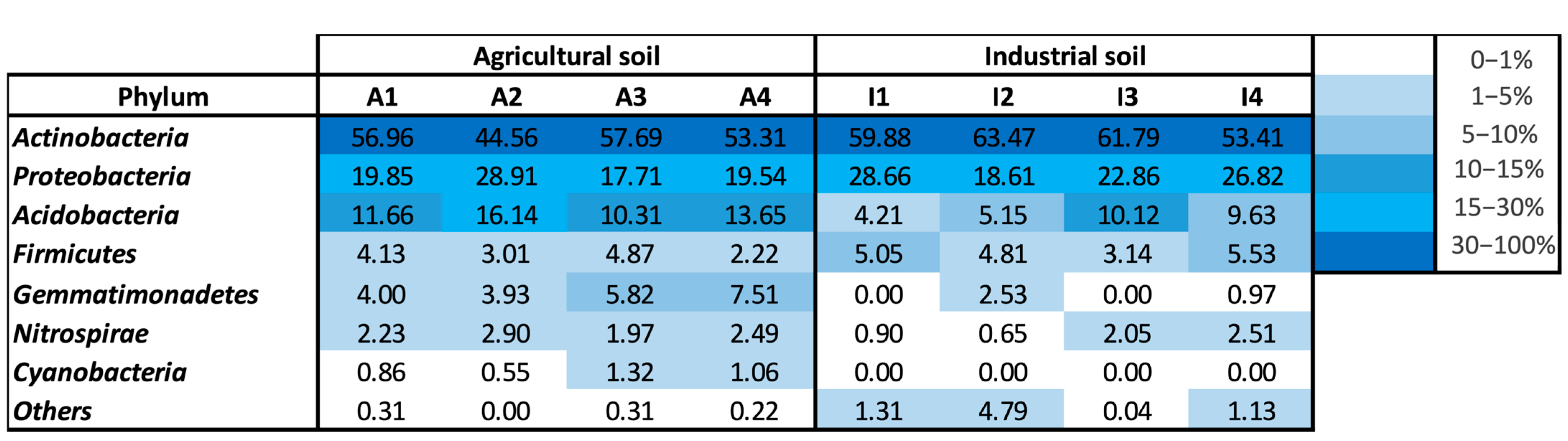
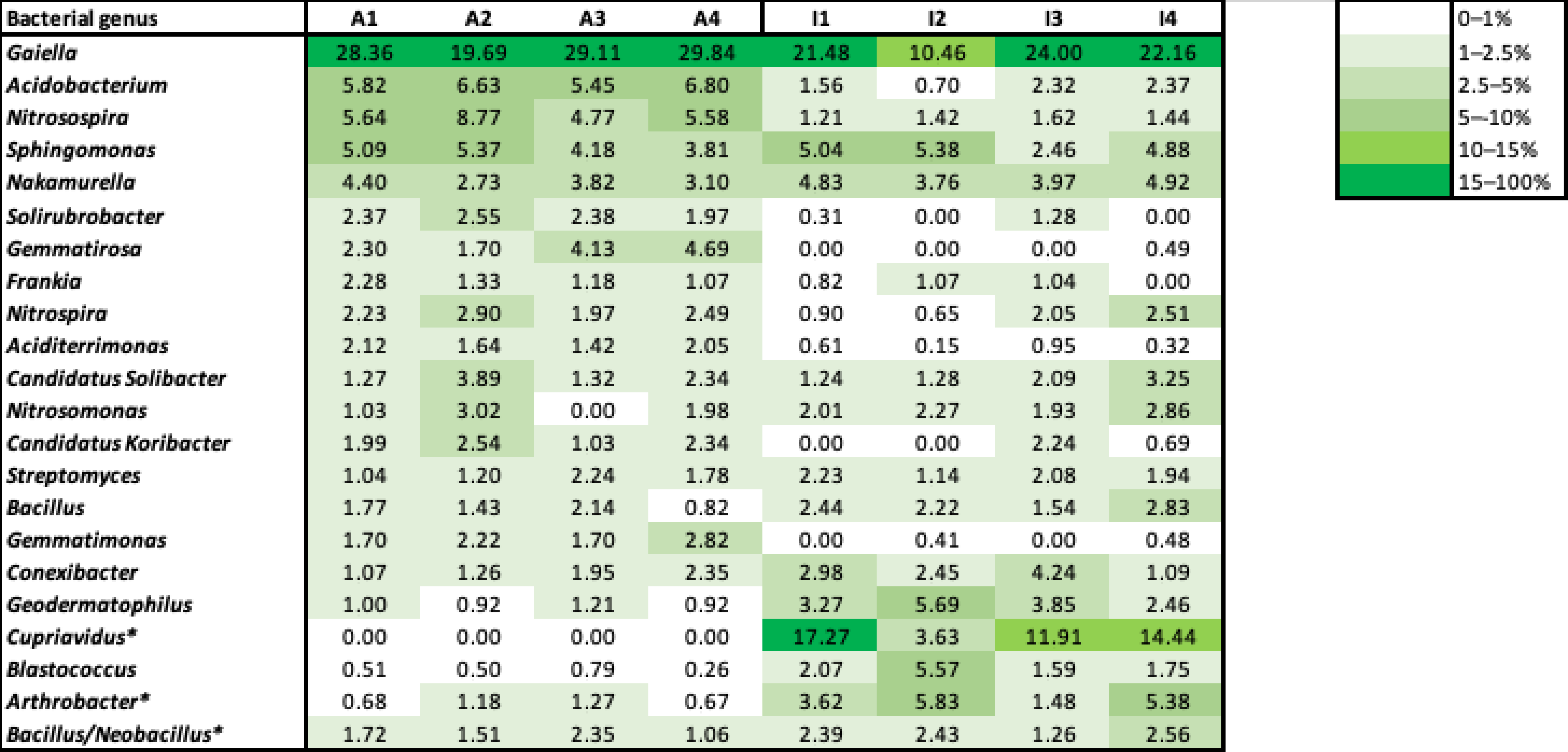
3.5. Monitoring of Bacterial Community Present in Soils
3.6. Mycorrhizal Colonization of Roots
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bermudez, G.M.A.; Jasan, R.; Plá, R.; Pignata, M.L. Heavy metals and trace elements in atmospheric fall-out: Their relationship with topsoil and wheat element composition. J. Hazard. Mater. 2012, 213–214, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Hu, C.; Lin, J.; Wang, X.; Tang, C.; Dai, Z.; Xu, J. Contamination with multiple heavy metals decreases microbial diversity and favors generalists as the keystones in microbial occurrence networks. Environ. Pollut. 2022, 306, 119406. [Google Scholar] [CrossRef]
- Xu, Y.; Seshadri, B.; Sarkar, B.; Wang, H.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; Yang, X.; Bolan, N. Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci. Total Environ. 2018, 621, 148–159. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-Up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Reddy, K.R.; Amaya-Santos, G.; Yargicoglu, E.; Cooper, D.E.; Negri, M.C. Phytoremediation of heavy metals and PAHs at slag fill site: Three-year field-scale investigation. Int. J. Geotech. Eng. 2019, 13, 32–47. [Google Scholar] [CrossRef]
- Willscher, S.; Mirgorodsky, D.; Jablonski, L.; Ollivier, D.; Merten, D.; Büchel, G.; Wittig, J.; Werner, P. Field scale phytoremediation experiments on a heavy metal and uranium contaminated site, and further utilization of the plant residues. Hydrometallurgy 2013, 131–132, 46–53. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Mench, M.; Garrbisu, C.; Kidd, P.; Castro, P.M.L. Phytomanagement of Metal(loid)-Contaminated Soils: Options, Efficiency and Value. Front. Environ. Sci. 2021, 9, 661423. [Google Scholar] [CrossRef]
- Agnello, A.C.; Potysz, A.; Fourdrin, C.; Huguenot, D.; Chauhan, P.S. Impact of pyrometallurgical slags on sunflower growth, metal accumulation and rhizosphere microbial communities. Chemosphere 2018, 208, 626–639. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Deram, A. Phytomanagement: A realistic approach to soil remediating phytotechnologies with new challenges for plant science (Mini review). Int. J. Plant Biol. Res. 2014, 2, 1023. [Google Scholar]
- Marques, A.P.G.C.; Moreira, H.; Franco, A.R.; Rangel, A.O.S.S.; Castro, P.M.L. Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria—Effects on phytoremediation strategies. Chemosphere 2013, 92, 74–83. [Google Scholar] [CrossRef]
- Abeed, A.H.A.; Mahdy, R.E.; Alshehri, D.; Hammami, I.; Eissa, M.A.; Abdel Latef, A.A.H.; Mahmoud, G.A. Induction of resilience strategies against biochemical deteriorations prompted by severe cadmium stress in sunflower plant when Trichoderma and bacterial inoculation were used as biofertilizers. Front. Plant Sci. 2022, 13, 1004173. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Liu, H.; Macdonald, C.A.; Singh, B.K. Application of microbial inoculants significantly enhances crop productivity: A meta-analysis of studies from 2010 to 2020. J. Sustain. Agric. Environ. 2022, 3, 216–225. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Mine land valorization through energy maize production enhanced by the application of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2016, 23, 6940–6950. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Bai, J.; Qin, H.; Wang, J.; Wang, J.; Lin, X. Intercropping with sunflower and inoculation with arbuscular mycorrhizal fungi promotes growth of garlic chive in metal-contaminated soil at a WEEE-recycling site. Ecotoxicol. Environ. Saf. 2019, 167, 376–384. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Gianinazzi-Pearson, V.; Cleyet-Marel, J.C.; Baudoin, E.; van Tuinen, D. Glomeromycota communities survive extreme levels of metal toxicity in an orphan mining site. Sci. Total Environ. 2017, 598, 121–128. [Google Scholar] [CrossRef]
- Read, S.E.; Smith, D. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I. Arbuscular mychorrizal fungi induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Moreira, H.; Argyras, K.; Castro, P.M.L.; Marques, A.P.G.C. Promotion of sunflower growth under saline water irrigation by the inoculation of beneficial microorganisms. Appl. Soil Ecol. 2016, 105, 36–47. [Google Scholar] [CrossRef]
- Jin, Z.; Deng, S.; Wen, Y.; Jin, Y.; Pan, L.; Zhang, Y.; Black, T.; Jones, K.C.; Zhang, H.; Zhang, D. Application of Simplicillium chinense for Cd and Pb biosorption and enhancing heavy metal phytoremediation of soils. Sci. Total Environ. 2019, 697, 134148. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Khan, M.S.I.; Shahid, N.; Aaliya, K. Bottlenecks in commercialisation and future prospects of PGPR. Appl. Soil Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- Marques, A.P.G.C.; Pires, C.; Moreira, H.; Rangel, A.O.S.S.; Castro, P.M.L. Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol. Biochem. 2010, 42, 1229–1235. [Google Scholar] [CrossRef]
- Pires, C. Bacteria in Heavy Metal Contaminated Soil: Diversity, Tolerance and Use in Remediation Systems; Cranfield University: Cranfield, UK, 2010. [Google Scholar]
- Balsamo, R.A.; Kelly, W.J.; Satrio, J.A.; Ruiz-Felix, M.N.; Fetterman, M.; Wynn, R.; Hagel, K. Utilization of Grasses for Potential Biofuel Production and Phytoremediation of Heavy Metal Contaminated Soils. Int. J. Phytoremediation 2015, 17, 448–455. [Google Scholar] [CrossRef]
- Olivares, A.R.; Carrillo-González, R.; González-Chávez, M.C.A.; Hernández, R.M.S. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. J. Environ. Manag. 2013, 114, 316–323. [Google Scholar] [CrossRef]
- Chami, Z.A.; Amer, N.; Smets, K.; Yperman, J.; Carleer, R.; Dumontet, S.; Vangronsveld, J. Evaluation of flash and slow pyrolysis applied on heavy metal contaminated Sorghum bicolor shoots resulting from phytoremediation. Biomass Bioenergy 2014, 63, 268–279. [Google Scholar] [CrossRef]
- Paulo, A.M.S.; Caetano, N.S.; Marques, A.P.G.C. Bioenergetic Products from Phytoremediation Derived Biomass: Assessment of the Potential of Sunflower Grown in Metal Contaminated Soils for the Production of Biofuels. 2023. Available online: http://dx.doi.org/10.2139/ssrn.4441114 (accessed on 26 July 2023).
- Marques, A.P.G.C.; Moreira, H.; Rangel, A.O.S.S.; Castro, P.M.L. Arsenic, lead and nickel accumulation in Rubus ulmifolius growing in contaminated soil in Portugal. J. Hazard. Mater. 2009, 165, 174–179. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Pereira, S.I.A.; Barbosa, L.; Castro, P.M.L. Rhizobacteria isolated from a metal-polluted area enhance plant growth in zinc and cadmium-contaminated soil. Int. J. Environ. Sci. Technol. 2015, 12, 2127–2142. [Google Scholar] [CrossRef]
- Wallinga, I.; Vark, W.; Houba, V.J.G.; Lee, J.J. Plant Analysis Procedures. In Syllabus; Department of Soil Science and Plant Nutrition, Wageningen Agricultural Univerdsity: Wageningen, The Netherlands, 1998. [Google Scholar]
- Mani, D.; Kumar, C.; Kumar Patel, N. Integrated micro-biochemical approach for phytoremediation of cadmium and zinc contaminated soils. Ecotoxicol. Environ. Saf. 2015, 111, 86–95. [Google Scholar] [CrossRef]
- Moreira, H.; Marques, A.P.G.C.; Franco, A.R.; Rangel, A.O.S.S.; Castro, P.M.L. Phytomanagement of Cd-contaminated soils using maize (Zea mays L.) assisted by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. 2014, 21, 9742–9753. [Google Scholar] [CrossRef]
- de Koe, T. Agrostis castellana and Agrostis delicatula on heavy metal and arsenic enriched sites in NE Portugal. Sci. Total Environ. 1994, 145, 103–109. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.W.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Kisand, V.; Cuadros, R.; Wikner, J. Phylogeny of culturable estuarine bacteria catabolizing riverine organic matter in the northern Baltic Sea. Appl. Environ. Microbiol. 2002, 68, 379–388. [Google Scholar] [CrossRef]
- Paulo, A.M.S.; Amorim, C.L.; Costa, J.; Mesquita, D.P.; Ferreira, E.C.; Castro, P.M.L. Long-term stability of a non-adapted aerobic granular sludge process treating fish canning wastewater associated to EPS producers in the core microbiome. Sci. Total Environ. 2021, 756, 144007. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Meers, E.; Ruttens, A.; Hopgood, M.; Lesage, E.; Tack, F.M.G. Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 2005, 61, 561–572. [Google Scholar] [CrossRef]
- Nehnevajova, E.; Herzig, R.; Bourigault, C.; Bangerter, S.; Schwitzguébel, J.-P. Stability of Enhanced Yield and Metal Uptake By Sunflower Mutants for Improved Phytoremediation. Int. J. Phytoremediation 2009, 11, 329–346. [Google Scholar] [CrossRef]
- Kloke, A.; Sauerbeck, D.R.; Vetter, H. The Contamination of Plants and Soils with Heavy Metals and the Transport of Metals in Terrestrial Food Chains. In Changing Metal Cycles and Human Health: Report of the Dahlem Workshop on Changing Metal Cycles and Human Health; Nriagu, J.O., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 1984; pp. 113–141. [Google Scholar] [CrossRef]
- Tomas, M.; Pagani, M.A.; Andreo, C.S.; Capdevila, M.; Atrian, S.; Bofill, R. Sunflower metallothionein family characterisation. Study of the Zn(II)- and Cd(II)-binding abilities of the HaMT1 and HaMT2 isoforms. J. Inorg. Biochem. 2015, 148, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Nehnevajova, E.; Lyubenova, L.; Herzig, R.; Schröder, P.; Schwitzguébel, J.P.; Schmülling, T. Metal accumulation and response of antioxidant enzymes in seedlings and adult sunflower mutants with improved metal removal traits on a metal-contaminated soil. Environ. Exp. Bot. 2012, 76, 39–48. [Google Scholar] [CrossRef]
- Tassi, E.; Pouget, J.; Petruzzelli, G.; Barbafieri, M. The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere 2008, 71, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.; Pereira, S.I.A.; Vega, A.; Castro, P.M.L.; Marques, A.P.G.C. Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J. Environ. Manag. 2020, 257, 109982. [Google Scholar] [CrossRef]
- Luo, Y.M.; Christie, P.; Baker, A.J.M. Soil solution Zn and pH dynamics in non-rhizosphere soil and in the rhizosphere of Thlaspi caerulescens grown in a Zn/Cd-contaminated soil. Chemosphere 2000, 41, 161–164. [Google Scholar] [CrossRef]
- Leung, H.M.; Wang, Z.W.; Ye, Z.H.; Yung, K.L.; Peng, X.L.; Cheung, K.C. Interactions Between Arbuscular Mycorrhizae and Plants in Phytoremediation of Metal-Contaminated Soils: A Review. Pedosphere 2013, 23, 549–563. [Google Scholar] [CrossRef]
- de Quadros, P.D.; Zhalnina, K.; Davis-Richardson, A.G.; Drew, J.C.; Menezes, F.B.; Camargo, F.A.O.; Triplett, E.W. Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 2016, 98, 195–203. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Zhelezova, A.D.; Chernov, T.I.; Dedysh, S.N. Linking ecology and systematics of acidobacteria: Distinct habitat preferences of the Acidobacteriia and Blastocatellia in tundra soils. PLoS ONE 2020, 15, e0230157. [Google Scholar] [CrossRef]
- Liu, P.; Jia, S.; He, X.; Zhang, X.; Ye, L. Different impacts of manure and chemical fertilizers on bacterial community structure and antibiotic resistance genes in arable soils. Chemosphere 2017, 188, 455–464. [Google Scholar] [CrossRef]
- Hu, Q.; Tan, L.; Gu, S.; Xiao, Y.; Xiong, X.; Zeng, W.A.; Feng, K.; Wei, Z.; Deng, Y. Network analysis infers the wilt pathogen invasion associated with non-detrimental bacteria. NPJ Biofilms Microbiomes 2020, 6, 8. [Google Scholar] [CrossRef]
- Nouioui, I.; Göker, M.; Carro, L.; Montero-Calasanz, M.d.C.; Rohde, M.; Woyke, T.; Kyrpides, N.C.; Klenk, H.P. High quality draft genome of Nakamurella lactea type strain, a rock actinobacterium, and emended description of Nakamurella lactea. Stand. Genom. Sci. 2017, 12, 4. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Gallo, I.F.L.; Furlan, J.P.R.; Sanchez, D.G.; Stehling, E.G. Heavy metal resistance genes and plasmid-mediated quinolone resistance genes in Arthrobacter sp. isolated from Brazilian soils. Antonie Van Leeuwenhoek 2019, 112, 1553–1558. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Nardi, P.; Laanbroek, H.J.; Nicol, G.W.; Renella, G.; Cardinale, M.; Pietramellara, G.; Weckwerth, W.; Trinchera, A.; Ghatak, A.; Nannipieri, P. Biological nitrification inhibition in the rhizosphere: Determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol. Rev. 2020, 037, 874–908. [Google Scholar] [CrossRef]
- Juan, Z.; Shi, Q.; Fan, S.; Zhang, Y.; Zhang, M.; Zhang, J. Distinction between Cr and other heavy–metal–resistant bacteria involved in C/N cycling in contaminated soils of copper producing sites. J. Hazard. Mater. 2021, 402, 123454. [Google Scholar] [CrossRef]
- Chaney, R.L. Toxic Element Accumulation in Soils and Crops: Protecting Soil Fertility and Agricultural Food-Chains. In Inorganic Contaminants in the Vadose Zone; Springer: Berlin/Heidelberg, Germany, 1989; pp. 140–158. [Google Scholar] [CrossRef]
- Underwood, E.J.; Suttle, N.F. The Mineral Nutrition of Livestock, 3rd ed.; CAB International: Wallingford, CT, USA, 1999. [Google Scholar]
- Marques, A.P.G.C.; Caetano, N.S.C.; Castro, P.M.L. Chapter 12: Strategies for Enhancing Soil Phytoremediation and Biomass Valorization. In Handbook of Environmental Remediation: Cloassic and Modern Techniques; Hussein, C.M., Ed.; Royal Society of Chemistry: London, UK, 2020; pp. 331–356. [Google Scholar] [CrossRef]



| Parameter | Agricultural | Industrial | (Method) |
|---|---|---|---|
| pH (1:2.5) | 6.52 ± 0.08 | 5.80 ± 0.06 | (potentiometric) |
| Organic content (%) | 3.0 ± 0.3 | 7.2 ± 0.1 | (Walkey–Black) |
| Total N (mg kg−1) | 1248 ± 62 | 2602 ± 968 | (Kjeldahl) |
| Total P (mg kg−1) | 1628 ± 34 | 2400 ± 23 | (colorimetric-ascorbic acid) |
| Extractable K (mg kg−1) | 98 ± 14 | 41 ± 12 | (Egner-Rhien) |
| Extractable Mg (mg kg−1) | 101 ± 11 | 45 ± 11 | (ammonium acetate) |
| Total Zn (mg kg−1) | 37 ± 3 | 599 ± 12 | (aqua regia-FAAS) |
| Total Cd (mg kg−1) | 0.5 ± 0.5 | 1.2 ± 0.5 | (aqua regia-FAAS) |
| Treatment | Biomass (g) | |||
|---|---|---|---|---|
| Root | Stem | Flower | Seeds | |
| Agricultural | 34 | 750 | 224 | 63 |
| Industrial | 19 | 620 | 199 | 52 |
| Treatment | Zn (mg kg−1 Dry Weight) | Cd (mg kg−1 Dry Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root | Stem | Flower | Seeds | Root | Stem | Flower | Seeds | |||
| Agricultural | 67 ± 3 A,c | 56 ± 5 A,c | 36 ± 11 A,b | 2 ± 1 A,a | *** F4,12 = 60.7 | 1.6 ± 0.2. A,b | 1.0 ± 0.1. A,b | n.d. A,a | n.d. A,a | *** F4,12 = 11.2 |
| Industrial | 434 ± 6 B,d | 343 ± 9 B,c | 129 ± 7 B,b | 4 ± 2 A,a | *** F4,12 = 2418 | 24 ± 2 B,d | 15 ± 2 B,c | 5.3 ± 0.6 B,b | 0.5 ± 0.2 A,a | *** F4,12 = 142 |
| t = 4.062 | t = 1.328 | t = 1.306 | t = 6.484 | t = 0.319 | t = 1.759 | t = 14.545 | t = 16 | |||
| Zn (mg kg−1) | Cd (mg kg−1) | |||||
| Beginning | End | Beginning | End | |||
| Agricultural | 18.8 ± 0.70 a,A | 25.2 ± 2.8 a,B | t = 4.189 | n.d. | n.d. | -- |
| Industrial | 69.4 ± 1.5 b,A | 82.3 ± 6.3 b,B | t = 9.445 | n.d. | n.d. | -- |
| t = 0.851 | t = 2.679 | -- | -- | |||
| % Colonization | |
|---|---|
| Agricultural | 27.3 ± 6.5 a |
| Industrial | 41.3 ± 4.7 b |
| t = 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulo, A.M.S.; Caetano, N.S.; Castro, P.M.L.; Marques, A.P.G.C. Phytomanagement of Zn- and Cd-Contaminated Soil: Helianthus annuus Biomass Production and Metal Remediation Abilities with Plant-Growth-Promoting Microbiota Assistance. Soil Syst. 2023, 7, 69. https://doi.org/10.3390/soilsystems7030069
Paulo AMS, Caetano NS, Castro PML, Marques APGC. Phytomanagement of Zn- and Cd-Contaminated Soil: Helianthus annuus Biomass Production and Metal Remediation Abilities with Plant-Growth-Promoting Microbiota Assistance. Soil Systems. 2023; 7(3):69. https://doi.org/10.3390/soilsystems7030069
Chicago/Turabian StylePaulo, Ana M. S., Nidia S. Caetano, Paula M. L. Castro, and Ana P. G. C. Marques. 2023. "Phytomanagement of Zn- and Cd-Contaminated Soil: Helianthus annuus Biomass Production and Metal Remediation Abilities with Plant-Growth-Promoting Microbiota Assistance" Soil Systems 7, no. 3: 69. https://doi.org/10.3390/soilsystems7030069
APA StylePaulo, A. M. S., Caetano, N. S., Castro, P. M. L., & Marques, A. P. G. C. (2023). Phytomanagement of Zn- and Cd-Contaminated Soil: Helianthus annuus Biomass Production and Metal Remediation Abilities with Plant-Growth-Promoting Microbiota Assistance. Soil Systems, 7(3), 69. https://doi.org/10.3390/soilsystems7030069