Abstract
The use of humic substances in agriculture has increased in recent years, and leonardite has been an important raw material in the manufacture of commercial products rich in humic and fulvic acids. Leonardite-based products have been used to improve soil properties and to help plants cope with abiotic and biotic stresses. In this study, the effects of two commercial leonardites and an organic compost, in addition to a control treatment, were assessed for pot-grown olive plants over a period of fourteen months on soil properties, tissue elemental composition and dry matter yield (DMY). Three organic amendments were applied at single and double rates of that set by the manufacturer. The study was arranged in two experiments: one containing the seven treatments mentioned above and the other containing the same treatments supplemented with mineral nitrogen (N), phosphorus (P) and potassium (K) fertilization. Overall, organic compost increased soil organic carbon by ~8% over the control. In the experiment without NPK supplementation, N concentrations in shoots and P in roots were the highest for the compost application (leaf N 12% and root P 32% higher than in the control), while in the experiment with NPK supplementation, no significant differences were observed between treatments. Total DMY was ~10% higher in the set of treatments with NPK in comparison to treatments without NPK. Leonardites did not affect significantly any measured variables in comparison to the control. In this study, a good management of the majority of environmental variables affecting plant growth may have reduced the possibility of obtaining a positive effect on plant nutritional status and growth from the use of commercial leonardites. The leonardites seemed to have caused a slight effect on biological N immobilization. This is not necessarily an advantage or a drawback; it is rather a feature that must be understood to help farmers make better use of these products.
1. Introduction
Soil organic matter is the most widely used soil fertility index and a measure of its suitability for plant growth. Organic matter improves physical, chemical and biological soil properties, resulting in increased water holding capacity and aeration, pH stabilization, trace element immobilization and enhanced microbial activity and nutrient bioavailability [1]. For centuries, the use of farmyard manure and other organic materials in agricultural fields was the main method of improving soil fertility and maintaining crop productivity. Currently, the addition of organic amendments continues to be a recommended practice to restore fertility and to increase crop growth and yield, particularly in degraded soils [2,3].
In recent years, agriculture has undergone increasing specialization, and the rearing of livestock has tended to separate itself from crop production. There are regions of the world where landless livestock production systems are dominant, which create complications in waste management, sometimes resulting in serious problems of environmental contamination [4,5]. In other regions, animals and their associated farmyard manures are practically nonexistent. Although organic materials resulting from agro-industrial activities are sometimes available, which can be composted and used in agriculture [6,7], their availability is always limited given the extent of cultivated areas that need them. This is one of the primary causes of the low levels of organic matter in soils, especially when associated with other inadequate soil management practices, such as excessive tillage [8,9].
In recent years, the use of plant biostimulants in agriculture has increased greatly. Plant biostimulants are materials of a diverse nature, the most salient of which are protein hydrolysates and other N-containing compounds, seaweed extracts, inorganic compounds, chitosan, humic and fulvic acids and beneficial microorganisms [10,11]. Although commercial uses and specifications may vary depending on the nature of the plant biostimulant, they are not characterized by providing nutrients directly to the plants due to the low rates in which they are used but rather by favouring processes in the soil or in the plants that result in increased nutrient use efficiency and/or tolerance to several abiotic and biotic stresses [12,13].
Products rich in humic substances (HS) are an important group within the category of plant biostimulants [10,11]. HS includes humic acid, fulvic acid and humin fractions. Due to the insoluble nature of humin fractions, the commercial biostimulant activity of HS is focused on humic and fulvic acids [14]. They are normally applied to the soil as fertigation and less directly to the crop as a foliar spray. The reduced availability of conventional organic amendments has increased interest in such products, since it is expected that they have an effect on soil properties that can make up for the lack of manure or compost [15,16].
Commercial HSs are complex mixtures of distinct types of biomolecules which render their mode of action difficult to understand. The mechanisms by which HS elicit biostimulatory activity on plants remain elusive despite great efforts made over recent years [14,17]. Although positive results from the use of humic substances are not guaranteed, since they seem to be dependent on several agro-ecological variables that are difficult to optimize, a great number of positive results in soils and/or plants has been recorded [18,19]. Enhancement of plant growth by the application of humic substances has often been attributed to increased nutrient use efficiency and nutrient cycling due to the stimulus of microbial activity [13,20]. HSs have also been related to crop protection against environmental stresses, since they can enhance the activity of key enzymes in the metabolism of phenylpropanoids, which play a central role in the production of phenolic compounds involved in secondary metabolism, providing a strong argument for stress response modulation [17,21]. The negative action of HS has been associated with inordinate interactions with essential proteins or with the presence of entrapped small soil phenols exhibiting strong phytotoxicity in plants [22].
Commercial HSs are obtained from different raw materials, such as composted by-products, vermicomposts, peat and leonardite. Due to natural abundance and cost-effectiveness and because it contains high amounts of humic acids, leonardite has become an important raw material for the manufacture of commercial products [16,23]. Some of the positive effects of HS contained in leonardite may be ascribed to a general improvement in soil fertility, resulting in higher nutrient availability for plants. However, more consistent results from the use of HS tend to be recorded when plants are subjected to some form of environmental stress [20,24].
Nursery-grown and potted plants are very demanding in their requirements, giving rise to many difficulties in managing the salinity of the medium and the availability of water and nutrients for plants [25,26]. Due to ease and low cost with which HS can be added to a growing medium, it was hypothesized for this study that the use of two HS-rich leonardites in potted olive cuttings could benefit plant growth and make the cultivation process easier to implement. Thus, the main goal of the study was to compare two commercial leonardites rich in humic and fulvic acids with a composted organic amendment and a control treatment. An experiment was carried out using pre-rooted olive cuttings and growing them until they reached commercial size. Two commercial leonardites and the organic amendment were applied at single and double rates of that set by the manufacturer. The experimental apparatus was arranged as two independent experiments: In one, organic materials were used alone; in the other, they were used as a supplement to mineral NPK fertilization. This experimental design allowed the integration of a considerable number of treatments, 14 in total, without excessively complicating the interpretation of results.
2. Materials and Methods
2.1. Experimental Setup
This study reports the results of two experiments that took place in Bragança, northeastern Portugal. According to the Köppen–Geiger climate classification, the region benefits from a warm summer Mediterranean climate (Csb). The mean annual temperature is 12.3 °C, and annual precipitation is 758.3 mm [27].
Pot experiments were carried out indoors in a greenhouse covered by a double-wall polycarbonate panel. Lateral and zenith openings ensured aeration of the greenhouse and thermal conditions suitable for cultivation. The greenhouse was also equipped by a reflective screen to assist in the regulation of internal temperature, which automatically slid across when the temperature reached 28 °C.
The pots were filled with 3 kg of dried and sieved (2 mm mesh) soil and mixed with 50 g of perlite. The soil used in these experiments was collected from the surface layer (0.0–0.20 m) in a plot left fallow during the previous year. It is classified as a Regosol of colluvial origin, and the texture is sandy-clay loam (24.2% clay, 21.7% silt and 54.2% sand). Soil organic carbon is low (11.7 g kg−1), and pH(H2O) is close to neutrality (6.8). The levels of extractable P and K (Egnér–Riehm) are both classified as medium, 85.7 mg P2O5 kg−1 and 94.0 mg K2O kg−1, respectively. Cation exchange capacity is 17.9 cmolc kg−1 (11.63, 4.24, 0.45 and 1.58 cmolc kg−1 of Ca, Mg, K and Na, respectively).
In these experiments, three organic amendments (Nutrimais®, Humitec® and Humic gold®) and a compound NPK (10% N, 10% P2O5, and 10% K2O) fertilizer were used. Nutrimais is a dehydrated and pelletized product, resulting from composting forestry, agro-industrial and domestic waste. It is recommended for agricultural use at rates of 3500 to 7000 l ha−1. Humitec and Humic gold are two commercial formulations of leonardite, obtained from fossilized forest wood from about 40 to 60 million years ago, and recommended as soil conditioners. Humitec is recommended for direct application to the soil at rates of 75 to 275 kg ha−1. Humic gold is recommended for application in fertigation at rates of 2 to 6 kg ha−1. The most relevant properties of these fertilizing materials are presented in Table 1.

Table 1.
Properties of the organic amendments used in these experiments (data provided by the manufacturers).
The experiments were conducted on a completely randomized design, with seven treatments each and four replicates. Nutrimais® (Nu), Humitec® (Ht) and Humic gold® (Hg) were applied at two rates (a single and a double rate of those set by the vendor). Thus, the rates used were 5 (Nu1) and 10 (Nu2) t ha−1, 250 (Ht1) and 500 (Ht2) kg ha−1 and 5 (Hg1) and 10 (Hg2) kg ha−1. The conversion of the rates of organic amendments recommended for use in the field to those used in pots took into account the individual area occupied by each pot. The pots were placed in a square with a 0.267 m side, each pot occupying the equivalent of 0.071 m2. Thus, each pot received organic amendments in an amount equivalent to a fraction of 1/140,000 of a hectare. Thus, each pot received 35.7 (Nu1), 71.4 (Nu2), 1.79 (Ht1), 3.57 (Ht2), 0.036 (Hg1) and 0.071 (Hg2) g of commercial product. A non-fertilized control (C) was also included in the experimental design.
The second experiment was arranged in the same manner. The seven treatments described for the first experiment were supplemented with mineral NPK fertilization. Thus, the treatments of this experiment were named as Nu1+, Nu2+, Ht1+, Ht2+, Hg1+, Hg2+ and C+. These pots received the same rates of organic amendments reported for the first experiments in addition to 5 g pot−1 of NPK fertilizer, which corresponds to a field fertilization of ~70 kg ha−1 of N, P2O5 and K2O.
Rooted cuttings of homogeneous size (~10 cm in height) of the cultivar Cobrançosa were used in this study. The trials were set up on 4 August 2019. Subsequently, the pots were kept free of weeds and watered regularly with 200 mL of water. Irrigation frequency was dependent on phenological stages and environmental conditions. The experiment was completed on 7 October 2020.
2.2. Sample Collection and Analysis
Soil samples were taken by carefully recovering all soil from the pots and by trying to avoid breaking the roots. Soil samples were then properly homogenized and 200 g subsamples sent to the laboratory. Afterwards, the roots were washed with a low-pressure water jet in a metallic box with a 1 mm mesh in an attempt to recover as many roots as possible. The plant was divided into roots, stems and leaves, and the three parts were dried and weighed separately. Plant tissues were oven-dried at 65 °C to a constant weight and then ground in a 1-millimetre mesh mill.
Soil samples were oven-dried at 40 °C and analyzed for pH (H2O and KCl) (soil: solution, 1:2.5), cation-exchange capacity (ammonium acetate, pH 7.0), organic carbon (wet digestion, Walkley–Black method) and extractable P and K (Egnér–Riehm method). Soil boron (B) was extracted by hot water, and the extracts were analysed by the azomethine-H method. For more details on these analytical procedures, the reader is referred to van Reeuwijk [28]. The availability of other soil micronutrients (copper (Cu), iron (Fe), zinc (Zn) and manganese (Mn)) was determined by atomic absorption spectrometry after extraction with ammonium acetate and EDTA, according to the method previously described by Lakanen and Erviö [29].
Elemental tissue analyses were performed by Kjeldahl (N), colorimetry (B and P), flame emission spectrometry (K) and atomic absorption spectrophotometry (Ca, Mg, Cu, Fe, Zn and Mn) methods after nitric digestion of the samples [30].
2.3. Data Analysis
Data were analysed for normality and homogeneity of variances using the Shapiro–Wilk and Bartlett’s test, respectively. Thereafter, one-way ANOVA was performed. When significant differences were found among treatments, the means were separated by Tukey HSD (α = 0.05) test.
3. Results
In the experiment without NPK supplementation, significant differences (p < 0.05) between treatments were found (Figure 1). The application of Nutrimais increased DMY in comparison to the control and other treatments such as Hu1 and Hg1. The differences were mainly due to the contribution of leaves to total DMY. In the experiment in which the application of organic amendments was supplemented with NPK, significant differences between treatments (p < 0.05) were not found; however, overall the DMY of these treatments was ~10% higher than that of treatments without NPK supplementation. In both experiments, the contribution of roots to DMY was approximately one-third of the contribution of stems and leaves.
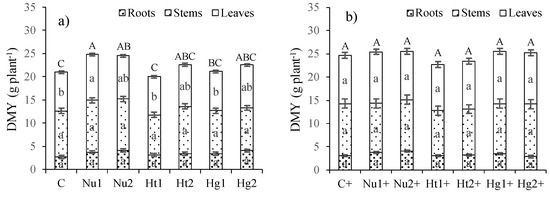
Figure 1.
Dry matter yield (DMY) in roots, stems, leaves and total in response to the application of organic amendments (C, Control; Nu, Nutrimais; Ht, Humitec; and Hg, Humic gold, applied at two rates (1,2)) in experiments (a) without and (b) with NPK(+) mineral fertilization. Lowercase letters show the results of analysis of variance and means separation (Tukey HSD, α = 0.05) for each plant part and uppercase letters for total DMY.
In the experiment without NPK addition, Nutrimais applied at the rate 2 (Nu2) produced the highest average leaf N concentration (23.7 g kg−1), a value significantly higher than that of the control (20.9 g kg−1) and other treatments such as Ht2 (17.5 g kg−1) and Hg2 (18.6 g kg−1) (Table 2). Comparing the two rates of Humitec and also the two rates of Humic gold, it seems that the higher rate reduced leaf N concentration, although without significant differences. In the experiment without NPK supplementation, no significant differences between treatments were found for any of the other nutrients. Leaf P and K, the other nutrients applied in the experimental design, varied from 1.5 to 2.0 g kg−1 and 8.2 to 9.2 g kg−1, respectively. In the experiment with NPK supplementation, no significant differences were recorded for any of the elements determined. The effect of organic amendments on leaf nutrient concentration was less than experimental variability. The range of variation of the macronutrients N, P and K was 23.4–24.5 g kg−1, 1.6–2.3 g kg−1 and 8.7–9.7 g kg−1, respectively.

Table 2.
Leaf nutrient concentration in response to the application of organic amendments (C, Control; Nu, Nutrimais; Ht, Humitec; and Hg, Humic gold, applied at two rates (1,2)) in the experiments without and with NPK(+) mineral fertilization. For each independent experiment, means followed by the same letter are not significantly different by Tukey HSD test (α = 0.05).
Stem N concentrations, although lower than those of the leaves, followed a somewhat similar pattern when comparing treatments (Table 3). The highest values were recorded for Nu2 (9.8 g kg−1), which were significantly higher than those of the Ht2 treatment (7.9 g kg−1). As in the leaves, there seems to be a tendency for higher rates of Humitec (Hu2) and Humic gold (Hg2) to produce stem N concentrations lower than those recorded in Hu1 and Hg1 treatments. For the other nutrients and for all nutrients in the experiment with NPK supplementation, significant differences between treatments were not observed. As mentioned for leaf nutrient concentration, the effect of organic amendments was not enough to stand out from experimental variability.

Table 3.
Stem nutrient concentration in response to the application of organic amendments (C, Control; Nu, Nutrimais; Ht, Humitec; and Hg, Humic gold, applied at two rates (1,2)) in experiments without and with NPK(+) mineral fertilization. For each independent experiment, means followed by the same letter are not significantly different by Tukey HSD test (α = 0.05).
As in aboveground tissue, average root N concentration was the highest in the Nu2 treatment (16.3 g kg−1), a value significantly higher than that of the Ht2 treatment (13.3 g kg−1) (Table 4). Moreover, as in the aboveground tissue, the average values of treatments Ht2 (13.3 g kg−1) and Hg2 (13.7 g kg−1) were lower than those of Ht1 (14.1 g kg−1) and Hg1 (15.6 g kg−1). Root P concentrations varied significantly between treatments, Nut2 displaying the highest value (2.5 g kg−1) and C, Ht1 and Ht2 displaying the lower one (1.7 g kg−1) in the experiment without NPK addition. Root Mg concentration was significantly higher in Nu2 (4.3 g kg−1) in comparison to the control (3.1 g kg−1). For the other nutrients and for all nutrients in the experiment with NPK supplementation, significant differences between treatments were not found. Root N, P and K concentrations in the experiment with NPK addition were in the ranges 15.4–18.4 g kg−1, 1.8–2.4 g kg−1 and 12.0–14.1 g kg−1, respectively.

Table 4.
Root nutrient concentration in response to the application of organic amendments (C, Control; Nu, Nutrimais; Ht, Humitec; and Hg, Humic gold, applied at two rates (1,2)), in experiments without and with NPK(+) mineral fertilization. For each independent experiment, means followed by the same letter are not significantly different by Tukey HSD test (α = 0.05).
Soil organic carbon varied significantly between treatments in the experiments without and with NPK addition (Table 5). Higher values were found in Nu2 (12.3 g kg−1) and Nu2+ (12.6 g kg−1) treatments in each of the experiments. Extractable P varied also between treatments, and Nu2 (167.6 mg P2O5 kg−1) and Nu2+ (226.0 mg P2O5 kg−1) showed higher values of each experiment. Overall, the values of extractable P in the experiment with NPK addition were higher than those of the experiment where only organic amendments were applied. Extractable K followed a trend similar to extractable P. The higher values of the experiments were found in treatments Nu2 (163.3 mg K2O kg−1) and Nu2+ (264.0 mg K2O kg−1). No significant differences were found between treatments for exchangeable Ca++ and Mg++ and CEC, as well as for extractable micronutrients. The effect of the treatments on these variables was poor and less than experimental variability.

Table 5.
Selected soil properties in response to the application of organic amendments (C, Control; Nu, Nutrimais; Ht, Humitec; and Hg, Humic gold, applied at two rates (1,2)), in experiments without and with NPK(+) mineral fertilization. For each independent experiment, means followed by the same letter are not significantly different by Tukey HSD test (α = 0.05).
4. Discussion
In the experiment without the addition of NPK, the application of Nutrimais increased DMY in comparison to the control. In the experiment with NPK addition, differences between treatments were not found, and the average values were ~10% higher than those of the treatments without NPK addition. The use of organic amendments can positively influence several soil properties, which may result in the enhancement of plant growth [2,7]. In these experiments, under controlled growing conditions, the positive effect of Nutrimais in DMY was probably due to the supply of nutrients, a thesis supported by the absence of a positive effect of Nutrimais on DMY when the pots were supplemented with the NPK fertilizer.
The analysis of the elemental composition of plant tissues showed that Nutrimais consistently increased tissue N levels (leaves, stems and roots). In the experiment with NPK supplementation, tissue N levels were globally higher than in the experiment without NPK but with no differences between treatments. As no differences in shoots were observed in the concentration of other nutrients, the result suggests that the positive effect of Nutrimais on DMY was due to the supply of N. Nitrogen is one of the main limiting factors for plant growth in most agricultural ecosystems [31,32,33,34]. Soils do not accumulate N in readily available forms, with plants being dependent on regular applications of N as a fertilizer [1]. The availability of N to plants from organic substrates depends on their N content and C/N ratio. C/N ratios above or below 25 are usually associated to net N immobilization or mineralization, respectively [1]. Nutrimais presented an N concentration of 24.1 g kg−1 and C/N ratio of 11.94 (Table 1), suggesting net mineralization in the short term, which was most likely the reason for the increase in N concentration in plant tissues and DMY.
Leaf P concentrations did not vary with the application of Nutrimais. Although P is a limiting factor in plant growth in many regions of the world, where crop yield is dependent on the regular application of P as a fertilizer [35,36,37]. In northeastern Portugal, in soils with similar properties to those used in this experiment, it has been difficult to observe a response in crops relative to P applications [38,39]. This suggests that these soils provide non-limiting amounts of P for plant growth. The roots from Nutrimais pots showed higher P concentrations than those of the other treatments. It has also been shown from previous studies that olive plants can accumulate P in roots when the nutrient is highly available in the soil, while the concentration of P in aboveground plant tissues is maintained at adequate levels [25,40]. This may have reduced the importance of P on plant growth in this experiment. Nevertheless, it is clear that Nutrimais provided the system with P.
The application of Nutrimais increased K levels in the soil, but this was not enough to increase the concentration of K in plant tissues. These soils normally supply K in adequate amounts to plants. In previous studies, there has also been a reduced response of plants observed relative to the application of K [41,42], as mentioned above for P.
Nutrimais increased the content of soil organic carbon in the pots with and without NPK supplementation. The increase in soil organic matter is an important objective in the use of organic amendments and stems from the direct supply of carbon and a slow process of mineralization in the soil [1]. Leonardites did not have such an effect because, at the rates they were used, the carbon added was not enough to be detected by soil analysis.
The leonardites Hu and Hg tended to reduce average tissue N concentrations at the higher rate they were used. This seems to be evidence of biological net N immobilization. Humic and fulvic acids, by stimulating soil microbiology, may have had this short-term effect, contributing to the immobilization of N. This is not necessarily negative. It is a property of organic amendments with little available N [1], but it is important to take this property into account to avoid N shortage during the growing season. In potted cultivation, especially in nurseries, it is common to use slow or controlled release fertilizers as a method of reducing leaching or other forms of nutrient loss [24]. These kinds of leonardites may have a similar role in regulating the N cycle.
Leonardites are also known for their ability to immobilize trace metals, as they contain oxidized functional groups that can bind different metal ions or heavy metals [15,43]. This effect, however, was not observed in this study, since the concentration of metals in plant tissues did not vary significantly with the experimental treatments. The reduced rate at which these commercial products are recommended and used in this particular study was probably the reason for the absence of any detectable effect.
Humic substances are recommended for increasing crop P uptake since they are capable of competing with P to be bound to soil adsorption complexes [44]. In agreement with this, Kaya et al. [45] observed that leonardite enhanced leaf P and yield in maize under P and water stress in calcareous soils. In this study, however, no benefits were found for P nutrition from the use of leonardites, perhaps because the plants were not grown under water stress conditions and these soils may have provided enough P for plant growth, even without P fertilization as above mentioned.
Although some studies have reported different beneficial effects for plants resulting from the use of HS [15,20,46], in other studies, it was shown that there is a possibility of HS exerting a negative impact on plant growth and development [22]. Atiyeh et al. [47] reported that the inhibitory effect of HS on plant growth is dosage dependent and that the negative action of HS was associated with inordinate interactions with essential proteins. It has also been hypothesized that another structural feature in terms of HS toxicity is the presence of entrapped small soil phenols, which exhibit a strong phytotoxicity by affecting glycolysis and pentose phosphate pathways in plants [22,48]. In this study, however, no relevant signs of positive or negative effects on plants or soil were detected when leonardites were used at single and double recommended rates.
5. Conclusions
Nutrimais produced typical results for an organic amendment, with an increase in organic carbon and nutrients in the soil, which, in turn, increased the concentrations of some nutrients in plant tissues and dry matter yield in the experiment without NPK addition. The positive effect on dry matter yield was probably due to the increase in nitrogen availability, the most limiting nutrient in these experiments.
In these pot experiments, a good control of the majority of environmental variables affecting plant growth may have been the reason for not having found any beneficial effects of the use of these commercial leonardites. Leonardites are rich in humic substances, and their effect on plants tends to become more evident when plants are grown under some kind of environmental stress.
Leonardites are not used to provide nutrients due to their low nutrient concentration and the rates at which they are usually applied. Used at such low rates in our experiment, they also did not cause any measurable effect on increasing soil organic carbon. Indeed, the results seem to show a slight effect of biological nitrogen immobilization from the use of leonardites, which may be due to some stimulus in soil biological activity. This is not necessarily an advantage or a drawback; it is simply a feature that must be understood. Despite there being promising indications about these products found in some studies, in others they have not been so clear; thus, more studies are needed to set safe guidelines for their use by farmers.
Author Contributions
Conceptualization, M.Â.R.; methodology, M.A.; investigation, S.F.d.A. and S.R.; resources, M.Â.R. and M.A.; data curation, M.Â.R. and L.d.S.D.; writing—original draft preparation, M.A. and S.F.d.A.; writing—review and editing, M.Â.R., M.A. and L.d.S.D.; supervision, M.A. and M.Â.R.; project administration, M.A.; funding acquisition, M.Â.R. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology (FCT, Portugal) and FEDER under Programme PT2020 for financial support to CIMO (UIDB/00690/2020). The research was integrated in the activities of the operational group “Novas práticas em olivais de sequeiro: estratégias de mitigação e adaptação às alterações climáticas”, funded by PT2020 and EAFRD (European Agricultural Fund for Rural Development).
Data Availability Statement
Data sharing not applicable. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weil, R.R.; Brady, N.C. Nature and Properties of Soils, 15th ed.; Pearson: London, UK, 2017. [Google Scholar]
- Obriot, F.; Stauffer, M.; Goubard, Y.; Cheviron, N.; Peres, G.; Eden, M.; Revallier, A.; Vieublé-Gonod, L.; Houot, S. Multi-criteria indices to evaluate the effects of repeated organic amendment applications on soil and crop quality. Agric. Ecosyst. Environ. 2016, 232, 165–178. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.M.; Domínguez, M.T.; Madejón, E.; Madejón, P.; Pastorelli, R.; Renella, G. Long-term effects of organic amendments on bacterial and fungal communities in a degraded Mediterranean soil. Geoderma 2018, 332, 20–28. [Google Scholar] [CrossRef]
- Francis, D.D.; Vigil, M.F.; Mosier, A.R. Gaseous losses of nitrogen other than through denitrification. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2008; pp. 255–279. [Google Scholar]
- Cao, X.; Reichel, R.; Wissel, H.; Kummer, S.; Brüggemann, N. High carbon amendments increase nitrogen retention in soil after slurry application—an incubation study with silty loam soil. J. Soil Sci. Plant Nutr. 2021. [Google Scholar] [CrossRef]
- Afonso, S.; Pereira, E.; Arrobas, M.; Rodrigues, M.A. Recycling nutrient-rich hop leaves by composting with wheat straw and farmyard manure in suitable mixtures. J. Environ. Manag. 2021, 284, 112105. [Google Scholar] [CrossRef] [PubMed]
- Arrobas, M.; Carvalho, J.T.N.; Raimundo, S.; Poddere, G.; Rodrigues, M.A. The safe use of compost derived from municipal solid waste depends on its composition and conditions of application. Soil Use Manag. 2021. [Google Scholar] [CrossRef]
- Almagro, M.; de Vente, J.; Boix-Fayos, C.; García-Franco, N.; Aguilar, J.M.; González, D.; Solé-Benet, A.; Martínez-Mena, M. Sustainable land management practices as providers of several ecosystem services under rainfed Mediterranean agroecosystems. Mitig Adapt. Strat. Glob. Chang. 2016, 21, 1029–1043. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Arrobas, M. Cover cropping for increasing fruit production and farming sustainability. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–295. [Google Scholar]
- du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs): An Introduction. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 3–29. [Google Scholar]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.S.; Selvaraj, V.; Gunupuru, L.R.; Rathor, P.K.; Prithiviraj, B. Combined application of Ascophyllum nodosum extract and chitosan synergistically activates host-defense of peas against powdery mildew. BMC Plant Biol. 2020, 20, 113. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, J.; Huo, L.; Li, Y.C.; Li, X.; Xia, L.; Zhou, Z.; Zhang, M.; Li, B. Humic acids derived from Leonardite to improve enzymatic activities and bioavailability of nutrients in a calcareous soil. Int. J. Agric. Biol. Eng. 2020, 13, 200–205. [Google Scholar] [CrossRef]
- Lamar, R.T. Possible role for electron shuttling capacity in elicitation of PB activity of humic substances on plant growth enhancement. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 97–121. [Google Scholar]
- Sugier, D.; Kołodziej, B.; Bielińska, E. The effect of leonardite application on Arnica montana L. yielding and chosen chemical properties and enzymatic activity of the soil. J. Geochem. Explor. 2013, 129, 76–82. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Denre, M.; Ghanti, G.; Sarkar, K. Effect of humic acids application on accumulation of mineral nutrition and pungency in garlic (Allium sativum L.). Int. J. Biotech. Mol. Biol. Res. 2014, 5, 7–12. [Google Scholar]
- Sandepogu, M.; Shukla, P.S.; Asiedu, S.; Yurgel, S.; Prithiviraj, B. Combination of Ascophyllum nodosum extract and humic acid improves early growth and reduces post-harvest loss of lettuce and spinach. Agriculture 2019, 9, 240. [Google Scholar] [CrossRef] [Green Version]
- Litardo, R.C.M.; Bendezú, S.J.G.; Zenteno, M.D.C.; Pérez-Almeida, I.B.; Parismoreno, L.L.; García, E.D.L. Effect of mineral and organic amendments on rice growth and yield in saline soils. J. Saudi Soc. Agric. Sci. 2021. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Yoon, H.Y.; Cha, J.-Y.; Kim, E.-K.; Kim, P.J.; Jeon, J.-R. Artificial humification of lignin architecture: Top-down and bottom-up approaches. Biotechnol. Adv. 2019, 37, 107416. [Google Scholar] [CrossRef] [PubMed]
- Özyazici, G. Yield and quality of black cumin (Nigella sativa L.) according to leonardite and nitrogen doses. Appl. Ecol. Environ. Res. 2020, 18, 7057–7075. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Lu, J.; Yuan, G. Effects of leonardite on the coastal saline soil improvement. Chem. Ecol. 2020, 36, 750–765. [Google Scholar] [CrossRef]
- Lopes, J.I.; Correia, C.M.; Gonçalves, A.; Silva, E.; Martins, S.; Arrobas, M.; Rodrigues, M.A. Arbuscular mycorrhizal fungi inoculation reduced the growth of pre-rooted olive cuttings in a greenhouse. Soil Syst. 2021, 5, 30. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Piroli, L.B.; Forcelini, D.; Raimundo, S.; Domingues, L.C.; Cassol, L.C.; Correia, C.M.; Arrobas, M. Use of commercial mycorrhizal fungi in stress-free growing conditions of potted olive cuttings. Sci. Hortic. 2021, 275, 109712. [Google Scholar] [CrossRef]
- IPMA (Instituto Português do Mar e da Atmosfera). Normais Climatológicas; Instituto Português do Mar e da Atmosfera: Lisabona, Portugal, 2021; Available online: https://www.ipma.pt/pt/oclima/normais.clima/ (accessed on 2 December 2021).
- van Reeuwijk, L.P. Procedures for Soil Analysis; Technical Paper 9; ISRIC FAO: Wageningen, The Netherlands, 2002. [Google Scholar]
- Lakanen, E.; Erviö, R. A Comparison of Eight Extractants for the Determination of Plant Available Micronutrients in Soils. Acta Agral. Fenn. 1971, 123, 223–232. [Google Scholar]
- Temminghoff, E.E.J.M.; Houba, V.G. Plant Analysis Procedures; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Rodrigues, M.A.; Afonso, S.; Ferreira, I.Q.; Arrobas, M. Response of stevia to nitrogen fertilization and harvesting regime in Northeastern Portugal. Arch. Agron. Soil Sci. 2017, 63, 626–637. [Google Scholar] [CrossRef]
- Arrobas, M.; Afonso, S.; Rodrigues, M.A. Diagnosing the nutritional condition of chestnut groves by soil and leaf analyses. Sci. Hortic. 2018, 228, 113–121. [Google Scholar] [CrossRef]
- Arrobas, M.; Santos, D.; Ribeiro, A.; Pereira, E.; Rodrigues, M.A. Soil and foliar nitrogen and boron fertilization of almond trees grown under rainfed conditions. Eur. J. Agron. 2019, 106, 39–48. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.M.; Rodrigues, M.A. The effect of nitrogen applications on the growth of young olive trees and nitrogen use efficiency. Turk. J. Agric. 2020, 44, 278–289. [Google Scholar] [CrossRef]
- Vistoso, E.; Iraira, S.; Sandaña, P. Phosphorus use efficiency in permanent pastures in Andisols. J. Soil Sci. Plant Nutr. 2021, 21, 2587–2599. [Google Scholar] [CrossRef]
- Li, G.; Huang, G.; Li, H.; van Ittersum, M.K.; Leffelaar, P.A.; Zhang, F. Identifying potential strategies in the key sectors of China’s food chain to implement sustainable phosphorus management: A review. Nutr. Cycl. Agroecosys. 2016, 104, 341–359. [Google Scholar] [CrossRef]
- Tian, D.; Li, Z.; O’Connor, D.; Shen, Z. The need to prioritize sustainable phosphate-based fertilizers. Soil Use Manag. 2020, 36, 351–354. [Google Scholar] [CrossRef]
- Arrobas, M.; Afonso, S.; Ferreira, I.Q.; Moutinho-Pereira, J.M.; Correia, C.M.; Rodrigues, M.A. Liming and application of nitrogen, phosphorus, potassium and boron on a young plantation of chestnut. Turk. J. Agric. 2017, 41, 441–451. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Ferreira, I.Q.; Afonso, S.; Arrobas, M. Sufficiency ranges and nutrient removals in lemon balm based on crop response to applied nitrogen, phosphorus, potassium and boron. J. Plant Nutr. 2018, 41, 996–1008. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.A.; Moutinho-Pereira, J.M.; Correia, C.; Arrobas, M. Olive tree response to applied phosphorus in field and pot experiments. Sci. Hortic. 2018, 234, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Arrobas, M.; Ferreira, I.Q.; Afonso, S.; Rodrigues, M.A. Sufficiency ranges and crop nutrient removals for peppermint (Mentha x piperita L.) established from field and pot fertilizer experiments. Commun Soil Sci. Plant Anal. 2018, 49, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.; Rodrigues, M.A. Olive response to potassium applications under different water regimes and cultivars. Nutr. Cycl. Agroecosys. 2018, 112, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Cieschi, M.T.; Lucena, J.L. Leonardite iron humate and synthetic iron chelate mixtures in Glycine max nutrition. J. Sci. Food Agric. 2021, 101, 4207–4219. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, B.H.; Wulandari, P.; Sulistyaningsih, E.; Utami, S.N.H.; Handayani, S. Improved corn yields when humic acid extracted from composted manure is applied to acid soils with phosphorus fertilizer. Appl. Environ. Soil Sci. 2021. [Google Scholar] [CrossRef]
- Kaya, C.; Şenbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salin, A.; Karadogan, T.; Tonguc, M. Effects of leonardite applications on yield and some quality parameters of potatoes (Solanum tuberosum L.). Turk. J. Field Crop. 2013, 18, 20–26. [Google Scholar]
- Atiyeh, R.M.; Lee, S.; Edwards, C.A.; Arancon, N.Q.; Metzger, J.D. The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Sidari, M. The effect of phenols on respiratory enzymes in seed germination. Respiratory enzyme activities during germination of Pinus laricio seeds treated with phenols extracted from different forest soils. Plant Growth Regul. 2001, 35, 31–35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).