Forest Soils—What’s Their Peculiarity?
Abstract
1. Introduction—What Are Forest Soils Expected to Be and to Deliver?
- To work out how physical, chemical and biological properties are interlinked in forest soils and how they define soil functions.
- To clarify the scale levels of soil functions and ecosystem services.
- Comparing soil properties under forests and other forms of land use to work out the peculiarity of forest soils.
- To collect the specific threats on forest soil functions through environmental change and/or management.
- To give hints for strategies to preserve forest soil functions.
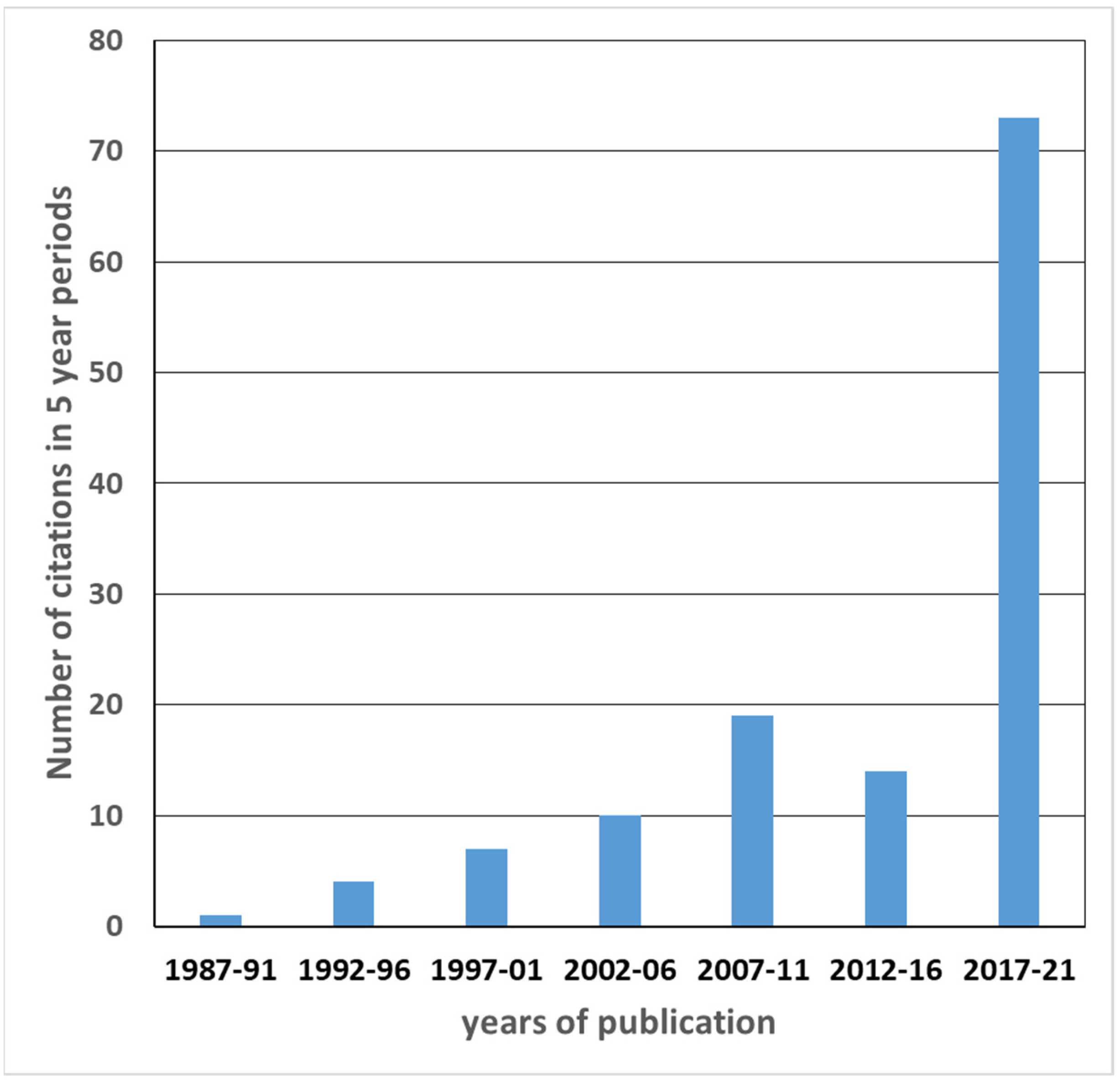
2. Materials and Methods—Perception of Forest Soils in the Scientific Literature
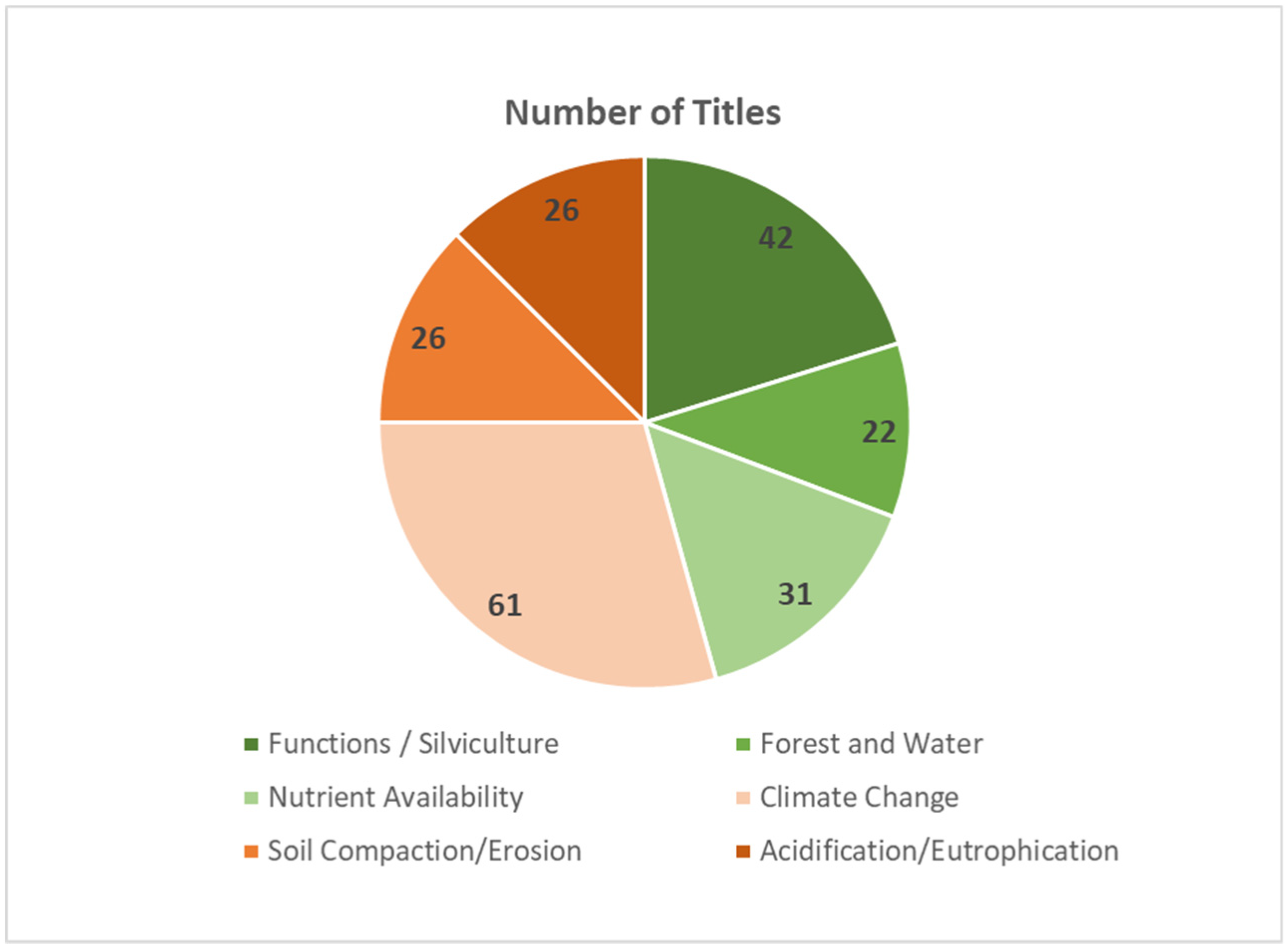
- Soil functions and silviculture: The effects of tree species and stand structures on soil chemical, soil physical and soil hydrological properties are dealt with in this field of interest. Since tree species selection and forest management systems, e.g., clear-cut vs. small-scaled harvesting regimes preserving ample crown cover over all stages of stand regeneration, these fundamental instruments of silviculture substantially influence soil processes [13] and soil characteristics. In this sense, silvicultural strategies can be taken as tools of long-term soil management [14].
- Forest and water: This field comprises the effect of forest soils on the quality and quantity of water yield. All over the world, forested areas are judged to be predominantly suited to provide high-quality drinking water [15]. The second important issue in this field is the function of forest soils as a store of plant-available water resources. This aspect is increasingly relevant under the actual increase of drought periods caused by climate change [16].
- Nutrient availability in forest soils: This item comprises the nutrient pools in forest soils, as well as processes governing the mobilization and availability of nutrients for forest trees.
- Climate change and forest soils: Forests and forest soils are concerned by climate change in two ways. On the one hand, forest soil functions are threatened by extreme weather events like droughts endangering continuous water and nutrient supplies for trees [17,18] or storms and storm floods causing wind throw and erosion damages. On the other hand, forest ecosystems and forest soils can contribute to lower greenhouse gas emissions through carbon sequestration or methane consumption in terrestrial forest soils [19].
- Soil compaction and erosion: Forest soils are in their natural stage over-proportionally unconsolidated and open-pored [12], and erosion is a seldom and subordinate process because of the coherent structure of the forest floor layer and the more-or-less continuous vegetation cover [20]. Therefore, soil compaction and erosion of forest soils are mainly manmade damages. They are caused by machine-bound harvesting techniques or inadequate management techniques like big clearcutting at steep slopes or forest roads and skidding tracks without sufficient water deduction facilities.
- Soil acidification and eutrophication: Soil processes caused by the deposition of acid compounds and nitrogen with precipitation seem to apparently be of minor relevance, since these problems have been somehow cursorily considered in the recent literature. This can be explained because, in the heavily industrialized regions, at least in Europe, the deposition of acidity was substantially reduced through effective filter techniques [21]. However, unnatural soil acidification and its after-effects remained as an inherited problem that still has to be counteracted by ecosystem-conforming measures aiming to rehabilitate the natural functions of forest soils [22,23].
3. Results—Forest Soils, the Basis for Multi-Functionality of Forest Ecosystems
3.1. Soil Properties and Processes Founding Forest Soil Functions
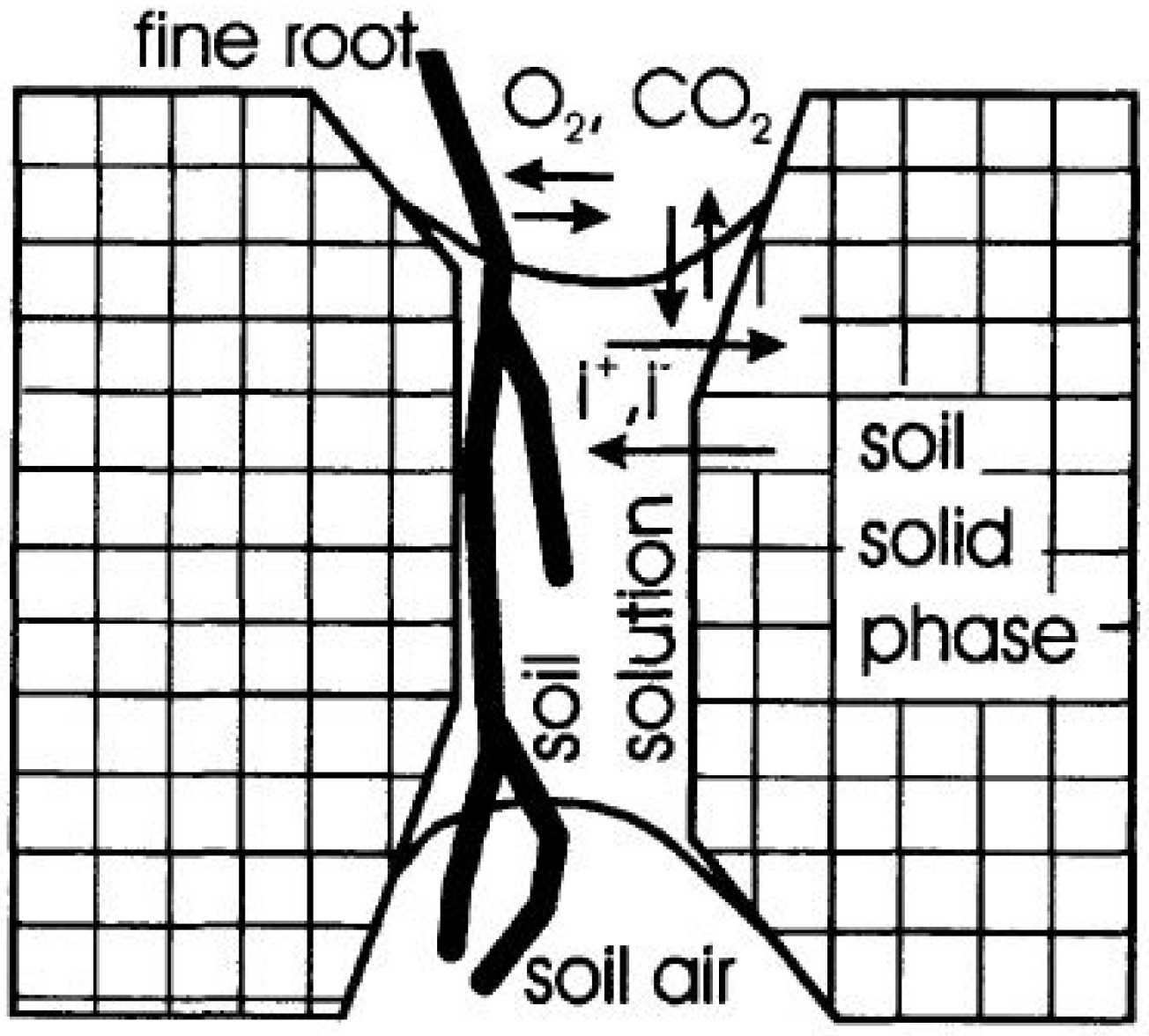
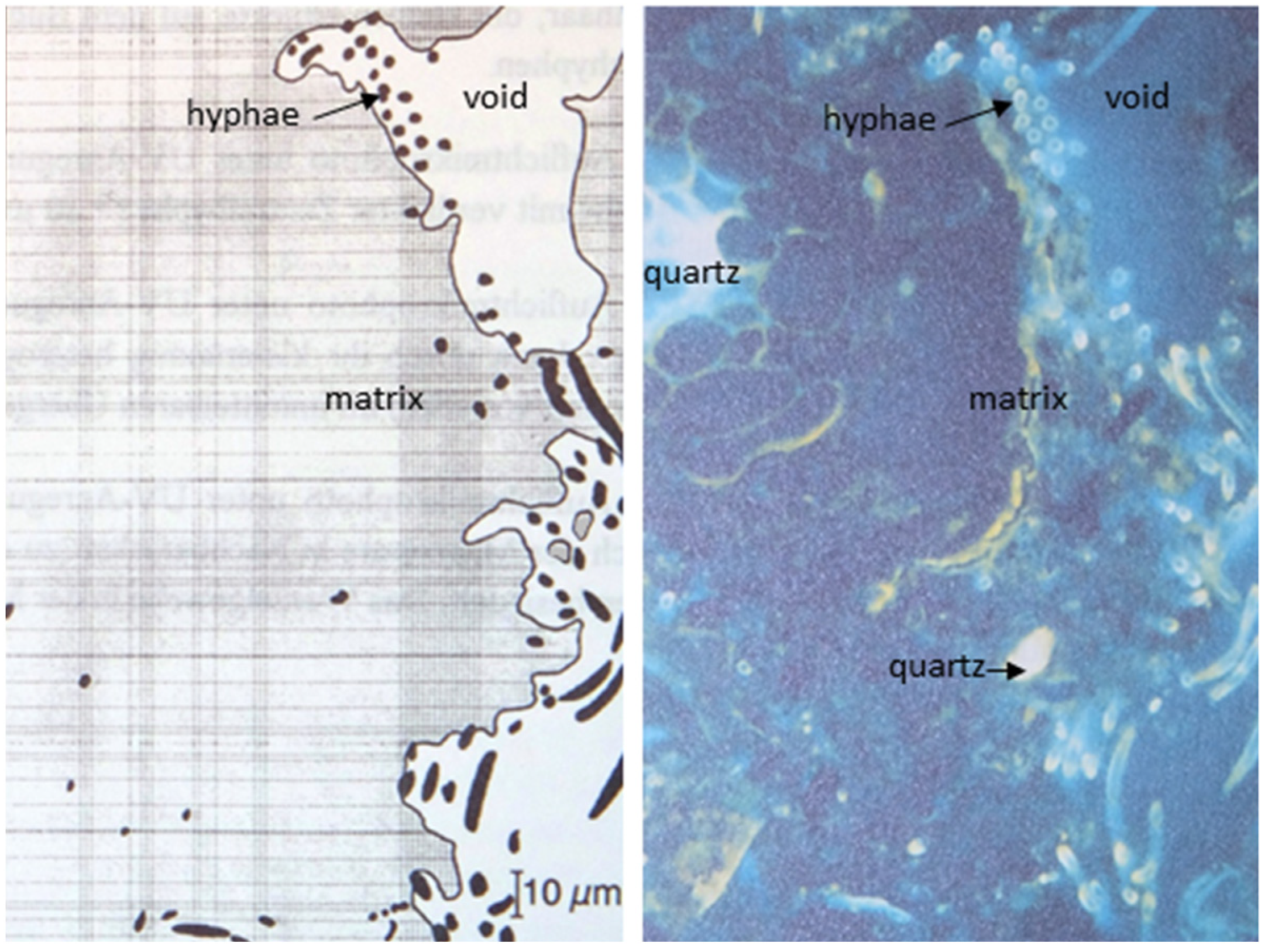
3.1.1. Secondary Soil Structure—The Spatial Frame of Soil Functions
3.1.2. Soil Chemical Status
3.2. Forest Ecosystem Services
3.2.1. Forest Soils as Basis for Growth and Existence of Forests
3.2.2. Secondary Ecosystem Services
4. Outlook and Conclusions
4.1. Threats to Forest Soil Functions and Ecosystem Services
4.2. Management Approaches for Protecting the Functionality of Forest Soils
4.2.1. Silvicultural Management Options
4.2.2. Technical Approaches for Forest Soil Preservation
4.3. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Regional Context | Climate Zone Köppen-Geiger | Titles Cited | Titles | % |
|---|---|---|---|---|
| World wide | all except EF, ET, BW | [20,26,27,28,48,87,125,127,128] | 9 | 7.0 |
| Europe wide | Dfa, Dfb, Dfc, Cfa, Cfb, Csa, Csb, BSk | [8,15,17,21,31,47,49,70,76,97,103,106] | 12 | 9.4 |
| N-America, Canada, subpolar, no dry season | Dfa, Dfb, Dfc | [71,73,121] | 3 | 2.3 |
| Scandinavia, subpolar, no dry season | Dfa, Dfb, Dfc | [36,72,104,119,120] | 5 | 3.9 |
| Scandinavia, cold, no dry season | Dfb, Dfc | [3] | 1 | 0.8 |
| Europe, cold, no dry season | Cfa, Cfb, Dfa, Dfb, Dfc | [1,2,4,5,22,24,25,34,35,43,46,51,61,62,64,69,77,84,86,90,92,101,105,107,111,112,117] | 27 | 21.1 |
| Europe, temperate humid | Cfa, Cfb, Csb | [6,9,10,11,12,13,14,16,18,19,23,29,30,37,38,42,44,45,50,52,53,54,55,56,57,58,63,75,82,83,85,89,93,94,95,96,98,100,109,110,113,115,116,118,122,123,124,126] | 48 | 37.5 |
| Asia, N-America temperate humid | Cfa, Csb, Dfc | [60,88,91] | 3 | 2.3 |
| Europe, semi arid | BSk, Csa, Csb, Cfa | [32,33,65,66,67,68,81,102,108,114] | 10 | 7.8 |
| Asia, Africa, semi arid | Bwk, Cwa, Cfa | [7,74,79,80] | 4 | 3.1 |
| Asia, Africa, S-America tropic | Af, Am, As, Aw, Cfa | [39,40,41,59,78,99] | 6 | 4.7 |
References
- Sokołowska, J.; Józefowska, A.; Woźnica, K.; Zaleski, T. Succession from meadow to mature forest: Impacts on soil biological, chemical and physical properties—Evidence from the Pieniny Mountains, Poland. Catena 2020, 189, 104503. [Google Scholar] [CrossRef]
- Ma, S.; De Frenne, P.; Boon, N.; Brunet, J.; Cousins, S.A.O.; Decocq, G.; Kolb, A.; Lemke, I.; Lemke, J.; Naaf, T.; et al. Plant species identity and soil characteristics determine rhizosphere soil bacteria community composition in European temperate forests. FEMS Microbiol. Ecol. 2019, 95, fiz063. [Google Scholar] [CrossRef] [PubMed]
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Orlinskiy, P.; Münze, R.; Beketov, M.; Gunold, R.; Paschke, A.; Knillmann, S.; Liess, M. Forested headwaters mitigate pesticide effects on macroinvertebrate communities in streams: Mechanisms and quantification. Sci. Total Environ. 2015, 524, 115–123. [Google Scholar] [CrossRef]
- Makowski, V.; Julich, S.; Feger, K.-H.; Julich, D. Soil Phosphorus Translocation via Preferential Flow Pathways: A Comparison of Two Sites with Different Phosphorus Stocks. Front. For. Glob. Chang. 2020, 3, 48. [Google Scholar] [CrossRef]
- Fiquepron, J.; Garcia, S.; Stenger, A. Land use impact on water quality: Valuing forest services in terms of the water supply sector. J. Environ. Manag. 2013, 126, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, X.; Rubinato, M.; Li, G.; Tian, Y.; Zhou, J. Impact of Multiple Vegetation Covers on Surface Runoff and Sediment Yield in the Small Basin of Nverzhai, Hunan Province, China. Forests 2020, 11, 329. [Google Scholar] [CrossRef]
- Waldner, P.; Thimonier, A.; Graf Pannatier, E.; Etzold, S.; Schmitt, M.; Marchetto, A.; Rautio, P.; Derome, K.; Nieminen, T.M.; Nevalainen, S.; et al. Exceedance of critical loads and of critical limits impacts tree nutrition across Europe. Ann. For. Sci. 2015, 72, 929–939. [Google Scholar] [CrossRef]
- Hildebrand, E.E.; Schack-Kirchner, H. The influence of compaction on soil structure and functions in forest sites. In Modern Trends in Applied Terrestrial Ecology; Ambasht, N.K., Ambasht, R.S., Eds.; Springer-Science+Business Media: New York, NY, USA, 2002; pp. 1–11. [Google Scholar]
- Gaertig, T.; Schack-Kirchner, H.; Hildebrand, E.E.; von Wilpert, K. The impact of soil aeration on oak decline in south-western Germany. For. Ecol. Manag. 2002, 159, 15–25. [Google Scholar] [CrossRef]
- Ampoorter, E. Soil Compaction Due to Mechanized Forest Harvesting: Quantification of Ecosystem Effects and Exploration of Recovery Potential. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2011; p. 182. [Google Scholar]
- Schäffer, J.; von Wilpert, K.; Kublin, E. Analysis of fine rooting below skid trails using linear and generalized additive models. Can. J. For. Res. 2009, 39, 2047–2058. [Google Scholar] [CrossRef]
- von Wilpert, K.; Zirlewagen, D.; Kohler, M. To what extent can silviculture enhance sustainability of forest sites under the immission regime in Central Europe? Water Air Soil Pollut. 2000, 122, 105–120. [Google Scholar] [CrossRef]
- von Wilpert, K.; Zirlewagen, D. Forestry Management options to maintain sustainability—element budgets at Level II sites in South—West Germany. In Forests in a Changing Environment—Results of 20 years ICP Forests Monitoring; Eichhorn, J., Ed.; Schriften aus der Forstlichen Fak. University Göttingen: Göttingen, Germany, 2007; Volume 142, pp. 170–179. [Google Scholar]
- Aubin, D.; Varone, F. The evolution of European water policy. In The Evolution of National Water Regimes in Europe. Transitions in Water Rights and Water Policies; Kissling-Näf, I., Kuks, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, UK, 2004; p. 4986. [Google Scholar]
- Boden, S.; Kahle, H.P.; von Wilpert, K.; Spiecker, H. Resilience of Norway spruce (Picea abies (L.) Karst) growth to changing climatic conditions in Southwest Germany. For. Ecol. Manag. 2014, 315, 12–21. [Google Scholar] [CrossRef]
- Mellert, K.H.; Lenoir, J.; Winter, S.; Kölling, C.; Čarni, A.; Dorado-Liñán, I.; Gégout, J.C.; Göttlein, A.; Hornstein, D.; Jantsch, M.; et al. Soil water storage appears to compensate for climatic aridity at the xeric margin of European tree species distribution. Eur. J. For. Res. 2018, 137, 79–92. [Google Scholar] [CrossRef]
- Chakraborty, T.; Reif, A.; Matzarakis, A.; Saha, S. How Does Radial Growth of Water-Stressed Populations of European Beech (Fagus sylvatica L.) Trees Vary under Multiple Drought Events? Forests 2021, 12, 129. [Google Scholar] [CrossRef]
- Maier, M.; Paulus, S.; Nicolai, C.; Stutz, K.P.; Nauer, P.A. Drivers of Plot-Scale Variability of CH4 Consumption in a Well-Aerated Pine Forest Soil. Forests 2017, 8, 193. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Soil Erosion Under Forests. In Principles of Soil Conservation and Management; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Banzhaf, S.; Schaap, M.; Kerschbaumer, A.; Reimer, E.; Stern, R.; van der Swaluw, E.; Builtjes, P. Wet Deposition: Model Development and Evaluation. In Air Pollution Modeling and its Application. Springer 2011, 21, 459–465. [Google Scholar]
- Navratil, T.; Kurz, D.; Kram, P.; Hofmeisteer, J.; Hruska, J. Acidification and recovery of soil at a heavily impacted forest catchment (Lysina, Czech Republic)—SAFE modeling and field results. Ecol. Model. 2007, 205, 464–474. [Google Scholar] [CrossRef]
- Johnson, J.; Graf Pannatier, E.; Carnicellt, S.; Cecchini, G.; Clarke, N.; Cools, N.; Hansen, K.; Meesenburg, H.; Nieminen, T.M.; Pihl-Karlsson, G. The response of soil solution chemistry in European forests to decreasing acid deposition. Glob. Chang. Biol. 2018, 24, 3603–3619. [Google Scholar] [CrossRef]
- Ulrich, B. Process hierarchy in forest ecosystems: An integrating ecosystem theory. In Effects of Acid Rain on Forest Processes; Godbold., D.L., Hüttermann., A., Eds.; Wiley-Liss: New York, NY, USA, 1994; pp. 353–397. [Google Scholar]
- Ulrich, B.; Bigham, J. The role of soil processes in the process hierarchy of forest ecosystems. In Soils and Environment—Soil Processes from Mineral to Landscape Scale; Auerswald, K., Stanjek, H., Eds.; Advances in Geoecology, Catena Verlag: Reiskirchen, Germany, 1997; Volume 30, pp. 11–22. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of Köppen-Geiger Climate Classification updated. Meteorol. Z. 2006, 15, 259–263. Available online: http://koeppen-geiger.vu-wien.ac.at (accessed on 11 September 2021). [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Global Forest Resources Assessment 2000. FAO Main Report. Forestry Paper 140; Rome, Italy, 2001; Available online: http://www.fao.org/forestry/site/7949/en/ (accessed on 19 April 2021).
- Food and Agriculture Organization of the United Nations (FAO). Soils Deliver Ecosystem Services that Enable Life on Earth. International Year of Soils (Poster) 2015. Available online: http://www.fao.org/soils-2015/en/ (accessed on 17 October 2021).
- Vanermen, I.; Muys, B.; Verheyen, K.; Vanwindekens, F.; Bouriaud, L.; Kardol, P.; Vranken, L. What do scientists and managers know about soil biodiversity? Comparative knowledge mapping for sustainable forest management. For. Policy Econ. 2020, 119, 102264. [Google Scholar] [CrossRef]
- Burst, M.; Chauchard, S.; Dambrine, E.; Dupouey, J.L.; Amiaud, B. Distribution of soil properties along forest-grassland interfaces: Influence of permanent environmental factors or land-use after-effects? Agric. Ecosyst. Environ. 2020, 289, 106739. [Google Scholar] [CrossRef]
- Hernández, L.; Jandl, R.; Blujdea, V.N.B.; Lehtonen, A.; Kriiska, K.; Alberdi, I.; Adermann, A.; Cañellas, I.; Marin, G.; Moreno-Fernandez, D.; et al. Towards complete and harmonized assessment of soil carbon stocks and balance in forests: The ability of the Yasso07 model across a wide gradient of climatic and forest conditions in Europe. Sci. Total Environ. 2017, 599, 1171–1180. [Google Scholar] [CrossRef]
- Charro, E.; Moyano, A.; Cabezón, R. The Potential of Juniperus thurifera to Sequester Carbon in Semi-Arid Forest Soil in Spain. Forests 2017, 8, 330. [Google Scholar] [CrossRef]
- Caddeo, A.; Marras, S.; Sallustio, L.; Spano, D.; Sirca, C. Soil organic carbon in Italian forests and agroecosystems: Estimating current stock and future changes with a spatial modelling approach. Agric. For. Meteorol. 2019, 278, 107654. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Szymańska, M.; Stępień, W.; Rutkowska, B.; Szulc, W. Soil N2O emissions under conventional tillage conditions and from forest soil. Soil Tillage Res. 2019, 190, 86–91. [Google Scholar] [CrossRef]
- Täumer, J.; Kolb, S.; Boeddinghaus, R.S.; Wang, H.; Schöning, I.; Schrumpf, M.; Ulrich, T.; Marhan, S. Divergent drivers of the microbial methane sink in temperate forest and grassland soils. Glob. Change Biol. 2021, 27, 929–940. [Google Scholar] [CrossRef]
- Räty, M.; Järvenranta, K.; Saarijärvi, E.; Koskiaho, J.; Virkajärvi, P. Losses of phosphorus, nitrogen, dissolved organic carbon and soil from a small agricultural and forested catchment in east-central Finland. Agric. Ecosyst. Environ. 2020, 302, 107075. [Google Scholar] [CrossRef]
- Zangerlé, A.; Hissler, C.; Lavelle, P. Effects of earthworms and plants on the soil structure, the physical stabilization of soil organic matter and the microbial abundance and diversity in soil aggregates in a long term study. In Proceedings of the EGU General Assembly, Vienna, Austria, 27 April–2 May 2014; Geophysical Research Abstracts. Volume 16, p. 16293. [Google Scholar]
- Schack-Kirchner, H.; Hildebrand, E.E. Changes in soil structure and aeration due to liming and acid irrigation. Plant Soil 1998, 199, 167–176. [Google Scholar] [CrossRef]
- Saputra, D.D.; Sari, R.R.; Hairiah, K.; Roshetko, J.M.; Suprayogo, D.; van Noordwijk, M. Can cocoa agroforestry restore degraded soil structure following conversion from forest to agricultural use? Agrofor. Syst. 2020, 94, 2261–2276. [Google Scholar] [CrossRef]
- Damptey, F.G.; Birkhofer, K.; Nsiah, Ü.K.; de la Riva, E.G. Soil Properties and Biomass Attributes in a Former Gravel Mine Area after Two Decades of Forest Restoration. Land 2020, 9, 209–227. [Google Scholar] [CrossRef]
- Zhang, J.; Bruijnzeel, L.A.; Quiñones, C.M.; Tripoli, R.; Asio, V.B.; van Meerveld, H.J. Soil physical characteristics of a degraded tropical grassland and a ‘reforest’: Implications for runoff generation. Geoderma 2019, 333, 163–177. [Google Scholar] [CrossRef]
- Schack-Kirchner, H.; von Wilpert, K.; Hildebrand, E.E. The spatial distribution of soil hyphae in structured spruce-forest soils. Plant Soil 2000, 224, 195–205. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- von Wilpert, K. Chemical deposition and seepage water quality in forests. In Forest Hydrology—Results of Research in Germany and Russia, Part 1; Puhlmann, H., Schwarze, R., Eds.; IHP/HWRP: Koblenz, Germany, 2007; Volume 6, pp. 23–35. [Google Scholar]
- Kohler, M.; Hildebrand, E.E. New Aspects of Element Cycling and Forest Nutrition. In Towards the Sustainable Use of Europe’s Forests—Forest Ecosystem and Landscape Research: Scientific Challenges and Opportunities; Andersson, F., Birot, Y., Päivinen, R., Eds.; European Forest Institute: Joensuu, Finland, 2004; EFI Proceedings; Volume 49, pp. 171–180. [Google Scholar]
- Lang, F.; Krüger, J.; Amelung, W.; Willbold, S.; Frossard, E.; Bünemann, E.K.; Bauhus, J.; Nitschke, R.; Kandeler, E.; Marhan, S.; et al. Soil phosphorus supply controls P nutrition strategies of beech forest ecosystems in Central Europe. Biogeochemistry 2017, 136, 5–29. [Google Scholar] [CrossRef]
- Rusek, J.; Marshall, V.G. Impacts of airborne pollutants on soil fauna. Annu. Rev. Ecol. Syst. 2000, 31, 395–423. [Google Scholar] [CrossRef]
- Ojha, R.B.; Devkota, D. Earthworms: Soil and Ecosystem Engineers—A Review. World J. Agric. Res. 2014, 2, 257–260. [Google Scholar] [CrossRef]
- Le Bayon, R.C.; Bullinger-Weber, G.; Schomburg, A.C.; Turberg, P.; Schlaepfer, R.; Guenat, C. Earthworms as ecosystem engineers: A review. In Earthworms. Types, Roles and Research; Clayton, H.G., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 129–177. ISBN 978-1-53612-176-6. [Google Scholar]
- HILDEBRAND, E.E. The Heterogeneous Distribution of Mobile Ions in the Rhizosphere of Acid Forest Soils: Facts, Causes, and Consequences. J. Environ. Sci. Health 1994, A29, 1973–1992. [Google Scholar] [CrossRef][Green Version]
- Walthert, L.; Graf Pannatier, E.; Meier, E.S. Shortage of nutrients and excess of toxic elements in soils limit the distribution of soil-sensitive tree species in temperate forests. For. Ecol. Manag. 2013, 297, 94–107. [Google Scholar] [CrossRef]
- Hildebrand, E.E. The spatial heterogeneity of chemical properties in acid forest soils and its importance for tree nutrition. Water Air Soil Pollut. 1990, 54, 183–191. [Google Scholar] [CrossRef]
- van Schöll, L.; Kuyper, T.W.; Smits, M.M.; Landeweert, R.; Hoffland, E.; van Breemen, N. Rock-eating mycorrhizas: Their role in plant nutrition and biogeochemical cycles. Plant Soil 2008, 303, 35–47. [Google Scholar] [CrossRef]
- Heisner, U.; Raber, B.; Hildebrand, E.E. The Importance of the Skeleton in Forest Sites of the Southern Black Forest. Eur. J.For. Res. 2004, 123, 249–257. [Google Scholar] [CrossRef]
- Koele, N.; Hildebrand, E.E.; Schack-Kirchner, H. Effects of weathering state of coarse soil fragments on tree-seedling nutrient uptake. J. Plant Nutr. Soil Sci. 2010, 173, 245–251. [Google Scholar] [CrossRef]
- Koele, N.; Storch, F.; Hildebrand, E.E. The coarse-soil fraction is the main living space of fungal hyphae in the BhBs horizon of a Podzol. J. Plant Nutr. Soil Sci. 2011, 174, 750–753. [Google Scholar] [CrossRef]
- Koele, N.; Hildebrand, E.E. The ecological significance of the coarse soil fraction for Picea abies (L.) Karst. seedling nutrition. Plant Soil 2008, 312, 163–174. [Google Scholar] [CrossRef]
- Kohler, M.; von Wilpert, K.; Hildebrand, E.E. The Soil Skeleton as a Source for the short-term Supply of “Basic Cations” in Forest Soils of the Black Forest (Germany). Water Air Soil Poll. 2000, 122, 37–48. [Google Scholar] [CrossRef]
- Soong, J.L.; Janssens, I.A.; Grau, O.; Margalef, O.; Stahl, C.; Van Langenhove, L.; Urbina, I.; Chave, J.; Dourdain, A.; Ferry, B.; et al. Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. Sci. Rep. 2020, 10, 2302. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Chen, G.; Guo, J.; Li, Y. Enzyme stoichiometry indicates the variation of microbial nutrient requirements at different soil depths in subtropical forests. PLoS ONE 2020, 15, e0220599. [Google Scholar] [CrossRef]
- Stahr, S.; Graf-Rosenfellner, M.; Klysubun, W.; Mikutta, R.; Prietzel, J.; Lang, F. Phosphorus speciation and C:N:P stoichiometry of functional organic matter fractions in temperate forest soils. Plant Soil 2018, 427, 53–69. [Google Scholar] [CrossRef]
- Rodionov, A.; Bauke, S.L.; von Sperber, C.; Hoeschen, C.; Kandeler, E.; Kruse, J.; Lewandowski, H.; Marhan, S.; Mueller, C.W.; Simon, M.; et al. Biogeochemical cycling of phosphorus in subsoils of temperate forest ecosystems. Biogeochemistry 2020, 150, 313–328. [Google Scholar] [CrossRef]
- Baumann, K.; Glaser, K.; Mutz, J.E.; Karsten, U.; MacLennan, A.; Hu, Y.; Michalik, D.; Kruse, J.; Eckhardt, K.U.; Schall, P. Biological soil crusts of temperate forests: Their role in P cycling. Soil Biol. Biochem. 2017, 109, 156–166. [Google Scholar] [CrossRef]
- Zederer, D.P.; Talkner, U. Organic P in temperate forest mineral soils as affected by humus form and mineralogical characteristics and its relationship to the foliar P content of European beech. Geoderma 2018, 325, 162–171. [Google Scholar] [CrossRef]
- Yang, F.; Magh, R.K.; Ivanković, M.; Lanšćak, M.; Haberstroh, S.; Du, B.; Dannenmann, M.; Rennenberg, H.; Herschbach, C. Foliar P nutrition of European beech (Fagus sylvatica L.) depends on the season but remains unaffected by co-cultivation with silver fir (Abies alba Mill.). Eur. J. For. Res. 2020, 139, 853–868. [Google Scholar] [CrossRef]
- Bueis, T.; Bravo, F.; Pando, V.; Kissi, Y.A.; Turrión, M.B. Phosphorus availability in relation to soil properties and forest productivity in Pinus sylvestris L. plantations. Ann. For. Sci. 2019, 79, 69102. [Google Scholar] [CrossRef]
- Salmon, S. Changes in humus forms, soil invertebrate communities and soil functioning with forest dynamics. Appl. Soil Ecol. 2018, 123, 345–354. [Google Scholar] [CrossRef]
- Landi, S.; d’Errico, G.; Binazzi, F.; Di Salvatore, U.; Gardin, L.; Marchi, M.; Mazza, G.; Roversi, P.F.; Simoncini, S.; Torrini, G.; et al. The Short-Term Impact of Different Silvicultural Thinnings on Soil Nematode and Microarthropod Biodiversity in Artificial Black Pine Stands. Forests 2020, 11, 1212. [Google Scholar] [CrossRef]
- Rożek, K.; Rola, K.; Błaszkowski, J.; Leski, T.; Zubek, S. How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil? For. Ecol. Manag. 2020, 465, 118091. [Google Scholar] [CrossRef]
- Galluzzi, M.; Giannetti, F.; Puletti, N.; Canullo, R.; Rocchini, D.; Bastrup-Birk, A.; Chirici, G. A plot-level exploratory analysis of European forest based on the results from the BioSoil Forest Biodiversity project. Eur. J. For. Res. 2019, 138, 831–845. [Google Scholar] [CrossRef]
- Giguère-Tremblay, R.; Laperriere, G.; de Grandpré, A.; Morneault, A.; Bisson, D.; Chagnon, P.L.; Germain, H.; Maire, V. Boreal Forest Multifunctionality Is Promoted by Low Soil Organic Matter Content and High Regional Bacterial Biodiversity in Northeastern Canada. Forests 2020, 11, 149–167. [Google Scholar] [CrossRef]
- Friggens, N.L.; Aspray, T.J.; Parker, T.C.; Subke, J.A.; Wookey, P.A. Spatial patterns in soil organic matter dynamics are shaped by mycorrhizosphere interactions in a treeline forest. Plant Soil 2020, 447, 521–535. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, A.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Suh, J.; Kim, S.M.; Yi, H.; Choi, Y. An Overview of GIS-Based Modeling and Assessment of Mining-Induced Hazards: Soil, Water, and Forest. Int. J. Environ. Res. Public Health 2017, 14, 1463. [Google Scholar] [CrossRef]
- Missong, A.; Holzmann, S.; Bol, R.; Nischwitz, V.; Puhlmann, H.; von Wilpert, K.; Siemens, J.; Klumpp, E. Leaching of natural colloids from forest topsoils and their relevance for phosphorus mobility. Sci. Total Environ. 2018, 634, 305–315. [Google Scholar] [CrossRef]
- Ambus, P.; Zechmeister-Boltenstern, S. Denitrification and N-Cycling in Forest Ecosystems. In Biology of the Nitrogen Cycle; Bothe, H., Ferguson, S.J., Newton, W.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 343–358. [Google Scholar]
- Sucker, C.; von Wilpert, K.; Puhlmann, H. Acidification reversal in low mountain range streams of Germany. Environ. Monit. Assess. 2011, 174, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Ilstedt, U.; Malmer, A.; Verbeeten, E.; Murdiyarso, D. The effect of afforestation on water infiltration in the tropics: A systematic review and meta-analysis. For. Ecol. Manag. 2007, 251, 45–51. [Google Scholar] [CrossRef]
- Mireille, N.M.; Mwangi, H.M.; Mwangi, J.K.; Gathenya, J.M. Analysis of Land Use Change and Its Impact on the Hydrology of Kakia and Esamburmbur Sub-Watersheds of Narok County, Kenya. Hydrology 2019, 6, 86. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, G.Q.; Li, Z.B.; Li, P. Experimental Study on Slope Runoff, Erosion and Sediment under Different Vegetation Types. Water Resour. Manag. 2014, 28, 2415–2433. [Google Scholar] [CrossRef]
- Pellis, G.; Chiti, T.; Rey, A.; Yuste, J.C.; Trotta, C.; Papale, D. The ecosystem carbon sink implications of mountain forest expansion into abandoned grazing land: The role of subsoil and climatic factors. Sci. Total Environ. 2019, 672, 106–120. [Google Scholar] [CrossRef]
- Kalks, F.; Liebmann, P.; Wordell-Dietrich, P.; Guggenberger, G.; Kalbitz, K.; Mikutta, R.; Helfrich, M.; Don, A. Fate and stability of dissolved organic carbon in topsoils and subsoils under beech forests. Biogeochemistry 2020, 148, 111–128. [Google Scholar] [CrossRef]
- Wordell-Dietrich, P.; Wotte, A.; Rethemeyer, J.; Bachmann, J.; Helfrich, M.; Kirfel, K.; Leuschner, C.; Don, A. Vertical partitioning of CO2 production in a forest soil. Biogeosciences 2020, 17, 6341–6356. [Google Scholar] [CrossRef]
- Zacháry, D.; Filep, T.; Jakab, G.; Molnár, M.; Kertész, T.; Király, C.; Hegyi, I.; Gáspár, L.; Szalai, Z. Carbon Isotope Measurements to Determine the Turnover of Soil Organic Matter Fractions in a Temperate Forest Soil. Agronomy 2020, 10, 1944. [Google Scholar] [CrossRef]
- Nitsch, P.; Kaupenjohann, M.; Wulf, M. Forest continuity, soil depth and tree species are important parameters for SOC stocks in an old forest (Templiner Buchheide, northeast Germany). Geoderma 2018, 310, 65–76. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bárcena, T.G.; Vesterdal, L. Tree species and time since afforestation drive soil C and N mineralization on former cropland. Geoderma 2017, 305, 153–161. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Morishita, T.; Sakata, T.; Takahashi, M.; Ishizuka, S.; Mizoguchi, T.; Inagaki, Y.; Terazawa, K.; Sawata, S.; Igarashi, M.; Yasuda, H.; et al. Methane uptake and nitrous oxide emission in Japanese forest soils and their relationship to soil and vegetation types. Soil Sci. Plant Nutr. 2007, 53, 678–691. [Google Scholar] [CrossRef]
- Jungkunst, H.F.; Flessa, H.; Scherber, C.; Fiedler, S. Groundwater level controls CO2, N2O and CH4 fluxes of three different hydromorphic soil types of a temperate forest ecosystem. Soil Biol. Biochem. 2008, 40, 2047–2054. [Google Scholar] [CrossRef]
- Schindler, T.; Mander, Ü.; Machacova, K.; Espenberg, M.; Krasnov, D.; Escuer-Gatius, J.; Veber, G.; Pärn, J.; Soosaar, K. Short-term flooding increases CH4 and N2O emissions from trees in a riparian forest soil-stem continuum. Sci. Rep. 2020, 10, 3204. [Google Scholar] [CrossRef] [PubMed]
- Arango, C.; Ponette-González, A.; Neziri, I.; Bailey, J. Western spruce budworm effects on throughfall N, P, and C fluxes and soil nutrient status in the Pacific Northwest. Can. J. For. Res. 2019, 49, 1207–1218. [Google Scholar] [CrossRef]
- Wasak, K.; Klimek, B.; Drewnik, M. Rapid effects of windfall on soil microbial activity and substrate utilization patterns in the forest belt in the Tatra Mountains. J. Soils Sediments 2020, 20, 801–815. [Google Scholar] [CrossRef]
- Matzner, E.; Murach, D. Soil changes induced by air pollutant deposition and their implication for forests in central Europe. Water Air Soil Pollut. 1995, 85, 63–76. [Google Scholar] [CrossRef]
- von Wilpert, K.; Schäffer, J. Ecological effects of soil compaction and initial recovery dynamics: A preliminary study. Eur. J. For. Res. 2006, 125, 129–138. [Google Scholar] [CrossRef]
- Teepe, R.; Brumme, R.; Beese, F.; Ludwig, B. Nitrous Oxide Emission and Methane Consumption Following Compaction of Forest Soils. Soil Sci. Soc. Am. J. 2004, 68, 605–611. [Google Scholar] [CrossRef]
- Warlo, H.; von Wilpert, K.; Lang, F.; Schack-Kirchner, H. Black Alder (Alnus glutinosa (L.) Gaertn.) on Compacted Skid Trails: A Trade-off between Greenhouse Gas Fluxes and Soil Structure Recovery? Forests 2019, 10, 726. [Google Scholar] [CrossRef]
- Büntgen, U.; Urban, O.; Krusic, P.J.; Rybníček, M.; Kolář, T.; Kyncl, T.; Ač, A.; Koňasová, E.; Čáslavský, J.; Esper, J.; et al. Recent European drought extremes beyond Common Era background variability. Nat. Geosci. 2021, 14, 190–196. [Google Scholar] [CrossRef]
- Fleck, S.; Ahrends, B.; Sutmöller, J.; Albert, M.; Evers, J.; Meesenburg, H. Is Biomass Accumulation in Forests an Option to Prevent Climate Change Induced Increases in Nitrate Concentrations in the North German Lowland? Forests 2017, 8, 219. [Google Scholar] [CrossRef]
- Hennings, N.; Becker, J.N.; Guillaume, T.; Damris, M.; Dippold, M.A.; Kuzyakov, Y. Riparian wetland properties counter the effect of land-use change on soil carbon stocks after rainforest conversion to plantations. Catena 2021, 196, 104941. [Google Scholar] [CrossRef]
- Puhlmann, H.; Schmidt-Walter, P.; Hartmann, P.; Meesenburg, H.; von Wilpert, K. Soil Water Budget and Drought Stress. In Status and Dynamics of Forests in Germany. Results of the National Forest Monitoring; Wellbrock, N., Bolte, A., Eds.; Springer Open, Ecological Studies: New York, NY, USA, 2019; Volume 237, Chapter 3; pp. 55–91. [Google Scholar]
- Wermelinger, B.; Rigling, A.; Schneider Mathis, D.; Kenis, M.; Gossner, M.M. Climate Change Effects on Trophic Interactions of Bark Beetles in Inner Alpine Scots Pine Forests. Forests 2021, 12, 136. [Google Scholar] [CrossRef]
- Rewald, B. Impact of Climate Change Induced Drought on Tree Root Hydraulic Properties and Competition Belowground. Ph.D. Thesis, Georg-August-Universität, Göttingen, Germany, 2008; p. 172. [Google Scholar]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Jönsson, A.M. Modeling Bark Beetle Responses to Climate Change. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Braun, S.; Tresch, S.; Augustin, S. Soil solution in Swiss forest stands: A 20 year’s time series. PLoS ONE 2020, 15, e0227530. [Google Scholar] [CrossRef]
- Daněk, P.; Šamonil, P.; Vrška, T. Four decades of the coexistence of beech and spruce in a Central European old-growth forest. Which succeeds on what soils and why? Plant Soil 2019, 437, 257–272. [Google Scholar] [CrossRef]
- Brunel, C.; Gros, R.; Ziarelli, F.; Da Silva, A.M.F. Additive or non-additive effect of mixing oak in pine stands on soil properties depends on the tree species in Mediterranean forests. Sci. Total Environ. 2017, 590, 676–685. [Google Scholar] [CrossRef]
- Zirlewagen, D.; von Wilpert, K. Using model scenarios to predict and evaluate forest management impacts on soil base saturation at landscape level. Eur. J. For. Res. 2004, 123, 269–282. [Google Scholar] [CrossRef]
- Zeller, B.; Legout, A.; Bienaimé, S.; Gratia, B.; Santenoise, P.; Bonnaud, P.; Ranger, J. Douglas fir stimulates nitrification in French forest soils. Sci. Rep. 2019, 9, 10687. [Google Scholar] [CrossRef]
- Mayer, M.; Matthews, B.; Rosinger, C.; Sanden, H.; Godbold, D.L.; Katzensteiner, K. Tree regeneration retards decomposition in a temperate mountain soil after forest gap disturbance. Soil Biol. Biochem. 2017, 115, 490–498. [Google Scholar] [CrossRef]
- Łabęda, D.; Kondras, M. Influence of forest management on soil organic carbon stocks. Soil Sci. Annu. 2020, 71, 165–173. [Google Scholar] [CrossRef]
- Wambsganss, J.; Stutz, K.P.; Lang, F. European beech deadwood can increase soil organic carbon sequestration in forest topsoils. For. Ecol. Manag. 2017, 405, 200–209. [Google Scholar] [CrossRef]
- Papaioannou, E.; Chatzistathis, T.; Menexes, G. The Impact of Management Practices on Soil Fertility and Foliar Nutrient Concentrations in a Spruce (Picea abies) Forest Ecosystem of Rodopi Mountainous Area, in Northern Greece. Not. Bot. Horti Agrobot. 2018, 46, 301–308. [Google Scholar] [CrossRef]
- Bolte, A.; Block, J.; Eichhorn, J.; Sanders, T.G.M.; Wellbrock, N. Sustainable Use and Development of Forests and Forest Soils: A Resume. In Status and Dynamics of Forests in Germany Results of the National Forest Monitoring; Wellbrock, N., Bolte, A., Eds.; Springer, Ecological Studies: New York, NY, USA, 2019; Volume 237, Chapter 12; pp. 355–374. [Google Scholar]
- Jansone, L.; von Wilpert, K.; Hartmann, P. Natural Recovery and Liming Effects in Acidified Forest Soils in SW-Germany. Soil Syst. 2020, 4, 38. [Google Scholar] [CrossRef]
- Berger, T.W.; Türtscher, S.; Berger, P.; Lindebner, L. A slight recovery of soils from Acid Rain over the last three decades is not reflected in the macro nutrition of beech (Fagus sylvatica) at 97 forest stands of the Vienna Woods. Environ. Pollut. 2016, 216, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Kohler, M.; Kunz, J.; Herrmann, J.; Hartmann, P.; Jansone, L.; Puhlmann, H.; von Wilpert, K.; Bauhus, J. The Potential of Liming to Improve Drought Tolerance of Norway Spruce [Picea abies (L.) Karst.]. Front. Plant Sci. 2019, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Lepilin, D.; Laurén, A.; Uusitalo, J.; Tuittila, E.S. Soil deformation and its recovery in logging trails of drained boreal peatlands. Can. J. For. Res. 2019, 49, 743–751. [Google Scholar] [CrossRef]
- Marra, E.; Cambi, E.; Fernandez-Lacruz, R.; Giannetti, F.; Marchi, E.; Nordfjell, T. Photogrammetric estimation of wheel rut dimensions and soil compaction after increasing numbers of forwarder passes. Scand. J. For. Res. 2018, 33, 613–620. [Google Scholar] [CrossRef]
- Green, P.Q.; Chung, W.; Leshchinsky, B.; Belart, F.; Sessions, J.; Fitzgerald, S.A.; Wimer, J.A.; Cushing, T.; Garland, J.J. Insight into the Productivity, Cost and Soil Impacts of Cable-assisted Harvester-forwarder Thinning in Western Oregon. For. Sci. 2019, 66, 82–96. [Google Scholar] [CrossRef]
- Bonnaud, P.; Santenoise, P.; Tisserand, D.; Nourrisson, G.; Ranger, J. Impact of compaction on two sensitive forest soils in Lorraine (France) assessed by the changes occurring in the perched water table. For. Ecol. Manag. 2019, 437, 380–395. [Google Scholar] [CrossRef]
- Flores Fernández, J.L.; Rubin, L.; Hartmann, P.; Puhlmann, H.; von Wilpert, K. Initial recovery of soil structure of a compacted forest soil can be enhanced by technical treatments and planting. For. Ecol. Manag. 2018, 431, 54–62. [Google Scholar] [CrossRef]
- Flores Fernández, J.L.; Hartmann, P.; Schäffer, J.; Puhlmann, H.; von Wilpert, K. Initial recovery of compacted soil—planting and technical treatments decrease CO2 concentrations in soil and promote root growth. Ann. For. Sci. 2017, 74, 73–85. [Google Scholar] [CrossRef]
- Reynolds, C.A.; Jackson, T.J.; Rawls, W.J. Estimating soil water-holding capacities by linking the Food and Agriculture Organization soil map of the world with global pedon databases and continuous pedotransfer functions. Water Resour. Res. 2000, 36, 3653–3662. [Google Scholar] [CrossRef]
- Puhlmann, H.; von Wilpert, K. Pedotransfer functions for water retention and unsaturated hydraulic conductivity of forest soils. J. Plant Nutr. Soil Sci. 2012, 175, 221–235. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Rome World Soil Resour. Rep. No 106; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015; 203p. [Google Scholar]
- Rabot, E.; Wiesmeier, M.; Schlüter, M.; Vogel, H.J. Soil structure as an indicator of soil functions: A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilpert, K.v. Forest Soils—What’s Their Peculiarity? Soil Syst. 2022, 6, 5. https://doi.org/10.3390/soilsystems6010005
Wilpert Kv. Forest Soils—What’s Their Peculiarity? Soil Systems. 2022; 6(1):5. https://doi.org/10.3390/soilsystems6010005
Chicago/Turabian StyleWilpert, Klaus von. 2022. "Forest Soils—What’s Their Peculiarity?" Soil Systems 6, no. 1: 5. https://doi.org/10.3390/soilsystems6010005
APA StyleWilpert, K. v. (2022). Forest Soils—What’s Their Peculiarity? Soil Systems, 6(1), 5. https://doi.org/10.3390/soilsystems6010005





