Do the Invasive Earthworms Amynthas agrestis (Oligochaeta: Megascolecidae) and Lumbricus rubellus (Oligochaeta: Lumbricidae) Stimulate Oxalate-Based Browser Defenses in Jack-in-the-Pulpit (Arisaema triphyllum) by Their Presence or Their Soil Biogeochemical Activity?
Abstract
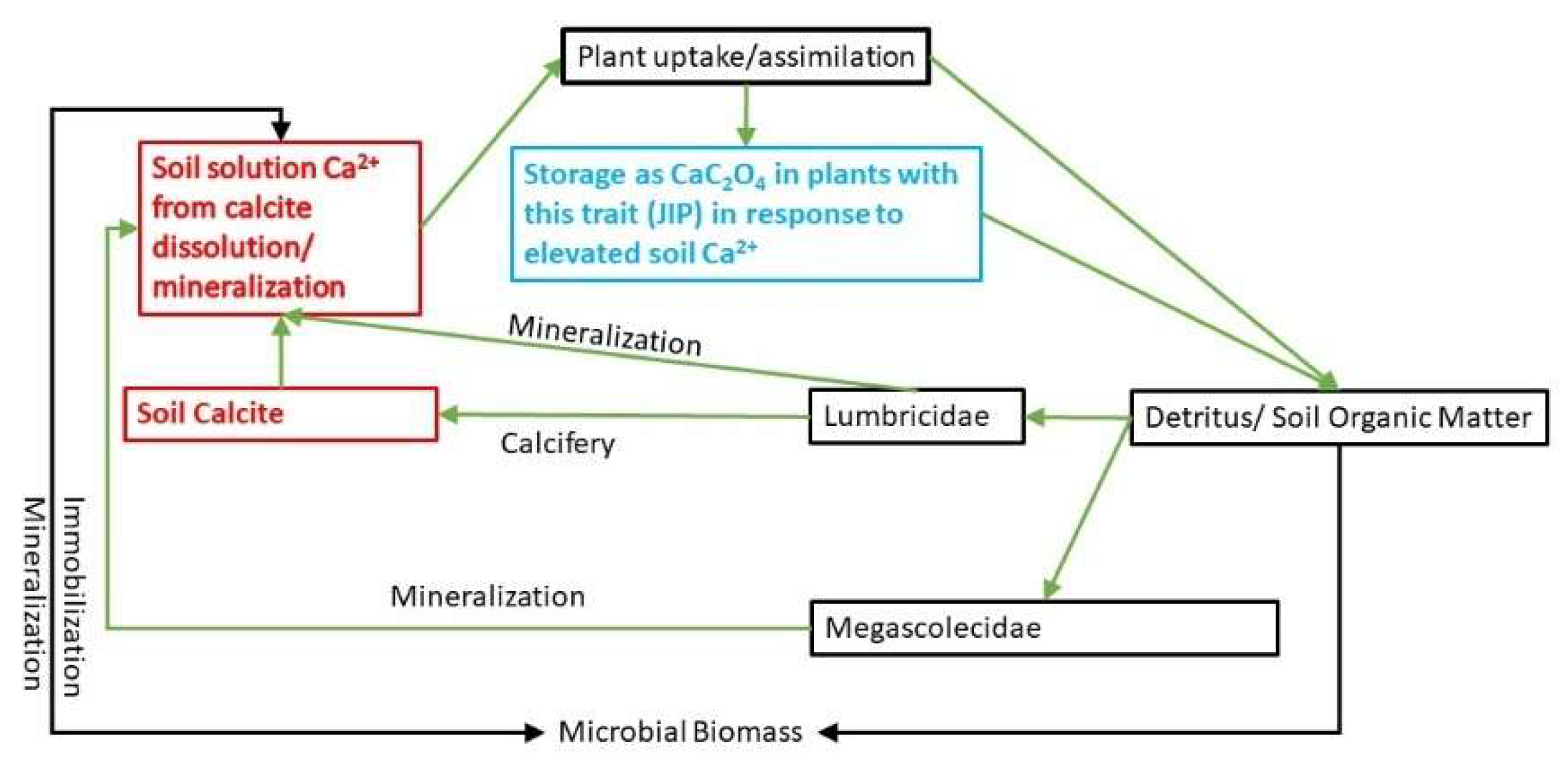
1. Introduction
2. Materials and Methods
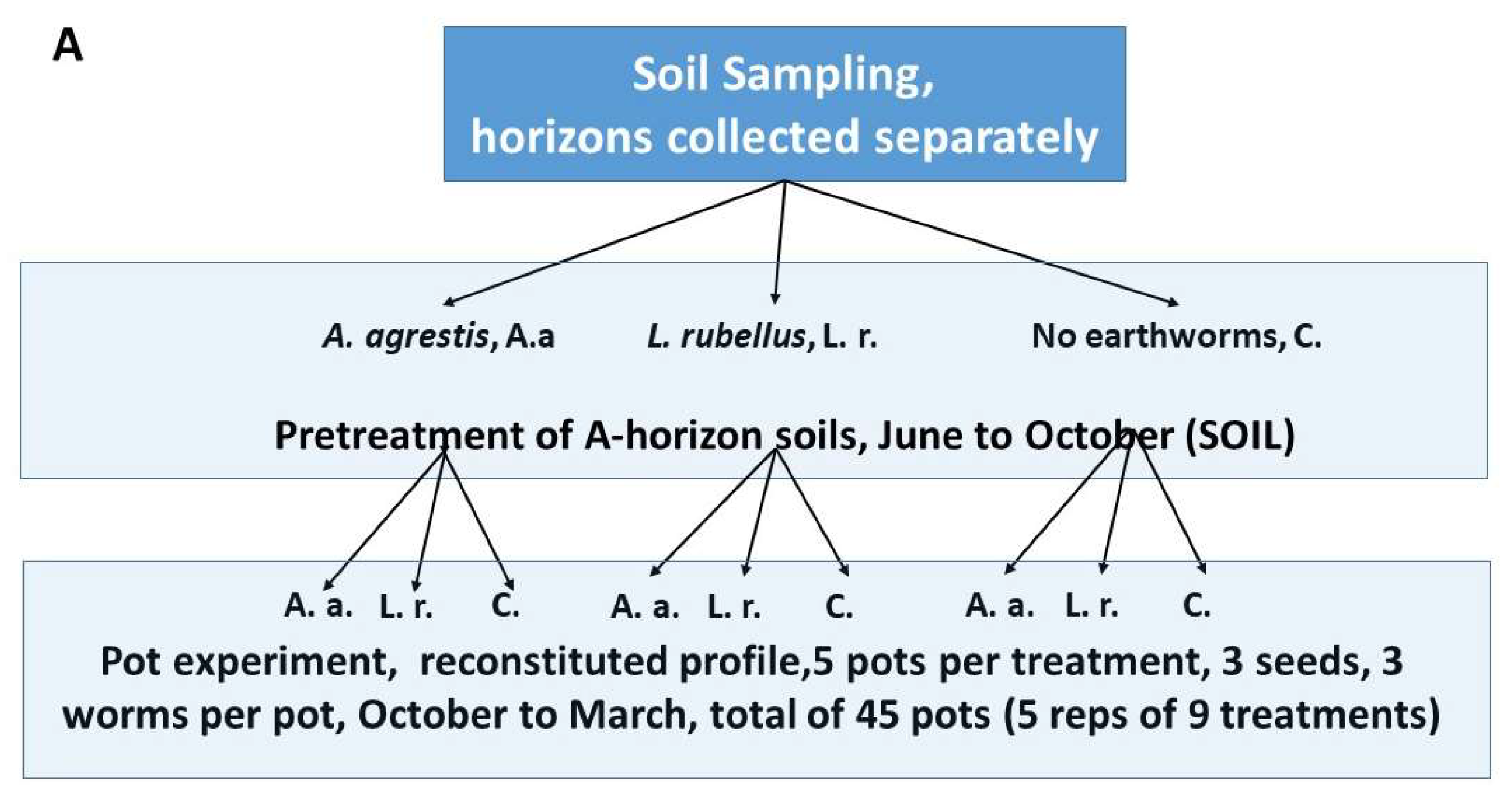
2.1. Earthworm and Soil Collection
2.2. Greenhouse Pot Trials
2.3. Oxalate Quantification
2.4. Aggregate-Scale Ca Concentrations
2.5. Statistics
3. Results
3.1. Oxalate Fractions
3.2. Bulk Soil Chemistry
3.3. Aggregate Scale Ca Concentrations
4. Discussion
4.1. Oxalate Production by JIP
4.1.1. Effect of Earthworms (WORM)
4.1.2. Effect of the Soil Pretreatment (SOIL)
4.1.3. Effect of Sequence of Earthworm Invasions
4.2. Effect of Soil Chemistry
4.3. Biological Significance of Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bohlen, P.J.; Scheu, S.; Hale, C.M.; McLean, M.A.; Migge, S.; Groffman, P.M.; Parkinson, D. Non-native invasive earthworms as agents of change in northern temperate forests. Front. Ecol. Environ. 2004, 2, 427–435. [Google Scholar] [CrossRef]
- Hendrix, P.F.; Baker, G.; Callaham, M.A.; Damoff, G.; Fragoso, C.; Gonzalez, G.; James, S.; Lachnicht, S.L.; Winsome, T.; Zou, X. Invasion of exotic earthworms into ecosystems inhabited by native earthworms. In Biological Invasions Belowground: Earthworms as Invasive Species; Springer: Dordrecht, The Netherlands, 2006; pp. 87–100. [Google Scholar]
- Frelich, L.E.; Hale, C.M.; Reich, P.B.; Holdsworth, A.R.; Scheu, S.; Heneghan, L.; Bohlen, P.J. Earthworm invasion into previously earthworm-free temperate and boreal forests. In Biological Invasions Belowground: Earthworms as Invasive Species; Springer: Dordrecht, The Netherlands, 2006; pp. 35–45. [Google Scholar]
- Tiunov, A.V.; Hale, C.M.; Holdsworth, A.R.; Vsevolodova-Perel, T.S. Invasion patterns of Lumbricidae into the previously earthworm-free areas of northeastern Europe and the western Great Lakes region of North America. In Biological Invasions Belowground: Earthworms as Invasive Species; Springer: Dordrecht, The Netherlands, 2006; pp. 23–34. [Google Scholar]
- Hale, C.M.; Frelich, L.E.; Reich, P.B.; Pastor, J. Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota, USA. Ecosystems 2005, 8, 911–927. [Google Scholar] [CrossRef]
- Frelich, L.E.; Blossey, B.; Cameron, E.K.; Dávalos, A.; Eisenhauer, N.; Fahey, T.; Ferlian, O.; Groffman, P.M.; Larson, E.; Loss, S.R.; et al. Side-swiped: Ecological cascades emanating from earthworm invasions. Front. Ecol. Environ. 2019, 17, 502–510. [Google Scholar] [CrossRef]
- Wackett, A.A.; Yoo, K.; Cameron, E.K.; Olid, C.; Klaminder, J. Global Wo’rming and Darwin Revisited: Quantifying Soil Mixing Rates by Non-native Earthworms in Fennoscandian Boreal and Arctic Ecosystems. In Proceedings of the AGU Fall Meeting Abstracts, New Orleans, LA, USA, 11–15 December 2017; p. B21F-2002. [Google Scholar]
- Makarova, O.; Kolesnikova, A. Earthworms (Oligochaeta, Lumbricidae) in the tundra of Eastern Europe. Biol. Bull. 2019, 46, 438–449. [Google Scholar] [CrossRef]
- Arvidsson, E. Invasive Earthworms and Their Effect on Soil Organic Matter: Impact on Soil Carbon ‘Quality’in Fennoscandian Tundra. 2021. Available online: https://www.diva-portal.org/smash/get/diva2:1565059/FULLTEXT01.pdf (accessed on 20 October 2021).
- Lejoly, J.; Quideau, S.; Laganière, J. Invasive earthworms affect soil morphological features and carbon stocks in boreal forests. Geoderma 2021, 404, 115262. [Google Scholar] [CrossRef]
- Lawrence, B.; Fisk, M.C.; Fahey, T.J.; Suarez, E.R. Influence of nonnative earthworms on mycorrhizal colonization of sugar maple (Acer saccharum). New Phytol. 2003, 157, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Longcore, T.; MacDonald, B.; McCormick, M.K.; Szlavecz, K.; Wilson, G.W.; Loss, S.R. Belowground interactions with aboveground consequences: Invasive earthworms and arbuscular mycorrhizal fungi. Ecology 2016, 97, 605–614. [Google Scholar] [CrossRef]
- Blume-Werry, G.; Krab, E.J.; Olofsson, J.; Sundqvist, M.K.; Väisänen, M.; Klaminder, J. Invasive earthworms unlock arctic plant nitrogen limitation. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Hale, C.M.; Frelich, L.E.; Reich, P.B. Changes in hardwood forest understory plant communities in response to European earthworm invasions. Ecology 2006, 87, 1637–1649. [Google Scholar] [CrossRef]
- Fisichelli, N.; Eisenhauer, N.; Frelich, L.; Reich, P. Deer and Earthworms Modify Forest Responses to Climate Change; NPS Climate Change Response Program: Washington, DC, USA, 2013. [Google Scholar]
- Reynolds, J.W. The earthworms of Tennessee (Oligochaeta). IV. Megascolecidae, with notes on distribution, biology and a key to the species in the state. Megadrilogica 1978, 3, 117–129. [Google Scholar]
- Reynolds, J.W.; Görres, J.H.; Knowles, M.E. A checklist by counties of earthworms (Oligochaeta: Acanthodrilidae, Lumbricidae and Megascolecidae) in the states of Maine, New Hampshire and Vermont, USA. Megadrilogica 2015, 17, 125–140. [Google Scholar]
- Alban, D.H.; Berry, E.C. Effects of earthworm invasion on morphology, carbon, and nitrogen of a forest soil. Appl. Soil Ecol. 1994, 1, 243–249. [Google Scholar] [CrossRef]
- Cameron, E.K.; Bayne, E.M.; Clapperton, M.J. Human-facilitated invasion of exotic earthworms into northern boreal forests. Ecoscience 2007, 14, 482–490. [Google Scholar] [CrossRef]
- Chang, C.-H.; Johnston, M.R.; Görres, J.H.; Dávalos, A.; McHugh, D.; Szlavecz, K. Co-invasion of three Asian earthworms, Metaphire hilgendorfi, Amynthas agrestis and Amynthas tokioensis in the USA. Biol. Invasions 2018, 20, 843–848. [Google Scholar] [CrossRef]
- Addison, J. Distribution and impacts of invasive earthworms in Canadian forest ecosystems. Biol. Invasions 2009, 11, 59–79. [Google Scholar] [CrossRef]
- Holdsworth, A.R.; Frelich, L.E.; Reich, P.B. Effects of earthworm invasion on plant species richness in northern hardwood forests. Conserv. Biol. 2007, 21, 997–1008. [Google Scholar] [CrossRef]
- Drouin, M.; Bradley, R.; Lapointe, L.; Whalen, J. Non-native anecic earthworms (Lumbricus terrestris L.) reduce seed germination and seedling survival of temperate and boreal trees species. Appl. Soil Ecol. 2014, 75, 145–149. [Google Scholar] [CrossRef]
- Nuzzo, V.; Dávalos, A.; Blossey, B. Invasive earthworms shape forest seed bank composition. Divers. Distrib. 2015, 21, 560–570. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Pérès, G.; Tondoh, J. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Corio, K.; Wolf, A.; Draney, M.; Fewless, G. Exotic earthworms of great lakes forests: A search for indicator plant species in maple forests. For. Ecol. Manag. 2009, 258, 1059–1066. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- Black, O. Calcium oxalate in the Dasheen. Am. J. Bot. 1918, 5, 447–451. [Google Scholar] [CrossRef]
- Mazen, A.; El Maghraby, O. Accumulation of cadmium, lead and strontium, and a role of calcium oxalate in water hyacinth tolerance. Biol. Plant. 1997, 40, 411–417. [Google Scholar] [CrossRef]
- Briones, M.; López, E.; Méndez, J.; Rodríguez, J.; Gago-Duport, L. Biological control over the formation and storage of amorphous calcium carbonate by earthworms. Mineral. Mag. 2008, 72, 227–231. [Google Scholar] [CrossRef]
- Lambkin, D.C.; Gwilliam, K.H.; Layton, C.; Canti, M.G.; Piearce, T.G.; Hodson, M.E. Production and dissolution rates of earthworm-secreted calcium carbonate. Pedobiologia 2011, 54, S119–S129. [Google Scholar] [CrossRef]
- Webb, M.A. Cell-mediated crystallization of calcium oxalate in plants. Plant Cell 1999, 11, 751–761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piearce, T. The calcium relations of selected Lumbricidae. J. Anim. Ecol. 1972, 41, 167–188. [Google Scholar] [CrossRef]
- Canti, M.G.; Piearce, T.G. Morphology and dynamics of calcium carbonate granules produced by different earthworm species: The 7th International Symposium on Earthworm Ecology·Cardiff·Wales·2002. Pedobiologia 2003, 47, 511–521. [Google Scholar]
- Bierzychudek, P. The demography of jack-in-the-pulpit, a forest perennial that changes sex. Ecol. Monogr. 1982, 52, 335–351. [Google Scholar] [CrossRef]
- Sims, G.; Ellsworth, T.; Mulvaney, R. Microscale determination of inorganic nitrogen in water and soil extracts. Commun. Soil Sci. Plant Anal. 1995, 26, 303–316. [Google Scholar] [CrossRef]
- Bellitürk, K.; Görres, J.H.; Kunkle, J.; Melnichuk, R.D.S. Can commercial mulches be reservoirs of invasive earthworms? Promotion of ligninolytic enzyme activity and survival of Amynthas agrestis (Goto and Hatai, 1899). Appl. Soil Ecol. 2015, 87, 27–31. [Google Scholar] [CrossRef]
- Chang, C.; Szlavecz, K.; Bernard, M.; Pitz, S. The second wave of earthworm invasion: Soil organic matter dynamics from the stable isotope perspective. In Proceedings of the 2013 AGU Fall Meeting, San Francisco, CA, USA, 9–13 December 2013. Abstracts B31C-0423. [Google Scholar]
- Plisko, J.D.; Nxele, T.C. An annotated key separating foreign earthworm species from the indigenous South African taxa (Oligochaeta: Acanthodrilidae, Eudrilidae, Glossoscolecidae, Lumbricidae, Megascolecidae, Microchaetidae, Ocnerodrilidae and Tritogeniidae). Afr. Invertebr. 2015, 56, 663–708. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: http://websoilsurvey.sc.egov.usda.gov/ (accessed on 10 October 2021).
- Moore, T. Patterns of dissolved organic matter in subarctic peatlands. Earth Surf. Processes Landf. 1987, 12, 387–397. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Turner, N.A.; Bollard, E.G. Problems in fractionating calcium in plant tissue. J. Sci. Food Agric. 1980, 31, 7–14. [Google Scholar] [CrossRef]
- Libert, B.; Franceschi, V.R. Oxalate in crop plants. J. Agric. Food Chem. 1987, 35, 926–938. [Google Scholar] [CrossRef]
- Zindler-Frank, E.; Hönow, R.; Hesse, A. Calcium and oxalate content of the leaves of Phaseolus vulgaris at different calcium supply in relation to calcium oxalate crystal formation. J. Plant Physiol. 2001, 158, 139–144. [Google Scholar] [CrossRef]
- Hönow, R.; Hesse, A. Comparison of extraction methods for the determination of soluble and total oxalate in foods by HPLC-enzyme-reactor. Food Chem. 2002, 78, 511–521. [Google Scholar] [CrossRef]
- Tecimen, H.B.; Gorres, J.H.; Melnichuk, R.D. Effect of Lumbricus rubellus and Amynthas agrestis earthworms on soil biogeochemistry at the aggregate scale in northern hardwood forests. J. Sustain. For. 2020, 40, 1–16. [Google Scholar] [CrossRef]
- Richardson, J.T. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Price-Christenson, G.J.; Johnston, M.R.; Herrick, B.M.; Yannarell, A.C. Influence of invasive earthworms (Amynthas spp.) on Wisconsin forest soil microbial communities and soil chemistry. Soil Biol. Biochem. 2020, 149, 107955. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 2008, 400, 356–368. [Google Scholar] [CrossRef]
- Cromack, K., Jr.; Sollins, P.; Todd, R.; Fogel, R.; Todd, A.; Fender, W.; Crossley, M.; Crossley, D., Jr. The role of oxalic acid and bicarbonate in calcium cycling by fungi and bacteria: Some possible implications for soil animals. Ecol. Bull. 1977, 25, 246–252. [Google Scholar]
- Parkin, T.B. Soil microsites as a source of denitrification variability. Soil Sci. Soc. Am. J. 1987, 51, 1194–1199. [Google Scholar] [CrossRef]
- Sexstone, A.J.; Revsbech, N.P.; Parkin, T.B.; Tiedje, J.M. Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci. Soc. Am. J. 1985, 49, 645–651. [Google Scholar] [CrossRef]
- Görres, J.H.; Savin, M.C.; Neher, D.A.; Weicht, T.R.; Amador, J.A. Grazing in a porous environment: 1. The effect of soil pore structure on C and N mineralization. Plant Soil 1999, 212, 75–83. [Google Scholar]
- Görres, J.H.; Savin, M.C.; Amador, J.A. Soil micropore structure and carbon mineralization in burrows and casts of an anecic earthworm (Lumbricus terrestris). Soil Biol. Biochem. 2001, 33, 1881–1887. [Google Scholar] [CrossRef]
- Amador, J.A.; Görres, J.H.; Savin, M.C. Carbon and Nitrogen Dynamics in (L.) Burrow Soil. Soil Sci. Soc. Am. J. 2003, 67, 1755–1762. [Google Scholar] [CrossRef]
- Burtelow, A.E.; Bohlen, P.J.; Groffman, P.M. Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Appl. Soil Ecol. 1998, 9, 197–202. [Google Scholar] [CrossRef]
- Parkin, T.B.; Berry, E.C. Microbial nitrogen transformations in earthworm burrows. Soil Biol. Biochem. 1999, 31, 1765–1771. [Google Scholar] [CrossRef]
- Amador, J.A.; Potts, D.A.; Savin, M.C.; Tomlinson, P.; Görres, J.H.; Nicosia, E.L. Mesocosm-scale evaluation of faunal and microbial communities of aerated and unaerated leachfield soil. J. Environ. Qual. 2006, 35, 1160–1169. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Morris, J.; Hester, A.; Nakata, P.A.; Hirschi, K.D. Genetically modified Medicago truncatula lacking calcium oxalate has increased calcium bioavailability and partially rescues vitamin D receptor knockout mice phenotypes. J. Bioequiv. Bioavailab. 2013, 5, 47–52. [Google Scholar] [CrossRef]
- Korth, K.L.; Doege, S.J.; Park, S.-H.; Goggin, F.L.; Wang, Q.; Gomez, S.K.; Liu, G.; Jia, L.; Nakata, P.A. Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol. 2006, 141, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Spiegel, M.; Saltz, D. Gazelle herbivory and interpopulation differences in calcium oxalate content of leaves of a desert lily. J. Chem. Ecol. 1997, 23, 333–346. [Google Scholar] [CrossRef]
- Konno, K.; Inoue, T.A.; Nakamura, M. Synergistic defensive function of raphides and protease through the needle effect. PLoS ONE 2014, 9, e91341. [Google Scholar] [CrossRef]




| Oxalate Extract (µg/g) | ||||||
|---|---|---|---|---|---|---|
| Full Model | Ethanol | Water | 5% Acetic Acid | 2 N HCl | Total | |
| df | 8,44 | 8,44 | 8,44 | 8,44 | 8,44 | |
| F-value | 1.113 | 3.954 | 0.322 | 2.073 | 3.150 | |
| p-value | 0.378 | 0.002 | 0.952 | 0.065 | 0.011 | |
| Effect tests | ||||||
| SOIL | df | 2,36 | 2,36 | 2,36 | 2,36 | 2,36 |
| F-value | 0.549 | 1.391 | 0.349 | 0.169 | 0.935 | |
| p-value | 0.582 | 0.262 | 0.708 | 0.845 | 0.402 | |
| η2% | 0.043 (S) | 0.015 (S) | 0.003 (S) | 0.031 (S) | ||
| Partial η2% | 0.074 (M) | 0.016 (S) | 0.004 (S) | 0.050 (S) | ||
| WORM | df | 2,36 | 2,36 | 2,36 | 2,36 | 2,36 |
| F-value | 0.046 | 6.266 | 0.205 | 4.563 | 4.247 | |
| p-value | 0.955 | 0.005 | 0.816 | 0.017 | 0.027 | |
| η2% | 0.189 (L) | 0.087 (M) | 0.185 (L) | 0.137 (M) | ||
| Partial η2% | 0.255 (L) | 0.092 (M) | 0.213 (L) | 0.187 (L) | ||
| SOIL*WORM | df | 4,36 | 4,36 | 4,36 | 4,36 | 4,36 |
| F-value | 1.928 | 4.079 | 0.368 | 1.780 | 3.709 | |
| p-value | 0.127 | 0.008 | 0.830 | 0.154 | 0.013 | |
| η2% | 0.248 (L) | 0.041 (S) | 0.129 (M) | 0.245 (L) | ||
| Partial η2% | 0.312 (L) | 0.042 (S) | 0.159 (L) | 0.292 (L) | ||
| Treatment Mean (SE) | ||||||
| SOIL | Control | 35.6(4.7) | 585.2(41.8) | 300.7(40.1) | 171.6(21.0) | 1093.1(80.7) |
| A. agrestis | 29.6(3.1) | 513.6(39.4) | 264.8(22.7) | 187.9(25.1) | 995.9(59.6) | |
| L. rubellus | 36.0(6.4) | 533.7(35.2) | 273.0(25.1) | 177.1(21.5) | 1019.7(39.7) | |
| WORM | Control | 33.0(5.3) | 453.7(39.8) a | 269.3(24.5) | 161.2(24.6) ab | 917.2(52.3) a |
| A. agrestis | 34.9(4.5) | 585.6(40.4) b | 296.0(40.1) | 147.1(14.0) a | 1063.6(69.8) ab | |
| L. rubellus | 33.3(5.0) | 593.1(25.5) b | 273.1(23.8) | 228.2(21.8) b | 1127.8(52.2) b | |
| Ca | K | Na | Al | Fe | Mn | Zn | Mg | NH4 | NO3 | 330 nm | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOIL | WORM | (mg/kg Soil) | SE | ||||||||||
| Control | Control | 939 ± 202 | 126 ± 23.6 | 46.3 ± 8.2 | 152 ± 31.9 | 36.3 ± 5.3 | 66.3 ± 13.9 | 7.08 ± 0.39 | 142 ± 31.6 | 33.1 ± 9.3 | 180 ± 40.1 | 0.267 | 0.049 |
| A. agrestis | 853 ± 65 | 129 ± 12.6 | 42.7 ± 6.4 | 164 ± 15.1 | 37.8 ± 2.9 | 68.3 ± 9.2 | 7.08 ± 0.20 | 142 ± 7.8 | 41.6 ± 10.5 | 230 ± 60.6 | 0.260 | 0.043 | |
| L. rubellus | 1002 ± 260 | 177 ± 38.1 | 60.7 ± 15.7 | 170 ± 8.2 | 35.7 ± 1.3 | 72.0 ± 17.1 | 7.03 ± 0.96 | 178 ± 41.7 | 64.1 ± 10.9 | 370 ± 111 | 0.232 | 0.035 | |
| A. agrestis | Control | 1135 ± 127 | 189 ± 25.8 | 52.4 ± 6.6 | 116 ± 20.4 | 25.4 ± 2.5 | 96.9 ± 13.5 | 7.52 ± 0.37 | 204 ± 18.4 | 55.6 ± 12.4 | 301 ± 80 | 0.493 | 0.070 |
| A. agrestis | 1102 ± 101 | 197 ± 31.6 | 73.4 ± 11.3 | 124 ± 15.6 | 26.6 ± 2.2 | 89.5 ± 11.7 | 7.95 ± 0.21 | 200 ± 19.5 | 67.6 ± 11.0 | 345 ± 61.3 | 0.442 | 0.059 | |
| L. rubellus | 1218 ± 109 | 189 ± 22.2 | 64.1 ± 13.7 | 119 ± 16.1 | 27.9 ± 5.0 | 102.0 ± 6.6 | 8.34 ± 0.53 | 219 ± 26.0 | 71.6 ± 17.4 | 366 ± 94.8 | 0.467 | 0.038 | |
| L. rubellus | Control | 959 ± 148 | 151 ± 30.5 | 41.1 ± 9.8 | 126 ± 25.9 | 29.7 ± 3.9 | 68.3 ± 11.7 | 7.06 ± 0.77 | 165 ± 27.3 | 39.0 ± 13.2 | 211 ± 37.7 | 0.339 | 0.036 |
| A. agrestis | 1146 ± 121 | 173 ± 22.5 | 55.8 ± 6.8 | 116 ± 14.1 | 30.7 ± 1.8 | 85.0 ± 5.3 | 7.46 ± 0.35 | 198 ± 21.3 | 54.5 ± 10.9 | 303 ± 65.8 | 0.395 | 0.051 | |
| L. rubellus | 1125 ± 141 | 191 ± 35.2 | 84.2 ± 14.0 | 171 ± 19.2 | 39.1 ± 2.9 | 90.0 ± 17.3 | 8.76 ± 0.66 | 198 ± 31.0 | 77.8 ± 13.5 | 588 ± 205 | 0.303 | 0.019 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnichuk, R.D.S.; Tecimen, H.B.; Görres, J.H. Do the Invasive Earthworms Amynthas agrestis (Oligochaeta: Megascolecidae) and Lumbricus rubellus (Oligochaeta: Lumbricidae) Stimulate Oxalate-Based Browser Defenses in Jack-in-the-Pulpit (Arisaema triphyllum) by Their Presence or Their Soil Biogeochemical Activity? Soil Syst. 2022, 6, 11. https://doi.org/10.3390/soilsystems6010011
Melnichuk RDS, Tecimen HB, Görres JH. Do the Invasive Earthworms Amynthas agrestis (Oligochaeta: Megascolecidae) and Lumbricus rubellus (Oligochaeta: Lumbricidae) Stimulate Oxalate-Based Browser Defenses in Jack-in-the-Pulpit (Arisaema triphyllum) by Their Presence or Their Soil Biogeochemical Activity? Soil Systems. 2022; 6(1):11. https://doi.org/10.3390/soilsystems6010011
Chicago/Turabian StyleMelnichuk, Ryan D. S., Hüseyin Barış Tecimen, and Josef H. Görres. 2022. "Do the Invasive Earthworms Amynthas agrestis (Oligochaeta: Megascolecidae) and Lumbricus rubellus (Oligochaeta: Lumbricidae) Stimulate Oxalate-Based Browser Defenses in Jack-in-the-Pulpit (Arisaema triphyllum) by Their Presence or Their Soil Biogeochemical Activity?" Soil Systems 6, no. 1: 11. https://doi.org/10.3390/soilsystems6010011
APA StyleMelnichuk, R. D. S., Tecimen, H. B., & Görres, J. H. (2022). Do the Invasive Earthworms Amynthas agrestis (Oligochaeta: Megascolecidae) and Lumbricus rubellus (Oligochaeta: Lumbricidae) Stimulate Oxalate-Based Browser Defenses in Jack-in-the-Pulpit (Arisaema triphyllum) by Their Presence or Their Soil Biogeochemical Activity? Soil Systems, 6(1), 11. https://doi.org/10.3390/soilsystems6010011






