Soil Lead Concentration and Speciation in Community Farms of Newark, New Jersey, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling
2.3. Metal Extractions
2.4. X-ray Absorption Fine Structure Spectroscopy (XAFS)
3. Results and Discussion
3.1. Single-Step Extraction
3.2. Sequential Extraction
3.3. Metal Hotspot and Pb in Particle Size Fractions
3.4. X-ray Absorption Fine Structure Spectroscopy (XAFS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulsen, M.N.; Hulland, K.R.S.; Gulas, C.A.; Pham, H.; Dalglish, S.L.; Wilkinson, R.K.; Winch, P.J. Growing an Urban Oasis: A Qualitative Study of the Perceived Benefits of Community Gardening in Baltimore, Maryland. CAFÉ 2014, 36, 69–82. [Google Scholar] [CrossRef]
- Brown, K.H.; Jameton, A.L. Public Health Implications of Urban Agriculture. J. Public Health Policy 2000, 21, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Siegner, A.; Sowerwine, J.; Acey, C. Does Urban Agriculture Improve Food Security? Examining the Nexus of Food Access and Distribution of Urban Produced Foods in the United States: A Systematic Review. Sustainability 2018, 10, 2988. [Google Scholar] [CrossRef]
- Lovell, S.T. Multifunctional Urban Agriculture for Sustainable Land Use Planning in the United States. Sustainability 2010, 2, 2499–2522. [Google Scholar] [CrossRef]
- National Gardening Association. Available online: https://garden.org/special/pdf/2014-NGA-Garden-to-Table.pdf (accessed on 14 October 2020).
- Attanayake, C.P.; Hettiarachchi, G.M.; Harms, A.; Presley, D.; Martin, S.; Pierzynski, G.M. Field evaluations on soil plant transfer of lead from an urban garden soil. J. Environ. Qual. 2014, 43, 475–487. [Google Scholar] [CrossRef]
- Chaney, R.; Mielke, H.W. Standards for soil lead limitations in the United States. Trace Subst. Environ. Health 1986, 20, 357–377. [Google Scholar]
- Mielke, H.W.; Reagan, P.L. Soil is an important pathway of human lead exposure. Environ. Health Perspect. 1998, 106 (Suppl. 1), 217–229. [Google Scholar] [CrossRef]
- Smith, D.B.; Cannon, W.F.; Woodruff, L.G.; Solano, F.; Kilburn, J.E.; Fey, D.L. Geochemical and Mineralogical Data for Soils of the Conterminous United States. 2013. Available online: https://pubs.usgs.gov/ds/801/pdf/ds801.pdf (accessed on 13 October 2020).
- Minca, K.K.; Basta, N.T. Comparison of plant nutrient and environmental soil tests to predict Pb in urban soils. Sci. Total Environ. 2013, 445–446, 57–63. [Google Scholar] [CrossRef]
- Datko-Williams, L.; Wilkie, A.; Richmond-Bryant, J. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci. Total Environ. 2014, 468–469, 854–863. [Google Scholar] [CrossRef]
- Mielke, H.W.; Anderson, J.C.; Berry, K.J.; Mielke, P.W.; Chaney, R.L.; Leech, M. Lead concentrations in inner-city soils as a factor in the child lead problem. Am. J. Public Health 1983, 73, 1366–1369. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Szlavecz, K.; Yesilonis, I.D.; Wong, C.P.; Murawski, L.; Marra, P.; Casey, R.E.; Lev, S. Multi-scale assessment of metal contamination in residential soil and soil fauna: A case study in the Baltimore–Washington metropolitan region, USA. Landscape Urban. Plann. 2015, 142, 7–17. [Google Scholar] [CrossRef]
- Pavilonis, B.; Maroko, A.; Cheng, Z. Lead in New York City’s soils: Population growth, land use, and contamination. Int. J. Hygiene Environ. Health 2020, 229, 113564. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.W.; Jenerette, G.D.; Bain, D.J. Urban legacies and soil management affect the concentration and speciation of trace metals in Los Angeles community garden soils. Environ. Pollut. 2015, 197, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.D.; Landsberger, S. Heavy Metals in Urban Soils of East St. Louis, IL, Part I: Total Concentration of Heavy Metals in Soils. J. Air Waste Manag. Assoc. 2000, 50, 1667–1679. [Google Scholar] [CrossRef]
- Mielke, H.W.; Laidlaw, M.A.S.; Gonzales, C. Lead (Pb) legacy from vehicle traffic in eight California urbanized areas: Continuing influence of lead dust on children’s health. Sci. Total Environ. 2010, 408, 3965–3975. [Google Scholar] [CrossRef]
- Attanayake, C.P.; Hettiarachchi, G.M.; Ma, Q.; Pierzynski, G.M.; Ransom, M.D. Lead Speciation and In Vitro Bioaccessibility of Compost-Amended Urban Garden Soils. J. Environ. Qual. 2017, 46, 1215–1224. [Google Scholar] [CrossRef]
- Filippelli, G.M.; Adamic, J.; Nichols, D.; Shukle, J.; Frix, E. Mapping the Urban Lead Exposome: A Detailed Analysis of Soil Metal Concentrations at the Household Scale Using Citizen Science. Int. J. Environ. Res. 2018, 15, 1531. [Google Scholar] [CrossRef]
- Finster, M.E.; Gray, K.A.; Binns, H.J. Lead levels of edibles grown in contaminated residential soils: A field survey. Sci. Total Environ. 2004, 320, 245–257. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Filippelli, G.M.; Brown, S.; Paz-Ferreiro, J.; Reichman, S.M.; Netherway, P.; Truskewycz, A.; Ball, A.S.; Mielke, H.W. Case studies and evidence-based approaches to addressing urban soil lead contamination. Appl. Geochem. 2017, 83, 14–30. [Google Scholar] [CrossRef]
- Mielke, H.W.; Blake, B.; Burroughs, S.; Hassinger, N. Urban lead levels in Minneapolis: The case of the Hmong children. Environ. Res. 1984, 34, 64–76. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Spliethoff, H.M.; Ribaudo, L.N.; Lopp, D.M.; Shayler, H.A.; Marquez-Bravo, L.G.; Lambert, V.T.; Ferenz, G.S.; Russell-Anelli, J.M.; Stone, E.B.; et al. Lead (Pb) and other metals in New York City community garden soils: Factors influencing contaminant distributions. Environ. Pollut. 2014, 187, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Moller, K.M.; Hartwell, J.G.; Simon-Friedt, B.R.; Wilson, M.J.; Wickliffe, J.K. Soil Contaminant Concentrations at Urban Agricultural Sites in New Orleans, Louisiana. J. Agric. Food Syst. Community Dev. 2018, 8, 139–149. [Google Scholar] [CrossRef]
- Defoe, P.P.; Hettiarachchi, G.M.; Benedict, C.; Martin, S. Safety of Gardening on Lead- and Arsenic-Contaminated Urban Brownfields. J. Environ. Qual. 2014, 43, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
- Shinn, N.J.; Bing-Canar, J.; Cailas, M.; Peneff, N.; Binns, H.J. Determination of Spatial Continuity of Soil Lead Levels in an Urban Residential Neighborhood. Environ. Res. 2000, 82, 46–52. [Google Scholar] [CrossRef]
- Sterrett, S.B.; Chaney, R.L.; Gifford, C.H.; Mielke, H.W. Influence of fertilizer and sewage sludge compost on yield and heavy metal accumulation by lettuce grown in urban soils. Environ. Geochem. Health 1996, 18, 135–142. [Google Scholar] [CrossRef]
- Wagner, T.; Langley-Turnbaugh, S. Case study: Examining the contribution of historical sources of lead in urban soils in Portland, Maine, USA. J. Environ. Plan. Manag. 2008, 51, 525–541. [Google Scholar] [CrossRef]
- Brown, S.L.; Chaney, R.L.; Hettiarachchi, G.M. Lead in Urban Soils: A Real or Perceived Concern for Urban Agriculture? J. Environ. Qual. 2016, 45, 26–36. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Protect Your Family from Exposures to Lead. Available online: https://www.epa.gov/lead/protect-your-family-exposures-lead (accessed on 22 May 2020).
- Agency for Toxic Substances and Disease Registry. Toxicological Profile: Lead. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=96&tid=22 (accessed on 14 May 2020).
- Nevidomskaya, D.; Minkina, T.; Soldatov, A.; Shuvaeva, V.; Zubavichus, Y.; Podkovyrina, Y. Comprehensive study of Pb (II) speciation in soil by X-ray absorption spectroscopy (XANES and EXAFS) and sequential fractionation. J. Soils Sediments 2016, 16, 1183–1192. [Google Scholar] [CrossRef]
- Wong, C.S.C.; Li, X.D. Pb contamination and isotopic composition of urban soils in Hong Kong. Sci. Total Environ. 2004, 319, 185–195. [Google Scholar] [CrossRef]
- Imperato, M.; Adamo, P.; Naimo, D.; Arienzo, M.; Stanzione, D.; Violante, P. Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ. Pollut. 2003, 124, 247–256. [Google Scholar] [CrossRef]
- Ryan, J.A.; Zhang, P.; Hesterberg, D.; Chou, J.; Sayers, D.E. Formation of Chloropyromorphite in a Lead-Contaminated Soil Amended with Hydroxyapatite. Environ. Sci. Technol. 2001, 35, 3798–3803. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Takaoka, M.; Oshita, K.; Tanida, H. Incomplete transformations of Pb to pyromorphite by phosphate-induced immobilization investigated by X-ray absorption fine structure (XAFS) spectroscopy. Chemosphere 2009, 76, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, G.M.; Pierzynski, G.M. Soil lead bioavailability and in situ remediation of lead-contaminated soils: A review. Environ. Prog. 2004, 23, 78–93. [Google Scholar] [CrossRef]
- Funasaka, K.; Tojo, T.; Katahira, K.; Shinya, M.; Miyazaki, T.; Kamiura, T.; Yamamoto, O.; Moriwaki, H.; Tanida, H.; Takaoka, M. Detection of Pb-LIII edge XANES spectra of urban atmospheric particles combined with simple acid extraction. Sci. Total Environ. 2008, 403, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, K.; Tojo, T.; Kaneco, S.; Takaoka, M. Different chemical properties of lead in atmospheric particles from urban roadside and residential areas. Atmos. Pollut. Res. 2013, 4, 362–369. [Google Scholar] [CrossRef][Green Version]
- Yi, K. Green Gardens Sprouting from Vacant Lots. Urban Farmers Hope to Grow City’s Economy. Available online: https://www.nj.com/essex/2019/12/green-gardens-sprouting-from-vacant-lots-urban-farmers-hope-to-grow-citys-economy.html (accessed on 22 May 2020).
- City of Newark. Available online: https://www.newarknj.gov/zoning (accessed on 29 October 2020).
- Weil, R.; Brady, N. The Nature and Properties of Soils, 15th ed.; Pearson Education: London, UK, 2017; ISBN 9780133254488. [Google Scholar]
- United States Environmental Protection Agency. Observational Economy Series Volume 1: Composite Sampling; EPA: Washington, DC, USA, 1995. Available online: https://www.epa.gov/sites/production/files/2016-03/documents/comp-samp.pdf (accessed on 24 December 2020).
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Chaney, R.L.; Sterrett, S.B.; Mielke, H.W. The potential for heavy metal exposure from urban gardens and soils. In Proceedings of the Symposium on Heavy Metals in Urban Gardens; University of District of Columbia Extension Service: Washington, DC, USA, 1984; pp. 37–84. Available online: https://www.researchgate.net/publication/242693949_THE_POTENTIAL_FOR_HEAVY_METAL_EXPOSURE_FROM_URBAN_GARDENS_AND_SOILS (accessed on 28 December 2020).
- N.J.A.C. 7:26D Remediation Standards. Available online: https://www.nj.gov/dep/rules/rules/njac7_26d.pdf (accessed on 17 October 2020).
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- John, D.A.; Leventhal, J.S. Bioavailability of Metals. Available online: https://pubs.usgs.gov/of/1995/ofr-95-0831/CHAP2.pdf (accessed on 17 October 2020).
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12. [Google Scholar] [CrossRef]
- McBride, M.B.; Mathur, R.R.; Baker, L.L. Chemical Extractability of Pb in Field-Contaminated Soils: Implications for Estimating Total Pb. Commun. Soil Sci. Plant. Anal. 2011, 42, 1581–1593. [Google Scholar] [CrossRef]
- Wharton, S.E.; Shayler, H.A.; Spliethoff, H.M.; Marquez-Bravo, L.G.; Ribaudo, L.; McBride, M.B. A Comparison of Screening Tests for Soil Pb. Soil Sci. 2012, 177, 650–654. [Google Scholar] [CrossRef]
- Kessler, R. Urban Gardening: Managing the Risks of Contaminated Soil. Environ. Health Perspect. 2013, 121, A326–A333. [Google Scholar] [CrossRef]
- Van Roosmalen, G.R.E.M.; Lustenhouwer, J.W.A.; Oosthoek, J.; Senden, M.M.G. Heavy metal sources and contamination mechanisms in compost production. Resour. Conserv. 1987, 14, 321–334. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Remeikaitė-Nikienė, N.; Garnaga-Budrė, G.; Lujanienė, G.; Jokšas, K.; Stankevičius, A.; Malejevas, V.; Barisevičiūtė, R. Distribution of metals and extent of contamination in sediments from the south-eastern Baltic Sea (Lithuanian zone). Oceanologia 2018, 60, 193–206. [Google Scholar] [CrossRef]
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Sanders, P.F. Ambient Levels of Metals in New Jersey Soils; NJDEP: Trenton, NJ, USA, 2003; Available online: https://www.state.nj.us/dep/dsr/research/ambient-levels-metal.pdf (accessed on 24 December 2020).
- Clark, H.F.; Brabander, D.J.; Erdil, R.M. Sources, Sinks, and Exposure Pathways of Lead in Urban Garden Soil. J. Environ. Qual. 2006, 35, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 402647. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Elder, J.F. Metal biogeochemistry in surface-water systems; a review of principles and concepts. Circular 1988. [Google Scholar] [CrossRef]
- Zimdahl, R.L.; Skogerboe, R.K. Behavior of lead in soil. Environ. Sci. Technol. 1977, 11, 1202–1207. [Google Scholar] [CrossRef]
- Harrison, R.M.; Laxen, D.P.H.; Wilson, S.J. Chemical associations of lead, cadmium, copper, and zinc in street dusts and roadside soils. Environ. Sci. Technol. 1981, 15, 1378–1383. [Google Scholar] [CrossRef]
- Sutherland, R.A.; Tack, F.M.G. Metal phase associations in soils from an urban watershed, Honolulu, Hawaii. Sci. Total Environ. 2000, 256, 103–113. [Google Scholar] [CrossRef]
- Norrström, A.C.; Jacks, G. Concentration and fractionation of heavy metals in roadside soils receiving de-icing salts. Sci. Total Environ. 1998, 218, 161–174. [Google Scholar] [CrossRef]
- Preer, J.R.; Akintoye, J.O.; Martin, M.L. Metals in downtown Washington, DC gardens. Biol. Trace Elem. Res. 1984, 6, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nirel, P.M.V.; Morel, F.M.M. Pitfalls of sequential extractions. Water Res. 1990, 24, 1055–1056. [Google Scholar] [CrossRef]
- Dollar, N.L.; Souch, C.J.; Filippelli, G.M.; Mastalerz, M. Chemical Fractionation of Metals in Wetland Sediments: Indiana Dunes National Lakeshore. Environ. Sci. Technol. 2001, 35, 3608–3615. [Google Scholar] [CrossRef]
- Mi, Y.; Zhan, F.; Li, B.; Qin, L.; Wang, J.; Zu, Y.; Li, Y. Distribution characteristics of cadmium and lead in particle size fractions of farmland soils in a lead–zinc mine area in Southwest China. Environ. Syst. Res. 2018, 7, 14. [Google Scholar] [CrossRef]
- Park, H.; Park, H.; Yang, H.I.; Park, S.; Lim, S.; Kwak, J.; Lee, G.; Lee, S.; Park, M.; Choi, W. Sorption of Pb in chemical and particle-size fractions of soils with different physico-chemical properties. J. Soils Sediments 2019, 19, 310–321. [Google Scholar] [CrossRef]
- Sharp, R.M.; Brabander, D.J. Lead (Pb) Bioaccessibility and Mobility Assessment of Urban Soils and Composts: Fingerprinting Sources and Refining Risks to Support Urban Agriculture. GeoHealth 2017, 1, 333–345. [Google Scholar] [CrossRef]
- Dermatas, D.; Chrysochoou, M. Lead particle size and its association with firing conditions and range maintenance: Implications for treatment. Environ. Geochem. Health 2007, 29, 347–355. [Google Scholar] [CrossRef]
- Manceau, A.; Boisset, M.; Sarret, G.; Hazemann, J.; Mench, M.; Cambier, P.; Prost, R. Direct Determination of Lead Speciation in Contaminated Soils by EXAFS Spectroscopy. Environ. Sci. Technol. 1996, 30, 1540–1552. [Google Scholar] [CrossRef]
- Ostergren, J.D.; Brown, G.E.; Parks, G.A.; Tingle, T.N. Quantitative Speciation of Lead in Selected Mine Tailings from Leadville, CO. Environ. Sci. Technol. 1999, 33, 1627–1636. [Google Scholar] [CrossRef]
- Rouff, A.A.; Elzinga, E.J.; Reeder, R.J.; Fisher, N.S. X-ray Absorption Spectroscopic Evidence for the Formation of Pb (II) Inner-Sphere Adsorption Complexes and Precipitates at the Calcite−Water Interface. Environ. Sci. Technol. 2004, 38, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Hamel, S.; Heckman, J.; Murphy, S. Lead Contaminated Soil: Minimizing Health Risks. Available online: https://njaes.rutgers.edu/fs336/ (accessed on 17 October 2020).
- Chaney, R.L.; Mielke, H.W.; Sterrett, S.B. Speciation, mobility, and bioavailability of Soil Lead. In Lead in Soils: Issues and Guidelines; Davies, B.E., Wixson, B.G., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 105–129. [Google Scholar]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Zahran, S.; Laidlaw, M.A.S.; McElmurry, S.P.; Filippelli, G.M.; Taylor, M. Linking Source and Effect: Resuspended Soil Lead, Air Lead, and Children’s Blood Lead Levels in Detroit, Michigan. Environ. Sci. Technol. 2013, 47, 2839–2845. [Google Scholar] [CrossRef]
- Pingitore, J.; Nicholas, E.; Clague, J.W.; Amaya, M.A.; Maciejewska, B.; Reynoso, J.J. Urban Airborne Lead: X-Ray Absorption Spectroscopy Establishes Soil as Dominant Source. PLoS ONE 2009, 4, e5019. [Google Scholar] [CrossRef]
- Burt, R.; Hernandez, L.; Shaw, R.; Tunstead, R.; Ferguson, R.; Peaslee, S. Trace element concentration and speciation in selected urban soils in New York City. Environ. Monit. Assess. 2014, 186, 195–215. [Google Scholar] [CrossRef]
- Essex County Trash Incinerator Unfairly Burdens Poor Critics Say. Available online: https://patch.com/new-jersey/newarknj/essex-county-trash-incinerator-unfairly-burdens-poor-critics-say (accessed on 7 October 2020).
- Essex Covanta. Available online: https://www.covanta.com/where-we-are/our-facilities/essex (accessed on 7 October 2020).
- Earthjustice: Newark Youth March on Covanta, Trash-Burning Incinerator Masquerading As ‘Energy Plant’. Available online: https://earthjustice.org/news/press/2018/newark-youth-march-on-covanta-trash-burning-incinerator-masquerading-as-energy-plant (accessed on 14 October 2020).
- Olson, D.E. Newark’s Lead Crisis Continues: Even Higher Levels in 2019. Available online: https://www.nrdc.org/experts/erik-d-olson/newarks-lead-crisis-continues-even-higher-levels-2019 (accessed on 21 October 2020).
- Yi, K. Newark Lead Levels Are Lower But Still Elevated, New Water Tests Show. Available online: https://www.nj.com/essex/2020/01/newark-lead-levels-are-lower-but-still-elevated-new-water-tests-show.html (accessed on 21 October 2020).
- Urban Agriculture: Practices to Improve Cities. Available online: https://urbanland.uli.org/news/urban-agriculture-practices-to-improve-cities/ (accessed on 22 May 2020).
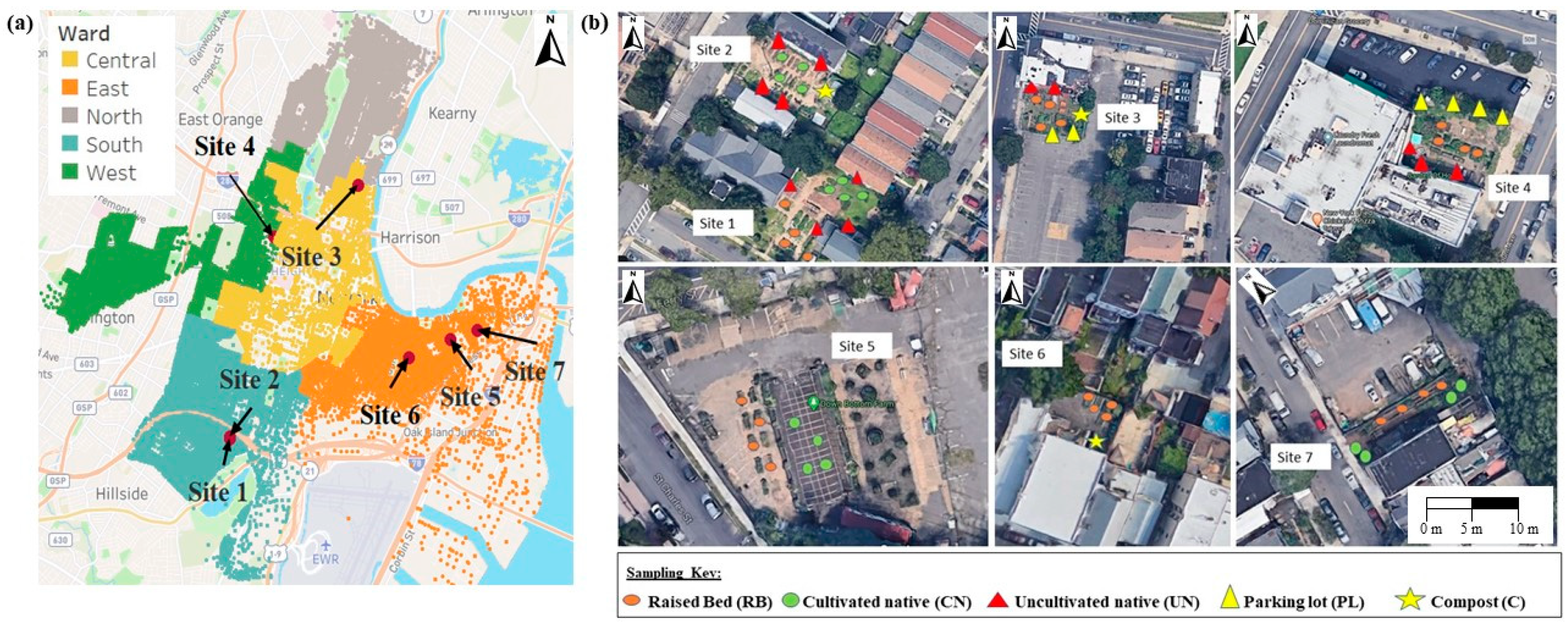
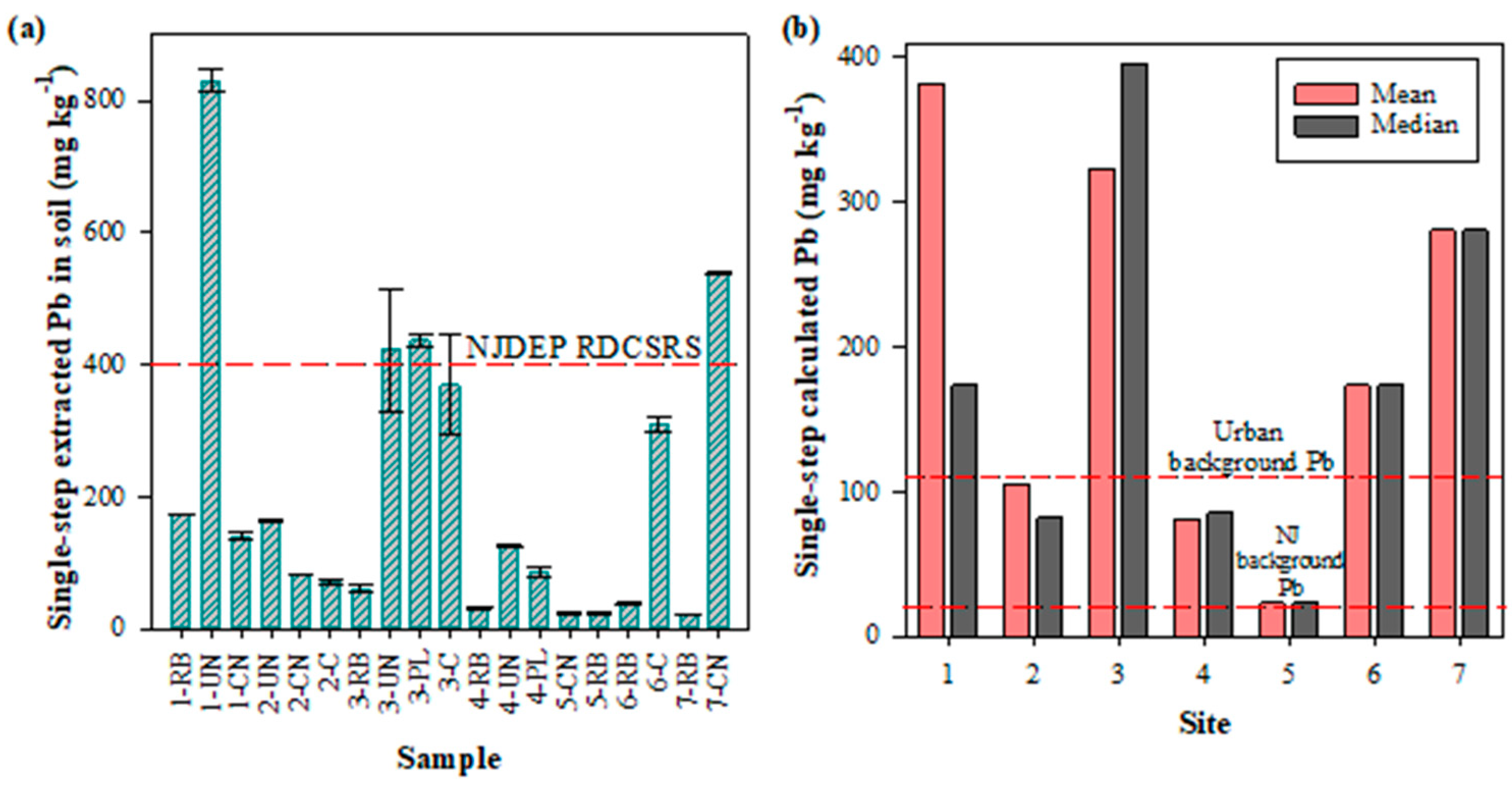

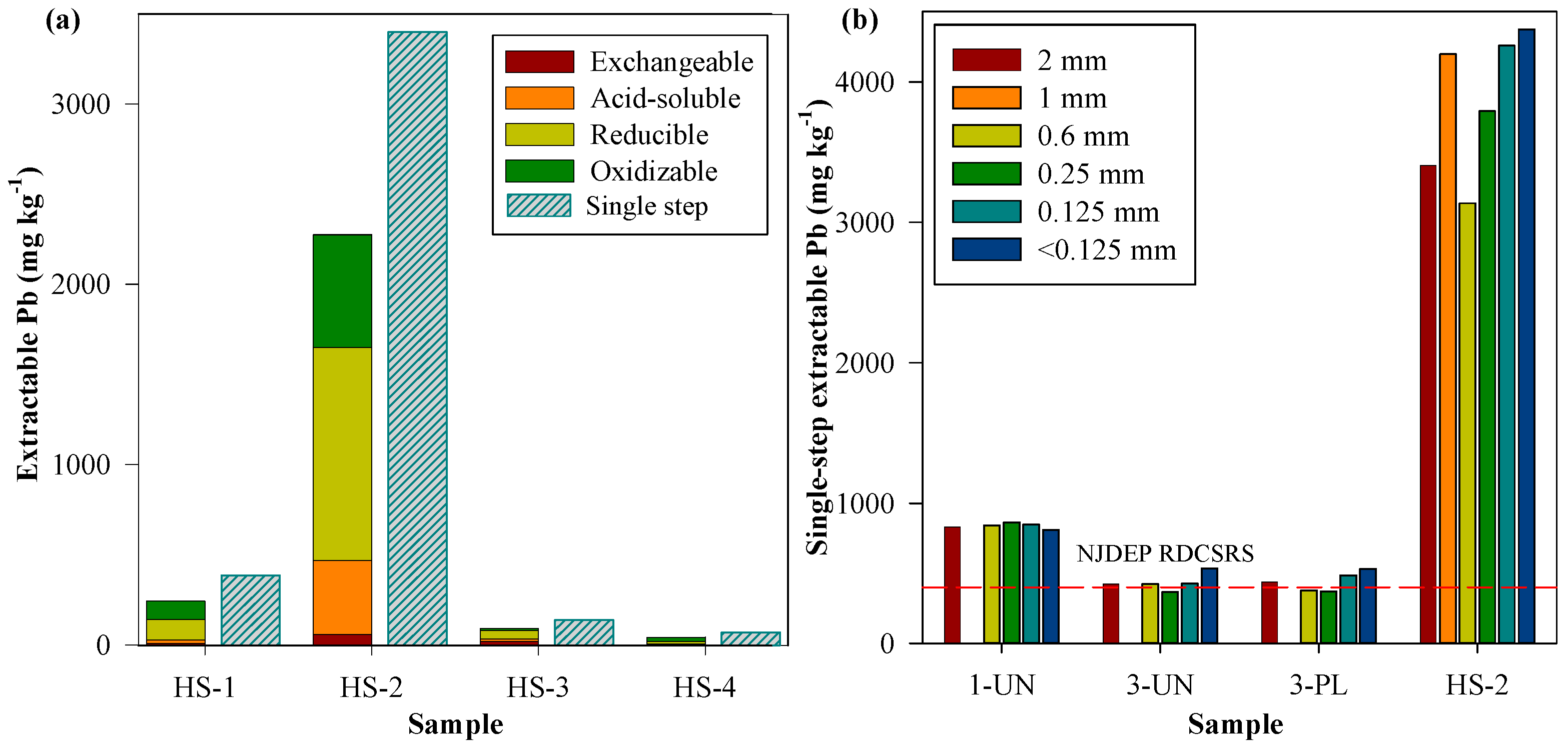
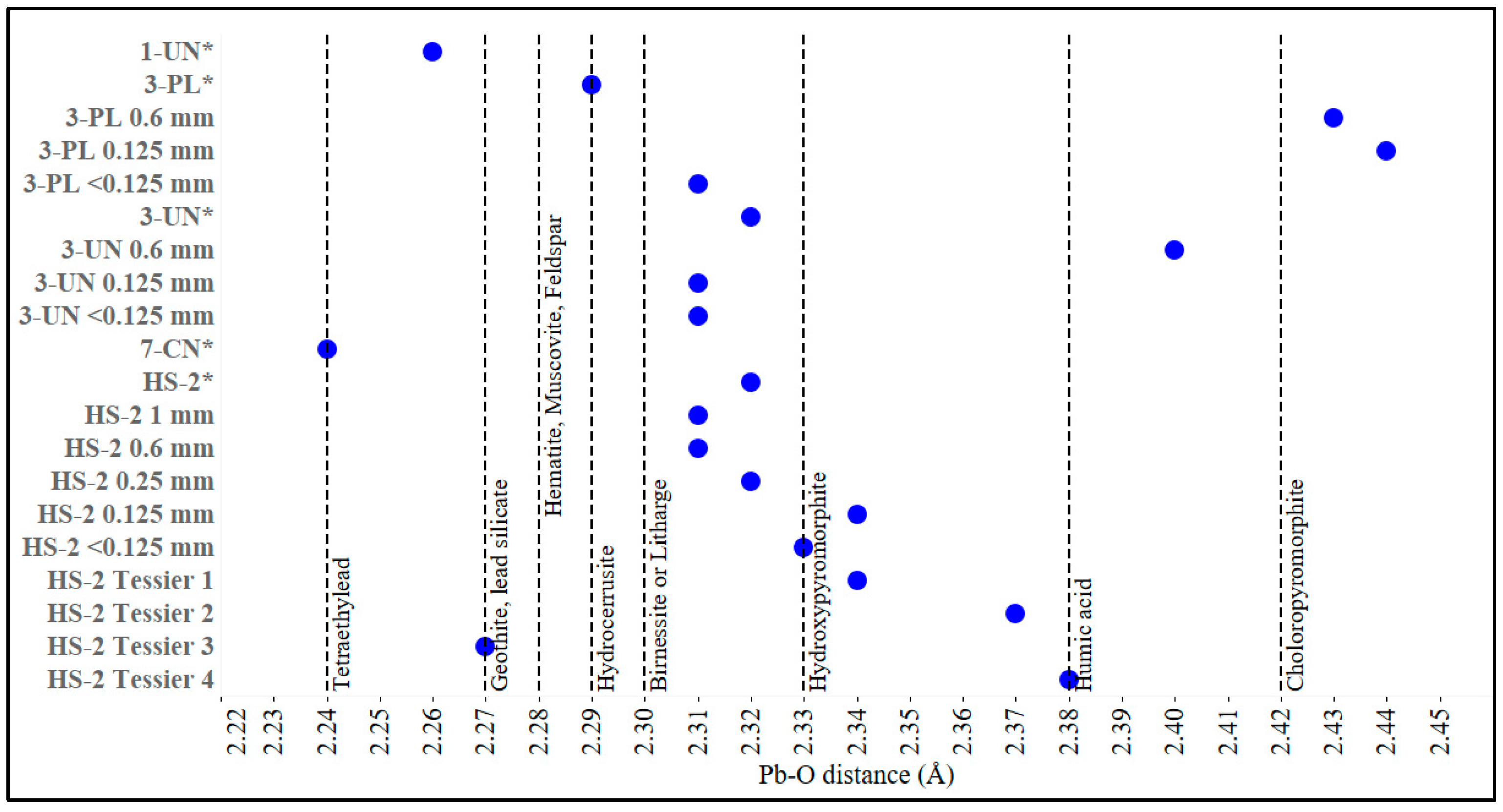
| Site | Area (m2) | Type of Location | Raised Beds | Cultivation in Native Soil | Composting |
|---|---|---|---|---|---|
| 1 | 474.1 | Community and regional commercial zone | √ | √ | × |
| 2 | 285.6 | × | √ | √ | |
| 3 | 112.9 | √ | × | √ | |
| 4 | 242.3 | √ | × | × | |
| 5 | 5801.3 | Heavy industrial zone | √ | √ | × |
| 6 | 156.5 | √ | × | √ | |
| 7 | 208.1 | √ | √ | × |
| Fraction | Extraction | Metal Speciation | Factors Influencing Metal Mobility |
|---|---|---|---|
| Exchangeable | 1 M MgCl2 pH 7.0 1 h at 25 °C | Dissolved cations | Changes in cation composition, sorption chemistry and ion exchange; high mobility |
| Acid-soluble | 1 M NaOAc pH 5 5 h at 25 °C | Bound to carbonates | Changes in soil pH; medium mobility |
| Reducible | 0.04 M NH2OH–HCl 25% HOAc 6 h at 96 °C | Bound to iron and manganese oxides | Changes in redox chemistry; medium mobility |
| Oxidizable | 0.02 M HNO3 30% H2O2 2 h at 85 °C 30% H2O2 3h at 85 °C 3.2 M NH4OAc 30 min at 25 °C | Bound to soil organic matter | Decomposition of organic matter; medium mobility |
| * Residual | 5:1 HF-HClO4 | Within mineral crystal structures | Weathering and mineral decomposition; low mobility |
| Sample | CF | CFAvg | CF Class | Igeo | Igeo Avg | IgeoClass |
|---|---|---|---|---|---|---|
| 2-C | 3.3 | 11.4 | Very high | 1.1 | 2.6 | Moderate to strongly contaminated |
| 3-C | 16.8 | 3.5 | ||||
| 6-C | 14.1 | 3.2 | ||||
| 1-CN | 6.4 | 8.9 | Very high | 2.1 | 1.8 | Moderately contaminated |
| 2-CN | 3.7 | 1.3 | ||||
| 5-CN | 1.1 | −0.5 | ||||
| 7-CN | 24.5 | 4 | ||||
| 3-PL | 19.9 | 11.9 | Very high | 3.7 | 2.6 | Moderate to strongly contaminated |
| 4-PL | 3.9 | 1.4 | ||||
| 1-RB | 7.9 | 2.7 | Moderate | 2.4 | 0.4 | Uncontaminated |
| 3-RB | 2.8 | 0.9 | ||||
| 4-RB | 1.4 | −0.1 | ||||
| 5-RB | 1.1 | −0.5 | ||||
| 6-RB | 1.7 | 0.2 | ||||
| 7-RB | 1 | −0.6 | ||||
| 1-UN | 37.7 | 17.5 | Very high | 4.7 | 3.2 | Strongly contaminated |
| 2-UN | 7.4 | 2.3 | ||||
| 3-UN | 9.2 | 3.7 | ||||
| 4-UN | 5.8 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, O.; Rouff, A.A. Soil Lead Concentration and Speciation in Community Farms of Newark, New Jersey, USA. Soil Syst. 2021, 5, 2. https://doi.org/10.3390/soilsystems5010002
Goswami O, Rouff AA. Soil Lead Concentration and Speciation in Community Farms of Newark, New Jersey, USA. Soil Systems. 2021; 5(1):2. https://doi.org/10.3390/soilsystems5010002
Chicago/Turabian StyleGoswami, Omanjana, and Ashaki A. Rouff. 2021. "Soil Lead Concentration and Speciation in Community Farms of Newark, New Jersey, USA" Soil Systems 5, no. 1: 2. https://doi.org/10.3390/soilsystems5010002
APA StyleGoswami, O., & Rouff, A. A. (2021). Soil Lead Concentration and Speciation in Community Farms of Newark, New Jersey, USA. Soil Systems, 5(1), 2. https://doi.org/10.3390/soilsystems5010002




