Biogeochemical Controls on the Potential for Long-Term Contaminant Leaching from Soils Developing on Historic Coal Mine Spoil
Abstract
1. Introduction
2. Materials and Methods
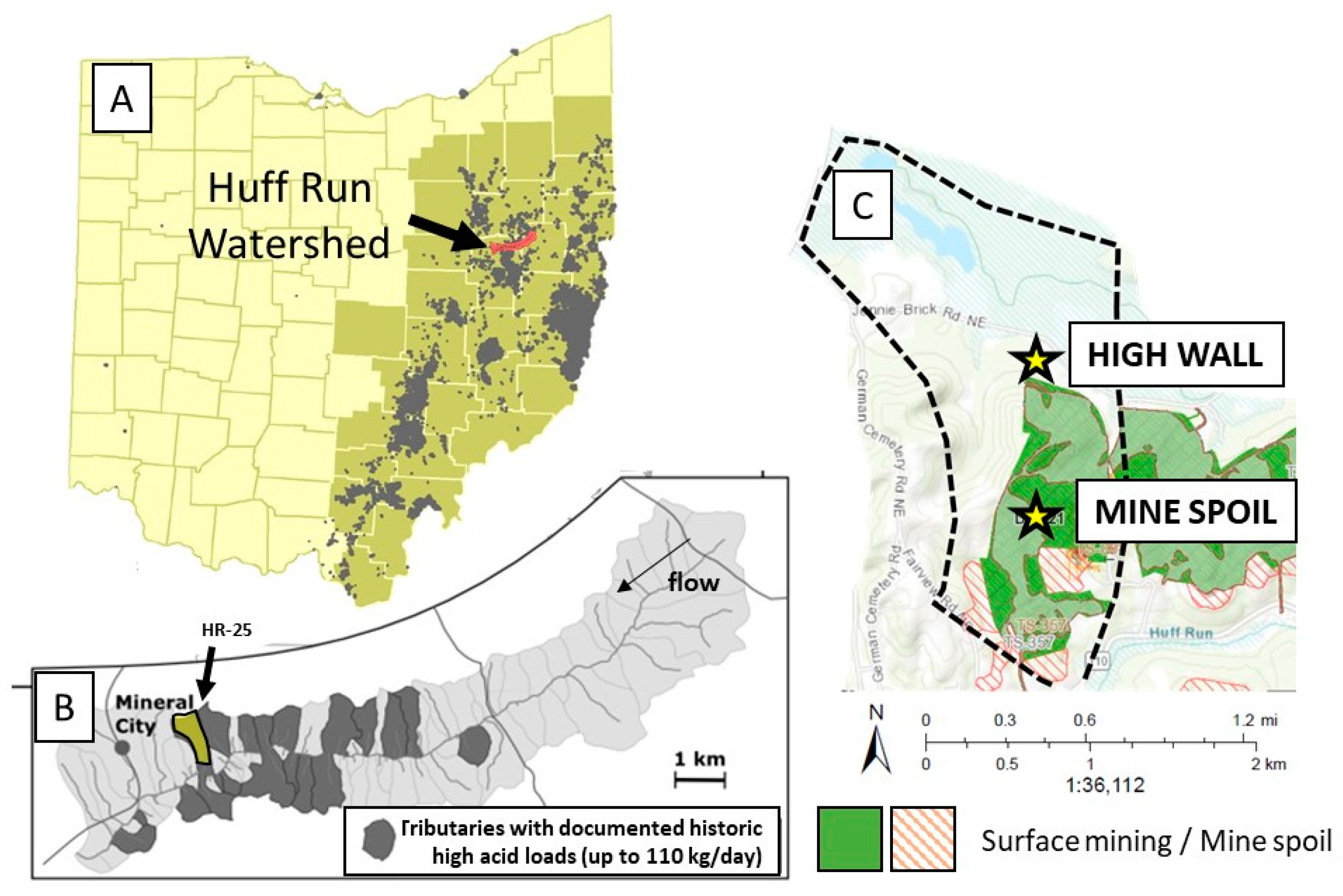
2.1. Site Description
2.2. Soil Sampling and Suction Lysimeter Installation
2.3. Solid-Phase Characterization
2.4. Pore Water Analyses
2.5. Sequential Extraction
2.6. Microbial Community Analysis
3. Results
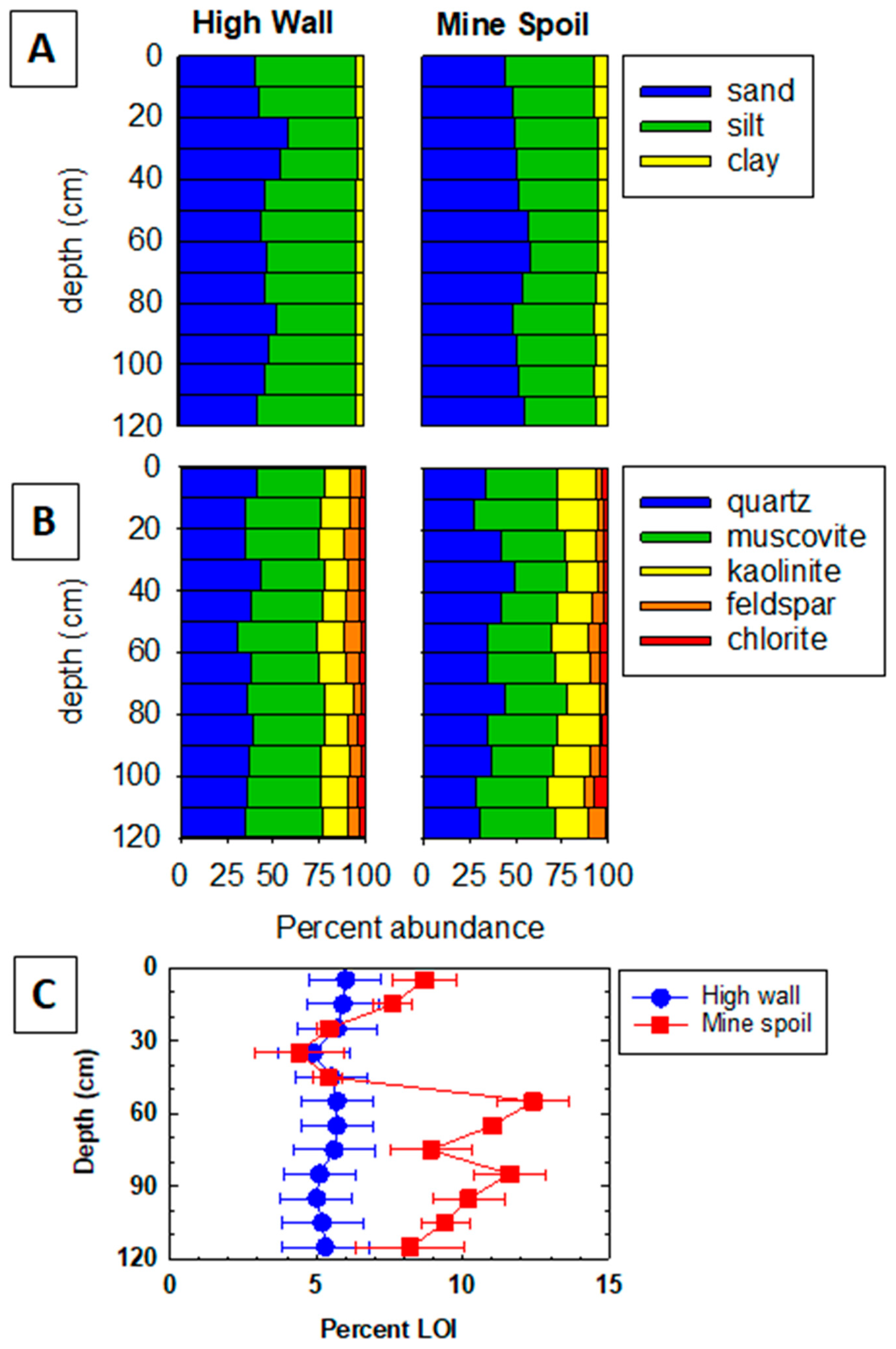
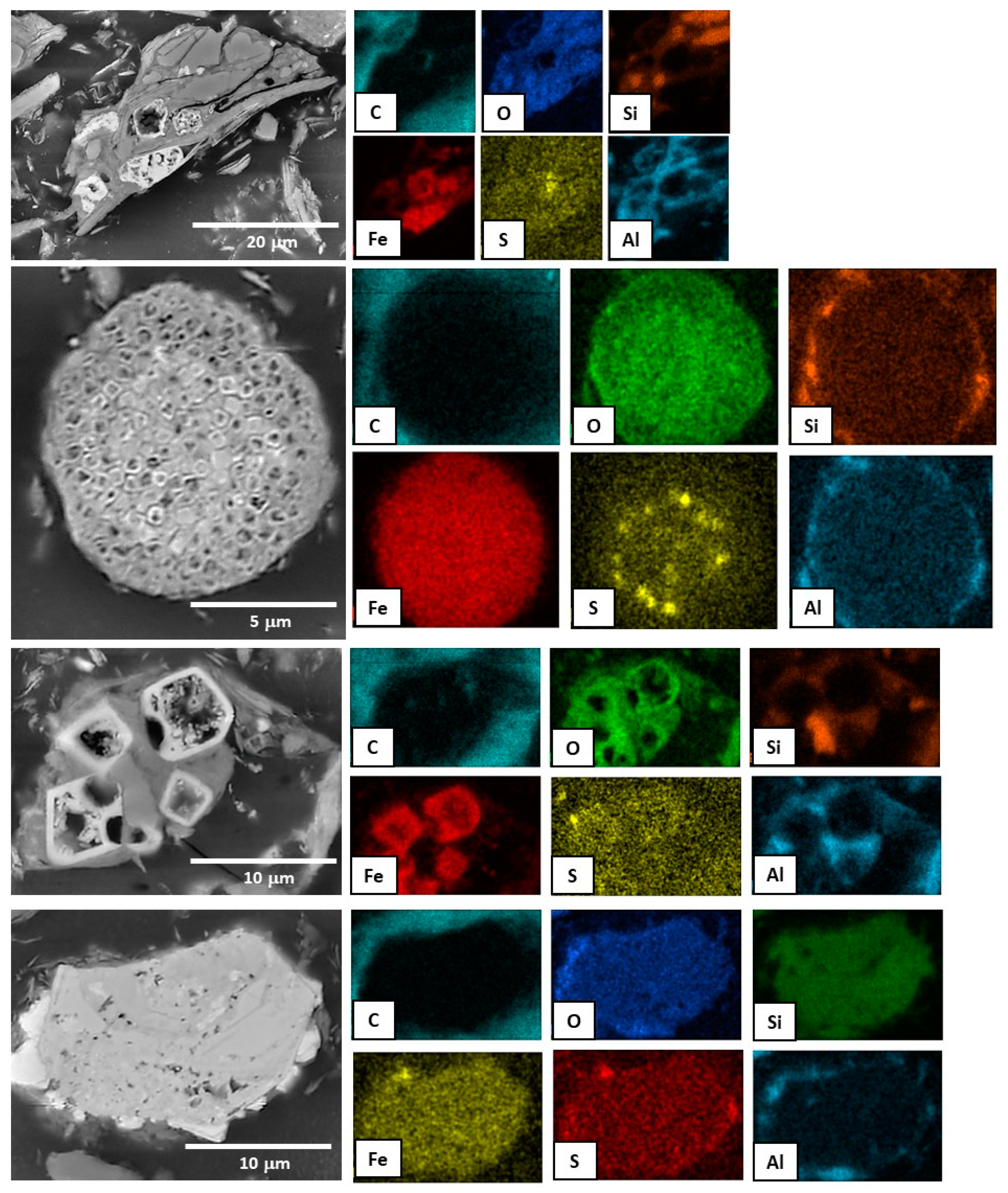
3.1. Bulk Composition and Analysis of Particle Size, Textures, and Morphology
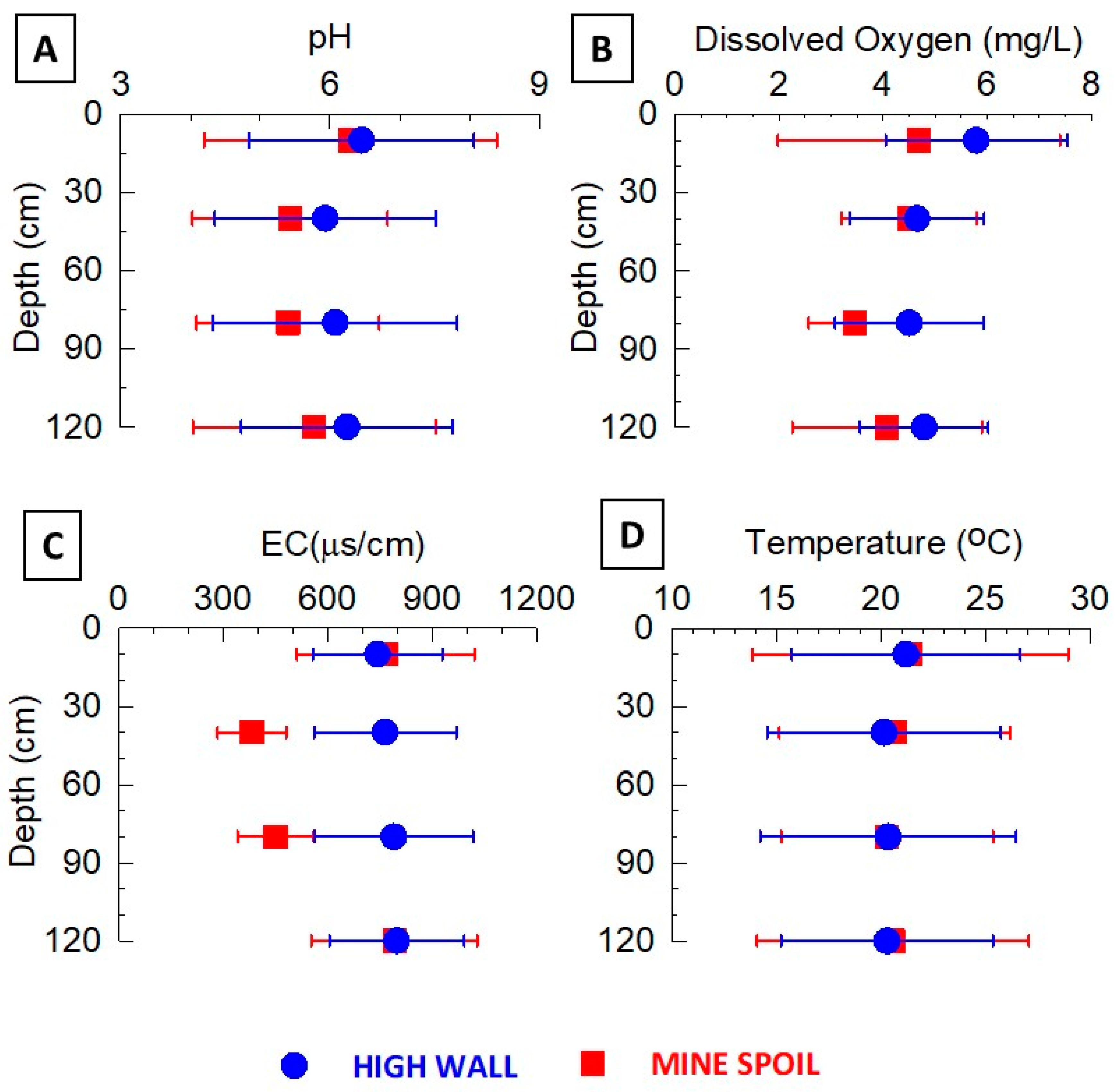
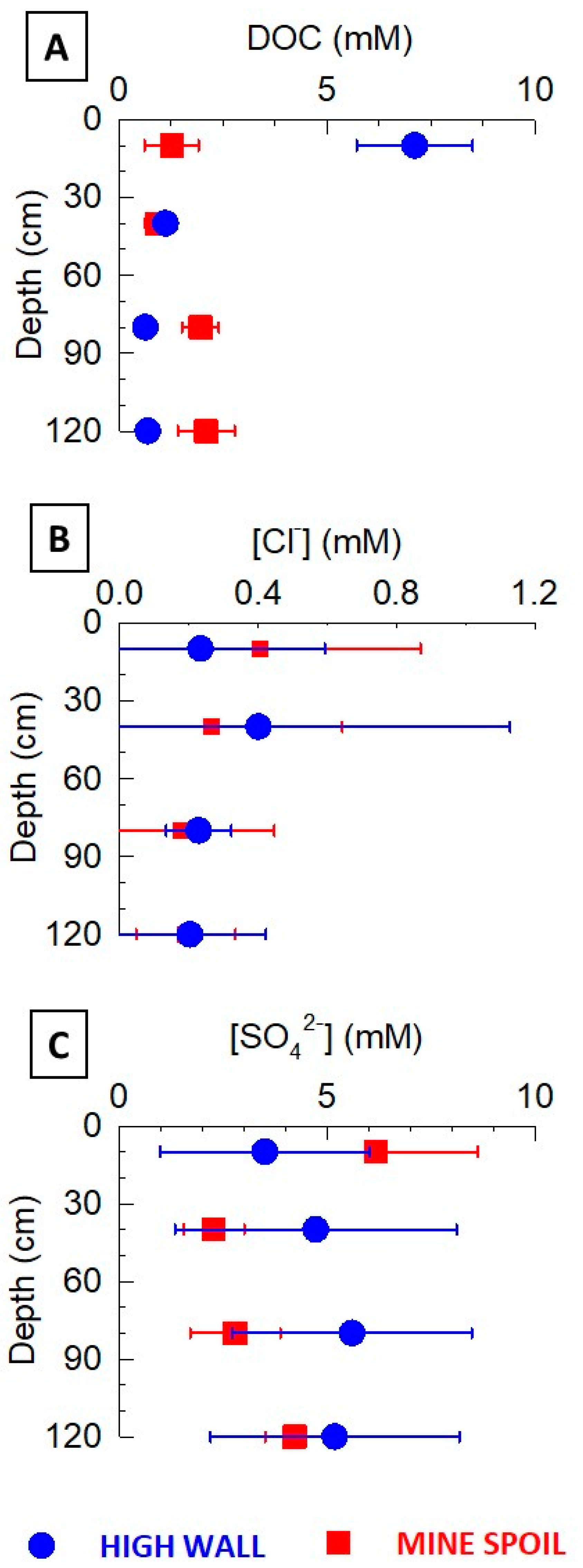
3.2. Lysimeter Pore Water Composition
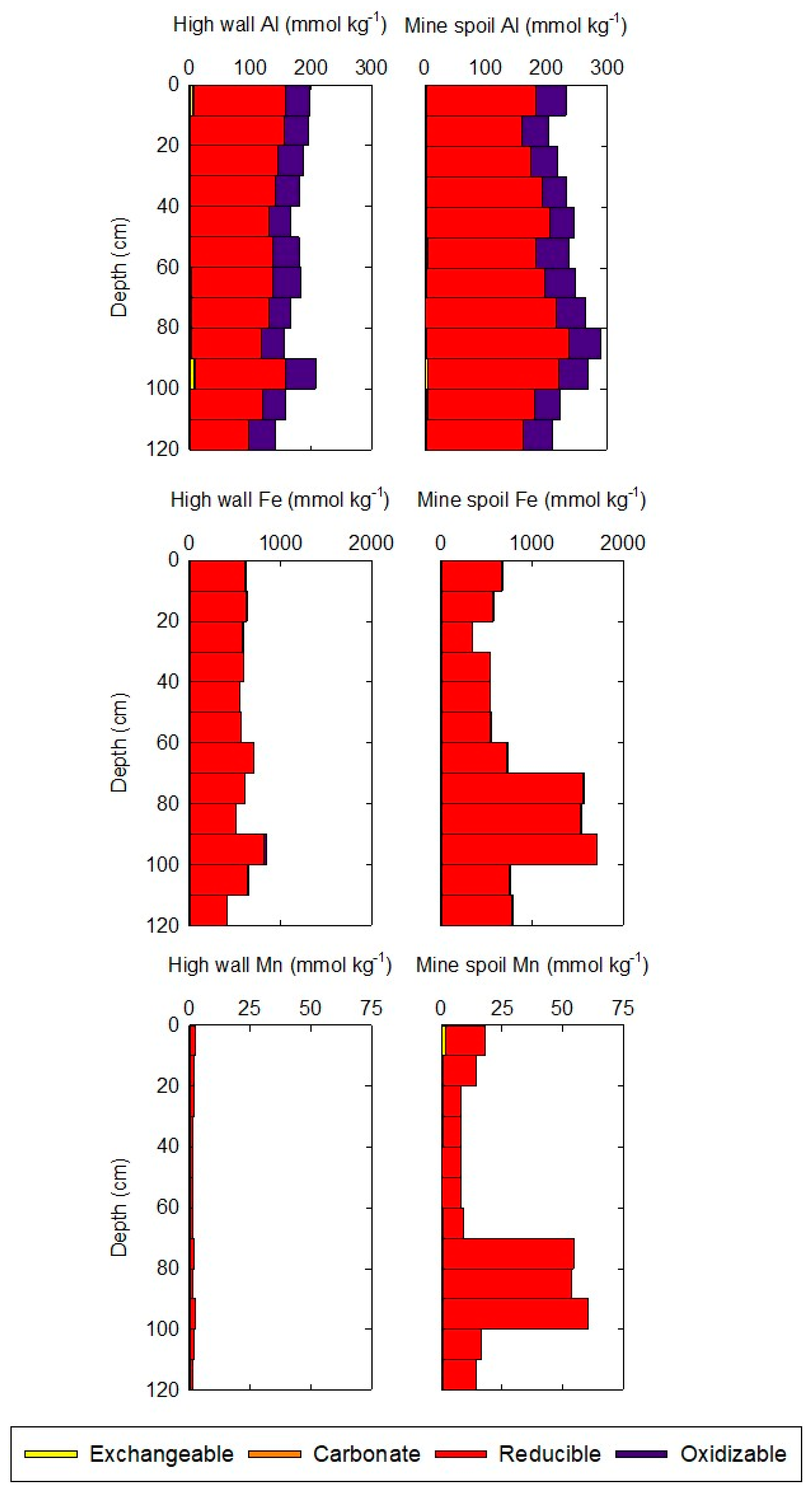
3.3. Extractable Al, Fe, and Mn
3.4. Microbial Community Analysis
4. Discussion
4.1. Indicators of Mineral Weathering Reaction Progress
4.2. Relationship between Soil Pore Water and Solid-Phase Composition
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ODNR. Economic Impact Analysis of the Ohio Abandoned Mine Land Program; Ohio Department of Natural Resources Division of Mineral Resources Management, Ohio University’sVoinovich School of Leadership and Public Affairs: Athens, GA, USA, 2014.
- Griffith, M.B.; Norton, S.B.; Alexander, L.C.; Pollard, A.I.; LeDuc, S.D. The effects of mountaintop mines and valley fills on the physicochemical quality of stream ecosystems in the central Appalachians: A review. Sci. Total Environ. 2012, 417, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sánchez España, J. Chapter 7—The Behavior of Iron and Aluminum in Acid Mine Drainage: Speciation, Mineralogy, and Environmental Significance. In Thermodynamics, Solubility and Environmental Issues; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 137–150. [Google Scholar]
- Nordstrom, D.K. Aqueous pyrite oxidation and the consequent formation of secondary iron minerals. Acid Sulfate Weather. 1982, 10, 37–56. [Google Scholar]
- Abraitis, P.; Pattrick, R.; Vaughan, D. Variations in the compositional, textural and electrical properties of natural pyrite: A review. Int. J. Miner. Process. 2004, 74, 41–59. [Google Scholar] [CrossRef]
- Cravotta, C.A.; Dugas, D.L.; Brady, K.B.; Kovalchuk, T. Effects of Selective Handling of Pyritic, Acid-Forming Materials on the Chemistry of Pore Gas and Ground Water at A Reclaimed Surface Coal Mine, Clarion County, PA. USA Bur. Mines Spec. Publ. SP A 1994, 6, 365–374. [Google Scholar] [CrossRef]
- Cravotta, C.A. Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 1: Constituent quantities and correlations. Appl. Geochem. 2008, 23, 166–202. [Google Scholar] [CrossRef]
- Singer, D.M.; Herndon, E.; Cole, K.; Koval, J.; Perdrial, N. Formation of secondary mineral coatings and the persistence of reduced metal-bearing phases in soils developing on historic coal mine spoil. Appl. Geochem. 2020, 121, 104711. [Google Scholar] [CrossRef]
- Herndon, E.; Yarger, B.; Frederick, H.; Singer, D.M. Iron and Manganese Biogeochemistry in Forested Coal Mine Spoil. Soil Syst. 2019, 3, 13. [Google Scholar] [CrossRef]
- Clark, E.V.; Daniels, W.L.; Zipper, C.E.; Eriksson, K. Mineralogical influences on water quality from weathering of surface coal mine spoils. Appl. Geochem. 2018, 91, 97–106. [Google Scholar] [CrossRef]
- Hartman, K.J.; Kaller, M.D.; Howell, J.W.; Sweka, J.A. How much do valley fills influence headwater streams? Hydrobiologia 2005, 532, 91–102. [Google Scholar] [CrossRef]
- Evans, D.M.; Zipper, C.E.; Donovan, P.F.; Daniels, W.L. Long-Term Trends of Specific Conductance in Waters Discharged by Coal-Mine Valley Fills in Central Appalachia, USA. JAWRA J. Am. Water Resour. Assoc. 2014, 50, 1449–1460. [Google Scholar] [CrossRef]
- Ross, M.R.; Nippgen, F.; Hassett, B.A.; McGlynn, B.L.; Bernhardt, E.S. Pyrite oxidation drives exceptionally high weathering rates and geologic CO2 release in mountaintop-mined landscapes. Glob. Biogeochem. Cycles 2018, 32, 1182–1194. [Google Scholar] [CrossRef]
- Förstner, U. Metal speciation and contamination of soil. Land contamination by metals: Global scope and magnitude of problem. Metal. Speciat. Contam. Soil 1995, 1, 1–33. [Google Scholar]
- Mccarty, D.K.; Moore, J.N.; Marcus, W.A. Mineralogy and trace element association in an acid mine drainage iron oxide precipitate; comparison of selective extractions. Appl. Geochem. 1998, 13, 165–176. [Google Scholar] [CrossRef]
- Blowes, D.; Ptacek, C.; Jambor, J.; Weisener, C. The geochemistry of acid mine drainage. Treatise Geochem. 2003, 9, 612. [Google Scholar]
- Schaider, L.A.; Senn, D.B.; Estes, E.R.; Brabander, D.J.; Shine, J.P. Sources and fates of heavy metals in a mining-impacted stream: Temporal variability and the role of iron oxides. Sci. Total Environ. 2014, 490, 456–466. [Google Scholar] [CrossRef]
- Lamborn, R.E. Geology of Tuscarawas County; Division of Geological Survey: Columbus, OH, USA, 1956; Volume 55.
- DeLong, R.M. Geology of the Malvern Quadrangle; Division of Geological Survey: Columbus, OH, USA, 1965.
- White, D.; Johnston, K.; Miller, M. Ohio river basin. Rivers N. Am. 2005, 1, 374–424. [Google Scholar] [CrossRef]
- Singer, D.M.; Jefferson, A.J.; Traub, E.L.; Perdrial, N. Mineralogical and geochemical variation in stream sediments impacted by acid mine drainage is related to hydro-geomorphic setting. Elementa 2018, 6, 16. [Google Scholar] [CrossRef]
- Singer, D.M.; Herndon, E.; Cole, K.; Burkey, M.; Morisson, S.; Cahill, M.; Bartucci, M.A. Micron-scale distribution controls metal(loid) release during simulated weathering of a Pennsylvanian coal shale. Geochim. Cosmochim. Acta 2020, 269, 117–135. [Google Scholar] [CrossRef]
- Wise, M. Huff Run Watershed Plan; Huff Run Watershed Restoration Partnership, Inc.: Mineral City, OH, USA, 2005. [Google Scholar]
- Haering, K.C.; Daniels, W.L.; Galbraith, J.M. Appalachian Mine Soil Morphology and Properties. Soil Sci. Soc. Am. J. 2004, 68, 1315–1325. [Google Scholar] [CrossRef]
- Daniels, W.L.; Haering, K.; Galbraith, J.; Thomas, J. Mine soil classification and mapping issues on pre- and post-smcra appalachian coal mined lands. Proc. Am. Soc. Min. Reclam. 2004, 2004, 450–479. [Google Scholar] [CrossRef]
- Daniels, W.L.; Zipper, C.E. Improving coal surface mine reclamation in the Central Appalachian region. Rehabil. Damaged Ecosyst. 1988, 1, 139–162. [Google Scholar]
- ODNR. Huff Run Watershed Acid Mine Drainage Abatement and Treatment Plan. Prepared for Ohio DNR by Gannett Fleming. Available online: Watersheddata.com (accessed on 29 October 2020).
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2011, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Perdrial, N.; Rivera, N.; Thompson, A.; O’Day, P.A.; Chorover, J. Trace contaminant concentration affects mineral transformation and pollutant fate in hydroxide-weathered Hanford sediments. J. Hazard. Mater. 2011, 197, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Gault, A.G.; Polya, D.A.; Lythgoe, P.R.; Farquhar, M.L.; Charnock, J.M.; Wogelius, R.A. Arsenic speciation in surface waters and sediments in a contaminated waterway: An IC-ICP-MS and XAS based study. Appl. Geochem. 2003, 18, 1387–1397. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Werner, J.J.; Koren, O.; Hugenholtz, P.; DeSantis, T.Z.; Walters, W.A.; Caporaso, J.G.; Angenent, L.T.; Knight, R.; Ley, R.E. Impact of training sets on classification of high-throughput bacterial 16s rRNA gene surveys. ISME J. 2012, 6, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Li, Y.; Wen, H.; Chen, L.; Yin, T. Succession of Bacterial Community Structure and Diversity in Soil along a Chronosequence of Reclamation and Re-Vegetation on Coal Mine Spoils in China. PLoS ONE 2014, 9, e115024. [Google Scholar] [CrossRef]
- Poncelet, D.M.; Cavender, N.; Cutright, T.J.; Senko, J.M. An assessment of microbial communities associated with surface mining-disturbed overburden. Environ. Monit. Assess. 2013, 186, 1917–1929. [Google Scholar] [CrossRef]
- Brooks, J.P.; Adeli, A.; Smith, R.K.; McGrew, R.; Lang, D.J.; Read, J.J. Bacterial Community Structure Recovery in Reclaimed Coal Mined Soil under Two Vegetative Regimes. J. Environ. Qual. 2019, 48, 1029–1037. [Google Scholar] [CrossRef]
- Youssef, N.H.; Elshahed, M.S. Diversity rankings among bacterial lineages in soil. ISME J. 2009, 3, 305–313. [Google Scholar] [CrossRef]
- Youssef, N.; Sheik, C.S.; Krumholz, L.R.; Najar, F.Z.; Roe, B.A.; Elshahed, M.S. Comparison of Species Richness Estimates Obtained Using Nearly Complete Fragments and Simulated Pyrosequencing-Generated Fragments in 16S rRNA Gene-Based Environmental Surveys. Appl. Environ. Microbiol. 2009, 75, 5227–5236. [Google Scholar] [CrossRef]
- Sheik, C.S.; Beasley, W.H.; Elshahed, M.S.; Zhou, X.; Luo, Y.; Krumholz, L.R. Effect of warming and drought on grassland microbial communities. ISME J. 2011, 5, 1692–1700. [Google Scholar] [CrossRef]
- Sharma, S.; Lee, M.; Reinmann, C.S.; Pumneo, J.; Cutright, T.J.; Senko, J.M. Impact of acid mine drainage chemistry and microbiology on the development of efficient Fe removal activities. Chemosphere 2020, 249, 126117. [Google Scholar] [CrossRef] [PubMed]
- Kuever, J.; Rainey, F.; Widdel, F. The family syntrophobacteraceae. Prokaryot. Deltaproteobact. Epsil. 2014, 1, 289–299. [Google Scholar]
- Garcia, R.; Müller, R. The Family Myxococcaceae. Prokaryotes 2014, 10, 191–212. [Google Scholar] [CrossRef]
- Roling, W. 12 The family Geobacteraceae. Prokaryot. Deltaproteobact. Epsil. 2014, 157–172. Available online: https://www.narcis.nl/publication/RecordID/oai:research.vu.nl:publications%2F617baa81-4beb-4ae2-b352-6b0db777a8d5 (accessed on 29 October 2020).
- King, G.M.; Garey, M.A. Ferric Iron Reduction by Bacteria Associated with the Roots of Freshwater and Marine Macrophytes. Appl. Environ. Microbiol. 1999, 65, 4393–4398. [Google Scholar] [CrossRef]
- Küsel, K.; Wagner, C.; Trinkwalter, T.; Gößner, A.S.; Bäumler, R.; Drake, H.L. Microbial reduction of Fe (III) and turnover of acetate in Hawaiian soils. FEMS Microbiol. Ecol. 2002, 40, 73–81. [Google Scholar]
- Lopes, A.R.; Bello, D.; Prieto-Fernández, Á.; Trasar-Cepeda, C.; Manaia, C.M.; Nunes, O.C. Relationships among bulk soil physicochemical, biochemical, and microbiological parameters in an organic alfalfa-rice rotation system. Environ. Sci. Pollut. Res. 2015, 22, 11690–11699. [Google Scholar] [CrossRef]
- Lopes, A.R.; Manaia, C.M.; Nunes, O.C. Bacterial community variations in an alfalfa-rice rotation system revealed by 16S rRNA gene 454-pyrosequencing. FEMS Microbiol. Ecol. 2014, 87, 650–663. [Google Scholar] [CrossRef]
- Walsh, F.; Smith, D.P.; Owens, S.M.; Duffy, B.; Frey, J.E. Restricted streptomycin use in apple orchards did not adversely alter the soil bacteria communities. Front. Microbiol. 2014, 4, 383. [Google Scholar] [CrossRef]
- Cavaletti, L.; Monciardini, P.; Bamonte, R.; Schumann, P.; Rohde, M.; Sosio, M.; Donadio, S. New Lineage of Filamentous, Spore-Forming, Gram-Positive Bacteria from Soil. Appl. Environ. Microbiol. 2006, 72, 4360–4369. [Google Scholar] [CrossRef]
- Yabe, S.; Sakai, Y.; Abe, K.; Yokota, A.; Také, A.; Matsumoto, A.; Sugiharto, A.; Susilowati, D.; Hamada, M.; Nara, K. Dictyobacter aurantiacus gen. nov., sp. nov., a member of the family Ktedonobacteraceae, isolated from soil, and emended description of the genus Thermosporothrix. Int. J. Syst. Evol. Microbiol. 2017, 67, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.; Aiba, Y.; Sakai, Y.; Hazaka, M.; Yokota, A. Thermogemmatispora onikobensis gen. nov., sp. nov. and Thermogemmatispora foliorum sp. nov., isolated from fallen leaves on geothermal soils, and description of Thermogemmatisporaceae fam. nov. and Thermogemmatisporales ord. nov. within the class Ktedonobacteria. Int. J. Syst. Evol. Microbiol. 2011, 61, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Sekiguchi, Y. The Phylum Gemmatimonadetes. Prokaryotes 2014, 11, 677–681. [Google Scholar] [CrossRef]
- Leitholf, A.M.; Fretz, C.E.; Mahanke, R.; Santangelo, Z.; Senko, J.M. An integrated microbiological and electrochemical approach to determine distributions of Fe metabolism in acid mine drainage-induced “iron mound” sediments. PLoS ONE 2019, 14, e0213807. [Google Scholar] [CrossRef]
- Wiggering, H. Weathering of clay minerals in waste dumps of Upper Carboniferous coal-bearing strata, the Ruhr area, West Germany. Appl. Clay Sci. 1987, 2, 353–361. [Google Scholar] [CrossRef]
- Vigil de la Villa, R.; Frías, M.; García-Giménez, R.; Martínez-Ramirez, S.; Fernández-Carrasco, L. Chemical and mineral transformations that occur in mine waste and washery rejects during pre-utilization calcination. Int. J. Coal Geol. 2014, 132, 123–130. [Google Scholar] [CrossRef]
- Miller, J.O.; Barton, C.; Agouridis, C.T.; Fogel, A.; Dowdy, T.; Angel, P. Evaluating Soil Genesis and Reforestation Success on a Surface Coal Mine in Appalachia. Soil Sci. Soc. Am. J. 2012, 76, 950–960. [Google Scholar] [CrossRef]
- Jha, A.; Singh, J. Spoil characteristics and vegetation development of an age series of mine spoils in a dry tropical environment. Vegetatio 1991, 97, 63–76. [Google Scholar]
- Dang, Z.; Liu, C.; Haigh, M.J. Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environ. Pollut. 2002, 118, 419–426. [Google Scholar] [CrossRef]
- Tuttle, M.L.; Breit, G.N. Weathering of the New Albany Shale, Kentucky, USA: I. Weathering zones defined by mineralogy and major-element composition. Appl. Geochem. 2009, 24, 1549–1564. [Google Scholar] [CrossRef]
- Jin, L.; Ravella, R.; Ketchum, B.; Bierman, P.R.; Heaney, P.; White, T.; Brantley, S.L. Mineral weathering and elemental transport during hillslope evolution at the Susquehanna/Shale Hills Critical Zone Observatory. Geochim. Cosmochim. Acta 2010, 74, 3669–3691. [Google Scholar] [CrossRef]
- Jin, L.; Mathur, R.; Rother, G.; Cole, D.R.; Bazilevskaya, E.; Williams, J.; Carone, A.; Brantley, S.L. Evolution of porosity and geochemistry in Marcellus Formation black shale during weathering. Chem. Geol. 2013, 356, 50–63. [Google Scholar] [CrossRef]
- Kang, C.-U.; Jeon, B.-H.; Park, S.-S.; Kang, J.-S.; Kim, K.-H.; Kim, D.-K.; Choi, U.-K.; Kim, S. Inhibition of pyrite oxidation by surface coating: A long-term field study. Environ. Geochem. Health 2016, 38, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Newman, D.K. Microbial iron respiration: Impacts on corrosion processes. Appl. Microbiol. Biotechnol. 2003, 62, 134–139. [Google Scholar] [CrossRef]
- Essilfie-Dughan, J.; Hendry, M.J.; Dynes, J.J.; Hu, Y.; Biswas, A.; Barbour, S.L.; Day, S. Geochemical and mineralogical characterization of sulfur and iron in coal waste rock, Elk Valley, British Columbia, Canada. Sci. Total Environ. 2017, 586, 753–769. [Google Scholar] [CrossRef]
- Keiluweit, M.; Wanzek, T.; Kleber, M.; Nico, P.; Fendorf, S. Anaerobic microsites have an unaccounted role in soil carbon stabilization. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Roden, E.E.; Kappler, A.; Bauer, I.; Jiang, J.; Paul, A.; Stoesser, R.; Konishi, H.; Xu, H. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat. Geosci. 2010, 3, 417–421. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Pandey, B.; Agrawal, S.; Singh, S. Ecological risk assessment of soil contamination by trace elements around coal mining area. J. Soils Sediments 2016, 16, 159–168. [Google Scholar] [CrossRef]
- Schatzel, S.J.; Stewart, B.W. Rare earth element sources and modification in the Lower Kittanning coal bed, Pennsylvania: Implications for the origin of coal mineral matter and rare earth element exposure in underground mines. Int. J. Coal Geol. 2003, 54, 223–251. [Google Scholar] [CrossRef]
- Scheel, T.; Dörfler, C.; Kalbitz, K. Precipitation of Dissolved Organic Matter by Aluminum Stabilizes Carbon in Acidic Forest Soils. Soil Sci. Soc. Am. J. 2007, 71, 64–74. [Google Scholar] [CrossRef]
- Berggren, D.; Mulder, J. The role of organic matter in controlling aluminum solubility in acidic mineral soil horizons. Geochim. Cosmochim. Acta 1995, 59, 4167–4180. [Google Scholar] [CrossRef]
- McDowell, W.H. Dissolved organic matter in soils—future directions and unanswered questions. Geoderma 2003, 113, 179–186. [Google Scholar] [CrossRef]
- Mastný, J.; Kaštovská, E.; Bárta, J.; Chroňáková, A.; Borovec, J.; Šantrůčková, H.; Urbanová, Z.; Edwards, K.R.; Picek, T. Quality of DOC produced during litter decomposition of peatland plant dominants. Soil Biol. Biochem. 2018, 121, 221–230. [Google Scholar] [CrossRef]
- Fisher-Power, L.M.; Cheng, T.; Rastghalam, Z.S. Cu and Zn adsorption to a heterogeneous natural sediment: Influence of leached cations and natural organic matter. Chemosphere 2016, 144, 1973–1979. [Google Scholar] [CrossRef]
- Toribio, M.; Romanyà, J. Leaching of heavy metals (Cu, Ni and Zn) and organic matter after sewage sludge application to Mediterranean forest soils. Sci. Total Environ. 2006, 363, 11–21. [Google Scholar] [CrossRef]
- Castillo-Meza, L.; Cravotta, C.; Tasker, T.; Warner, N.; Daniels, W.; Orndorff, Z.; Bergstresser, T.; Douglass, A.; Kimble, G.; Streczywilk, J.; et al. Batch extraction method to estimate total dissolved solids (TDS) release from coal refuse and overburden. Appl. Geochem. 2020, 115, 104540. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, G.; Williard, K.; Schoonover, J.; Kang, J. Monitoring of Water and Solute Transport in the Vadose Zone: A Review. Vadose Zone J. 2017, 17, 160058. [Google Scholar] [CrossRef]
- Swistock, B.R.; Yamona, J.J.; Dewalle, D.R.; Sharpe, W.E. Comparison of soil water chemistry and sample size requirements for pan vs tension lysimeters. Water Air Soil Pollut. 1990, 50, 387–396. [Google Scholar] [CrossRef]
- Agouridis, C.T.; Angel, P.N.; Taylor, T.J.; Barton, C.; Warner, R.C.; Yu, X.; Wood, C. Water Quality Characteristics of Discharge from Reforested Loose-Dumped Mine Spoil in Eastern Kentucky. J. Environ. Qual. 2012, 41, 454–468. [Google Scholar] [CrossRef]
- Mayanna, S.; Peacock, C.L.; Schäffner, F.; Grawunder, A.; Merten, D.; Kothe, E.; Büchel, G. Biogenic precipitation of manganese oxides and enrichment of heavy metals at acidic soil pH. Chem. Geol. 2015, 402, 6–17. [Google Scholar] [CrossRef]
- Herndon, E.M.; Jin, L.; Andrews, D.M.; Eissenstat, D.M.; Brantley, S.L. Importance of vegetation for manganese cycling in temperate forested watersheds. Glob. Biogeochem. Cycles 2015, 29, 160–174. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Harmon, M.E.; Mao, J.; Pett-Ridge, J.; Kleber, M. Long-term litter decomposition controlled by manganese redox cycling. Proc. Natl. Acad. Sci. USA 2015, 112, E5253–E5260. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.; Mann, R. Origin of high manganese concentrations in coal mine drainage, eastern Tennessee. J. Geochem. Explor. 2005, 86, 143–163. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, D.; Herndon, E.; Zemanek, L.; Cole, K.; Sanda, T.; Senko, J.; Perdrial, N. Biogeochemical Controls on the Potential for Long-Term Contaminant Leaching from Soils Developing on Historic Coal Mine Spoil. Soil Syst. 2021, 5, 3. https://doi.org/10.3390/soilsystems5010003
Singer D, Herndon E, Zemanek L, Cole K, Sanda T, Senko J, Perdrial N. Biogeochemical Controls on the Potential for Long-Term Contaminant Leaching from Soils Developing on Historic Coal Mine Spoil. Soil Systems. 2021; 5(1):3. https://doi.org/10.3390/soilsystems5010003
Chicago/Turabian StyleSinger, David, Elizabeth Herndon, Laura Zemanek, Kortney Cole, Tyler Sanda, John Senko, and Nicolas Perdrial. 2021. "Biogeochemical Controls on the Potential for Long-Term Contaminant Leaching from Soils Developing on Historic Coal Mine Spoil" Soil Systems 5, no. 1: 3. https://doi.org/10.3390/soilsystems5010003
APA StyleSinger, D., Herndon, E., Zemanek, L., Cole, K., Sanda, T., Senko, J., & Perdrial, N. (2021). Biogeochemical Controls on the Potential for Long-Term Contaminant Leaching from Soils Developing on Historic Coal Mine Spoil. Soil Systems, 5(1), 3. https://doi.org/10.3390/soilsystems5010003



