Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health
Abstract
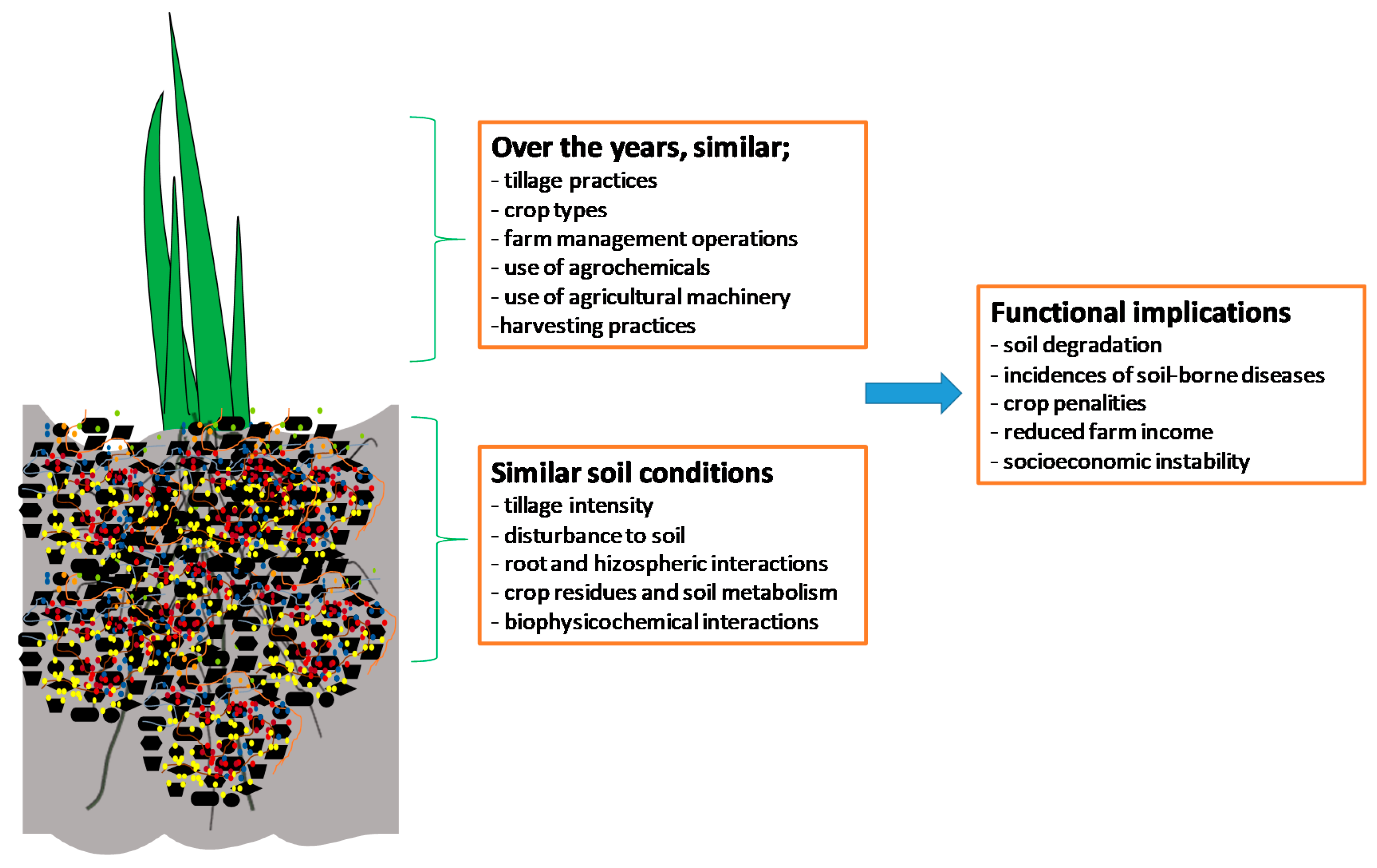
:1. Introduction
2. Indicators of Soil Health
3. Biotic Indicators of Soil Health
3.1. Microbiomic Indicators
3.1.1. Microbial Biomass or Abundance
3.1.2. Microbial Community Composition and Diversity
3.2. Soil Enzyme Activities
3.3. Abundance, Composition, and Diversity of Soil Macro-Organisms
3.3.1. Soil Nematodes
3.3.2. Soil Earthworms
3.3.3. Soil Mites and Other Organisms
3.4. Increased Incidences of Soil-Borne Diseases and Evolution of Disease Suppressive Soils
3.4.1. Soil-Borne Fungal Diseases
3.4.2. Soil-Borne Bacterial Diseases
3.4.3. Soil-Borne Nematode Infections
3.4.4. Evolution of Disease Resistance
4. Abiotic Indicators of Soil Health
4.1. Soil Aggregation and Structure
4.2. Soil Organic Matter and Organic C Contents
4.3. Mineralization and Cycling of C and N
4.4. Soil Physicochemical Properties and Nutrient Deficiencies
4.5. Accumulation of Plant- and Microbe-Derived Exudates in Soil
5. Conclusions: Alteration in Soil Physicochemical and Food Web Interactions
Author Contributions
Funding
Conflicts of Interest
References
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montanarella, L.; Pennock, D.J.; McKenzie, N.; Badraou, M.; Chude, V.; Baptista, I.; Mamo, T.; Yemefack, M.; Aulakh, M.S.; Yagi, K.; et al. World’s soils are under threat. Soil 2016. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.E.; Lemerle, D. Continuous cropping systems and weed selection. Euphytica 2006, 148, 61–73. [Google Scholar] [CrossRef]
- Plourde, J.D.; Pijanowski, B.C.; Pekin, B.K. Evidence for increased monoculture cropping in the Central United States. Agric. Ecosyst. Environ. 2013, 165, 50–59. [Google Scholar] [CrossRef]
- Shipton, P.J. Monoculture and soilborne plant pathogens. Monocult. Soilborne Plant Pathog. 1977, 15, 387–407. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Xu, Y.; Wei, W.; Yao, Q.; Pan, F. Diversity of parasitic fungi from soybean cyst nematode associated with long-term continuous cropping of soybean in black soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 432–442. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Bai, C.; Wu, D.; Song, Q.; Gao, D.; Dong, Z.; Cheng, X.; Dong, Q.; Zhang, Y.; et al. Effects of different mechanized soil fertilization methods on corn soil fertility under continuous cropping. IOP Conf. Ser. Earth Environ. Sci. 2017, 64, 012109. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, Z.-Z.; Zhao, Q.; Huang, X.-Y.; Huang, K.-F. Effect of continuous cropping on the rhizosphere soil and growth of common buckwheat. Plant Prod. Sci. 2020, 23, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Wang, Y.; Liu, Q.; Zhang, Y.; Li, X.; Li, H.; Li, W. Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Appl. Soil Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Liu, Q.; Li, W.; Li, H.; Li, X.; Zhang, Y. Transcriptomic analysis reveals recovery strategies in strawberry roots after using a soil amendment in continuous cropping soil. BMC Plant Biol. 2020, 20, 5. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Qi, G.; Luo, T.; Zhang, H.; Jiang, Q.; Wang, R.; Zhao, X. Continuous-cropping tobacco caused variance of chemical properties and structure of bacterial network in soils. Land Degrad. Dev. 2018, 29, 4106–4120. [Google Scholar] [CrossRef]
- Treder, K.; Jastrzębska, M.; Kostrzewska, M.K.; Makowski, P. Do Long-Term Continuous Cropping and Pesticides Affect Earthworm Communities? Agronomy 2020, 10, 586. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Liu, T.; Wang, H.; Yang, Y.; Chen, X.; Zhu, S. Analysis of bacterial and fungal communities in continuous-cropping ramie (Boehmeria nivea L. Gaud) fields in different areas in China. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Li, T.; Zhao, D.; Liao, Y. Tillage practices with different soil disturbance shape the rhizosphere bacterial community throughout crop growth. Soil Tillage Res. 2020, 197, 104501. [Google Scholar] [CrossRef]
- Yang, J.; Ruegger, P.M.; McKenry, M.V.; Becker, J.O.; Borneman, J. Correlations between Root-Associated Microorganisms and Peach Replant Disease Symptoms in a California Soil. PLoS ONE 2012, 7, e46420. [Google Scholar] [CrossRef]
- Seifert, C.A.; Roberts, M.J.; Lobell, D.B. Continuous Corn and Soybean Yield Penalties across Hundreds of Thousands of Fields. Agron. J. 2017, 109, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.-L.; Jiao, X.-Y.; Fan, F.-F.; Wang, J.-S.; Guo, J.; Dong, E.-W.; Wang, L.-G.; Shen, X.-M. Effect of continuous sorghum cropping on the rhizosphere microbial community and the role of Bacillus amyloliquefaciens in altering the microbial composition. Plant Growth Regul 2019, 89, 299–308. [Google Scholar] [CrossRef]
- Li, X.; Lewis, E.E.; Liu, Q.; Li, H.; Bai, C.; Wang, Y. Effects of long-term continuous cropping on soil nematode community and soil condition associated with replant problem in strawberry habitat. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, K.; Schindelbeck, R.; Kurtz, K.S.M.; van Es, H. Soil health assessment. In Soil Analysis: Recent Trends and Applications; Rakshit, A., Ghosh, S., Chakraborty, S., Philip, V., Datta, A., Eds.; Springer: Singapore, 2020; pp. 199–219. ISBN 978-981-15-2039-6. [Google Scholar]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Tiemann, L.K.; Grandy, A.S. Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 2014, 24, 560–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDaniel, M.D.; Grandy, A.S. Soil microbial biomass and function are altered by 12 years of crop rotation. Soil 2016, 2, 583–599. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.R.; Vas Es, H.M.; Schindelbeck, R.; Ristow, A.J.; Ryan, M. No-till and cropping system diversification improve soil health and crop yield. Geoderma 2018, 328, 30–43. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More Than the Sum of Its Parts: Microbiome Biodiversity as a Driver of Plant Growth and Soil Health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Saleem, M. Microbiome Community Ecology: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-11665-5. [Google Scholar]
- Weisberger, D.; Nichols, V.; Liebman, M. Does diversifying crop rotations suppress weeds? A meta-analysis. PLoS ONE 2019, 14, e0219847. [Google Scholar] [CrossRef] [Green Version]
- Abouziena, H.F.; Haggag, W.M.; Abouziena, H.F.; Haggag, W.M. Weed Control in Clean Agriculture: A Review 1. Planta Daninha 2016, 34, 377–392. [Google Scholar] [CrossRef]
- Titi, A.E. Soil Tillage in Agroecosystems; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-1-4200-4060-9. [Google Scholar]
- Hamblin, A.P. The effect of tillage on soil surface properties and the water balance of a xeralfic alfisol. Soil Tillage Res. 1984, 4, 543–559. [Google Scholar] [CrossRef]
- Ward, N.K.; Maureira, F.; Stöckle, C.O.; Brooks, E.S.; Painter, K.M.; Yourek, M.A.; Gasch, C.K. Simulating field-scale variability and precision management with a 3D hydrologic cropping systems model. Precis. Agric. 2018, 19, 293–313. [Google Scholar] [CrossRef] [Green Version]
- Kahimba, F.C.; Sri Ranjan, R.; Froese, J.; Entz, M.; Nason, R. Cover Crop Effects on Infiltration, Soil Temperature, and Soil Moisture Distribution in the Canadian Prairies. Appl. Eng. Agric. 2008, 24, 321–333. [Google Scholar] [CrossRef]
- Dexter, A.R. Soil physical quality: Part, I. Theory, effects of soil texture, density, and organic matter, and effects on root growth. Geoderma 2004, 120, 201–214. [Google Scholar] [CrossRef]
- Khademalrasoul, A.; Naveed, M.; Heckrath, G.; Kumari, K.G.I.D.; de Jonge, L.W.; Elsgaard, L.; Vogel, H.-J.; Iversen, B.V. Biochar Effects on Soil Aggregate Properties Under No-Till Maize. Soil Sci. 2014, 179, 273–283. [Google Scholar] [CrossRef]
- Lal, R. Soil quality changes under continuous cropping for seventeen seasons of an alfisol in western Nigeria. Land Degrad. Dev. 1998, 9, 259–274. [Google Scholar] [CrossRef]
- Hati, K.M.; Swarup, A.; Singh, D.; Misra, A.K.; Ghosh, P.K. Long-term continuous cropping, fertilisation, and manuring effects on physical properties and organic carbon content of a sandy loam soil. Soil Res. 2006, 44, 487–495. [Google Scholar] [CrossRef]
- Rieu, M.; Sposito, G. Fractal Fragmentation, Soil Porosity, and Soil Water Properties: I. Theory. Soil Sci. Soc. Am. J. 1991, 55, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Głąb, T.; Kulig, B. Effect of mulch and tillage system on soil porosity under wheat (Triticum aestivum). Soil Tillage Res. 2008, 99, 169–178. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Kundu, S.; Shanker, A.K.; Naik, R.P.; Vanaja, M.; Venkanna, K.; Maruthi Sankar, G.R.; Rao, V.U.M. Continuous cropping under elevated CO2: Differential effects on C4 and C3 crops, soil properties and carbon dynamics in semi-arid alfisols. Agric. Ecosyst. Environ. 2016, 218, 73–86. [Google Scholar] [CrossRef]
- Hansen, V.; Hauggaard-Nielsen, H.; Petersen, C.T.; Mikkelsen, T.N.; Müller-Stöver, D. Effects of gasification biochar on plant-available water capacity and plant growth in two contrasting soil types. Soil Tillage Res. 2016, 161, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chalise, K.S.; Singh, S.; Wegner, B.R.; Kumar, S.; Pérez-Gutiérrez, J.D.; Osborne, S.L.; Nleya, T.; Guzman, J.; Rohila, J.S. Cover Crops and Returning Residue Impact on Soil Organic Carbon, Bulk Density, Penetration Resistance, Water Retention, Infiltration, and Soybean Yield. Agron. J. 2019, 111, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Nouri, A.; Lee, J.; Yin, X.; Saxton, A.M.; Tyler, D.D.; Sykes, V.R.; Arelli, P. Crop species in no-tilled summer cropping rotation affect soil quality and yield in Alfisols, southeastern USA. Geoderma 2019, 345, 51–62. [Google Scholar] [CrossRef]
- Feng, G.; Sharratt, B.; Young, F. Influence of long-term tillage and crop rotations on soil hydraulic properties in the US Pacific Northwest. J. Soil Water Conserv. 2011, 66, 233–241. [Google Scholar] [CrossRef]
- Anwar, U.; Schulte, L.A.; Helmers, M.; Kolka, R.K. The Effect of Five Biomass Cropping Systems on Soil-Saturated Hydraulic Conductivity Across a Topographic Gradient. Bioenerg. Res. 2017, 10, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.; Gregorich, E.G.; McLaughlin, N.B.; Zhang, X.; Guo, Y.; Liang, A.; Fan, R.; Sun, B. No-tillage with continuous maize cropping enhances soil aggregation and organic carbon storage in Northeast China. Geoderma 2018, 330, 204–211. [Google Scholar] [CrossRef]
- Stirzaker, R.J.; Passioura, J.B.; Wilms, Y. Soil structure and plant growth: Impact of bulk density and biopores. Plant Soil 1996, 185, 151–162. [Google Scholar] [CrossRef]
- Saleem, M.; Pervaiz, Z.H.; Contreras, J.; Lindenberger, J.H.; Hupp, B.M.; Chen, D.; Zhang, Q.; Wang, C.; Iqbal, J.; Twigg, P. Cover crop diversity improves multiple soil properties via altering root architectural traits. Rhizosphere 2020, 100248. [Google Scholar] [CrossRef]
- Ryu, J.; Na, J.-S.; Hwang, N.-Y. Study on the Cause of Injury by Continuous Cropping of Peanut. Korean J. Soil Sci. Fertil. 1992, 25, 270–274. [Google Scholar]
- Tsunekawa, A.; Kar, A.; Yanai, J.; Tanaka, U.; Miyazaki, T. Influence of continuous cultivation on the soil properties affecting crop productivity in the Thar Desert, India. J. Arid Environ. 1997, 36, 367–384. [Google Scholar] [CrossRef]
- Van Eerd, L.L.; Congreves, K.A.; Hayes, A.; Verhallen, A.; Hooker, D.C. Long-term tillage and crop rotation effects on soil quality, organic carbon, and total nitrogen. Can. J. Soil Sci. 2014, 94, 303–315. [Google Scholar] [CrossRef]
- Idowu, O.J.; Van Es, H.M.; Abawi, G.S.; Wolfe, D.W.; Schindelbeck, R.R.; Moebius-Clune, B.N.; Gugino, B.K. Use of an integrative soil health test for evaluation of soil management impacts. Renew. Agric. Food Syst. 2009, 24, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.F.; Anderson, R.L. Aggregate stability and glomalin in alternative crop rotations for the central Great Plains. Biol. Fertil. Soils 2000, 31, 249–253. [Google Scholar] [CrossRef]
- Sithole, N.J.; Magwaza, L.S.; Thibaud, G.R. Long-term impact of no-till conservation agriculture and N-fertilizer on soil aggregate stability, infiltration and distribution of C in different size fractions. Soil Tillage Res. 2019, 190, 147–156. [Google Scholar] [CrossRef]
- García, G.V.; Wyngaard, N.; Calvo, N.I.R.; San Martino, S.; Covacevich, F.; Studdert, G.A. Soil survey reveals a positive relationship between aggregate stability and anaerobically mineralizable nitrogen. Ecol. Indic. 2020, 117, 106640. [Google Scholar] [CrossRef]
- Heyman, H.; Bassuk, N.; Bonhotal, J.; Walter, T. Compost Quality Recommendations for Remediating Urban Soils. Int. J. Environ. Res. Public Health 2019, 16, 3191. [Google Scholar] [CrossRef] [Green Version]
- Declerck, S.; Risede, J.M.; Rufyikiri, G.; Delvaux, B. Effects of arbuscular mycorrhizal fungi on severity of root rot of bananas caused by Cylindrocladium spathiphylli. Plant Pathol. 2002, 51, 109–115. [Google Scholar] [CrossRef]
- Roarty, S.; Hackett, R.A.; Schmidt, O. Earthworm populations in twelve cover crop and weed management combinations. Appl. Soil Ecol. 2017, 114, 142–151. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Allen, F.L.; Tyler, D.D.; Pote, D.H.; Shipitalo, M.J. Earthworm populations are affected from long-term crop sequences and bio-covers under no-tillage. Pedobiologia 2017, 60, 27–33. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Mao, Z.; Xie, B. Variation and Dynamics of Soil Nematode Communities in Greenhouses with Different Continuous Cropping Periods. Hortic. Plant J. 2020. [Google Scholar] [CrossRef]
- Jordan, D.L.; Corbett, T.; Bogle, C.; Shew, B.; Brandenburg, R.; Ye, W. Effect of Previous Rotation on Plant Parasitic Nematode Population in Peanut and Crop Yield. Crop Forage Turfgrass Manag. 2017, 3. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Vetsch, J.A.; Chen, S. Effects of Fertilizer, Nematicide, and Tillage on Plant–Parasitic Nematodes and Yield in Corn and Soybean. Agron. J. 2017, 109, 1651–1662. [Google Scholar] [CrossRef]
- Mahal, N.K.; Castellano, M.J.; Miguez, F.E. Potentially mineralizable nitrogen: A soil health indicator. Crop. Soils 2019, 52, 8–10. [Google Scholar] [CrossRef]
- Mahal, N.K.; Castellano, M.J.; Miguez, F.E. Conservation Agriculture Practices Increase Potentially Mineralizable Nitrogen: A Meta-Analysis. Soil Sci. Soc. Am. J. 2018, 82, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Fujii, K.; Hayakawa, C.; Inagaki, Y.; Kosaki, T. Effects of land use change on turnover and storage of soil organic matter in a tropical forest. Plant Soil 2020, 446, 425–439. [Google Scholar] [CrossRef]
- Stakhurlova, L.D.; Stulin, A.F. Biodynamics of black soils leached under different agrotechnical practices in long-term field experiments. Russ. Agric. Sci. 2017, 43, 35–39. [Google Scholar] [CrossRef]
- Smith, J.D.; Strauss, J.A.; Hardie, A.G. Effects of long-term grazed crop and pasture systems under no-till on organic matter fractions and selected quality parameters of soil in the Overberg, South Africa. S. Afr. J. Plant Soil 2020, 37, 1–10. [Google Scholar] [CrossRef]
- Hua, K.; Wang, D.; Guo, Z. Effects of long-term application of various organic amendments on soil particulate organic matter storage and chemical stabilisation of vertisol soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 505–514. [Google Scholar] [CrossRef]
- Hazra, K.K.; Nath, C.P.; Singh, U.; Praharaj, C.S.; Kumar, N.; Singh, S.S.; Singh, N.P. Diversification of maize-wheat cropping system with legumes and integrated nutrient management increases soil aggregation and carbon sequestration. Geoderma 2019, 353, 308–319. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, T.; Das, S.; Meena, S.K. Weed seed bank: Impacts and management for future crop production. In Agronomic Crops; Volume 2: Management Practices; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 207–223. ISBN 978-981-329-783-8. [Google Scholar]
- Pan, J.; Zhang, L.; Wang, L.; Fu, S. Effects of long-term fertilization treatments on the weed seed bank in a wheat-soybean rotation system. Glob. Ecol. Conserv. 2020, 21, e00870. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Liang, B.C.; Drury, C.F.; Mackenzie, A.F.; McGill, W.B. Elucidation of the source and turnover of water soluble and microbial biomass carbon in agricultural soils. Soil Biol. Biochem. 2000, 32, 581–587. [Google Scholar] [CrossRef]
- Li, L.; Wilson, C.B.; He, H.; Zhang, X.; Zhou, F.; Schaeffer, S.M. Physical, biochemical, and microbial controls on amino sugar accumulation in soils under long-term cover cropping and no-tillage farming. Soil Biol. Biochem. 2019, 135, 369–378. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Saleem, M.; Wang, B.; Hu, S.; Bai, Y.; Pan, Q.; Chen, D. Ecological clusters based on responses of soil microbial phylotypes to precipitation explain ecosystem functions. Soil Biol. Biochem. 2020, 142, 107717. [Google Scholar] [CrossRef]
- Guo, Z.C.; Zhang, Z.B.; Zhou, H.; Rahman, M.T.; Wang, D.Z.; Guo, X.S.; Li, L.J.; Peng, X.H. Long-term animal manure application promoted biological binding agents but not soil aggregation in a Vertisol. Soil Tillage Res. 2018, 180, 232–237. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.; Gregory, A.S.; White, R.P.; Watts, C.W. Soil organic carbon, extracellular polymeric substances (EPS), and soil structural stability as affected by previous and current land-use. Geoderma 2020, 363, 114143. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Yu, Z.; Yao, Q.; Zhang, W.; Mi, G.; Liang, A.; Li, L.; Chen, X.; Jin, J.; et al. Continuous cropping of soybean induced a more fluctuating fungal network and intensive pathogenic fungal interactions in a Mollisol of Northeast China. Soil Sci. Soc. Am. J. 2020, 84, 775–783. [Google Scholar] [CrossRef]
- Sun, T.; Miao, J.; Saleem, M.; Zhang, H.; Yang, Y.; Zhang, Q. Bacterial compatibility and immobilization with biochar improved tebuconazole degradation, soil microbiome composition and functioning. J. Hazard Mater. 2020, 398, 122941. [Google Scholar] [CrossRef]
- Sun, J.; Zou, L.; Li, W.; Wang, Y.; Xia, Q.; Peng, M.; Sun, J.; Zou, L.; Li, W.; Wang, Y.; et al. Soil microbial and chemical properties influenced by continuous cropping of banana. Sci. Agric. 2018, 75, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Rankoth, L.M.; Udawatta, R.P.; Gantzer, C.J.; Jose, S.; Veum, K.; Dewanto, H.A. Cover Crops on Temporal and Spatial Variations in Soil Microbial Communities by Phospholipid Fatty Acid Profiling. Agron. J. 2019, 111, 1693–1703. [Google Scholar] [CrossRef]
- Finney, D.M.; Buyer, J.S.; Kaye, J.P. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Sun, T.; Li, M.; Saleem, M.; Zhang, Q.; Wang, C. Soil-applied biochar increases microbial diversity and wheat plant performance under herbicide fomesafen stress. Ecotoxicol. Environ. Saf. 2019, 171, 75–83. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleem, M.; Wang, C. Probiotic strain Stenotrophomonas acidaminiphila BJ1 degrades and reduces chlorothalonil toxicity to soil enzymes, microbial communities and plant roots. AMB Express 2017, 7, 227. [Google Scholar] [CrossRef]
- Chen, W.; Teng, Y.; Li, Z.; Liu, W.; Ren, W.; Luo, Y.; Christie, P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil Ecol. 2018, 128, 23–34. [Google Scholar] [CrossRef]
- Kou, X.; Su, T.; Ma, N.; Li, Q.; Wang, P.; Wu, Z.; Liang, W.; Cheng, W. Soil micro-food web interactions and rhizosphere priming effect. Plant Soil 2018, 432, 129–142. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, C.; Chen, X.; Bonkowski, M.; Griffiths, B.; Chen, F.; Zhu, J.; Hu, S.; Hu, F.; Liu, M. Responses of rice paddy micro-food webs to elevated CO2 are modulated by nitrogen fertilization and crop cultivars. Soil Biol. Biochem. 2017, 114, 104–113. [Google Scholar] [CrossRef]
- De Vries, F.T.; Wallenstein, M.D. Below-ground connections underlying above-ground food production: A framework for optimising ecological connections in the rhizosphere. J. Ecol. 2017, 105, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Gou, X.; Cai, Y.; Wang, C.; Li, B.; Zhang, Y.; Tang, X.; Shen, J.; Cai, Z. Effects of different long-term cropping systems on phosphorus adsorption and desorption characteristics in red soils. J Soils Sediments 2020, 20, 1371–1382. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Zeng, S.; Wu, D.; Jacobs, D.F.; Sloan, J.L. Soil pH, organic matter, and nutrient content change with the continuous cropping of Cunninghamia lanceolata plantations in South China. J Soils Sediments 2017, 17, 2230–2238. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic Acid Fertilizer Improved Soil Properties and Soil Microbial Diversity of Continuous Cropping Peanut: A Three-Year Experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R. The potassium paradox: Implications for soil fertility, crop production and human health. Renew. Agric. Food Syst. 2014, 29, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Srinivasarao, C.; Kundu, S.; Sharma, K.L.; Reddy, S.; Pharande, A.L.; Vijayasankarbabu, M.; Satish, A.; Singh, R.P.; Singh, S.R.; Chary, G.R.; et al. Magnesium balance in four permanent manurial experiments under rainfed agro-ecosystems of India. Crop Pasture Sci. 2016, 66, 1230–1240. [Google Scholar] [CrossRef]
- Behera, S.K.; Singh, D.; Dwivedi, B.S. Changes in Fractions of Iron, Manganese, Copper, and Zinc in Soil under Continuous Cropping for More Than Three Decades. Commun. Soil Sci. Plant Anal. 2009, 40, 1380–1407. [Google Scholar] [CrossRef]
- Haruna, S.I.; Nkongolo, N.V. Influence of Cover Crop, Tillage, and Crop Rotation Management on Soil Nutrients. Agriculture 2020, 10, 225. [Google Scholar] [CrossRef]
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Growth and K Nutrition of Sesame (Sesamum indicum L.) Seedlings as Affected by Balancing Soil Exchangeable Cations Ca, Mg, and K of Continuously Monocropped Soil from Upland Fields Converted Paddy. Agronomy 2019, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Dhaliwal, S.S. Effect of Sewage Sludge and Rice Straw Compost on Yield, Micronutrient Availability and Soil Quality under Rice–Wheat System. Commun. Soil Sci. Plant Anal. 2019, 50, 1943–1954. [Google Scholar] [CrossRef]
- Moreira, A.; Sfredo, G.J.; Moraes, L.A.C.; Fageria, N.K. Lime and Cattle Manure in Soil Fertility and Soybean Grain Yield Cultivated in Tropical Soil. Commun. Soil Sci. Plant Anal. 2015, 46, 1157–1169. [Google Scholar] [CrossRef]
- Kalkhoran, S.S.; Pannell, D.J.; Thamo, T.; White, B.; Polyakov, M. Soil acidity, lime application, nitrogen fertility, and greenhouse gas emissions: Optimizing their joint economic management. Agric. Syst. 2019, 176, 102684. [Google Scholar] [CrossRef]
- Rengel, Z. Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-91231-7. [Google Scholar]
- Ghafoor, A.; Qadir, M.; Murtaza, G. Salt-Affected Soils: Principles of Management; Allied Book Centre: Lahore, Pakistan, 2004. [Google Scholar]
- Liu, X.; Song, Q.; Tang, Y.; Li, W.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil–vegetable system: A multi-medium analysis. Sci. Total Environ. 2013, 463–464, 530–540. [Google Scholar] [CrossRef]
- Karim, Z.; Qureshi, B.A. Health Risk Assessment of Heavy Metals in Urban Soil of Karachi, Pakistan. Hum. Ecol. Risk Assess. Int. J. 2014, 20, 658–667. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Han, W.; Kemmitt, S.J.; Brookes, P.C. Soil microbial biomass and activity in Chinese tea gardens of varying stand age and productivity. Soil Biol. Biochem. 2007, 39, 1468–1478. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, X.; Yamada, S.; Zhou, M.; Inoue, M.; Inosako, K. Cucumber Productivity and Soil Degradation in Recropping System in Greenhouse. Commun. Soil Sci. Plant Anal. 2012, 43, 1743–1748. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M.; Wong, P.T.W.; Mead, A.; Howe, G.; Newell, M. Field studies on the biofumigation of take-all by Brassica break crops. Aust. J. Agric. Res. 2000, 51, 445–456. [Google Scholar] [CrossRef]
- Meriles, J.M.; Gil, S.V.; Conforto, C.; Figoni, G.; Lovera, E.; March, G.J.; Guzmán, C.A. Soil microbial communities under different soybean cropping systems: Characterization of microbial population dynamics, soil microbial activity, microbial biomass, and fatty acid profiles. Soil Tillage Res. 2009, 103, 271–281. [Google Scholar] [CrossRef]
- Pérez-Brandán, C.; Huidobro, J.; Grümberg, B.; Scandiani, M.M.; Luque, A.G.; Meriles, J.M.; Vargas-Gil, S. Soybean fungal soil-borne diseases: A parameter for measuring the effect of agricultural intensification on soil health. Can. J. Microbiol. 2013, 60, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Katsalirou, E.; Deng, S.; Gerakis, A.; Nofziger, D.L. Long-term management effects on soil P, microbial biomass P, and phosphatase activities in prairie soils. Eur. J. Soil Biol. 2016, 76, 61–69. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, D.; Liu, J.; Qiao, P.; Zhou, X.; Lu, H.; Wu, X.; Liu, D.; Jin, X.; Wu, F. Changes in rhizosphere soil microbial communities in a continuously monocropped cucumber (Cucumis sativus L.) system. Eur. J. Soil Biol. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Carbon assimilation and microbial activity in soil. Z. Pflanz. Bodenkd. 1986, 149, 457–468. [Google Scholar] [CrossRef]
- Insam, H.; Parkinson, D.; Domsch, K.H. Influence of macroclimate on soil microbial biomass. Soil Biol. Biochem. 1989, 21, 211–221. [Google Scholar] [CrossRef]
- Wardle, D.A. A Comparative Assessment of Factors Which Influence Microbial Biomass Carbon and Nitrogen Levels in Soil. Biol. Rev. 1992, 67, 321–358. [Google Scholar] [CrossRef]
- Sahib, M.R.; Pervaiz, Z.H.; Williams, M.A.; Saleem, M.; De Bolt, S. Rhizobacterial species richness improves sorghum growth and soil nutrient synergism in a nutrient-poor greenhouse soil. Sci. Rep. 2020, 13, 41598. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kätterer, T.; Thornton, B.; Börjesson, G. Dynamics of fungal and bacterial groups and their carbon sources during the growing season of maize in a long-term experiment. Biol. Fertil. Soils 2020. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Saleem, M.; Cheng, J.; Mi, J.; Chu, P.; Tuvshintogtokh, I.; Hu, S.; Bai, Y. Effects of aridity on soil microbial communities and functions across soil depths on the Mongolian Plateau. Funct. Ecol. 2019. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Xu, X.; Liu, T.; Wu, D.; Zheng, X.; Tang, S.; Dai, Q. Potential use of high-throughput sequencing of soil microbial communities for estimating the adverse effects of continuous cropping on ramie (Boehmeria nivea L. Gaud). PLoS ONE 2018, 13, e0197095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, J.; Yu, Z.; Yao, Q.; Li, Y.; Liang, A.; Zhang, W.; Mi, G.; Jin, J.; Liu, X.; et al. Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Tillage Res. 2020, 197, 104503. [Google Scholar] [CrossRef]
- Xue, D.; Yao, H.; Huang, C. Microbial Biomass, N Mineralization and Nitrification, Enzyme Activities, and Microbial Community Diversity in Tea Orchard Soils. Plant Soil 2006, 288, 319–331. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Shi, F.Y.; Zhang, R.; Wu, Y.P. Comparison of bacterial diversity in rotational and continuous soybean cropping soils in Heilongjiang. Acta Phytophylacica Sin. 2014, 41, 403–409. [Google Scholar]
- Zhao, J.; Wu, X.; Nie, C.; Wu, T.; Dai, W.; Liu, H.; Yang, R. Analysis of unculturable bacterial communities in tea orchard soils based on nested PCR-DGGE. World J. Microbiol. Biotechnol. 2012, 28, 1967–1979. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, C.; Ma, J.; Yu, M.; Li, Y.; Wang, G.; Zhang, L. Prokaryotic diversity in continuous cropping and rotational cropping soybean soil. FEMS Microbiol. Lett. 2009, 298, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.-Y.; Bell, T.H.; Hamel, C.; Harker, K.N.; Mohr, R.; Greer, C.W.; Yergeau, É.; St-Arnaud, M. Canola Root–Associated Microbiomes in the Canadian Prairies. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Xi, H.; Shen, J.; Qu, Z.; Yang, D.; Liu, S.; Nie, X.; Zhu, L. Effects of Long-term Cotton Continuous Cropping on Soil Microbiome. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, X.; Kong, W.; Wu, Y.; Wang, J. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Li, X.; Ding, C.; Zhang, T.; Wang, X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol. Biochem. 2014, 72, 11–18. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different Continuous Cropping Spans Significantly Affect Microbial Community Membership and Structure in a Vanilla-Grown Soil as Revealed by Deep Pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Ji, X. Diversity and composition of rhizospheric soil and root endogenous bacteria in Panax notoginseng during continuous cropping practices. J. Basic Microbiol. 2017, 57, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Jiang, X.; Wright, A.L.; Wang, J.; Li, Z. Long-term tillage effects on the distribution patterns of microbial biomass and activities within soil aggregates. Catena 2011, 87, 276–280. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of Pesticides on Soil Microbial Diversity, Enzymes, and Biochemical Reactions; Academic Press: Cambridge, MA, USA, 2009; Chapter 5; Volume 102, pp. 159–200. ISBN 0065-2113. [Google Scholar]
- Wang, S.; Ren, J.; Huang, T.; Qiao, G.; Hu, H.; Li, W.; Wu, F.; Pan, K. Evaluation of soil enzyme activities and microbial communities in tomato continuous cropping soil treated with Jerusalem artichoke residues. Commun. Soil Sci. Plant Anal. 2018, 49, 2727–2740. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Turner, B.L.; Whitaker, J.; Ostle, N.; Bardgett, R.D.; McNamara, N.P.; Salinas, N.; Meir, P. Temperature sensitivity of soil enzymes along an elevation gradient in the Peruvian Andes. Biogeochemistry 2016, 127, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Martínez, V.; Burow, G.; Zobeck, T.M.; Allen, V.G. Soil Microbial Communities and Function in Alternative Systems to Continuous Cotton. Soil Sci. Soc. Am. J. 2010, 74, 1181–1192. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Yeboah, S.; Cao, L.; Zhang, J.; Shi, S.; Liu, Y. Breaking continuous potato cropping with legumes improves soil microbial communities, enzyme activities and tuber yield. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Dou, F.; Wright, A.L.; Mylavarapu, R.S.; Jiang, X.; Matocha, J.E. Soil Enzyme Activities and Organic Matter Composition Affected by 26 Years of Continuous Cropping. Pedosphere 2016, 26, 618–625. [Google Scholar] [CrossRef]
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Ishigaki, T.; Handa, T.; Kato, M.; Tenywa, M.M.; Masunaga, T.; Yamamoto, S.; et al. Imbalanced Soil Chemical Properties and Mineral Nutrition in Relation to Growth and Yield Decline of Sesame on Different Continuously Cropped Upland Fields Converted Paddy. Agronomy 2019, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Bünemann, E.K.; Schwenke, G.D.; Zwieten, L.V. Impact of agricultural inputs on soil organisms—A review. Soil Res. 2006, 44, 379–406. [Google Scholar] [CrossRef] [Green Version]
- Lata, J.-C.; Barot, S.; Leloup, J.; Lerch, T.; Nunan, N.; Raynaud, X. Biodiversity and ecological functioning of soils. In Soils as a Key Component of the Critical Zone 6; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 39–56. ISBN 978-1-119-43827-4. [Google Scholar]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S.; Subedi, A. Sensitivity of Nematode Community Analysis to Agricultural Management Practices and Inoculation with Local Effective Microorganisms in the Southeastern United States. Soil Syst. 2019, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Pulavarty, A.; Horgan, K.; Kakouli-Duarte, T. Effect of an Alltech soil health product on entomopathogenic nematodes, root-knot nematodes and on the growth of tomato plants in the greenhouse. J. Nematol. 2020, 52, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhang, W.; Wang, K.; Song, T.; Du, H. Responses of the soil nematode community to management of hybrid napiergrass: The trade-off between positive and negative effects. Appl. Soil Ecol. 2014, 75, 134–144. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Q.Z.; Wang, Y.Z.; Sun, H.Y.; Bai, C.Q.; Lewis, E.E. Different changes of soil nematode communities in replant and continuous-planting peach orchards and their indicative value for peach replant problem. Helminthologia 2015, 52, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Zhao, X.; Mao, Z.; Xie, B. Dynamics of Soil Nematode Communities Revealed Significant Variation in Greenhouse with Different Continuous Cropping Years. bioRxiv 2019, 593541. [Google Scholar] [CrossRef] [Green Version]
- Amador, J.A.; Görres, J.H. Microbiological characterization of the structures built by earthworms and ants in an agricultural field. Soil Biol. Biochem. 2007, 39, 2070–2077. [Google Scholar] [CrossRef]
- Hickman, Z.A.; Reid, B.J. Increased microbial catabolic activity in diesel contaminated soil following addition of earthworms (Dendrobaena veneta) and compost. Soil Biol. Biochem. 2008, 40, 2970–2976. [Google Scholar] [CrossRef]
- Edwards, C.A. Earthworm ecology in cultivated soils. In Earthworm Ecology: From Darwin to Vermiculture; Satchell, J.E., Ed.; Springer: Dordrecht, The Netherlands, 1983; pp. 123–137. ISBN 978-94-009-5965-1. [Google Scholar]
- Curry, J.P.; Byrne, D. The role of earthworms in straw decomposition and nitrogen turnover in arable land in ireland. Soil Biol. Biochem. 1992, 24, 1409–1412. [Google Scholar] [CrossRef]
- Crittenden, S.J.; Eswaramurthy, T.; De Goede, R.G.M.; Brussaard, L.; Pulleman, M.M. Effect of tillage on earthworms over short- and medium-term in conventional and organic farming. Appl. Soil Ecol. 2014, 83, 140–148. [Google Scholar] [CrossRef]
- Lavelle, P. Earthworm activities and the soil system. Biol. Fert. Soils 1988, 6, 237–251. [Google Scholar] [CrossRef]
- Fraser, P.M.; Haynes, R.J.; Williams, P.H. Effects of pasture improvement and intensive cultivation on microbial biomass, enzyme activities, and composition and size of earthworm populations. Biol. Fertil. Soils 1994, 17, 185–190. [Google Scholar] [CrossRef]
- Spurgeon, D.J.; Hopkin, S.P. Risk assessment of the threat of secondary poisoning by metals to predators of earthworms in the vicinity of a primary smelting works. Sci. Total Environ. 1996, 187, 167–183. [Google Scholar] [CrossRef]
- Chen, J.; Saleem, M.; Wang, C.; Liang, W.; Zhang, Q. Individual and combined effects of herbicide tribenuron-methyl and fungicide tebuconazole on soil earthworm Eisenia fetida. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleem, M.; Wang, C. Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci. Total Environ. 2019, 671, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Churchfield, S.; Hollier, J.; Brown, V.K. The Effects of Small Mammal Predators on Grassland Invertebrates, Investigated by Field Exclosure Experiment. Oikos 1991, 60, 283–290. [Google Scholar] [CrossRef]
- Emmerling, C. Response of earthworm communities to different types of soil tillage. Appl. Soil Ecol. 2001, 17, 91–96. [Google Scholar] [CrossRef]
- Henneron, L.; Bernard, L.; Hedde, M.; Pelosi, C.; Villenave, C.; Chenu, C.; Bertrand, M.; Girardin, C.; Blanchart, E. Fourteen years of evidence for positive effects of conservation agriculture and organic farming on soil life. Agron. Sustain. Dev. 2015, 35, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, L.; Chen, Q.; Wen, X.; Liao, Y. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron. Sustain. Dev. 2016, 36, 28. [Google Scholar] [CrossRef] [Green Version]
- Bilalis, D.; Sidiras, N.; Vavoulidou, E.; Konstantas, A. Earthworm populations as affected by crop practices on clay loam soil in a Mediterranean climate. Acta Agric. Scand. Sect. B Soil Plant Sci. 2009, 59, 440–446. [Google Scholar] [CrossRef]
- Schmidt, O.; Curry, J.P. Population dynamics of earthworms (Lumbricidae) and their role in nitrogen turnover in wheat and wheatclover cropping systems. Pedobiologia 2001, 45, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Feledyn-Szewczyk, B.; Radzikowski, P.; Stalenga, J.; Matyka, M. Comparison of the Effect of Perennial Energy Crops and Arable Crops on Earthworm Populations. Agronomy 2019, 9, 675. [Google Scholar] [CrossRef] [Green Version]
- Gulvik, M. Mites (Acari) as indicators of soil biodiversity and land use monitoring—A review. Pol. J. Ecol. 2007, 55, 415–440. [Google Scholar]
- Koehler, H.H. Predatory mites (Gamasina, Mesostigmata). Agric. Ecosyst. Environ. 1999, 74, 395–410. [Google Scholar] [CrossRef]
- Neher, D.A.; Barbercheck, M.E. Soil Microarthropods and Soil Health: Intersection of Decomposition and Pest Suppression in Agroecosystems. Insects 2019, 10, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badejo, M.A.; Woas, S.; Beck, L. Redescription of Archegozetes magnus [Sellinick, 1925] [Trhypochthonioidea] from Brazil and description of two new species of nanhermanniid mites: Bicyrthermannia nigeriana and Masthermannia seropedica [Nanhermannioidea] [Acari: Oribatida]. Genus. Int. J. Invertebr. Taxon. 2003, 14, 125–149. [Google Scholar]
- Gruss, I.; Twardowski, J.P.; Hurej, M. Influence of 90-year potato and winter rye monocultures under different fertilisation on soil mites. Plant Prot. Sci. 2017, 54, 31–38. [Google Scholar] [CrossRef]
- Gruss, I.; Twardowski, J. The assemblages of soil-dwelling springtails (Collembola) in winter rye under long-term monoculture and crop rotation. Žemdirbystė Agric. 2016, 103, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Twardowski, J.P.; Hurej, M.; Gruss, I. Diversity and abundance of springtails (Hexapoda: Collembola) in soil under 90-year potato monoculture in relation to crop rotation. Arch. Agron. Soil Sci. 2016, 62, 1158–1168. [Google Scholar] [CrossRef]
- Liu, D.; Sun, H.; Ma, H. Deciphering Microbiome Related to Rusty Roots of Panax ginseng and Evaluation of Antagonists against Pathogenic Ilyonectria. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Dong, L.; Xu, J.; Feng, G.; Li, X.; Chen, S. Soil bacterial and fungal community dynamics in relation to Panax notoginseng death rate in a continuous cropping system. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jie, W.; Bai, L.; Yu, W.; Cai, B. Analysis of interspecific relationships between Funneliformis mosseae and Fusarium oxysporum in the continuous cropping of soybean rhizosphere soil during the branching period. Biocontrol Sci. Technol. 2015, 25, 1036–1051. [Google Scholar] [CrossRef]
- Hornby, D. Take-All Disease of Cereals: A Regional Perspective; CAB International: Wallingford, UK, 1998. [Google Scholar]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial Populations Responsible for Specific Soil Suppressiveness to Plant Pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.J. Take-all of wheat. Physiol. Mol. Plant Pathol. 2003, 62, 73–86. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Yang, Q.; Chi, X.; Pan, L.; Chen, N.; Yang, Z.; Wang, T.; Wang, M.; Yu, S. Soil Eukaryotic Microorganism Succession as Affected by Continuous Cropping of Peanut—Pathogenic and Beneficial Fungi were Selected. PLoS ONE 2012, 7, e40659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Cui, J.; Jie, W.; Cai, B. Analysis of the community compositions of rhizosphere fungi in soybeans continuous cropping fields. Microbiol. Res. 2015, 180, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.; Ruan, Y.; Li, R.; Shen, Q. Banana Fusarium Wilt Disease Incidence Is Influenced by Shifts of Soil Microbial Communities Under Different Monoculture Spans. Microb. Ecol 2018, 75, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, M.; Hu, Y.; Li, Z.; Liu, C.; Wang, X.; Tian, Q.; Jiao, W.; Hu, J.; Liu, L.; et al. Effects of Continuous Cropping of Sweet Potato on the Fungal Community Structure in Rhizospheric Soil. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ciampi-Panno, L.; Fernandez, C.; Bustamante, P.; Andrade, N.; Ojeda, S.; Contreras, A. Biological control of bacterial wilt of potatoes caused by Pseudomonas solanacearum. Am. Potato J. 1989, 66, 315–332. [Google Scholar] [CrossRef]
- Niu, J.; Rang, Z.; Zhang, C.; Chen, W.; Tian, F.; Yin, H.; Dai, L. The succession pattern of soil microbial communities and its relationship with tobacco bacterial wilt. BMC Microbiol. 2016, 16, 233. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Zhang, S.; Liu, X.; Jiang, Q.; Ding, W. Soil acidification amendments change the rhizosphere bacterial community of tobacco in a bacterial wilt affected field. Appl. Microbiol. Biotechnol. 2018, 102, 9781–9791. [Google Scholar] [CrossRef] [Green Version]
- Luan, F.G.; Zhang, L.L.; Lou, Y.Y.; Wang, L.; Liu, Y.N.; Zhang, H.Y. Analysis of microbial diversity and niche in rhizosphere soil of healthy and diseased cotton at the flowering stage in southern Xinjiang. Genet. Mol. Res. 2015, 14, 1602–1611. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, B.-H.; Jin, Z.; Li, Z.; Song, H.; Ding, Y.-Q. Analysis of bacterial communities in rhizosphere soil of healthy and diseased cotton (Gossypium sp.) at different plant growth stages. Plant Soil 2011, 339, 447–455. [Google Scholar] [CrossRef]
- Wu, Z.; Hao, Z.; Zeng, Y.; Guo, L.; Huang, L.; Chen, B. Molecular characterization of microbial communities in the rhizosphere soils and roots of diseased and healthy Panax notoginseng. Antonie Van Leeuwenhoek 2015, 108, 1059–1074. [Google Scholar] [CrossRef]
- Govers, G.; Merckx, R.; Wesemael van, B.; Oost van, K. Soil conservation in the 21st century: Why we need smart agricultural intensification. Soil 2017, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, R.; Chen, S.; Qi, G.; He, Z.; Zhao, X. Microbial taxa and functional genes shift in degraded soil with bacterial wilt. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- She, S.; Niu, J.; Zhang, C.; Xiao, Y.; Chen, W.; Dai, L.; Liu, X.; Yin, H. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch. Microbiol. 2017, 199, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, Y.; Lv, F.; Hu, L.; Wei, L.; Yuan, Z.; Lin, H. Bacterial community structure and functional potential of rhizosphere soils as influenced by nitrogen addition and bacterial wilt disease under continuous sesame cropping. Appl. Soil Ecol. 2018, 125, 117–127. [Google Scholar] [CrossRef]
- Niblack, T.L. Soybean Cyst Nematode Management Reconsidered. Plant Dis. 2005, 89, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Li, S.; Wei, W.; Xu, Y.; Yao, Q. Assessment of parasitic fungi for reducing soybean cyst nematode with suppressive soil in soybean fields of northeast China. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 67, 730–736. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Sanguin, H.; Sarniguet, A.; Gazengel, K.; Moënne-Loccoz, Y.; Grundmann, G.L. Rhizosphere bacterial communities associated with disease suppressiveness stages of take-all decline in wheat monoculture. New Phytol. 2009, 184, 694–707. [Google Scholar] [CrossRef]
- Wei, W.; Xu, Y.; Li, S.; Zhu, L.; Song, J. Developing suppressive soil for root diseases of soybean with continuous long-term cropping of soybean in black soil of Northeast China. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 279–285. [Google Scholar] [CrossRef]
- Hamid, M.I.; Hussain, M.; Wu, Y.; Zhang, X.; Xiang, M.; Liu, X. Successive soybean-monoculture cropping assembles rhizosphere microbial communities for the soil suppression of soybean cyst nematode. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [Green Version]
- Garbeva, P.; Van Veen, J.A.; Van Elsas, J.D. Microbial Diversity in Soil: Selection of Microbial Populations by Plant and Soil Type and Implications for Disease Suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Dexter, A.R. Advances in characterization of soil structure. Soil Tillage Res. 1988, 11, 199–238. [Google Scholar] [CrossRef]
- Nunan, N.; Wu, K.; Young, I.M.; Crawford, J.W.; Ritz, K. Spatial distribution of bacterial communities and their relationships with the micro-architecture of soil. FEMS Microbiol. Ecol. 2003, 44, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Rabot, E.; Wiesmeier, M.; Schlüter, S.; Vogel, H.-J. Soil structure as an indicator of soil functions—A review. Geoderma 2018, 314, 122–137. [Google Scholar] [CrossRef]
- Amézketa, E. Soil Aggregate Stability—A review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Almajmaie, A.; Hardie, M.; Acuna, T.; Birch, C. Evaluation of methods for determining soil aggregate stability. Soil Tillage Res. 2017, 167, 39–45. [Google Scholar] [CrossRef]
- Karlen, D.L.; Hurley, E.G.; Andrews, S.S.; Cambardella, C.A.; Meek, D.W.; Duffy, M.D.; Mallarino, A.P. Crop Rotation Effects on Soil Quality at Three Northern Corn/Soybean Belt Locations. Agron. J. 2006, 98, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Liu, C.; Wang, J.; Meng, Q.; Yuan, Y.; Ma, X.; Liu, X.; Zhu, Y.; Ding, G.; Zhang, J.; et al. Soil aggregates stability and storage of soil organic carbon respond to cropping systems on Black Soils of Northeast China. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Acikgoz, S.; Anderson, S.H.; Gantzer, C.J.; Thompson, A.L.; Miles, R.J. 125 Years of Soil and Crop Management on Sanborn Field: Effects on Soil Physical Properties Related to Soil Erodibility. Soil Sci. 2017, 182, 172–180. [Google Scholar] [CrossRef]
- Miao, S.; Qiao, Y.; Li, P.; Han, X.; Tang, C. Fallow associated with autumn-plough favors structure stability and storage of soil organic carbon compared to continuous maize cropping in Mollisols. Plant Soil 2017, 416, 27–38. [Google Scholar] [CrossRef]
- Abe, S.S.; Hashi, I.; Masunaga, T.; Yamamoto, S.; Honna, T.; Wakatsuki, T. Soil Profile Alteration in a Brown Forest Soil under High-Input Tea Cultivation. Plant Prod. Sci. 2006, 9, 457–461. [Google Scholar] [CrossRef]
- Oh, K.; Kato, T.; Li, Z.-P.; Li, F.-Y. Environmental Problems From Tea Cultivation in Japan and a Control Measure Using Calcium Cyanamide1 1Project supported by the National Natural Science Foundation of China (No. 40471066) and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KZCX3-SW-417). Pedosphere 2006, 16, 770–777. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhattacharyya, R.; Dalal, R.C.; Wang, P.; Menzies, N.W.; Kopittke, P.M. Impact of land use change and soil type on total phosphorus and its fractions in soil aggregates. Land Degrad. Dev. 2020, 31, 828–841. [Google Scholar] [CrossRef]
- Hati, K.M.; Swarup, A.; Dwivedi, A.K.; Misra, A.K.; Bandyopadhyay, K.K. Changes in soil physical properties and organic carbon status at the topsoil horizon of a vertisol of central India after 28 years of continuous cropping, fertilization and manuring. Agric. Ecosyst. Environ. 2007, 119, 127–134. [Google Scholar] [CrossRef]
- Aboim, M.C.R.; Coutinho, H.L.C.; Peixoto, R.S.; Barbosa, J.C.; Rosado, A.S. Soil bacterial community structure and soil quality in a slash-and-burn cultivation system in Southeastern Brazil. Appl. Soil Ecol. 2008, 38, 100–108. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Wang, X.; Gao, X.; Liu, G. Erosion effect on the productivity of black soil in Northeast China. Sci. China Ser. D Earth Sci. 2009, 52, 1005–1021. [Google Scholar] [CrossRef]
- Jia, Z.; Kuzyakov, Y.; Myrold, D.; Tiedje, J. Soil Organic Carbon in a Changing World. Pedosphere 2017, 27, 789–791. [Google Scholar] [CrossRef]
- Kay, B.D. Soil Structure and Organic Carbon—A Review. Available online: https://www.taylorfrancis.com/ (accessed on 8 April 2020).
- Nunan, N.; Schmidt, H.; Raynaud, X. The ecology of heterogeneity: Soil bacterial communities and C dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190249. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Miao, S.; Li, Y.; Zhong, X. Chemical composition of soil organic carbon changed by long-term monoculture cropping system in Chinese black soil. Plant Soil Environ. 2018, 64, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Börjesson, G.; Bolinder, M.A.; Kirchmann, H.; Kätterer, T. Organic carbon stocks in topsoil and subsoil in long-term ley and cereal monoculture rotations. Biol. Fertil. Soils 2018, 54, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.J.; Allen, F.L.; Wight, J.P.; Saxton, A.M.; Tyler, D.D.; Sams, C.E. Soil Organic Carbon Sequestration Rates under Crop Sequence Diversity, Bio-Covers, and No-Tillage. Soil Sci. Soc. Am. J. 2014, 78, 1726–1733. [Google Scholar] [CrossRef]
- Lal, R. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad. Dev. 2006, 17, 197–209. [Google Scholar] [CrossRef]
- Fiorini, A.; Boselli, R.; Maris, S.C.; Santelli, S.; Ardenti, F.; Capra, F.; Tabaglio, V. May conservation tillage enhance soil C and N accumulation without decreasing yield in intensive irrigated croplands? Results from an eight-year maize monoculture. Agric. Ecosyst. Environ. 2020, 296, 106926. [Google Scholar] [CrossRef]
- Kätterer, T.; Bolinder, M.A.; Andrén, O.; Kirchmann, H.; Menichetti, L. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011, 141, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Kirchmann, H.; Schön, M.; Börjesson, G.; Hamnér, K.; Kätterer, T. Properties of soils in the Swedish long-term fertility experiments: VII. Changes in topsoil and upper subsoil at Örja and Fors after 50 years of nitrogen fertilization and manure application. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 25–36. [Google Scholar] [CrossRef]
- Blackmer, A.M. Nitrogen Fertilizer Recommendations for Corn in Iowa; FAO: Roma, Italy, 1997. [Google Scholar]
- Ding, W.; Hume, D.J.; Vyn, T.J.; Beauchamp, E.G. N credit of soybean to a following corn crop in central Ontario. Can. J. Plant Sci. 1998, 78, 29–33. [Google Scholar] [CrossRef]
- Gentry, L.F.; Ruffo, M.L.; Below, F.E. Identifying Factors Controlling the Continuous Corn Yield Penalty. Agron. J. 2013, 105, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S. Impact of inoculation with local effective microorganisms on soil nitrogen cycling and legume productivity using composted broiler litter. Appl. Soil Ecol. 2020, 154, 103567. [Google Scholar] [CrossRef]
- Mahal, N.; Osterholz, W.; Miguez, F.; Poffenbarger, H.; Sawyer, J.; Olk, D.; Archontoulis, S.; Castellano, M. Nitrogen Fertilizer Suppresses Mineralization of Soil Organic Matter in Maize Agroecosystems. Front. Ecol. Evol. 2019, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Kaboneka, S.; Sabbe, W.E.; Mauromoustakos, A. Carbon decomposition kinetics and nitrogen mineralization from corn, soybean, and wheat residues. Commun. Soil Sci. Plant Anal. 1997, 28, 1359–1373. [Google Scholar] [CrossRef]
- Gentry, L.E.; Below, F.E.; David, M.B.; Bergerou, J.A. Source of the soybean N credit in maize production. Plant Soil 2001, 236, 175–184. [Google Scholar] [CrossRef]
- Meng, Y.; Chua-Ona, T.; Thompson, M.L. Short-term Nitrogen Mineralization Potential in Soils of Biofuel Cropping Systems. Soil Sci. 2016, 181, 503–512. [Google Scholar] [CrossRef]
- Cai, A.; Xu, H.; Shao, X.; Zhu, P.; Zhang, W.; Xu, M.; Murphy, D.V. Carbon and Nitrogen Mineralization in Relation to Soil Particle-Size Fractions after 32 Years of Chemical and Manure Application in a Continuous Maize Cropping System. PLoS ONE 2016, 11, e0152521. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, W.W.; Wortmann, C.S. Tillage and Rotation Interactions for Corn and Soybean Grain Yield as Affected by Precipitation and Air Temperature. Agron. J. 2004, 96, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Hall, S.J.; Russell, A.E.; Moore, A.R. Do corn-soybean rotations enhance decomposition of soil organic matter? Plant Soil 2019, 444, 427–442. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Bhardwaj, A.K.; Basso, B.; Robertson, G.P.; Hamilton, S.K. Nitrate Leaching from Continuous Corn, Perennial Grasses, and Poplar in the US Midwest. J. Environ. Qual. 2019, 48, 1849–1855. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.E.; Willson, T.C.; Kizilkaya, K.; Parker, E.; Harwood, R.R. Enhancing the Mineralizable Nitrogen Pool Through Substrate Diversity in Long Term Cropping Systems. Soil Sci. Soc. Am. J. 2001, 65, 1442–1447. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Z.; Liu, H.; Xue, C.; Zhang, R.; Wu, H.; Li, R.; Shen, Q. The Effect of Long-Term Continuous Cropping of Black Pepper on Soil Bacterial Communities as Determined by 454 Pyrosequencing. PLoS ONE 2015, 10, e0136946. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, Y.; Wang, J.; Yang, L.; Zhang, S.; Xu, C.; Ding, W. Soil Acidification Aggravates the Occurrence of Bacterial Wilt in South China. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Z.; Arafat, Y.; Lin, W. Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Ann. Microbiol. 2020, 70, 7. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, J.; Corso, M.R.D.; Figueiredo, B.; Nambi, E.; Raun, W. Soil Organic Carbon, Total Nitrogen, and Soil pH, in a Long-Term Continuous Winter Wheat (Triticum aestivum, L.) Experiment. Commun. Soil Sci. Plant Anal. 2018, 49, 803–813. [Google Scholar] [CrossRef]
- Sakuma, F.; Maeda, M.; Takahashi, M.; Hashizume, K.; Kondo, N. Suppression of Common Scab of Potato Caused by Streptomyces turgidiscabies Using Lopsided Oat Green Manure. Plant Dis. 2011, 95, 1124–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juo, A.S.; Franzluebbers, K.; Dabiri, A.; Ikhile, B. Changes in soil properties during long-term fallow and continuous cultivation after forest clearing in Nigeria. Agric. Ecosyst. Environ. 1995, 56, 9–18. [Google Scholar] [CrossRef]
- Heidari, G.; Mohammadi, K.; Sohrabi, Y. Responses of Soil Microbial Biomass and Enzyme Activities to Tillage and Fertilization Systems in Soybean (Glycine max L.) Production. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011; ISBN 978-0-12-384906-9. [Google Scholar]
- Nyamadzawo, G.; Chikowo, R.; Nyamugafata, P.; Giller, K.E. Improved legume tree fallows and tillage effects on structural stability and infiltration rates of a kaolinitic sandy soil from central Zimbabwe. Soil Tillage Res. 2007, 96, 182–194. [Google Scholar] [CrossRef]
- Agegnehu, G.; Lakew, B.; Nelson, P.N. Cropping sequence and nitrogen fertilizer effects on the productivity and quality of malting barley and soil fertility in the Ethiopian highlands. Arch. Agron. Soil Sci. 2014, 60, 1261–1275. [Google Scholar] [CrossRef]
- Jiao, X.-L.; Zhang, X.-S.; Lu, X.-H.; Qin, R.; Bi, Y.-M.; Gao, W.-W. Effects of maize rotation on the physicochemical properties and microbial communities of American ginseng cultivated soil. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Malhi, S.S.; Nyborg, M.; Harapiak, J.T. Effects of long-term N fertilizer-induced acidification and liming on micronutrients in soil and in bromegrass hay. Soil Tillage Res. 1998, 48, 91–101. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Owens, P.R.; Allen, F.L. Long-term cropping systems management influences soil strength and nutrient cycling. Geoderma 2020, 361, 114062. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Contreras, J.; Hupp, B.M.; Lindenberger, J.; Chen, D.; Zhang, Q.; Wang, C.; Twigg, P.; Saleem, M. Root microbiome changes with root branching order and root chemistry in peach rhizosphere soil. Rhizosphere 2020, 100249. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Chomel, M.; Fernandez, C.; Bousquet-Mélou, A.; Gers, C.; Monnier, Y.; Santonja, M.; Gauquelin, T.; Gros, R.; Lecareux, C.; Baldy, V. Secondary metabolites of Pinus halepensis alter decomposer organisms and litter decomposition during afforestation of abandoned agricultural zones. J. Ecol. 2014, 102, 411–424. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.-Y.; Kong, C.-H.; Wang, J.-G.; Wang, Y.-F. Rhizosphere isoflavones (daidzein and genistein) levels and their relation to the microbial community structure of mono-cropped soybean soil in field and controlled conditions. Soil Biol. Biochem. 2011, 43, 2257–2264. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Zhang, J.; Yao, T.; Wei, D.; Wang, Y.; Wang, J. Effect of root exudates on beneficial microorganisms—Evidence from a continuous soybean monoculture. Plant Ecol. 2012, 213, 1883–1892. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Y.; Zhu, W.; Tang, J.; Hu, S.; Zhou, T.; Chen, X. Baicalin Released from Scutellaria baicalensis Induces Autotoxicity and Promotes Soilborn Pathogens. J. Chem. Ecol. 2010, 36, 329–338. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, H.; Ge, X.; Wu, F. Effects of vanillin on the community structures and abundances of Fusarium and Trichoderma spp. in cucumber seedling rhizosphere. J. Plant Interact. 2018, 13, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Kitazawa, H.; Asao, T.; Ban, T.; Pramanik, M.H.R.; Hosoki, T. Autotoxicity of root exudates from strawberry in hydroponic culture. J. Hortic. Sci. Biotechnol. 2005, 80, 677–680. [Google Scholar] [CrossRef]
- Gadelha, I.C.N.; Fonseca, N.B.S.; Oloris, S.C.S.; Melo, M.M.; Soto-Blanco, B. Gossypol Toxicity from Cottonseed Products. Available online: https://www.hindawi.com/journals/tswj/2014/231635/ (accessed on 28 March 2020).
- Zhou, X.; Wu, F. p-Coumaric Acid Influenced Cucumber Rhizosphere Soil Microbial Communities and the Growth of Fusarium oxysporum f. sp. cucumerinum Owen. PLoS ONE 2012, 7, e48288. [Google Scholar] [CrossRef] [Green Version]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M. Bacteria-Protist Interactions in the Context of Biodiversity and Ecosystem Functioning Research. Ph.D. Thesis, University of Leipzig, Leipzig, Germany, 2012. [Google Scholar]

| Soil Indicators | Functions | Monoculture Cropping | Measures to Improve | Relevant Literature |
|---|---|---|---|---|
| Visual indicators | ||||
| Weeds | Low weed coverage or presence is an indicator of healthy soil. | Promotes weeds. | Weeding, cover crops, cleaned use of farm machinery, increasing cropping diversity. | [28,29] |
| Soil surface | Relatively even soil surface and less shallow patches are indicators of healthy soil. | Alters the degree of soil surface evenness. | Precise use of farm machinery, proper land leveling | [30,31] |
| Water or moisture distribution | Relatively even moisture and fewer water ponds are indicators of healthy soil. | May alter moisture distribution in soil. | Proper land leveling, cover cropping, even distribution of crop residues and snow. | [32,33] |
| Physical indicators | ||||
| Soil texture | Loam-textured soils are considered productive. Sandy or clay-textured soils challenge plant growth. | Though no effect on texture, it may intensify textural effects. | Organic amendments, crop residues, biochar, livestock, and poultry waste, could improve soil conditions. | [34] |
| Bulk density (BD) | Soil with BD 1.3–1.6 g/cm3 is considered good for roots. | May increase BD. | Same as above, reduced use of farm machinery. No-till agriculture. | [35,36,37] |
| Soil porosity | Macro-, meso-, and micro-porosities regulate nutrient, gas, and water mobility and improve soil functions. | May reduce soil porosity. | Same as above, reduced use of farm machinery. No-till agriculture. | [38,39] |
| Available water capacity (AWC) | The AWC determines soil functioning. | May alter AWC. | Water conservation practices, mulching, organic amendments, crop residues. | [40,41] |
| Penetration resistance | It determines root penetration into soil and root-microbe (e.g., mycorrhizae) interactions. | May increase penetration resistance. | Organic amendments, no-till agriculture, cover cropping. | [42,43] |
| Saturated hydraulic conductivity | It may influence water and nutrient movement. | May alter or reduce hydraulic conductivity. | Organic amendments, no-till agriculture, cover cropping. | [44,45] |
| Soil aggregate size classes, their composition | These determine soil structure, nutrient dynamics, microbial activities, plant growth, and carbon (C) storage. | May reduce soil aggregation or aggregate composition. | No-till, cover cropping, organic amendments, cropping diversity. | [46,47,48] |
| Soil surface hardness | It is a measure of the maximum soil surface (0–15 cm depth) penetration resistance (psi), and it is determined by a field penetrometer. It must not exceed 300 psi. Low values are good for soil functioning and root growth. | May increase soil hardiness | No-till, crop rotation, organic amendment, crop residues, and reduced use of farm machinery. | [49,50,51] |
| Subsurface Hardness: | It is also a measure of the maximum resistance (psi) in soil between 15 to 45 cm depths using a field penetrometer. | May increase soil hardiness | Same as above. | [49,50,51,52] |
| Soil aggregate Stability | It is a measure of soil aggregates’ resistance to disintegration under raindrop impact. It is determined by testing the impact of simulated rainfall on aggregates in the soil sieves (0.25–2.0 mm). The proportion of soil that stays on the sieve reflects the percentage of aggregate stability. Healthy soils should have >50% stable aggregates. | May reduce soil aggregate stability. | No-till, crop rotation, organic amendment, cover cropping mulching, and reduced use of machinery. | [53,54,55] |
| Biological indicators | ||||
| Root pathogen pressure assessment | It is an estimation of pathogen load on plant roots and it is rated from 2 to 9. The higher numbers show higher plant sensitivity and pathogen damage. | May increase root pathogen pressure. | The mixture, inter-cropping, cover cropping, organic amendments, and biopesticides. | [56,57] |
| Beneficial nematode population | A higher abundance of beneficial nematodes is considered as a good indicator of soil health. | May reduce their abundance. | The mixture, inter-cropping, cover cropping, cropping diversity, organic amendments, | [12,58,59] |
| Earthworms | A higher abundance of earthworms is considered a good indicator of soil health. | May reduce their abundance. | The mixture, inter-cropping, cover cropping, cropping diversity, and organic amendments. | [12,58,59] |
| Parasitic nematode population | A low abundance of parasitic nematodes is considered a good indicator of soil health. | May increase their abundance | Biopesticides, mixture, inter-cropping, cover cropping, and crop rotation. | [60,61,62] |
| Potentially mineralizable N (PMN) | It is defined as the fraction of organic N that is available to plants. | May reduce PMN. | Cropping diversity, crop rotation, cover cropping, organic amendments, and crop residues. | [63,64] |
| Cellulose decomposition rate | Higher decomposition leads to greater C and nutrient availability to soil organisms and health. | May reduce decomposition. | Organic amendments, crop rotation, cover cropping, crop residues, and organic amendments. | [65,66] |
| Particulate organic matter (POM) | POM is a fundamental indicator of soil health. | May reduce POM. | The mixture, inter-cropping, cover cropping, and organic amendments. | [67,68] |
| Active C | It is a small portion of SOM that is readily available for soil organisms and regulates soil food web interactions. | May activate C. | Organic amendments, cropping diversity, cover cropping, and crop residues. | [25,69] |
| Weed seed bank | A higher weed seed bank may lead to weed invasion, and it is detrimental to soil health. | May increase weed seed bank. | Cropping diversity, cover cropping, and crop rotation | [70,71] |
| Microbial respiration rate | It is an indicator of microbial activities and soil functions. It also determines soil C storage and sequestration. | May alter respiration. | Organic amendments, crop residues, crop rotation, cover cropping, and cropping diversity. | [72,73,74] |
| Soil protein contents. | These determine the quality of SOM, and the level of soil biological activities. | Crop residues, organic amendments, cover cropping, and crop rotation. | [75,76] | |
| Microbiomic indicators | ||||
| Microbial networks, interactions | Microbial networks and teamwork regulate multiple soil functions. | May alter or deplete microbial networks. | Cropping diversity, crop rotation, cover cropping, biofertilizers, organic amendments, biochar, and crop residues. | [11,77,78] |
| Microbial abundance, biomass | Higher microbial biomass contents are considered an indicator of healthy soil. | May reduce or alter microbial biomass. | Same as above. | [79,80] |
| Community composition | Higher values of community composition such as species evenness are indicators of healthy soil. | May reduce or alter the microbial community structure. | Same as above. | [17,81] |
| Diversity | Higher values of microbial diversity such as species richness are indicators of healthy soil. | May reduce or alter microbial species richness. | Same as above. Microbial probiotics | [17,81,82,83] |
| Beneficial properties | Soils with microbes of diverse properties are considered healthy soils. | May reduce the abundance of beneficial microbes. | Same as above. Microbial probiotics. | [82,84] |
| Food web interactions | Soils with a greater abundance of different trophic level organisms and interactions are considered healthy soils. | May alter soil trophic complexity and interactions. | Same as above. Microbial probiotics. | [85,86,87] |
| Chemical indicators | ||||
| P | The optimum level of P (30–50 ppm) is also an indicator of healthy soil. | May reduce soil P contents. | Organic amendments, biofertilizers, crop residues, and cropping diversity. | [88,89,90] |
| N | The optimum level of N (50–75 mg-N/kg soil) is also an indicator of healthy soil. | May alter soil N contents. | Organic amendments, biofertilizers, crop residues, and legume cover cropping. | [88,89,90] |
| K | The optimum level of K (50–75 mg-N/kg soil) is also an indicator of healthy soil. | May reduce soil K contents. | Organic amendments, biofertilizers, crop residues, and cover crops. | [91] |
| pH | Near neutral pH is a sign of healthy soils while low and high pH damage soil health by causing acidity and salinity. | May alter soil pH. In slight acidic and saline soils, it may increase soil acidity and salinity, respectively. | Crop rotation, cover cropping, organic amendments, and crop residues. | [89] |
| Magnesium (Mg) | The optimum level of Mg is also an indicator of healthy soil. | May alter or reduce soil Mg contents. | Dolomitic limestone, quality irrigation water, cover cropping, and crop residues. | [92,93] |
| Calcium (Ca) | The optimum level of Ca determines soil structure and pH, and are an indicator of healthy soil. | May alter or reduce soil Ca contents. | Limestone, gypsum, irrigation water, and crop residues. | [93,94,95] |
| Iron (Fe) | The optimum level of soil Fe contents is an indicator of healthy soil. | May alter or reduce soil Fe contents. | Iron chelates, manure or sewage sludge, foliar spray of ferrous sulfate solution | [93,94,95,96] |
| Aluminum | [93,94,95,96] | |||
| Manganese (Mn) | The optimum levels of soil Mn contents are an indicator of healthy soil. | May alter or reduce soil Mn contents. | Crop residues, organic amendments, and conservation tillage. | [93,94,95,96,97] |
| Zinc (Zn) | The optimum levels of soil Zn contents are an indicator of healthy soil. | May alter or reduce soil Zn contents. | Crop residues, zinc sulfate, zinc chelates, biofertilizers, | [93,94,95,96,97] |
| Copper (Cu) | The optimum levels of soil Cu contents are an indicator of healthy soil. | May alter or reduce soil Cu contents. | Organic matter, organic amendments, biofertilizers, and crop residues. | [66,67,68,69] |
| Exchangeable acidity | It is defined as the sum of the concentrations of Al and hydrogen (H) ions in the soil exchange complex, and it is inversely related to the pH and base saturation of the soil. | May alter it and soil pH | Lime, organic matter, manure, crop rotation, and cover cropping. | [98,99] |
| Salinity/sodicity | Higher salt contents damage soil structure and salinity is linked to soil degradation. | May increase soil salinity in arid zones. | Organic amendments, cover cropping, crop residues, and crop rotation. | [100] |
| Heavy metals | Higher heavy metal contents damage soil health. Lower concentrations are considered as an indicator of healthy soil. | May increase or alter heavy metal contents. | Heavy metal-free organic amendments, crop residues, | [101,102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil Syst. 2020, 4, 59. https://doi.org/10.3390/soilsystems4040059
Pervaiz ZH, Iqbal J, Zhang Q, Chen D, Wei H, Saleem M. Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil Systems. 2020; 4(4):59. https://doi.org/10.3390/soilsystems4040059
Chicago/Turabian StylePervaiz, Zahida H., Javed Iqbal, Qingming Zhang, Dima Chen, Hui Wei, and Muhammad Saleem. 2020. "Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health" Soil Systems 4, no. 4: 59. https://doi.org/10.3390/soilsystems4040059
APA StylePervaiz, Z. H., Iqbal, J., Zhang, Q., Chen, D., Wei, H., & Saleem, M. (2020). Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil Systems, 4(4), 59. https://doi.org/10.3390/soilsystems4040059





