Landscape Influence on the Browning of a Lake Watershed in the Adirondack Region of New York, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Hydrology and Chemistry
2.4. Optical Measurements
2.5. Statistical Analyses
3. Results
3.1. Hydrology
3.2. Parafac Model Components
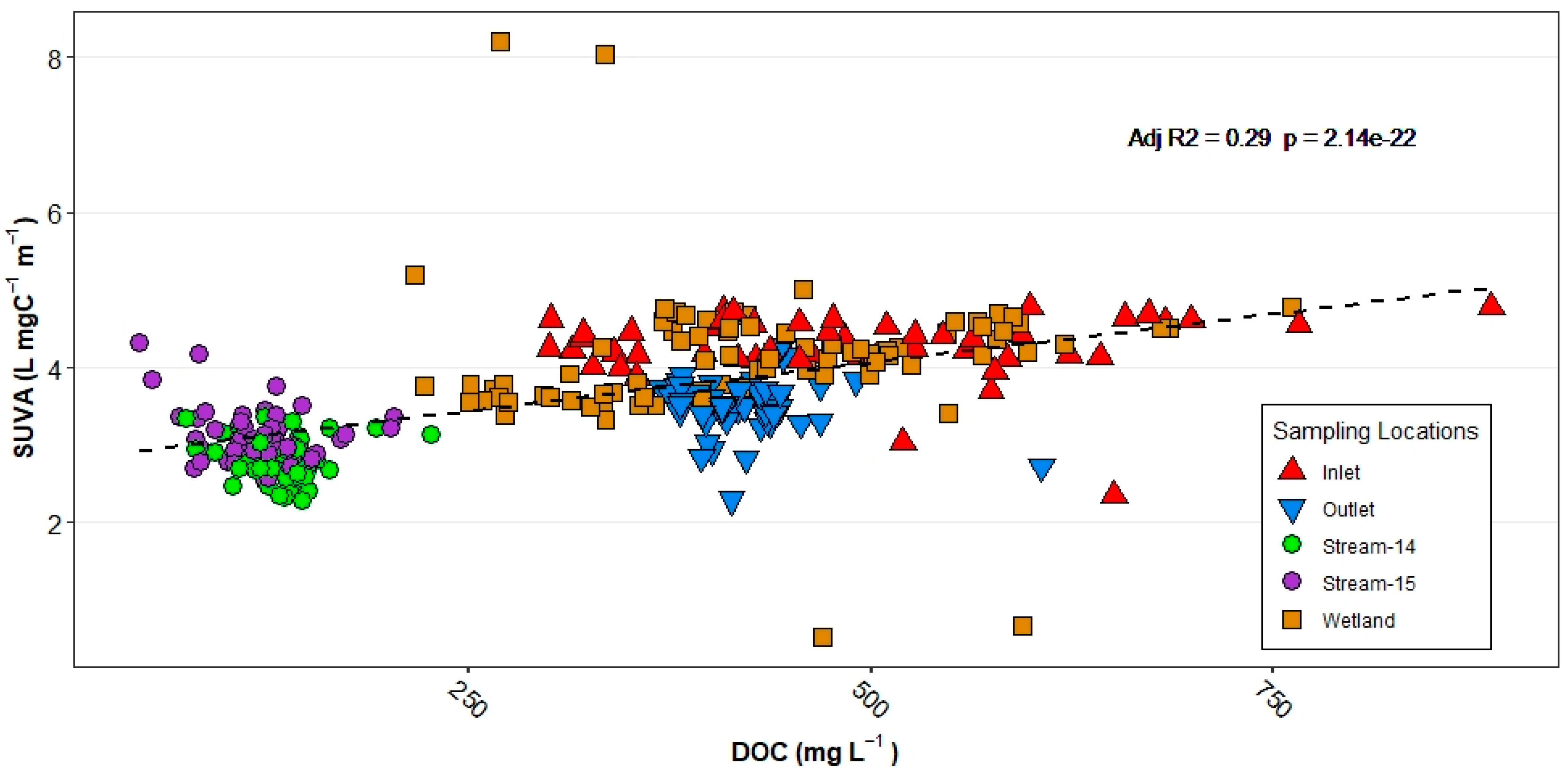
3.3. Spatial Patterns
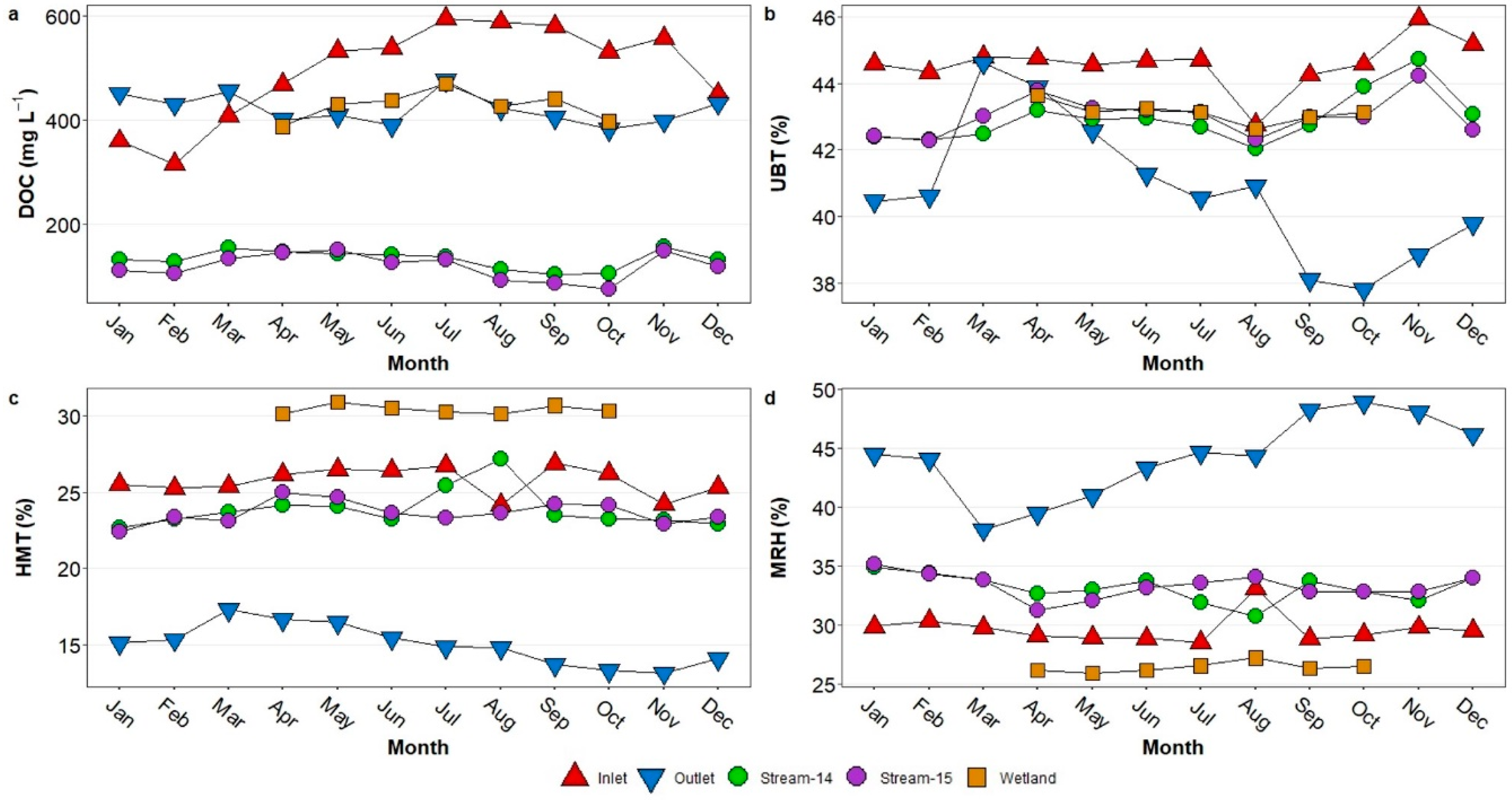
3.4. Seasonal Patterns
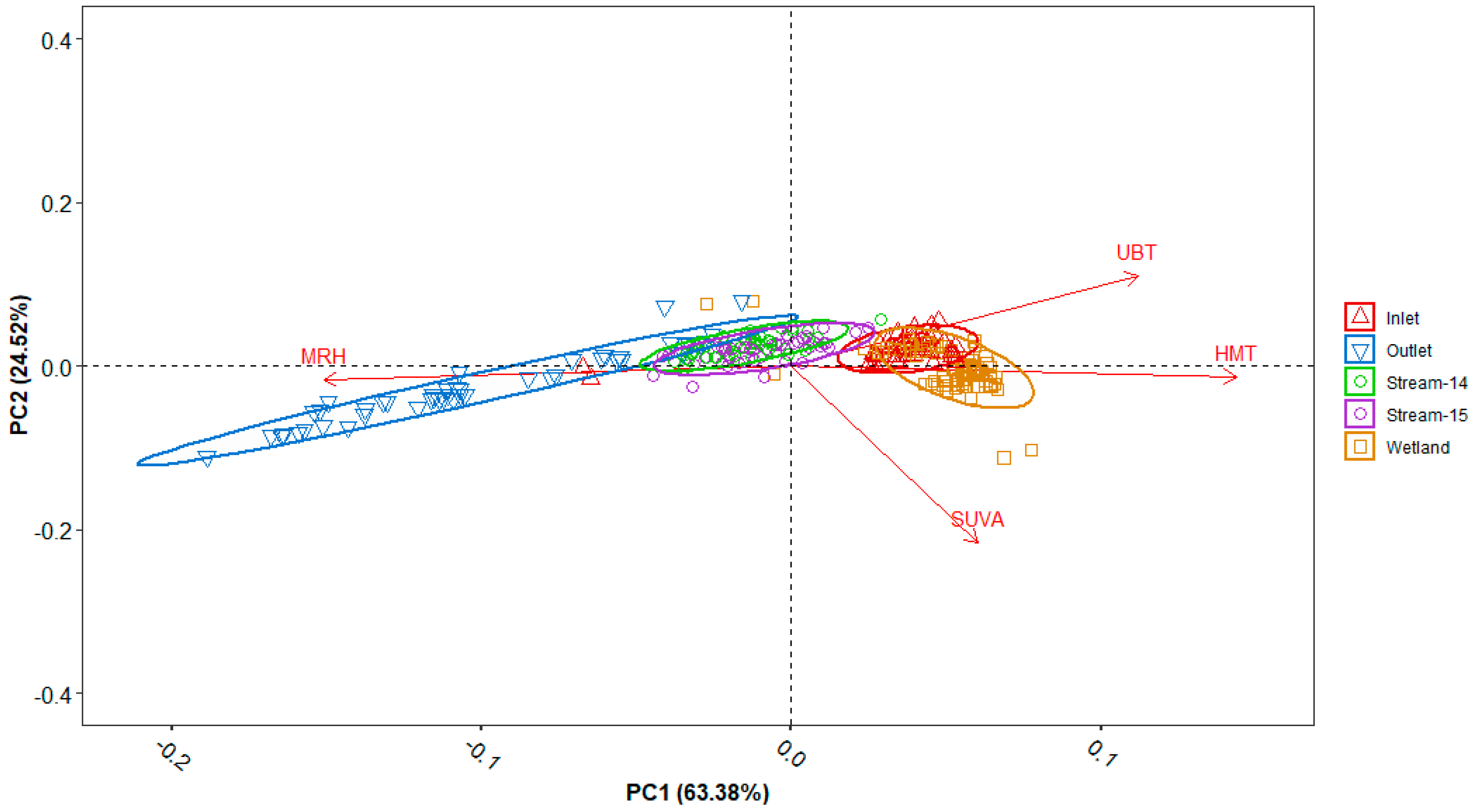
3.5. Principal Component Analysis (PCA)
4. Discussion
4.1. Parafac Component Interpretation
4.2. Upland Streams
4.3. Wetland Drainage
4.4. Within-Lake Processing
4.5. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Season. | Species | S14 | S15 | Wetland | Inlet | Outlet |
|---|---|---|---|---|---|---|
| Winter | Ca2+ (µmol L−1) | 740.0 (138.0) | 266.4 (76.9) | — | 153.4 (52.4) | 161.6 (79.1) |
| DOC (mg C L−1) | 1.7 (0.4) | 1.4 (0.4) | — | 4.4 (1.1) | 5.4 (0.2) | |
| SUVA (L·mg C−1 m−1) | 2.9 (0.3) | 3.2 (0.4) | — | 4.2 (0.2) | 3.6 (0.3) | |
| UBT (%) | 42.4 (0.4) | 42.6 (0.6) | — | 44.6 (0.4) | 42.1 (2.1) | |
| HMT (%) | 23.2 (1.1) | 23.0 (1.8) | — | 25.4 (0.4) | 16.0 (1.1) | |
| MRH (%) | 34.4 (1.4) | 34.4 (2.03 | — | 30.0 (0.5) | 41.9 (3.2) | |
| Snowmelt | Ca2+ (µmol L−1) | 637.4 (71.0) | 174.1 (40.6) | 131.8 (19.3) | 110.1 (15.9) | 104.6 (7.0) |
| DOC (mg C L−1) | 1.8 (0.1) | 1.7 (0.2) | 4.7 (1.0) | 5.6 (1.1) | 4.8 (0.4) | |
| SUVA (L·mg C−1 m−1) | 2.6 (0.3) | 3.0 (0.3) | 3.9 (0.3) | 4.3 (0.1) | 3.8 (0.2) | |
| UBT (%) | 43.2 (0.7) | 43.8 (0.4) | 43.6 (0.6) | 44.8 (0.4) | 43.9 (0.8) | |
| HMT (%) | 24.1 (0.2) | 25.0 (0.4) | 30.1 (1.7) | 26.1 (0.8) | 16.6 (0.4) | |
| MRH (%) | 32.7 (0.8) | 31.3 (0.5) | 26.2 (1.2) | 29.1 (0.5) | 39.5 (1.1) | |
| Spring | Ca2+ (µmol L−1) | 774.7 (66.8) | 219.5 (70.6) | 165.2 (23.2) | 148.1 (24.9) | 103.7 (11.0) |
| DOC (mgl C L−1) | 1.7 (0.1) | 1.6 (0.2) | 5.2 (1.1) | 6.4 (0.8) | 4.8 (0.2) | |
| SUVA (L·mg C−1 m−1) | 2.7 (0.2) | 3.0 (0.2) | 4 (0.4) | 4.3 (0.4) | 3.6 (0.2) | |
| UBT (%) | 43.0 (0.4) | 43.2 (0.3) | 43.2 (0.5) | 44.6 (0.3) | 41.8 (1.1) | |
| HMT (%) | 23.6 (0.9) | 24.1 (0.9) | 30.7 (1.6) | 26.5 (0.5) | 15.9 (0.8) | |
| MRH (%) | 33.4 (0.9) | 32.7 (1.1) | 26.1 (1.3) | 28.9 (0.7) | 42.3 (1.9) | |
| Summer | Ca2+ (µmol L−1) | 828.8 (69.9) | 365.6 (99.6) | 204.0 (25.5) | 173.5 (14.2) | 100.5 (2.7) |
| DOC (mg C L−1) | 1.4 (0.3) | 1.2 (0.4) | 5.3 (1.6) | 7.0 (1.7) | 5.2 (0.6) | |
| SUVA (L·mg C−1 m−1) | 7.7 (9.6) | 3.0 (0.3) | 4.3 (1.3) | 4.3 (0.7) | 3.4 (0.5) | |
| UBT (%) | 42.5 (0.5) | 42.8 (0.5) | 42.9 (0.6) | 43.8 (1.4) | 39.9 (2.8) | |
| HMT (%) | 25.5 (4.7) | 23.7 (0.9) | 30.4 (2.7) | 25.8 (2.5) | 14.5 (1.3) | |
| MRH (%) | 32.0 (4.7) | 33.5 (1.0) | 26.7 (2.5) | 30.4 (3.8) | 45.6 (4.1) | |
| Fall | Ca2+ (µmol L−1) | 809.5 (74.8) | 343.2 (120.1) | 221.9 (21.2) | 188.1 (47.2) | 123.2 (27.4) |
| DOC (mg C L−1) | 1.6 (0.3) | 1.4 (0.5) | 4.8 (1.5) | 6.1 (1.2) | 4.9 (0.3) | |
| SUVA (L·mg C−1 m−1) | 3.0 (0.2) | 3.3 (0.4) | 4.3 (0.5) | 4.3 (0.2) | 3.3 (0.3) | |
| UBT (%) | 43.8 (0.8) | 43.2 (0.9) | 43.1 (0.4) | 45.2 (0.7) | 38.9 (0.9) | |
| HMT (%) | 23.1 (1.3) | 23.5 (1.1) | 30.3 (2.1) | 25.3 (1.2) | 13.6 (0.5) | |
| MRH (%) | 33.1 (1.5) | 33.3 (1.5) | 26.5 (1.8) | 29.5 (0.8) | 47.5 (1.3) |
References
- Driscoll, C.T.; Lawrence, G.B.; Bulger, A.J.; Butler, T.J.; Cronan, C.S.; Eagar, C.; Lambert, K.F.; Likens, G.E.; Stoddard, J.L.; Weathers, K.C. Acidic Deposition in the Northeastern United States: Sources and Inputs, Ecosystem Effects, and Management Strategies. BioScience 2001, 51, 180. [Google Scholar] [CrossRef]
- Greaver, T.L.; Sullivan, T.J.; Herrick, J.D.; Barber, M.C.; Baron, J.S.; Cosby, B.J.; Deerhake, M.E.; Dennis, R.L.; Dubois, J.-J.B.; Goodale, C.L.; et al. Ecological Effects of Nitrogen and Sulfur Air Pollution in the US: What Do We Know? Front. Ecol. Environ. 2012, 10, 365–372. [Google Scholar] [CrossRef]
- Bailey, S.W.; Hornbeck, J.W.; Driscoll, C.T.; Gaudette, H.E. Calcium Inputs and Transport in A Base-Poor Forest Ecosystem as Interpreted by Sr Isotopes. Water Resour. Res. 1996, 32, 707–719. [Google Scholar] [CrossRef]
- Lawrence, G.B.; David, M.B.; Lovett, G.M.; Murdoch, P.S.; Burns, D.A.; Stoddard, J.L.; Baldigo, B.P.; Porter, J.H.; Thompson, A.W. Soil Calcium Status and the Response of Stream Chemistry to Changing Acidic Deposition Rates. Ecol. Appl. 1999, 9, 1059–1072. [Google Scholar] [CrossRef]
- Likens, A.G.E.; Driscoll, C.T.; Buso, D.C. Long-Term Effects of Acid Rain Response and Recovery of a Forest Ecosystem. Science 1996, 272, 244–246. [Google Scholar] [CrossRef]
- Cronan, C.S.; Schofield, C.L. Relationships between Aqueous Aluminum and Acidic Deposition in Forested Watersheds of North America and Northern Europe. Envrion. Sci. Technol. 1990, 24, 1100–1105. [Google Scholar] [CrossRef]
- Hawley, G.J.; Schaberg, P.G.; Eagar, C.; Borer, C.H. Calcium Addition at the Hubbard Brook Experimental Forest Reduced Winter Injury to Red Spruce in a High-Injury Year. Can. J. Forest Res. 2006, 36, 2544–2549. [Google Scholar] [CrossRef]
- Kunito, T.; Isomura, I.; Sumi, H.; Park, H.D.; Toda, H.; Otsuka, S.; Nagaoka, K.; Saeki, K.; Senoo, K. Aluminum and Acidity Suppress Microbial Activity and Biomass in Acidic Forest Soils. Soil Biol. Biochem. 2016, 97, 23–30. [Google Scholar] [CrossRef]
- Baker, J.P.; Schofield, C.L. Aluminum toxicity to fish in acidic waters. Water Air Soil Pollut. 1982, 18, 289–309. [Google Scholar] [CrossRef]
- Li, W.; Johnson, C.E. Relationships among PH, Aluminum Solubility and Aluminum Complexation with Organic Matter in Acid Forest Soils of the Northeastern United States. Geoderma 2016, 271, 234–242. [Google Scholar] [CrossRef]
- Tonitto, C.; Goodale, C.L.; Weiss, M.S.; Frey, S.D.; Ollinger, S.V. The Effect of Nitrogen Addition on Soil Organic Matter Dynamics: A Model Analysis of the Harvard Forest Chronic Nitrogen Amendment Study and Soil Carbon Response to Anthropogenic N Deposition. Biogeochemistry 2014, 11, 431–454. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Driscoll, K.M.; Fakhraei, H.; Civerolo, K. Long-Term Temporal Trends and Spatial Patterns in the Acid-Base Chemistry of Lakes in the Adirondack Region of New York in Response to Decreases in Acidic Deposition. Atmos. Environ. 2016, 146, 5–14. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Shortle, W.C.; David, M.B.; Smith, K.T.; Warby, R.A.F.; Lapenis, A.G. Early Indications of Soil Recovery from Acidic Deposition in U.S. Red Spruce Forests. Soil Sci. Soc. Am. J. 2012, 76, 1407–1417. [Google Scholar] [CrossRef]
- Wason, J.W.; Beier, C.M.; Battles, J.J.; Dovciak, M. Acidic Deposition and Climate Warming as Drivers of Tree Growth in High-Elevation Spruce-Fir Forests of the Northeastern US. Front. For. Glob. Chang. 2019, 2, 63. [Google Scholar] [CrossRef]
- Kosiba, A.M.; Schaberg, P.G.; Rayback, S.A.; Hawley, G.J. The Surprising Recovery of Red Spruce Growth Shows Links to Decreased Acid Deposition and Elevated Temperature. Sci. Total Environ. 2018, 637–638, 1480–1491. [Google Scholar] [CrossRef]
- Baldigo, B.P.; George, S.D.; Lawrence, G.B.; Paul, E.A. Declining Aluminum Toxicity and the Role of Exposure Duration on Brook Trout Mortality in Acidified Streams of the Adirondack Mountains, New York, USA. Environ. Toxicol. Chem. 2020, 39, 623–636. [Google Scholar] [CrossRef]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; de Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J.; et al. Dissolved Organic Carbon Trends Resulting from Changes in Atmospheric Deposition Chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef]
- Fahey, T.J.; Siccama, T.G.; Driscoll, C.T.; Likens, G.E.; Campbell, J.; Johnson, C.E.; Battles, J.J.; Aber, J.D.; Cole, J.J.; Fisk, M.C.; et al. The Biogeochemistry of Carbon at Hubbard Brook. Biogeochemistry 2005, 75, 109–176. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Battin, T.J.; Kaplan, L.A.; Findlay, S.; Hopkinson, C.S.; Marti, E.; Packman, A.I.; Newbold, J.D.; Sabater, F. Biophysical Controls on Organic Carbon Fluxes in Fluvial Networks. Nat. Geosci. 2008, 1, 95–100. [Google Scholar] [CrossRef]
- Ussiri, D.A.N.; Johnson, C.E. Characterization of Organic Matter in a Northern Hardwood Forest Soil by 13C NMR Spectroscopy and Chemical Methods. Geoderma 2003, 111, 123–149. [Google Scholar] [CrossRef]
- Jaffé, R.; McKnight, D.; Maie, N.; Cory, R.; McDowell, W.H.; Campbell, J.L. Spatial and Temporal Variations in DOM Composition in Ecosystems: The Importance of Long-Term Monitoring of Optical Properties: Variations in DOM Composition in Ecosystems. J. Geophys. Res. Biogeosci. 2008, 113. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling Downwards—Dissolved Organic Matter in Soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- SanClements, M.D.; Oelsner, G.P.; McKnight, D.M.; Stoddard, J.L.; Nelson, S.J. New Insights into the Source of Decadal Increases of Dissolved Organic Matter in Acid-Sensitive Lakes of the Northeastern United States. Environ. Sci. Technol. 2012, 46, 3212–3219. [Google Scholar] [CrossRef]
- Brothers, S.; Köhler, J.; Attermeyer, K.; Grossart, H.P.; Mehner, T.; Meyer, N.; Scharnweber, K.; Hilt, S. A Feedback Loop Links Brownification and Anoxia in a Temperate, Shallow Lake. Limnol. Oceanogr. 2014, 59, 1388–1398. [Google Scholar] [CrossRef]
- Solomon, C.T. Dissolved Organic Matter Causes Genetic Damage in Lake Zooplankton via Oxidative Stress. Funct. Ecol. 2017, 31, 806–807. [Google Scholar] [CrossRef]
- Williamson, C.E.; Overholt, E.P.; Pilla, R.M.; Leach, T.H.; Brentrup, J.A.; Knoll, L.B.; Mette, E.M.; Moeller, R.E. Ecological Consequences of Long-Term Browning in Lakes. Sci. Rep. 2016, 5. [Google Scholar] [CrossRef]
- Dittman, J.A.; Shanley, J.B.; Driscoll, C.T.; Aiken, G.R.; Chalmers, A.T.; Towse, J.E.; Selvendiran, P. Mercury Dynamics in Relation to Dissolved Organic Carbon Concentration and Quality during High Flow Events in Three Northeastern U.S. Streams. Water Resourc. Res. 2010, 46. [Google Scholar] [CrossRef]
- Schartup, A.T.; Ndu, U.; Balcom, P.H.; Mason, R.P.; Sunderland, E.M. Contrasting Effects of Marine and Terrestrially Derived Dissolved Organic Matter on Mercury Speciation and Bioavailability in Seawater. Environ. Sci. Technol. 2015, 49, 5965–5972. [Google Scholar] [CrossRef]
- Piatek, K.B.; Christopher, S.F.; Mitchell, M.J. Spatial and Temporal Dynamics of Stream Chemistry in a Forested Watershed. Hydrol. Earth Syst. Sci. 2009, 13, 423–439. [Google Scholar] [CrossRef]
- Kang, P.G.; Mitchell, M.J. Bioavailability and Size-Fraction of Dissolved Organic Carbon, Nitrogen, and Sulfur at the Arbutus Lake Watershed, Adirondack Mountains, NY. Biogeochemistry 2013, 115, 213–234. [Google Scholar] [CrossRef]
- Kang, P.G.; Mitchell, M.J.; McHale, P.J.; Driscoll, C.T.; Inamdar, S.; Park, J.-H. Importance of Within-Lake Processes in Affecting the Dynamics of Dissolved Organic Carbon and Dissolved Organic and Inorganic Nitrogen in an Adirondack Forested Lake/Watershed. Biogeosciences 2016, 13, 2787–2801. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence Spectroscopy and Multi-Way Techniques. PARAFAC. Anal. Methods 2013, 5, 6557. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R.; Colin, A. Stedmon and Rasmus Bro. Characterizing Dissolved Organic Matter Fluorescence with Parallel Factor Analysis: A Tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Park, J.H.; Mitchell, M.J.; Driscoll, C.T. Winter-Time Climatic Control on Dissolved Organic Carbon Export and Surface Water Chemistry in an Adirondack Forested Watershed. Environ. Sci. Technol. 2005, 39, 6993–6998. [Google Scholar] [CrossRef]
- New York State Energy Research and Development Authority (NYSERDA). Importance of Acidic and Mercury Deposition in Relation to Climate Change in the Adirondack Mountains: Biogeochemical Responses. N. Y. Stat Energy Res. Dev. Auth. Rep. 2015, 15-04s, 9. [Google Scholar]
- McHale, M.R.; Mitchell, M.J.; McDonnel, J.J.; Cirmo, C.P. Nitrogen Solutes in an Adirondack Forested Watershed: Importance of Dissolved Organic Nitrogen. Biogeochemistry 2000, 48, 165–184. [Google Scholar] [CrossRef]
- Bischoff, J.M.; Bukaveckas, P.; Mitchell, M.J.; Hurd, T. N Storage and Cycling in Vegetation of a Forested Wetland: Implications for Watershed N Processing. Water Air Soil Pollut. 2001, 128, 97–114. [Google Scholar] [CrossRef]
- Owen, J.S.; Mitchell, M.J.; Michener, R.H. Stable Nitrogen and Carbon Isotopic Composition of Seston and Sediment in Two Adirondack Lakes. Can. J. Fish. Aquat. Sci. 1999, 56, 2186–2192. [Google Scholar] [CrossRef]
- Driscoll, C.T.; van Dreason, R. Seasonal and long-term temporal patterns in the chemistry of Adirondack Lakes. Water Air Soil Pollut. 1993, 67, 319–344. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of Specific Ultraviolet Absorbance as an Indicator of the Chemical Composition and Reactivity of Dissolved Organic Carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; He, B.; Nover, D.; Yang, G.; Chen, W.; Meng, H.; Zou, S.; Liu, C. Water Quality Assessment and Pollution Source Identification of the Eastern Poyang Lake Basin Using Multivariate Statistical Methods. Sustainability 2016, 8, 133. [Google Scholar] [CrossRef]
- Walker, S.A.; Amon, R.M.W.; Stedmon, C.A. Variations in High-Latitude Riverine Fluorescent Dissolved Organic Matter: A Comparison of Large Arctic Rivers: FDOM in Large Arctic Rivers. J. Geophys. Res. Biogeosci. 2013, 118, 1689–1702. [Google Scholar] [CrossRef]
- Kothawala, D.N.; von Wachenfeldt, E.; Koehler, B.; Tranvik, L.J. Selective Loss and Preservation of Lake Water Dissolved Organic Matter Fluorescence during Long-Term Dark Incubations. Sci. Total Environ. 2012, 433, 238–246. [Google Scholar] [CrossRef]
- Shutova, Y.; Baker, A.; Bridgeman, J.; Henderson, R.K. Spectroscopic Characterization of Dissolved Organic Matter Changes in Drinking Water Treatment: From PARAFAC Analysis to Online Monitoring Wavelengths. Water Res. 2014, 54, 159–169. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of Marine and Terrestrial DOM in Seawater Using Excitation-Emission Matrix Spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Santín, C.; Yamashita, Y.; Otero, X.L.; Álvarez, M.Á.; Jaffé, R. Characterizing Humic Substances from Estuarine Soils and Sediments by Excitation-Emission Matrix Spectroscopy and Parallel Factor Analysis. Biogeochemistry 2009, 96, 131–147. [Google Scholar] [CrossRef]
- Yamashita, Y.; Scinto, L.J.; Maie, N.; Jaffé, R. Dissolved Organic Matter Characteristics Across a Subtropical Wetland’s Landscape: Application of Optical Properties in the Assessment of Environmental Dynamics. Ecosystems 2010, 13, 1006–1019. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kloeppel, B.D.; Knoepp, J.; Zausen, G.L.; Jaffé, R. Effects of Watershed History on Dissolved Organic Matter Characteristics in Headwater Streams. Ecosystems 2011, 14, 1110–1122. [Google Scholar] [CrossRef]
- Cawley, K.M.; Campbell, J.; Zwilling, M.; Jaffé, R. Evaluation of Forest Disturbance Legacy Effects on Dissolved Organic Matter Characteristics in Streams at the Hubbard Brook Experimental Forest, New Hampshire. Aquat. Sci. 2014, 76, 611–622. [Google Scholar] [CrossRef]



| Season. | Dates Included | Precipitation | Inlet | Outlet | |||
|---|---|---|---|---|---|---|---|
| Fall | Nov ’16 – Dec ’16, Oct ‘17 | 260.9 | (22%) | 126.6 | (15%) | 120.6 | (15%) |
| Winter | Jan ’17 – Mar ‘17 | 296.9 | (24%) | 237.8 | (28%) | 195.8 | (25%) |
| Snowmelt | Apr ‘17 | 94.8 | (8%) | 194.9 | (23%) | 169.2 | (22%) |
| Spring | May ’17 – Jun ‘17 | 289.3 | (24%) | 171.6 | (20%) | 172.5 | (22%) |
| Summer | July ’17 – Sep ‘17 | 272.2 | (22%) | 132.1 | (15%) | 128.7 | (16%) |
| Total | Nov ’16 – Oct ‘17 | 1214.2 | 863.0 | 786.8 | |||
| PARAFAC Component | Ex. Max (nm) | Em. Max (nm) | Traditional Classification (Peaks) | Walker et al. 2013 | Shutova et al. 2013 | Kothawala et al. 2012 | Sourcing | Description |
|---|---|---|---|---|---|---|---|---|
| UC1 | 265 (345) | 455 | A+C | — | C1 | C1 | Allochthonous | Humic-like |
| UC2 | 275 (400) | 510 | — | C3 | — | — | Allochthonous | High Molecular Weight |
| UC3 | 315 | 405 | M | — | C2 | C2 | Allochthonous | Humic-like; reprocessed |
| LC1 | 265 (365) | 465 | A+C | C2 | C1 | — | Allochthonous | Humic-like |
| LC2 | 310 | 425 | M | C1 | C2 | — | Allochthonous | Humic-like; reprocessed |
| LC3 | 280 (420) | 510 | — | C3 | — | — | Allochthonous | High Molecular Weight |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. LoRusso, N.; McHale, M.; McHale, P.; Montesdeoca, M.; Zeng, T.; T. Driscoll, C. Landscape Influence on the Browning of a Lake Watershed in the Adirondack Region of New York, USA. Soil Syst. 2020, 4, 50. https://doi.org/10.3390/soilsystems4030050
A. LoRusso N, McHale M, McHale P, Montesdeoca M, Zeng T, T. Driscoll C. Landscape Influence on the Browning of a Lake Watershed in the Adirondack Region of New York, USA. Soil Systems. 2020; 4(3):50. https://doi.org/10.3390/soilsystems4030050
Chicago/Turabian StyleA. LoRusso, Nicholas, Marykate McHale, Patrick McHale, Mario Montesdeoca, Teng Zeng, and Charles T. Driscoll. 2020. "Landscape Influence on the Browning of a Lake Watershed in the Adirondack Region of New York, USA" Soil Systems 4, no. 3: 50. https://doi.org/10.3390/soilsystems4030050
APA StyleA. LoRusso, N., McHale, M., McHale, P., Montesdeoca, M., Zeng, T., & T. Driscoll, C. (2020). Landscape Influence on the Browning of a Lake Watershed in the Adirondack Region of New York, USA. Soil Systems, 4(3), 50. https://doi.org/10.3390/soilsystems4030050







