Novel Application of Hybrid Anion Exchange Resin for Phosphate Desorption Kinetics in Soils: Minimizing Re-Adsorption of Desorbed Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
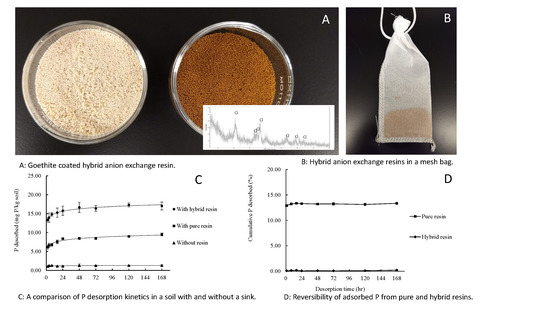
2.2. Anion Exchange Resin and Hybrid Resin
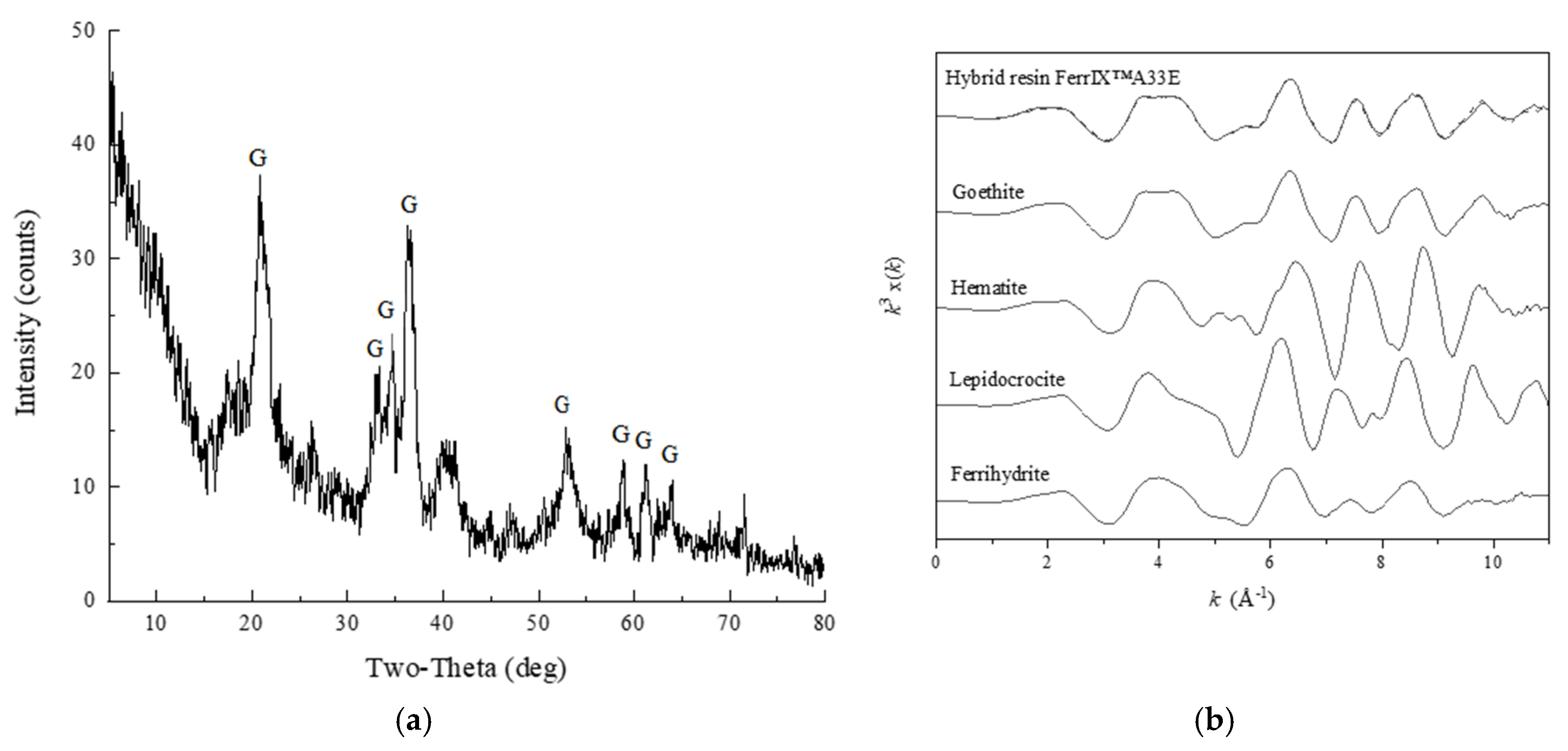
2.3. Mineralogical Characterization of the Iron Oxide Coating of the Hybrid Resin
2.4. Physicochemical Characterization of Soils
2.5. Total P, Inorganic P (IP), and Organic P (OP) Fractionation of Soils
2.6. Phosphate Adsorption Isotherm in the Anion Exchange Resin and the Hybrid Resin
2.7. Irreversibility of Adsorbed Phosphate in Resins
2.8. Phosphate Desorption Kinetics in Soils without P Sink
2.9. Phosphate Desorption Kinetics in Soils Using Resin Bags
2.10. Stability of Fe Oxide Coatings in the Hybrid Resin
3. Results and Discussion
3.1. Characterization of Soils and Resins
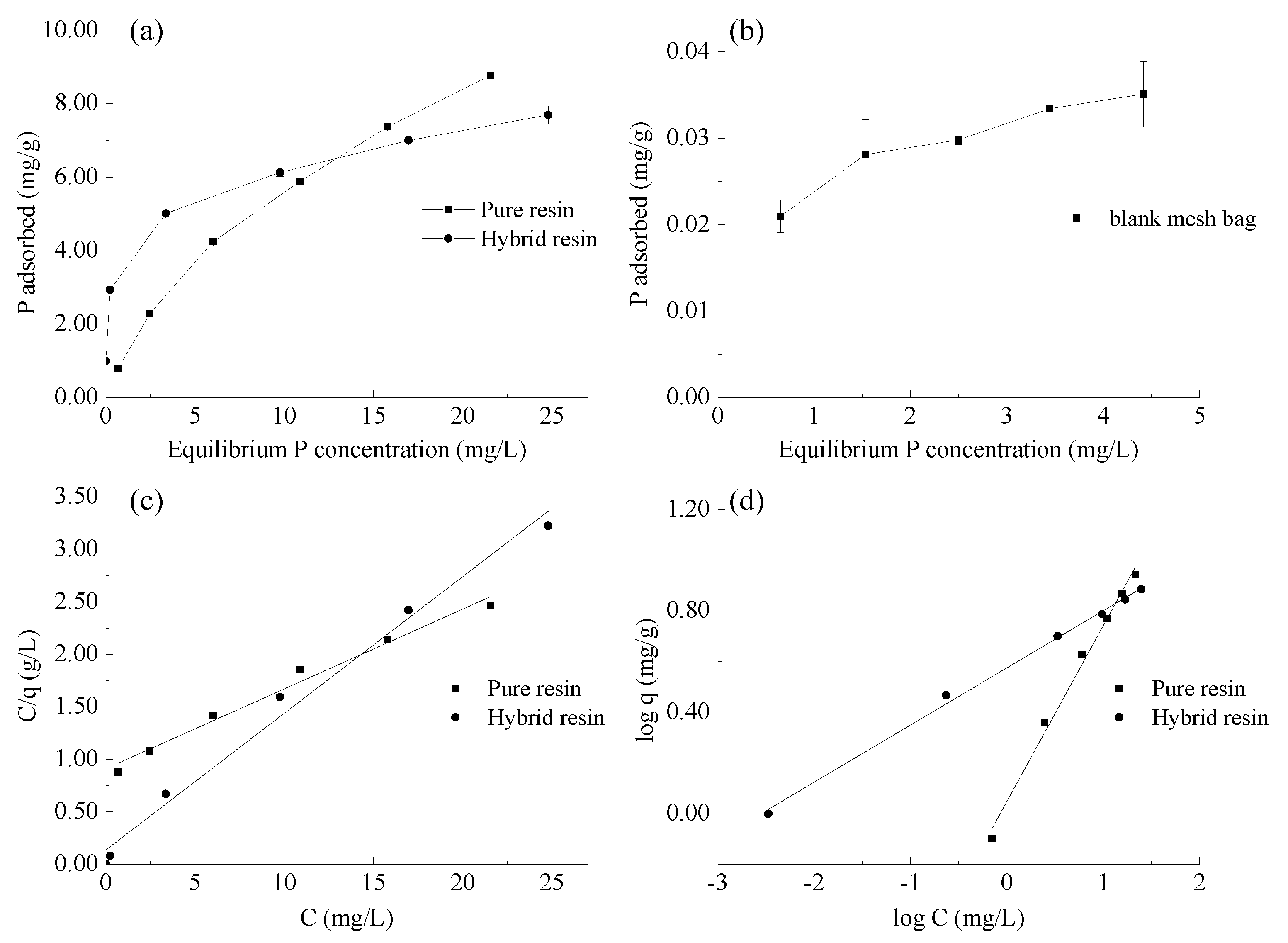
3.2. Phosphate Adsorption Isotherm in the Resins
3.3. Phosphate Irreversibility Tests in the Resins
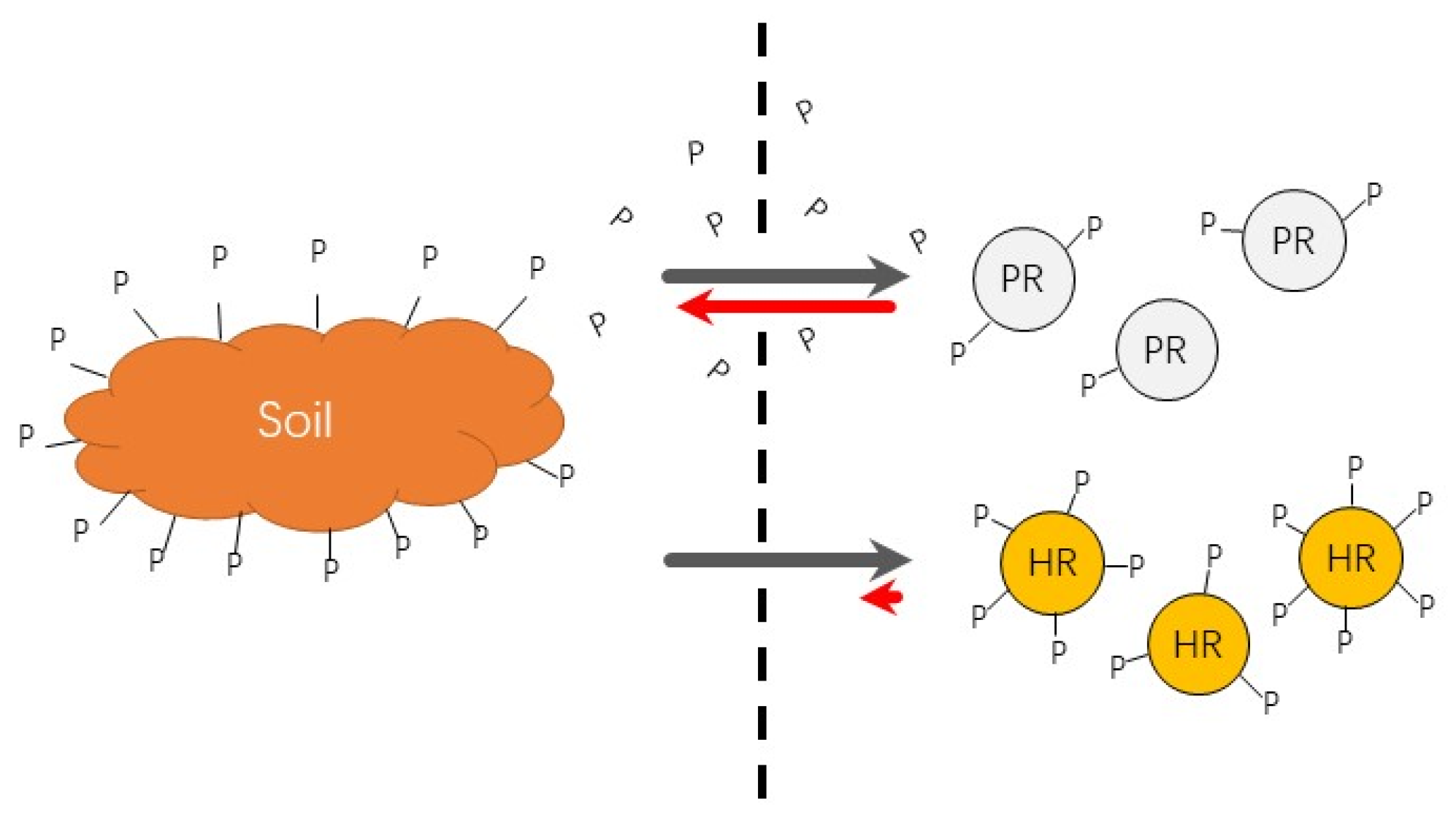
3.4. Phosphate Desorption in Soils
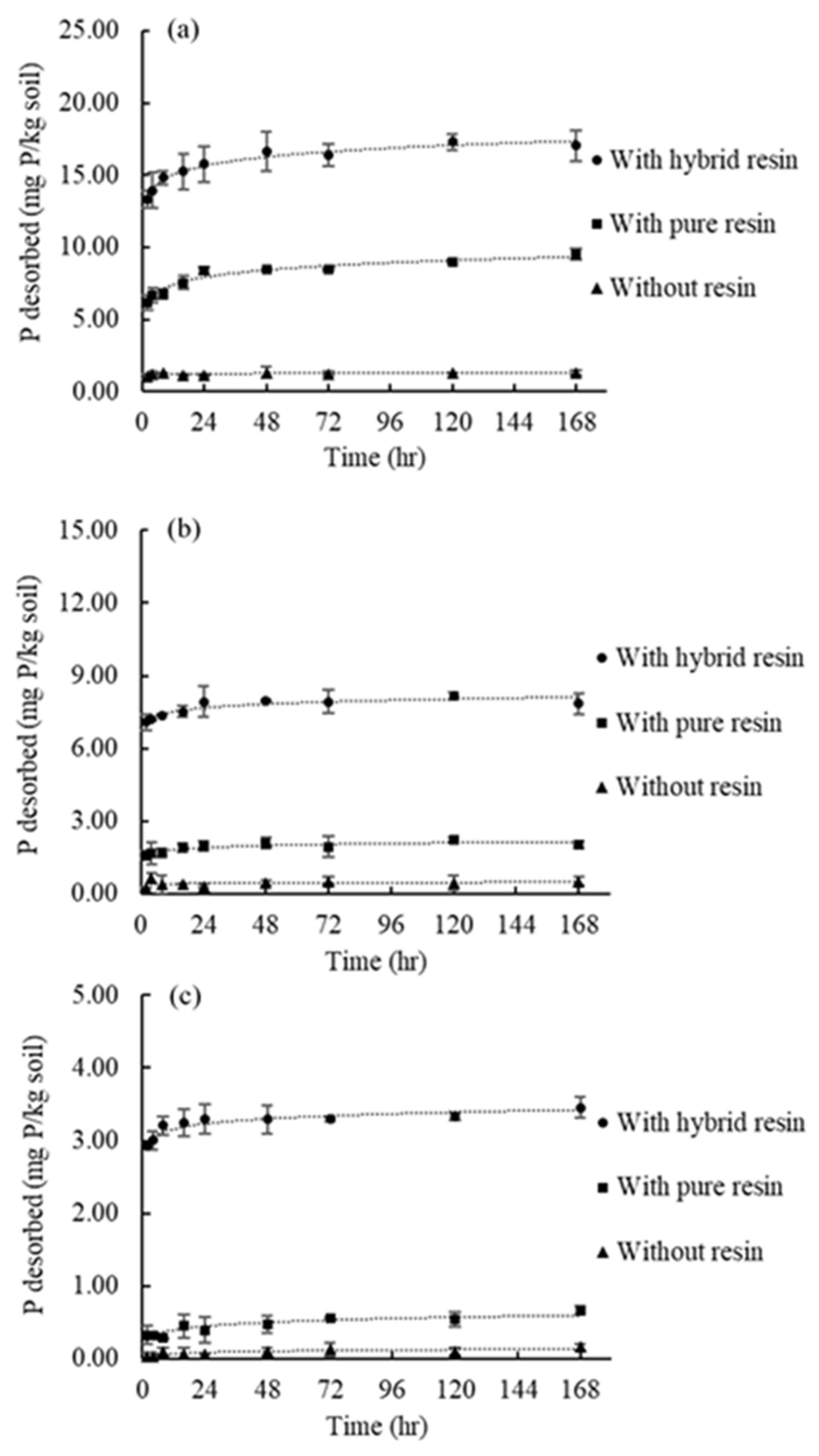
3.4.1. The Extent of P Desorption from Soils
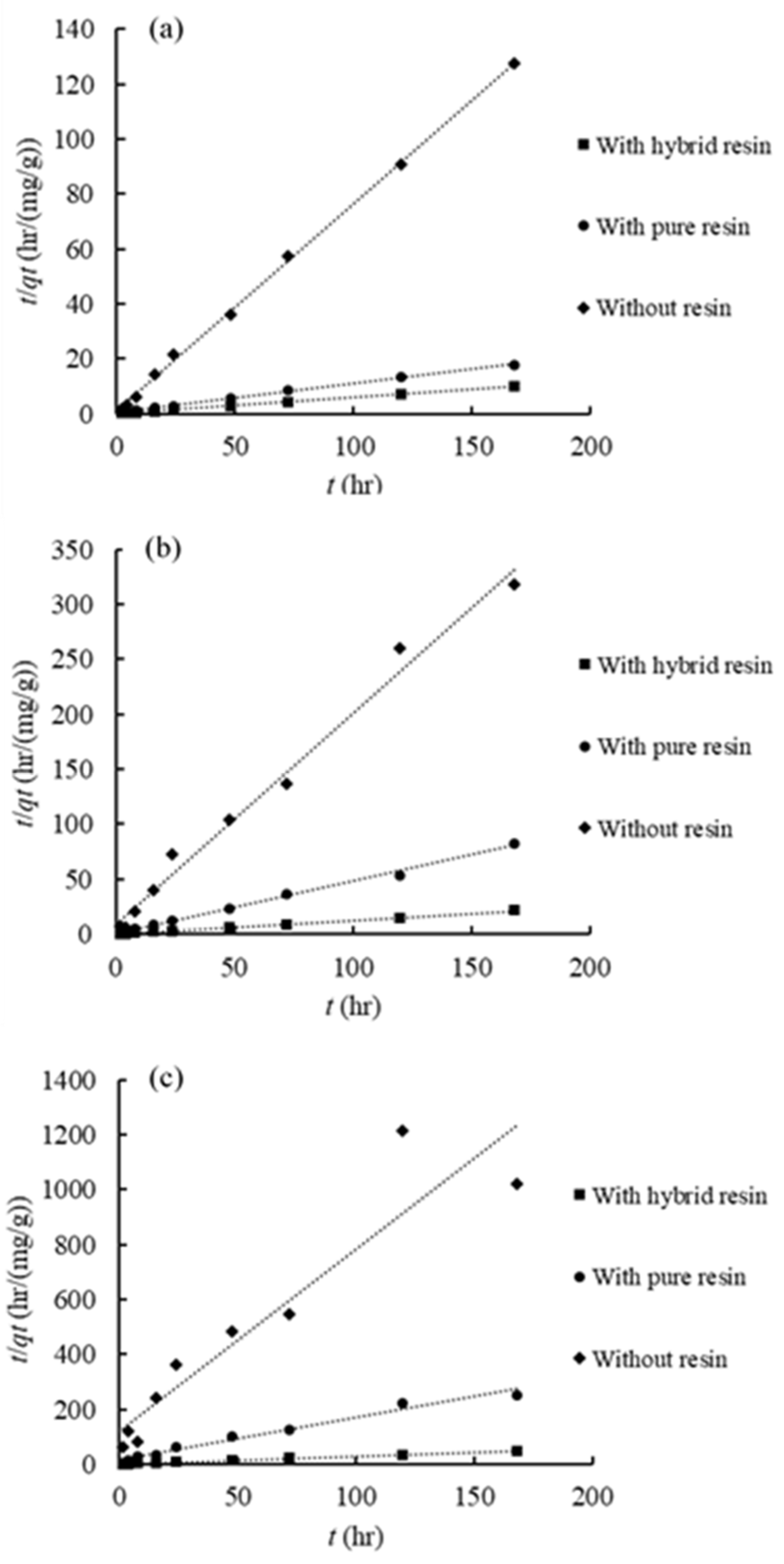
3.4.2. Phosphate Desorption Kinetics
3.4.3. Phosphate Desorption Kinetic Rate Affected by the Mass of Resin
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sparks, D.L. Kinetics of Soil Chemical Processes, 1st ed.; Academic Press, Inc.: San Diego, CA, USA, 1989; pp. 51–53. [Google Scholar]
- Arai, Y.; Livi, K.J. Underassessed phosphorus fixation mechanisms in soil sand fraction. Geoderma 2013, 192, 422–429. [Google Scholar] [CrossRef]
- Harris, W.G.; Rhue, R.D.; Kidder, G.; Brown, R.B.; Littell, R. Phosphorus retention as related to morphology of sandy coastal plain soil materials. Soil Sci. Soc. Am. J. 1996, 60, 1513–1521. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Lavkulich, L.M. Effect of sesquioxide coatings on surface charge of standard mineral and soil samples. Soil Sci. Soc. Am. J. 1983, 47, 1252–1260. [Google Scholar] [CrossRef]
- Zasoski, R.J.; Burau, R.G. A technique for studying the kinetics of adsorption in suspensions. Soil Sci. Soc. Am. J. 1978, 42, 372–374. [Google Scholar] [CrossRef]
- Zhao, D.; Sengupta, A.K. Ultimate removal of phosphate from wastewater using a new class of polymeric ion exchangers. Water Res. 1998, 32, 1613–1625. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Huang, F.; Zhang, J. Removal of phosphorus from anaerobic membrane bioreactor effluent by ion exchange resin. Sep. Sci. Technol. 2016, 51, 2833–2843. [Google Scholar] [CrossRef]
- Das Gupta, M.; Loganathan, P.; Vigneswaran, S. Adsorptive Removal of Nitrate and Phosphate from Water by a Purolite Ion Exchange Resin and Hydrous Ferric Oxide Columns in Series. Sep. Sci. Technol. 2012, 47, 1785–1792. [Google Scholar] [CrossRef]
- Awual, M.R.; Jyo, A. Assessing of phosphorus removal by polymeric anion exchangers. Desalination 2011, 281, 111–117. [Google Scholar] [CrossRef]
- Van Raij, B.; Quaggio, J.A.; da Silva, N.M. Extraction of phosphorus, potassium, calcium, and magnesium from soils by an ion-exchange resin procedure. Commun. Soil Sci. Plant Anal. 1986, 17, 547–566. [Google Scholar] [CrossRef]
- Somasiri, L.L.W.; Edwards, A.C. An Ion Exchange Resin Method for Nutrient Extraction of Agricultural Advisory Soil Samples. Commun. Soil Sci. Plant Anal. 1992, 23, 645–657. [Google Scholar] [CrossRef]
- Sendrowski, A.; Boyer, T.H. Phosphate removal from urine using hybrid anion exchange resin. Desalination 2013, 322, 104–112. [Google Scholar] [CrossRef]
- You, X.; Guaya, D.; Farran, A.; Valderrama, C.; Cortina, J.L. Phosphate removal from aqueous solution using a hybrid impregnated polymeric sorbent containing hydrated ferric oxide (HFO). J. Chem. Technol. Biotechnol. 2016, 91, 693–704. [Google Scholar] [CrossRef]
- Pan, B.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.; Zheng, S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009, 151, 19–29. [Google Scholar] [CrossRef]
- Sengupta, S.; Pandit, A. Selective removal of phosphorus from wastewater combined with its recovery as a solid-phase fertilizer. Water Res. 2011, 45, 3318–3330. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Tejedor, M.I.; Anderson, M.A. Protonation of Phosphate on the Surface of Goethite As Studied by CIR-FTIR and Electrophoretic Mobility. Langmuir 1990, 6, 602–611. [Google Scholar] [CrossRef]
- Arai, Y.; Sparks, D.L. ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. J. Colloid Interface Sci. 2001, 241, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Elzinga, E.J.; Sparks, D.L. Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J. Colloid Interface Sci. 2007, 308, 53–70. [Google Scholar] [CrossRef]
- Gasparatos, D.; Massas, I.; Godelitsas, A. Fe-Mn concretions and nodules formation in redoximorphic soils and their role on soil phosphorus dynamics: Current knowledge and gaps. Catena 2019, 182, 104106. [Google Scholar] [CrossRef]
- Pan, B.; Wu, J.; Pan, B.; Lv, L.; Zhang, W.; Xiao, L.; Wang, X.; Tao, X.; Zheng, S. Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents. Water Res. 2009, 43, 4421–4429. [Google Scholar] [CrossRef]
- Johir, M.A.H.; Nguyen, T.T.; Mahatheva, K.; Pradhan, M.; Ngo, H.H.; Guo, W.; Vigneswaran, S. Removal of phosphorus by a high rate membrane adsorption hybrid system. Bioresour. Technol. 2016, 201, 365–369. [Google Scholar] [CrossRef]
- Blaney, L.M.; Cinar, S.; SenGupta, A.K. Hybrid anion exchanger for trace phosphate removal from water and wastewater. Water Res. 2007, 41, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, J.A.; Boyer, T.H. Phosphate recovery using hybrid anion exchange: Applications to source-separated urine and combined wastewater streams. Water Res. 2013, 47, 5003–5017. [Google Scholar] [CrossRef] [PubMed]
- Nur, T.; Johir, M.A.H.; Loganathan, P.; Nguyen, T.; Vigneswaran, S.; Kandasamy, J. Phosphate removal from water using an iron oxide impregnated strong base anion exchange resin. J. Ind. Eng. Chem. 2014, 20, 1301–1307. [Google Scholar] [CrossRef] [Green Version]
- Bottini, A.; Rizzo, L. Phosphorus Recovery from Urban Wastewater Treatment Plant Sludge Liquor by Ion Exchange. Sep. Sci. Technol. 2012, 47, 613–620. [Google Scholar] [CrossRef]
- Boyer, T.H.; Persaud, A.; Banerjee, P.; Palomino, P. Comparison of low-cost and engineered materials for phosphorus removal from organic-rich surface water. Water Res. 2011, 45, 4803–4814. [Google Scholar] [CrossRef]
- USDA Soil Survey. Soil Survey of Douglas County; Natural Accessibility Conservation Service: IL, USA, 2006; Web Soil Survey. Available online: https://www.blogs.nrcs.usda.gov/Internet/FSE_MANUSCRIPTS/illinois/IL041/0/Douglas_IL.pdf (accessed on 1 May 2020).
- Klute, A.; Carter, D.L.; Mortland, M.M.; Kemper, W.D. Specific Surface; John Wiley & Sons, Inc.: Weinheim, Germany, 1986; pp. 413–423. [Google Scholar]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization, 2nd ed.; John Wiley & Sons, Inc.: Weinheim, Germany, 2000. [Google Scholar]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.W. Soil pH and soil acidity. In Methods Soil Analysis Part 3—Chemical Methods, 1st ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- McLean, E.O. Soil pH and lime requirement determination. In Methods of Soil Analysis Part 2 Chemical and Microbiological Properties, 1st ed.; Page, A.L., Ed.; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Schulte, E.E.; Hopkins, B.G. Estimation of soil organic matter by weight loss-on-ignition. In Soil Organic Matter: Analysis and Interpretation, 1st ed.; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Jr., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; Volume 46, pp. 21–31. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle size analysis. In Methods Soil Analysis Part 1—Physical Mineralogical Methods, 2nd ed.; Klute, A., Ed.; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1986; Volume 9, pp. 901–926. [Google Scholar]
- Ross, D.S.; Ketterings, Q. Recommended methods for determining soil cation exchange capacity. In Recommended Soil Testing Procedures for the Northeastern United States; Sims, J.T., Wolf, A., Eds.; University of Delaware: Newark, DE, USA, 1995; pp. 62–69. [Google Scholar]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- He, Z.; Baligar, V.; Ritchey, K.; Martens, D. Determination of Soluble Phosphorus in the Presence of Organic Ligands or Fluoride. Soil Sci. Soc. Am. J. 1998, 62, 1538–1541. [Google Scholar] [CrossRef]
- Kuo, S. Phosphorus. In Methods of Soil Analysis Part 3 Chemical Method, 1st ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Ho, Y.S.; McKay, G. The sorption of lead(II) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of chemisorption of gases on solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Weber, W.; Morris, J. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Upping, E.; Thompson, D.W.; Ohnstad, M.; Hetherington, N.B. Effects of ph on the release of metals from naturally-occurring oxides of mn and fe. Environ. Technol. Lett. 1986, 7, 109–114. [Google Scholar]
- Sager, M. Chemical speciation and environmental mobility of heavy metals in sediments and soils. Tech. Instrum. Anal. Chem. 1992, 12, 133–175. [Google Scholar]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef]
- Xu, S.; Gentry, L.; Chen, K.; Arai, Y. Intensive agricultural management induced subsurface accumulation of labile phosphorus in tile line dominated Midwestern agricultural soils. Soil Sci. Soc. Am. J. 2020. [Google Scholar] [CrossRef]
- Fernández, F.; Hoeft, R. Managing soil pH and crop nutrients. In Illinois Agronomy Handbook, 24th ed.; University of Illinois at Urbana-Champaign, College of Agriculture, Cooperative Extension Service: Champaign, IL, USA, 2009; pp. 91–112. [Google Scholar]
- Li, Z.; Arai, Y. Comprehensive evaluation of mineral adsorbents for phosphate removal in agricultural water. Adv. Agron. 2020, 164. in press. [Google Scholar]
- Hongshao, Z.; Stanforth, R. Competitive adsorption of phosphate and arsenate on goethite. Environ. Sci. Technol. 2001, 35, 4753–4757. [Google Scholar] [CrossRef]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]



| Soil Sample ID | Depth | pH | %IC † | %OC † | CEC † | %base † | M3P † | B1P † | OM † | Texture | TIP † | TOP † | TP † |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | ------% by wt.------ | cmolc/kg | % | -------mg/kg------- | % | ---------------mg/kg------------- | |||||||
| S_18 | 0–18 | 6.95 | 0.11 (±0.03) ‡ | 2.25 (±0.01) | 26.72 (±2.86) | 95.69 (±0.01) | 14.50 (±0.71) | 10.00 (±1.41) | 4.27 (±0.02) | SC § | 251.90 (±0.18) | 139.79 (±17.98) | 391.69 (±18.16) |
| S_90 | 72–90 | 8.03 | 0.27 (±0.06) | 0.80 (±0.01) | 23.23 (±0.46) | 96.05 (±0.31) | 5.00 (±0.00) | 2.00 (±0.00) | 2.12 (±0.02) | SCL § | 363.74 (±11.38) | 54.77 (±16.30) | 418.51 (±4.91) |
| S_180 | 162–180 | 8.20 | 2.18 (±0.11) | 0.54 (±0.02) | 28.80 (±1.19) | 96.37 (±0.03) | 1.00 (±0.00) | <1 | 1.39 (±0.01) | SC | 189.35 (±7.17) | 7.05 (±1.11) | 196.41 (±8.28) |
| Resin | Matrix | Structure Type | Functional Groups | Physical Form | Particle Size (µm) | Iron Content (mg/g) | Specific Surface Areas (m2/g) |
|---|---|---|---|---|---|---|---|
| AMBERLITE™ HPR9200, pure anion exchange resin | Polystyrene-DVB | Macroporous | Strong-base | White, spherical beads | 640 ± 50 | 0 | 1625.1 ± 20.9 |
| FerrIX™A33E, hybrid resin | Polystyrene-DVB | Macroporous | Strong-base | Brown, spherical beads | 750 ± 150 | 196.0 ± 3.0 | 810.4 ± 38.2 |
| Resin | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | Km (L/g) | R2 | n | Kf | R2 | |
| AMBERLITE™ HPR9200 (Pure resin) | 13.16 | 1.10 | 0.983 | 1.44 | 1.12 | 0.993 |
| FerrIX™A33E (Hybrid HPR9200) | 7.69 | 7.29 | 0.989 | 4.43 | 3.76 | 0.997 |
| Soil Sample ID | Desorption Systems | Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | 24 | 48 | 72 | 120 | 168 | ||
| S_18 | 1 g of hybrid resin | 13.34 (3.4) | 13.95 (3.6) | 14.82 (3.8) | 15.25 (3.9) | 15.77 (4) | 16.64 (4.2) | 16.38 (4.2) | 17.29 (4.4) | 17.03 (4.3) |
| 1 g of pure resin | 6.15 (1.6) | 6.67 (1.7) | 6.75 (1.7) | 7.56 (1.9) | 8.37 (2.1) | 8.46 (2.2) | 8.46 (2.2) | 8.98 (2.3) | 9.5 (2.4) | |
| No resin | 1.06 (0.3) | 1.25 (0.3) | 1.32 (0.3) | 1.12 (0.3) | 1.12 (0.3) | 1.32 (0.3) | 1.25 (0.3) | 1.32 (0.3) | 1.32 (0.3) | |
| 0.5 g hybrid resin | 12.38 (3.2) | 12.87 (3.3) | 13.97 (3.6) | 14.79 (3.8) | 15.44 (3.9) | 15.97 (4.1) | 16.46 (4.2) | 16.71 (4.3) | 16.87 (4.3) | |
| 2 g of hybrid resin | 13.40 (3.4) | 14.26 (3.6) | 14.95 (3.8) | 15.65 (4.0) | 15.97 (4.1) | 16.67 (4.3) | 16.99 (4.3) | 17.12 (4.4) | 17.32 (4.4) | |
| S_90 | 1 g of hybrid resin | 7.09 (1.7) | 7.19 (1.7) | 7.37 (1.8) | 7.53 (1.8) | 7.94 (1.9) | 7.95 (1.9) | 7.93 (1.9) | 8.15 (1.9) | 7.85 (1.9) |
| 1 g of pure resin | 1.6 (0.4) | 1.66 (0.4) | 1.69 (0.4) | 1.91 (0.5) | 1.99 (0.5) | 2.1 (0.5) | 1.96 (0.5) | 2.24 (0.5) | 2.03 (0.5) | |
| No resin | 0.26 (0.1) | 0.66 (0.2) | 0.4 (0.1) | 0.4 (0.1) | 0.33 (0.1) | 0.46 (0.1) | 0.53 (0.1) | 0.46 (0.1) | 0.53 (0.1) | |
| S_180 | 1 g of hybrid resin | 2.93 (1.5) | 3 (1.5) | 3.21 (1.6) | 3.24 (1.7) | 3.29 (1.7) | 3.29 (1.7) | 3.29 (1.7) | 3.33 (1.7) | 3.45 (1.8) |
| 1 g of pure resin | 0.33 (0.2) | 0.32 (0.2) | 0.29 (0.1) | 0.46 (0.2) | 0.4 (0.2) | 0.48 (0.2) | 0.56 (0.3) | 0.54 (0.3) | 0.67 (0.3) | |
| No resin | 0.03 (0) | 0.03 (0) | 0.1 (0) | 0.07 (0) | 0.07 (0) | 0.1 (0) | 0.13 (0.1) | 0.1 (0) | 0.16 (0.1) | |
| Soil Sample ID | Desorption Systems | Pseudo-First-Order Model | Pseudo-Second-Order Model | Elovich Model | Intra-Particle Diffusion Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (1/h) | qe (mg/g) | R2 | k2 (g/(mg h)) | qe (mg/g) | k2qe2 (mg/(g h)) | R2 | α (mg/(L h)) | β (L/mg) | R2 | kd (mg/(g h1/2)) | C | R2 | ||
| S_18 | 1 g of hybrid resin | 0.031 | 3.014 | 0.482 | 0.037 | 17.241 | 11.099 | 1.000 | 1.72 × 106 | 1.128 | 0.972 | 0.311 | 13.726 | 0.844 |
| 1 g of pure resin | 0.038 | 4.734 | 0.763 | 0.029 | 9.461 | 2.601 | 0.998 | 1.45× 103 | 1.360 | 0.957 | 0.266 | 6.269 | 0.882 | |
| No resin | 0.026 | 0.059 | 0.342 | 0.466 | 1.328 | 0.821 | 1.000 | 5.93× 1010 | 25.316 | 0.338 | 0.015 | 1.141 | 0.335 | |
| 0.5 g resin | 0.043 | 5.362 | 0.905 | 0.031 | 17.007 | 8.873 | 1.000 | 5.59× 104 | 0.929 | 0.982 | 0.376 | 12.778 | 0.843 | |
| 2.0 g resin | 0.041 | 4.278 | 0.883 | 0.039 | 17.391 | 11.848 | 1.000 | 2.08× 106 | 1.125 | 0.982 | 0.309 | 13.946 | 0.834 | |
| S_90 | 1 g of hybrid resin | 0.021 | 0.659 | 0.326 | 0.495 | 7.962 | 31.348 | 1.000 | 3.73× 1012 | 4.376 | 0.845 | 0.076 | 7.206 | 0.660 |
| 1 g of pure resin | 0.019 | 0.461 | 0.316 | 0.443 | 2.097 | 1.949 | 0.996 | 1.74× 104 | 7.837 | 0.821 | 0.044 | 1.644 | 0.675 | |
| No resin | 0.031 | 0.272 | 0.581 | 0.429 | 0.520 | 0.116 | 0.988 | 3.01× 105 | 43.668 | 0.089 | 0.009 | 0.391 | 0.103 | |
| S_180 | 1 g of hybrid resin | 0.027 | 0.502 | 0.729 | 0.258 | 3.423 | 3.029 | 0.999 | 5.08× 1011 | 10.040 | 0.879 | 0.034 | 3.020 | 0.727 |
| 1 g of pure resin | 0.028 | 0.506 | 0.753 | 0.158 | 0.651 | 0.067 | 0.979 | 1.201 | 13.158 | 0.831 | 0.030 | 0.268 | 0.889 | |
| No resin | 0.022 | 0.152 | 0.700 | 0.367 | 0.151 | 0.008 | 0.882 | 0.040 | 41.322 | 0.724 | 0.009 | 0.032 | 0.733 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, S.; Li, Y.; Arai, Y. Novel Application of Hybrid Anion Exchange Resin for Phosphate Desorption Kinetics in Soils: Minimizing Re-Adsorption of Desorbed Ions. Soil Syst. 2020, 4, 36. https://doi.org/10.3390/soilsystems4020036
Li Z, Xu S, Li Y, Arai Y. Novel Application of Hybrid Anion Exchange Resin for Phosphate Desorption Kinetics in Soils: Minimizing Re-Adsorption of Desorbed Ions. Soil Systems. 2020; 4(2):36. https://doi.org/10.3390/soilsystems4020036
Chicago/Turabian StyleLi, Zhe, Suwei Xu, Ying Li, and Yuji Arai. 2020. "Novel Application of Hybrid Anion Exchange Resin for Phosphate Desorption Kinetics in Soils: Minimizing Re-Adsorption of Desorbed Ions" Soil Systems 4, no. 2: 36. https://doi.org/10.3390/soilsystems4020036
APA StyleLi, Z., Xu, S., Li, Y., & Arai, Y. (2020). Novel Application of Hybrid Anion Exchange Resin for Phosphate Desorption Kinetics in Soils: Minimizing Re-Adsorption of Desorbed Ions. Soil Systems, 4(2), 36. https://doi.org/10.3390/soilsystems4020036