Methane and Nitrous Oxide Emission Fluxes Along Water Level Gradients in Littoral Zones of Constructed Surface Water Bodies in a Rewetted Extracted Peatland in Sweden
Abstract
1. Introduction
2. Materials and Methods
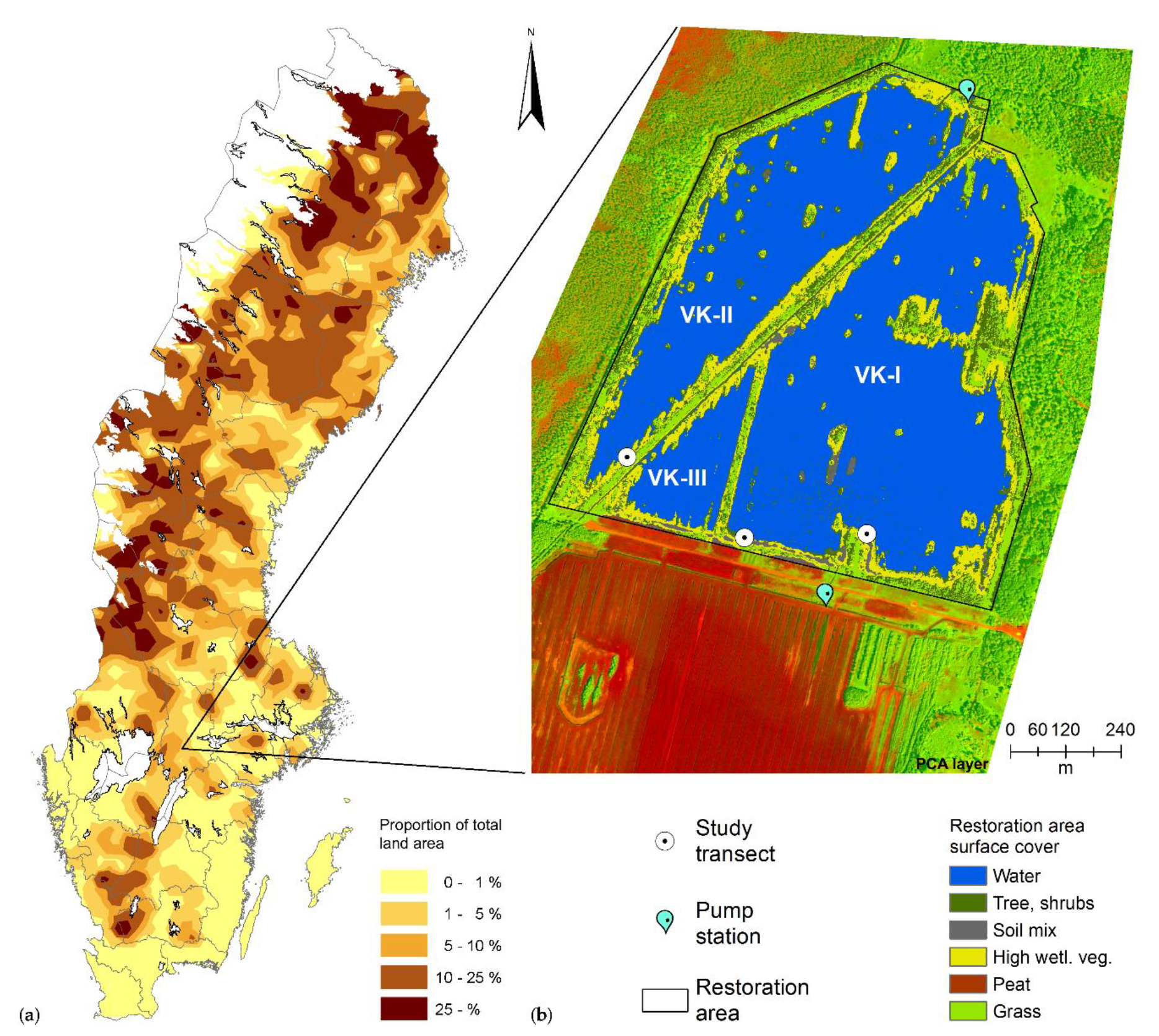
2.1. Site Description
2.2. Field Sampling and Measurements
2.3. Greenhouse Gas (GHG) Flux Determination
2.3.1. Chamber Sampling and Measurement of GHG Concentration
2.3.2. Flux Estimation and Evaluation
2.3.3. GHG Flux Detection Limits
2.4. Statistical Analyses of CH4 Flux
3. Results and Discussions
3.1. Soil Physical and Chemical Conditions
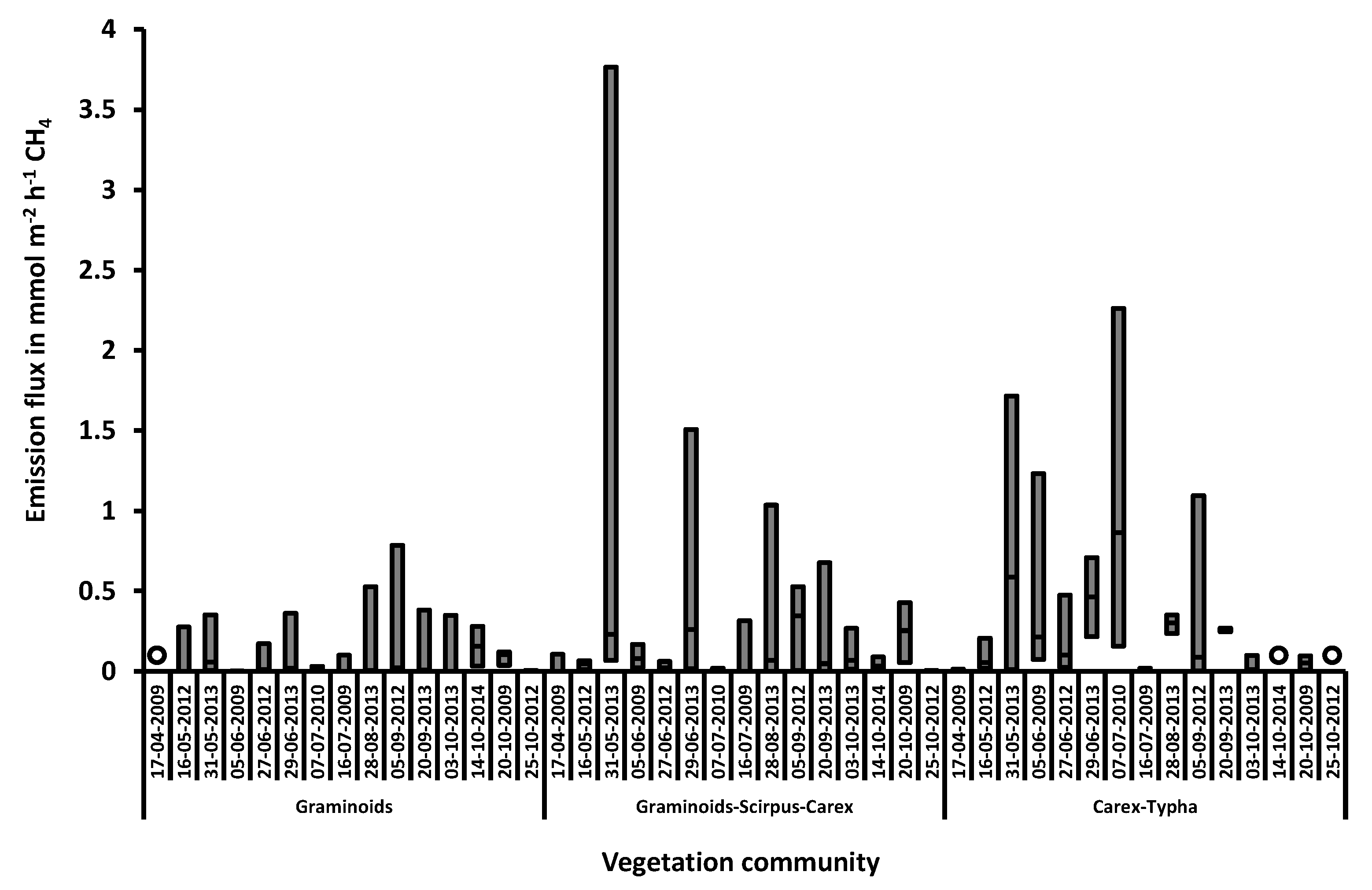
3.2. Methane Fluxes
3.2.1. Linear Mixed Effects Analysis
3.2.2. The Model Outcome: Influence of Water Level, Vegetation Community, and Soil Temperature on Methane Fluxes
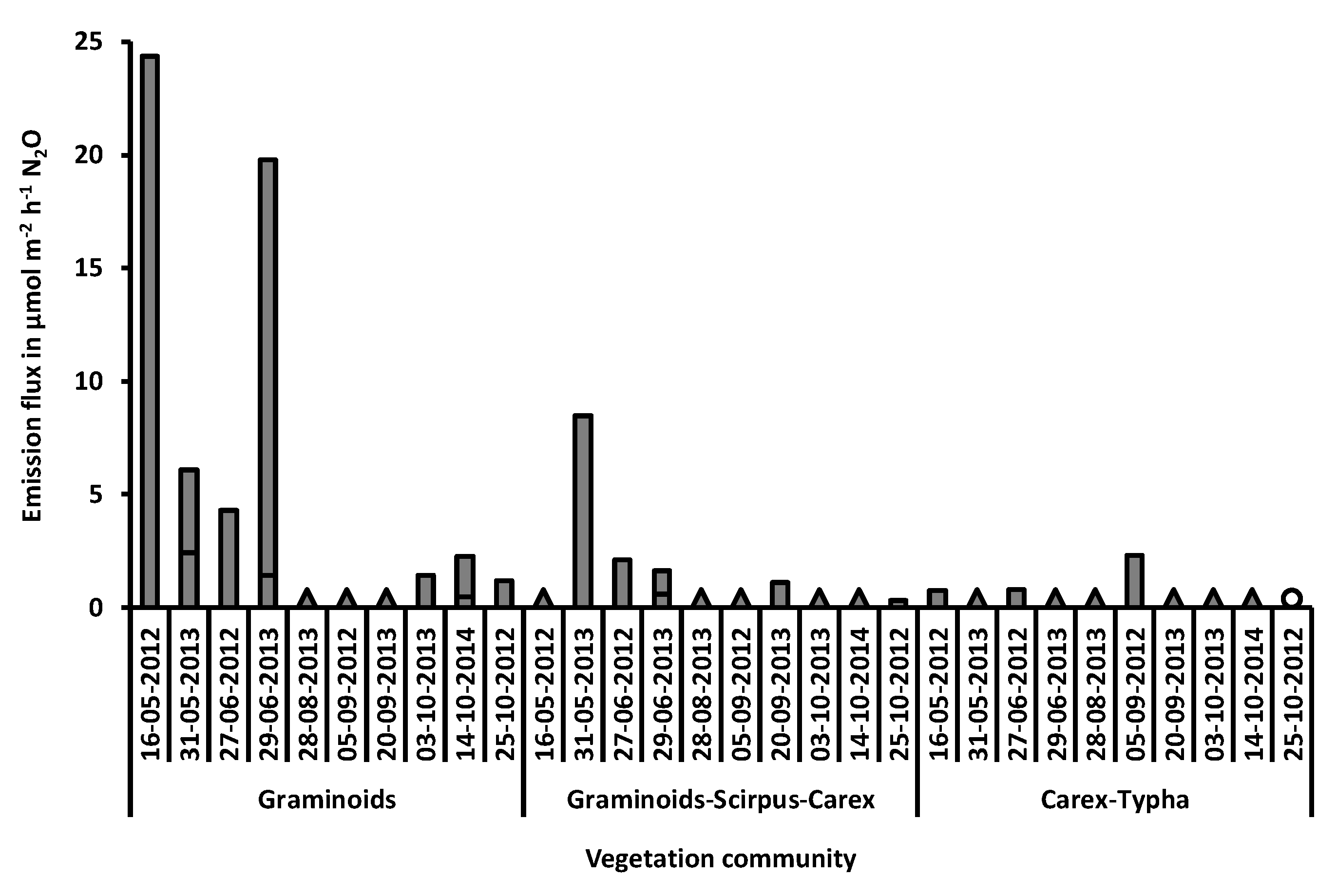
3.3. N2O Fluxes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanneberger, F.; Tegetmeyer, C.; Busse, S.; Barthelmes, A. The peatland map of Europe. Mires Peat 2017, 19, 22:1–22:17. [Google Scholar] [CrossRef]
- Juutinen, A.; Saarimaa, M.; Ojanen, P.; Sarkkola, S.; Haara, A.; Karhu, J.; Nieminen, M.; Minkkinen, K.; Penttilä, T.; Laatikainen, M.; et al. Trade-offs between economic returns, biodiversity, and ecosystem services in the selection of energy peat production sites. Ecosyst. Serv. 2019, 40, 1–14. [Google Scholar] [CrossRef]
- Glatzel, S.; Forbrich, I.; Krüger, C.; Lemke, S.; Gerold, G. Small scale controls of greenhouse gas release under elevated N deposition rates in a restoring peat bog in NW Germany. Biogeosciences 2008, 5, 925–935. [Google Scholar] [CrossRef]
- Quinty, F.; Rochefort, L. Plant reintroduction on a harvested peat bog. In Northern Forested Wetlands: Ecology and Management; Trettin, C.C., Jurgensen, M.F., Grigal, D.F., Gale, M.R., Jeglum, J.K., Eds.; CRC Lewis: Boca Raton, FL, USA, 1997; pp. 133–145. [Google Scholar]
- Quinty, F.; Rochefort, L. Peatland Restoration Guide, 2nd ed.; Canadian Sphagnum Peat Moss Association and New Brunswick Department of Natural Resources and Energy: Ville de Québec, QC, Canada, 2003; Available online: http://www.gret-perg.ulaval.ca/uploads/tx_centrerecherche/Peatland_Restoration_guide_2ndEd_01.pdf (accessed on 15 January 2020).
- Blankenburg, J.; Tonnis, W.J. Guidelines for Wetland Restoration of Peat Cutting Areas—Results of the Bridge-Project; Institute of Soil Technology, Geological Survey of Lower Saxony: Bremen, Germany, 2004. [Google Scholar]
- Joosten, H.; Tapio-Biström, M.-L.; Tol, S. Peatlands—Guidance for Climate Change Mitigation Through Conservation, Rehabilitation and Sustainable Use, 2nd ed.; Mitigation of Climate Change in Agriculture Series; Food and Agriculture Organization of the United Nations and Wetlands International: Roma, Italy, 2012; Volume 5. [Google Scholar]
- Wilson, D.; Farrell, C.; Mueller, C.; Hepp, S.; Renou-Wilson, F. Rewetted industrial cutaway peatlands in western Ireland: A prime location for climate change mitigation? Mires Peat 2013, 11, 1–22. Available online: http://www.mires-and-peat.net/pages/volumes/map11/map1101.php (accessed on 23 March 2020).
- Hiraishi, T.; Krug, T.; Tanabe, K.; Srivastava, N.; Baasansuren, J.; Fukuda, M.; Troxler, T.G. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas. Inventories: Wetlands; Intergovernmental Panel on Climate Change: Genève, Switzerland, 2014; Available online: http://www.ipcc-nggip.iges.or.jp/public/wetlands/index.html (accessed on 16 January 2020).
- Zeitz, J.; Velty, S. Soil properties of drained and rewetted fen soils. J. Plant Nutr. Soil Sci. 2002, 165, 618–626. [Google Scholar] [CrossRef]
- Höper, H.; Augustin, J.; Cagampan, J.P.; Drösler, M.; Lundin, L.; Moors, E.; Vasander, H.; Waddington, J.M.; Wilson, D. Restoration of peatlands and greenhouse gas balances. In Peatlands and Climate Change; Strack, M., Ed.; International Peat Society: Jyväskylä, Finland, 2008; pp. 182–210. [Google Scholar]
- Camarsa, G.; Toland, J.; Hudson, T.; Nottingham, S.; Jones, W.; Eldridge, J.; Severon, M.; Rose, C.; Sliva, J.; Joosten, H.; et al. Life and Climate Change Mitigation; LIFE Environment Publications; European Union: Ville de Luxembourg, Grand Duchy of Luxembourg, 2015. [Google Scholar]
- Malloy, S.; Price, J.S. Fen restoration on a bog harvested down to sedge peat: A hydrological assessment. Ecol. Eng. 2014, 64, 151–160. [Google Scholar] [CrossRef]
- Vasander, H.; Tuittila, E.-S.; Lode, E.; Lundin, L.; Ilomets, M.; Sallantaus, T.; Heikkilä, R.; Pitkänen, M.-L.; Laine, J. Status and restoration of peatlands in northern Europe. Wetl. Ecol. Manag. 2003, 11, 51–63. [Google Scholar] [CrossRef]
- Jordan, S.; Strömgren, M.; Fiedler, J.; Lundin, L.; Lode, E.; Nilsson, T. Ecosystem respiration, methane and nitrous oxide fluxes from ecotopes in a rewetted extracted peatland in Sweden. Mires Peat 2016, 17, 7:1–7:23. [Google Scholar] [CrossRef]
- Lundin, L.; Lode, E.; Nilsson, T.; Strömgren, M.; Jordan, S.; Koslov, S. Effekter vid Restaurering av Avslutade Torvtäkter Genom Återvätning; Undersökningar vid Porla, Toftmossen och Västkärr (Effects on Restoration of Terminated Peat Cuttings by Rewetting; Investigations at Porla, Toftmossen and Västkärr); Projektrapport; Stiftelsen Svensk Torvforskning: Johanneshov, Sweden, 2016; Volume 18, Available online: https://www.svensktorv.se/Homepage/Download-File/f/1067186/h/d6b79d231ac37a2d94368544c5be6b75/Rapport_restaruering_nr18_160608 (accessed on 16 January 2020)(In Swedish with English Summary).
- Lundin, L.; Nilsson, T.; Jordan, S.; Lode, E.; Strömgren, M. Impacts of rewetting on peat, hydrology and water chemical composition over 15 years in two finished peat extraction areas in Sweden. Wetl. Ecol. Manag. 2017, 25, 405–419. [Google Scholar] [CrossRef]
- Wilson, D.; Farrell, C.A.; Fallon, D.; Moser, G.; Müller, C.; Renou-Wilson, F. Multiyear greenhouse gas balances at a rewetted temperate peatland. Glob. Chang. Biol. 2016, 22, 4080–4095. [Google Scholar] [CrossRef]
- Mahmood, M.S.; Strack, M. Methane dynamics of recolonized cutover minerotrophic peatland: Implications for restoration. Ecol. Eng. 2011, 37, 1859–1868. [Google Scholar] [CrossRef]
- Lode, E. Wetland Restauration: A Survey of Options for Restoring Peatlands; Studia Forestalia Suecica; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1999; Volume 205. [Google Scholar]
- Jordan, S.; Lundin, L.; Strömgren, M.; Lode, E.; Nilsson, T. Peatland restoration in Sweden: From peat cutting areas to shallow lakes—After-use of industrial peat sites. Peatl. Int. 2009, 1, 42–43. [Google Scholar]
- Wilson, D.; Blain, D.; Couwenberg, J.; Evans, C.D.; Murdiyarso, D.; Page, S.E.; Renou-Wilson, F.; Rieley, J.O.; Sirin, A.; Strack, M.; et al. Greenhouse gas emission factors associated with rewetting of organic soils. Mires Peat 2016, 17, 4:1–4:28. [Google Scholar] [CrossRef]
- Audet, J.; Elsgaard, L.; Kjaergaard, C.; Larsen, S.E.; Hoffmann, C.C. Greenhouse gas emissions from a Danish riparian wetland before and after restoration. Ecol. Eng. 2013, 57, 170–182. [Google Scholar] [CrossRef]
- Juszczak, R.; Augustin, J. Exchange of the greenhouse gases methane and nitrous oxide between the atmosphere and a temperate peatland in central Europe. Wetlands 2013, 33, 895–907. [Google Scholar] [CrossRef]
- Hahn, J.; Köhler, S.; Glatzel, S.; Jurasinski, G. Methane Exchange in a Coastal Fen in the First Year after Flooding—A Systems Shift. PLoS ONE 2015, 10, e0140657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef]
- Bartlett, K.B.; Harriss, R.C. Review and assessment of methane emissions from wetlands. Chemosphere 1993, 26, 261–320. [Google Scholar] [CrossRef]
- Couwenberg, J. Methane Emissions from Peat Soils (Organic Soils, Histosols)—Facts, MRV-Ability, Emission Factors; Wetlands International: Ede, The Netherlands, 2009; Available online: https://www.wetlands.org/download/4809/ (accessed on 16 January 2020).
- Höper, H. Treibhausgasemissionen aus Mooren und Möglichkeiten der Verringerung (Greenhouse gas emissions of peatlands and measures for reduction). TELMA 2015, Beiheft 5, 133–158. (In German) [Google Scholar]
- Vanselow-Algan, M.; Schmidt, S.R.; Greven, M.; Fiencke, C.; Kutzbach, L.; Pfeiffer, E.-M. High methane emissions dominated annual greenhouse gas balances 30 years after bog rewetting. Biogeosciences 2015, 12, 4361–4371. [Google Scholar] [CrossRef]
- Augustin, J.; Joosten, H. Peatland rewetting and the greenhouse effect. Int. Mire Conserv. Group Newsl. 2007, 3, 29–30. [Google Scholar]
- Hahn-Schöfl, M.; Zak, D.; Minke, M.; Gelbrecht, J.; Augustin, J.; Freibauer, A. Organic sediment formed during inundation of a degraded fen grassland emits large fluxes of CH4 and CO2. Biogeosciences 2011, 8, 1539–1550. [Google Scholar] [CrossRef]
- Couwenberg, J.; Thiele, A.; Tanneberger, F.; Augustin, J.; Bärisch, S.; Dubovik, D.; Liashchynskaya, N.; Michaelis, D.; Minke, M.; Skuratovich, A.; et al. Assessing greenhouse gas emissions from peatlands using vegetation as a proxy. Hydrobiologia 2011, 674, 67–89. [Google Scholar] [CrossRef]
- Tuittila, E.-S.; Komulainen, V.-M.; Vasander, H.; Nykänen, H.; Martikainen, P.J.; Laine, J. Methane dynamics of a restored cut-away peatland. Glob. Chang. Biol. 2000, 6, 569–581. [Google Scholar] [CrossRef]
- Günther, A.; Jurasinski, G.; Huth, V.; Glatzel, S. Opaque closed chambers underestimate methane fluxes of Phragmites australis (Cav.) Trin. ex Steud. Environ. Monit. Assess. 2014, 186, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Marinier, M.; Glatzel, S.; Moore, T.R. The role of cotton-grass (Eriophorum vaginatum) in the exchange of CO2 and CH4 at two restored peatlands, eastern Canada. Ecoscience 2004, 11, 141–149. [Google Scholar] [CrossRef]
- Waddington, J.M.; Strack, M.; Greenwood, M.J. Toward restoring the net carbon sink function of degraded peatlands: Short-term response in CO2 exchange to ecosystem-scale restoration. J. Geophys. Res. 2010, 115, G01008:1–G01008:13. [Google Scholar] [CrossRef]
- Strack, M.; Zuback, Y.C.A. Annual carbon balance of a peatland 10 yr following restoration. Biogeosciences 2013, 10, 2885–2896. [Google Scholar] [CrossRef]
- Wilson, D.; Tuittila, E.-S.; Alm, J.; Laine, J.; Farrell, E.P.; Byrne, K.A. Carbon dioxide dynamics of a restored maritime peatland. Ecoscience 2007, 14, 71–80. [Google Scholar] [CrossRef]
- Järveoja, J.; Peichl, M.; Maddison, M.; Soosaar, K.; Vellak, K.; Karofeld, E.; Teemusk, A.; Mander, Ü. Impact of water table level on annual carbon and greenhouse gas balances of a restored peat extraction area. Biogeosciences 2016, 13, 2637–2651. [Google Scholar] [CrossRef]
- Minke, M.; Augustin, J.; Burlo, A.; Yarmashuk, T.; Chuvashova, H.; Thiele, A.; Freibauer, A.; Tikhonov, V.; Hoffmann, M. Water level, vegetation composition, and plant productivity explain greenhouse gas fluxes in temperate cutover fens after inundation. Biogeosciences 2016, 13, 3945–3970. [Google Scholar] [CrossRef]
- Strack, M.; Keith, A.M.; Xu, B. Growing season carbon dioxide and methane exchange at a restored peatland on the Western Boreal Plain. Ecol. Eng. 2014, 64, 231–239. [Google Scholar] [CrossRef]
- Strack, M.; Cagampan, J.; Hassanpour Fard, G.; Keith, A.M.; Nugent, K.; Rankin, T.; Robinson, C.; Strachan, I.B.; Waddington, J.M.; Xu, B. Controls on plot-scale growing season CO2 and CH4 fluxes in restored peatlands: Do they differ from unrestored and natural sites? Mires Peat 2016, 17, 5:1–5:18. [Google Scholar] [CrossRef]
- Juutinen, S.; Alm, J.; Larmola, T.; Huttunen, J.T.; Morero, M.; Martikainen, P.J.; Silvola, J. Major implication of the littoral zone for methane release from boreal lakes. Glob. Biogeochem. Cycles 2003, 17, 28:1–28:11. [Google Scholar] [CrossRef]
- Wilson, D.; Alm, J.; Laine, J.; Byrne, K.A.; Farrell, E.P.; Tuittila, E.-S. Rewetting of cutaway peatlands: Are we re-creating hot spots of methane emissions? Restor. Ecol. 2009, 17, 796–806. [Google Scholar] [CrossRef]
- Franz, D.; Koebsch, F.; Larmanou, E.; Augustin, J.; Sachs, T. High net CO2 and CH4 release at a eutrophic shallow lake on a formerly drained fen. Biogeosciences 2016, 13, 3051–3070. [Google Scholar] [CrossRef]
- Kozlov, S.A.; Lundin, L.; Avetov, N.A. Plant cover development on two rewetted extracted peatlands restored to wetlands. Mires Peat 2016, 18, 5:1–5:17. [Google Scholar] [CrossRef]
- Köppen, W. Das Geographische System der Klimate (The geographic system of the climates). In Handbuch der Klimatologie, Band 1: Allgemeine Klimalehre (Handbook of Climatology, Volume 1: General Climatology); Köppen, W., Geiger, R., Eds.; Gebrüder Borntraeger: Berlin, Germany, 1936; pp. C:1–C:44. (In German) [Google Scholar]
- Raab, B.; Vedin, H. Klimat, Sjöar och Vattendrag. Sveriges Nationalatlas (Climate, Lakes and Watercourses. Sweden’s National Atlas); SNA Förlag: Stockholm, Sweden, 1995. (In Swedish) [Google Scholar]
- Odin, H.; Eriksson, B.; Perttu, K. Temperaturklimatkartor för Svenskt Skogsbruk (Temperature Climate Maps for Swedish Forestry); Rapporter i Skogsekologi och Skoglig Marklära, Sveriges Lantbruksuniversitet: Uppsala, Sweden, 1983; Volume 45. [Google Scholar]
- SMHI LuftWebb. RT-90-Coordinates: 1433350-6545600. Sveriges Meteorologiska och Hydrologiska Institut (Sweden’s Meteorological and Hydrological Institute). Available online: http://luftwebb.smhi.se (accessed on 16 January 2020).
- Von Post, L. Das Genetische System der Organogenen Bildungen Schwedens (The Genetic System of the Organogenic Formations of Sweden). In Mémoires sur la Nomenclature et la Classification des Sols (Memoirs on the Nomenclature and the Classification of the Soils); Comité International de Pédologie, IVème Commission (Commission Pour la Nomenclature et la Classification des sols, Commission Pour l’Europe, Président: B. Frosterus); Comité International de Pédologie: Helsingfors/Helsinki, Finland, 1924; pp. 287–304. (In German) [Google Scholar]
- Hånell, B.; Lundin, L.; Magnusson, T. The peat resources in Sweden. In Proceedings of the 13th International Peat Congress, Tullamore, Ireland, 8–13 June 2008; Farrell, C., Feehan, J., Eds.; International Peat Society: Jyväskylä, Finland, 2008; Volume 1, pp. 109–113. [Google Scholar]
- ISO 10694. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis); Organisation Internationale de Normalisation (International Organisation for Standardisation): Vernier, Switzerland, 1995; (In French and English). [Google Scholar]
- ISO 13878. Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis); Organisation Internationale de Normalisation (International Organisation for Standardisation): Vernier, Switzerland, 1998; (In French and English). [Google Scholar]
- EN 1484. Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC); Comité Européen de Normalisation (European Committee for Standardization): Bruxelles, Belgium, 1997; (In French, English and German). [Google Scholar]
- EN 27888. Water Quality—Determination of Electrical Conductivity (ISO 7888:1985); Comité Européen de Normalisation (European Committee for Standardization): Bruxelles, Belgium, 1993; (In French, English and German). [Google Scholar]
- ISO 10523. Water Quality—Determination of pH; Organisation Internationale de Normalisation (International Organisation for Standardisation): Vernier, Switzerland, 2008; (In French and English). [Google Scholar]
- Parkin, T.B.; Venterea, R.T. USDA-ARS GRACEnet Project Protocols, Chapter 3, Chamber-based trace gas flux measurements. In GRACEnet Sampling Protocols; Follett, R.F., Ed.; Agricultural Research Service, United States Department of Agriculture: Washington, DC, USA, 2010. Available online: https://www.ars.usda.gov/SP2UserFiles/Program/212/Chapter%203.%20GRACEnet%20Trace%20Gas%20Sampling%20Protocols.pdf (accessed on 16 January 2020).
- Pumpanen, J.; Longdoz, B.; Kutsch, W.L. Field measurements of soil respiration: Principles and constraints, potentials and limitations of different methods. In Soil Carbon Dynamics—An Integrated Methodology; Kutsch, W.L., Bahn, M., Heinemeyer, A., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 16–33. [Google Scholar]
- Hutchinson, G.L.; Livingston, G.P. Vents and seals in non-steady-state chambers used for measuring gas exchange between soil and the atmosphere. Eur. J. Soil Sci. 2001, 52, 675–682. [Google Scholar] [CrossRef]
- Davidson, E.A.; Savage, K.; Verchot, L.V.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Calvert, J.G. Glossary of atmospheric chemistry terms (recommendations 1990 of IUPAC Commission on Atmospheric Chemistry). Pure Appl. Chem. 1990, 62, 2167–2219. [Google Scholar] [CrossRef]
- Möller, D. Luft—Chemie, Physik, Biologie, Reinhaltung, Recht (Air—Chemistry, Physics, Biology, Air Pollution Control, Law); W. de Gruyter: Berlin, Germany, 2003. (In German) [Google Scholar]
- International Union of Pure and Applied Chemistry. Compendium of Chemical Terminology (the “Gold Book”), 2nd ed.; Compiled by McNaught A.D. and Wilkinson, A.; Blackwell Science: Oxford, UK, 1997; Web 2.0 Version 2019. [Google Scholar] [CrossRef]
- Pihlatie, M.K.; Christiansen, J.R.; Aaltonen, H.; Korhonen, J.F.J.; Nordbo, A.; Rasilo, T.; Benanti, G.; Giebels, M.; Helmy, M.; Sheehy, J.; et al. Comparison of static chambers to measure CH4 emissions from soils. Agric. For. Meteorol. 2013, 171, 124–136. [Google Scholar] [CrossRef]
- Koskinen, M.; Minkkinen, K.; Ojanen, P.; Kämäräinen, M.; Laurila, T.; Lohila, A. Measurements of CO2 exchange with an automated chamber system throughout the year: Challenges in measuring night-time respiration on porous peat soil. Biogeosciences 2014, 11, 347–363. [Google Scholar] [CrossRef]
- Kutzbach, L.; Schneider, J.; Sachs, T.; Giebels, M.; Nykänen, H.; Shurpali, N.J.; Martikainen, P.J.; Alm, J.; Wilmking, M. CO2 flux determination by closed-chamber methods can be seriously biased by inappropriate application of linear regression. Biogeosciences 2007, 4, 1005–1025. [Google Scholar] [CrossRef]
- De Klein, C.A.M.; Harvey, M.J. Nitrous Oxide Chamber Methodology Guidelines; Version 1.1; Global Research Alliance on Agricultural Greenhouse Gases, Ministry for Primary Industries: Wellington, New Zeeland, 2015; Available online: http://globalresearchalliance.org/wp-content/uploads/2015/11/Chamber_Methodology_Guidelines_Final-V1.1-2015.pdf (accessed on 16 January 2020).
- Doerffel, K. Statistik in der Analytischen Chemie (Statistics in Analytical Chemistry), 5th ed.; VEB Deutscher Verlag für Grundstoffindustrie: Leipzig, Germany, 1990. (In German) [Google Scholar]
- VDI 2449. Part 1—Measurement methods test criteria—Determination of performance characteristics for the measurement of gaseous pollutants (immission). In VDI-Handbuch Reinhaltung der Luft, Band 5: Analysen- und Meßverfahren (VDI/DIN Manual Air Pollution Prevention, Volume 5: Analysis and Measurement Methods); Kommission Reinhaltung der Luft im VDI und DIN—Normenausschuß KRdL, Ed.; Beuth: Berlin, Germany, 1995; (In German and English). [Google Scholar]
- Baker, J.; Doyle, G.; McCarty, G.; Mosier, A.; Parkin, T.; Reicosky, D.; Smith, J.; Venterea, R. GRACEnet—Chamber-Based Trace Gas Flux Measurement Protocol; Trace Gas Protocol Development Committee, Agricultural Research Service, United States Department of Agriculture: Ames, IA, USA, 2003. Available online: https://www.ars.usda.gov/SP2UserFiles/person/31831/2003GRACEnetTraceGasProtocol.pdf (accessed on 16 January 2020).
- Bates, D. lme4: Mixed-Effects Modeling with R. Chapter Drafts; lme4—Mixed-Effects Models Project, R Forge. 2010. Available online: http://lme4.r-forge.r-project.org/lMMwR/lrgprt.pdf (accessed on 25 September 2016).
- Gries, S.T. Statistische Modellierung (Statistical Modelling). Z. Ger. Linguist. 2012, 40, 38–67. (In German) [Google Scholar]
- Winter, B. Linear Models and Linear Mixed Effects Models in R with Linguistic Applications. arXiv 2013, arXiv:1308.5499. Available online: http://arxiv.org/pdf/1308.5499.pdf (accessed on 16 January 2020).
- Fox, J.; Weisberg, S. Car—Companion to Applied Regression. R Package, Version 2.0 26 (2015 08 06). Available online: https://CRAN.R-project.org/package=car (accessed on 25 September 2016).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Lme4—Linear Mixed-Effects Models Using ‘Eigen’ and S4. R Package, Version 1.1 8 (2015 06 22). Available online: https://CRAN.R-project.org/package=lme4 (accessed on 25 September 2016).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Wien, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 24 August 2016).
- Holleman, A.F.; Wiberg, E. Lehrbuch der Anorganischen Chemie (Textbook of Inorganic Chemistry), 40th to 46th ed.; W. de Gruyter: Berlin, Germany, 1958. (In German) [Google Scholar]
- Rõõm, E.-I.; Nõges, P.; Feldmann, T.; Tuvikene, L.; Kisand, A.; Teearu, H.; Nõges, T. Years are not brothers: Two-year comparison of greenhouse gas fluxes in large shallow Lake Võrtsjärv, Estonia. J. Hydrol. 2014, 519, 1594–1606. [Google Scholar] [CrossRef]
- Komulainen, V.-M.; Nykänen, H.; Martikainen, P.J.; Laine, J. Short-term effect of restoration on vegetation change and methane emissions from peatlands drained for forestry in southern Finland. Can. J. For. Res. 1998, 28, 402–411. [Google Scholar] [CrossRef]
- Frenzel, P.; Rudolph, J. Methane emission from a wetland plant: The role of CH4 oxidation in Eriophorum. Plant Soil 1998, 202, 27–32. [Google Scholar] [CrossRef]
- Huttunen, J.T.; Alm, J.; Liikanen, A.; Juutinen, S.; Larmola, T.; Hammar, T.; Silvola, J.; Martikainen, P.J. Fluxes of methane, carbon dioxide and nitrous oxide in boreal lakes and potential anthropogenic effects on the aquatic greenhouse gas emissions. Chemosphere 2003, 52, 609–621. [Google Scholar] [CrossRef]
- Günther, A.; Huth, V.; Jurasinski, G.; Glatzel, S. The effect of biomass harvesting on greenhouse gas emissions from a rewetted temperate fen. GCB Bioenergy 2015, 7, 1092–1106. [Google Scholar] [CrossRef]
- Schulz, S.; Matsuyama, H.; Conrad, R. Temperature dependence on methane production from different precursors in a profundal sediment (Lake Constance). FEMS Microbiol. Ecol. 1997, 22, 207–213. [Google Scholar] [CrossRef]
- Bergström, I.; Mäkelä, S.; Kankaala, P.; Kortelainen, P. Methane efflux from littoral vegetation stands of southern boreal lakes: An upscaled regional estimate. Atmos. Environ. 2007, 41, 339–351. [Google Scholar] [CrossRef]
- Lundin, L.; Jordan, S.; Lode, E.; Nilsson, T.; Strömgren, M. Restoration of Terminated Peat Cuttings by Rewetting. Peatl. Int. 2018, 4, 27–31. [Google Scholar]
- Günther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. bioRxiv 2019. [Google Scholar] [CrossRef]
- Martikainen, P.J.; Nykanen, H.; Crill, P.; Silvola, J. Effect of a lowered water table on nitrous oxide fluxes from northern peatlands. Nature 1993, 366, 51–53. [Google Scholar] [CrossRef]
- Regina, K.; Nykänen, H.; Silvola, J.; Martikainen, P.J. Fluxes of nitrous oxide from boreal peatlands as affected by peatland type, water table level and nitrification capacity. Biogeochemistry 1996, 35, 401–418. [Google Scholar] [CrossRef]
- Klemedtsson, L.; von Arnold, K.; Weslien, P.; Gundersen, P. Soil CN ratio as a scalar parameter to predict nitrous oxide emissions. Glob. Chang. Biol. 2005, 11, 1142–1147. [Google Scholar] [CrossRef]
- Kasimir Klemedtsson, Å.; Weslien, P.; Klemedtsson, L. Methane and nitrous oxide fluxes from a farmed Swedish Histosol. Eur. J. Soil Sci. 2009, 60, 321–331. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. Lond. B 2013, 368, 1–13. [Google Scholar] [CrossRef]
- Berglund, Ö.; Berglund, K.; Jordan, S.; Norberg, L. Carbon capture efficiency, yield, nutrient uptake and trafficability of different grass species on a cultivated peat soil. Catena 2019, 173, 175–182. [Google Scholar] [CrossRef]
- Silvan, N.; Tuittila, E.-S.; Kitunen, V.; Vasander, H.; Laine, J. Nitrate uptake by Eriophorum vaginatum controls N2O production in a restored peatland. Soil Biol. Biochem. 2005, 37, 1519–1526. [Google Scholar] [CrossRef]

| Soil Property | Lake VK II | Lake VK I | Adjacent Peat Extraction Site | |||
|---|---|---|---|---|---|---|
| (west) | (east) | |||||
| 0–22 cm | 23–35 cm | Lake Bottom 5–22 cm | Lake Bottom 0–10 cm | Lake Bottom 0–12 cm | Loose Peat | |
| Median C/N ratio (min.-max.) | 20.1 (19.7–20.3) | 22.5 (21.6–23.0) | 21.4 | 24.1 | 22.2 | n.d.; 96% loss on ignition |
| Median bulk density in g cm−3 (min.-max.) | 0.36 (0.27–0.42) | 0.17 (0.17–0.23) | n.d. | n.d. | n.d. | n.d. |
| pH in water | 5.0 | 5.6 | 5.9 | 5.4 | 5.3 | 5.2 |
| Al in g kg−1 | 5.5 | 2.2 | 13.4 | 6.6 | 9.7 | 3.2 |
| Ca in g kg−1 | 12.1 | 11.8 | 8.8 | 13.9 | 12.5 | 3.8 |
| Fe in g kg−1 | 14.9 | 4.7 | 18.9 | 10.7 | 10.4 | 2.2 |
| K in g kg−1 | 0.27 | 0.09 | 3.05 | 0.42 | 0.62 | 0.12 |
| Mg in g kg−1 | 0.64 | 0.50 | 4.48 | 1.14 | 1.20 | 0.25 |
| Mn in g kg−1 | 0.83 | 0.51 | 0.61 | 0.49 | 0.34 | 0.08 |
| Na in g kg−1 | 0.08 | 0.07 | 0.27 | 0.12 | 0.10 | 0.06 |
| P in g kg−1 | 0.68 | 0.31 | 0.41 | 0.55 | 0.51 | 0.25 |
| S in g kg−1 | 3.5 | 3.2 | 2.2 | 5.1 | 5.2 | 2.8 |
| Measurement Occasion (month-year) | Vegetation Community | Water | |||||
|---|---|---|---|---|---|---|---|
| Graminoids | Graminoids-Scirpus-Carex | Carex-Typha | |||||
| 04-2009 | 7.1 | i | 4.2 | i&ni | 10.9 | i | 12.3 |
| 06-2009 | 10.2 | ni | 10.0 | ni | 10.4 | i&ni | 12.5 |
| 07-2009 | 21.6 | i | 20.3 | i | 21.0 | i | 21.5 |
| 10-2009 | 5.0 | i&ni | 5.0 | i | 5.2 | i | 5.1 |
| 07-2010 | 17.0 | ni | 15.1 | ni | 16.8 | ni | 23.9 |
| 05-2012 | 10.3 | i&ni | 12.1 | i | 16.7 | i | 17.3 |
| 06-2012 | 14.3 | i&ni | 13.4 | i&ni | 18.3 | i | 18.0 |
| 09-2012 | 13.8 | i&ni | 13.6 | i&ni | 14.3 | i | 14.3 |
| 10-2012 | 5.7 | i | 5.8 | i | n.d. | i | 5.7 |
| 05-2013 | 15.2 | i&ni | 16.1 | i&ni | 22.0 | i | 21.5 |
| 06-2013 | 15.3 | i&ni | 14.6 | i&ni | 16.9 | i | 15.5 |
| 08-2013 | 13.0 | i&ni | 12.8 | i&ni | 12.9 | i | 11.2 |
| 09-2013 | 10.2 | ni | 10.1 | i&ni | 9.2 | i | 8.0 |
| 10-2013 | 6.0 | ni | 5.8 | i&ni | 4.0 | i&ni | 2.7 |
| 10-2014 | 7.7 | i&ni | 7.6 | i | 7.0 | i | 7.4 |
| Coefficient | a1= aj | b1 | b2 | b3 | b4 | Σ bj | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Effect | Ti | vc | vc × wlc | wlc | intercept | ||||||||||||
| Pr (> chi-square) | 1.3 × 10−4 | 4.9 × 10−11 | 1.4 × 10−5 | 4.2 × 10−2 | |||||||||||||
| vc | wlc | est. | SE | t | est. | SE | t | est. | SE | t | est. | SE | t | est. | SE | t | |
| Water without vegetation | i | 0.11 | 0.029 | 3.8 | −1.4 | 0.76 | −1.8 | 1.9 | 0.50 | 3.9 | 0.1 | 0.69 | 0.2 | 0.7 | |||
| Carex-Typha | ni | 0.11 | 0.029 | 3.8 | 3.9 | 0.55 | 7.0 | 0.1 | 0.69 | 0.2 | 4.0 | ||||||
| Carex-Typha | i | 0.11 | 0.029 | 3.8 | −3.5 | 0.76 | −4.7 | 1.9 | 0.50 | 3.9 | 0.1 | 0.69 | 0.2 | 2.4 | |||
| Graminoids-Scirpus-Carex | ni | 0.11 | 0.029 | 3.8 | 2.0 | 0.39 | 5.3 | 0.1 | 0.69 | 0.2 | 2.2 | ||||||
| Graminoids-Scirpus-Carex | i | 0.11 | 0.029 | 3.8 | −1.0 | 0.69 | −1.5 | 1.9 | 0.50 | 3.9 | 0.1 | 0.69 | 0.2 | 3.1 | |||
| Graminoids | ni | 0.11 | 0.029 | 3.8 | 0.1 | 0.69 | 0.2 | 0.1 | |||||||||
| Graminoids | i | 0.11 | 0.029 | 3.8 | 1.9 | 0.50 | 3.9 | 0.1 | 0.69 | 0.2 | 2.0 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordan, S.; Strömgren, M.; Fiedler, J.; Lode, E.; Nilsson, T.; Lundin, L. Methane and Nitrous Oxide Emission Fluxes Along Water Level Gradients in Littoral Zones of Constructed Surface Water Bodies in a Rewetted Extracted Peatland in Sweden. Soil Syst. 2020, 4, 17. https://doi.org/10.3390/soilsystems4010017
Jordan S, Strömgren M, Fiedler J, Lode E, Nilsson T, Lundin L. Methane and Nitrous Oxide Emission Fluxes Along Water Level Gradients in Littoral Zones of Constructed Surface Water Bodies in a Rewetted Extracted Peatland in Sweden. Soil Systems. 2020; 4(1):17. https://doi.org/10.3390/soilsystems4010017
Chicago/Turabian StyleJordan, Sabine, Monika Strömgren, Jan Fiedler, Elve Lode, Torbjörn Nilsson, and Lars Lundin. 2020. "Methane and Nitrous Oxide Emission Fluxes Along Water Level Gradients in Littoral Zones of Constructed Surface Water Bodies in a Rewetted Extracted Peatland in Sweden" Soil Systems 4, no. 1: 17. https://doi.org/10.3390/soilsystems4010017
APA StyleJordan, S., Strömgren, M., Fiedler, J., Lode, E., Nilsson, T., & Lundin, L. (2020). Methane and Nitrous Oxide Emission Fluxes Along Water Level Gradients in Littoral Zones of Constructed Surface Water Bodies in a Rewetted Extracted Peatland in Sweden. Soil Systems, 4(1), 17. https://doi.org/10.3390/soilsystems4010017




