Physical Properties of Soils Altered by Invasive Pheretimoid Earthworms: Does Their Casting Layer Create Thermal Refuges?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites and Soil Sampling
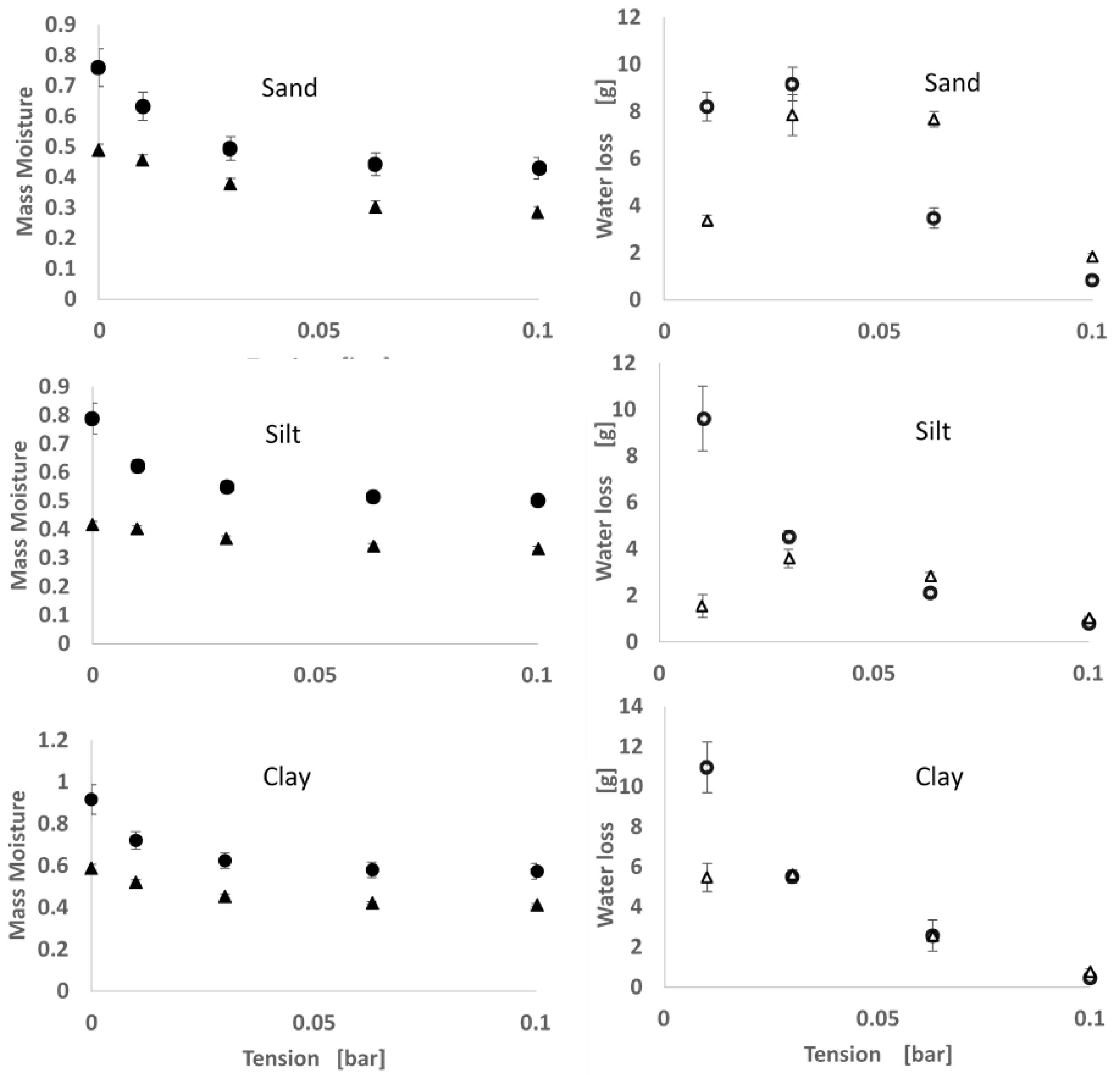
2.2. Soil Moisture Release Curve
2.3. Thermal Property Measurements
2.4. Pheretimoid Collections and Abundance
2.5. Statistical Analysis
3. Results
3.1. Moisture Release
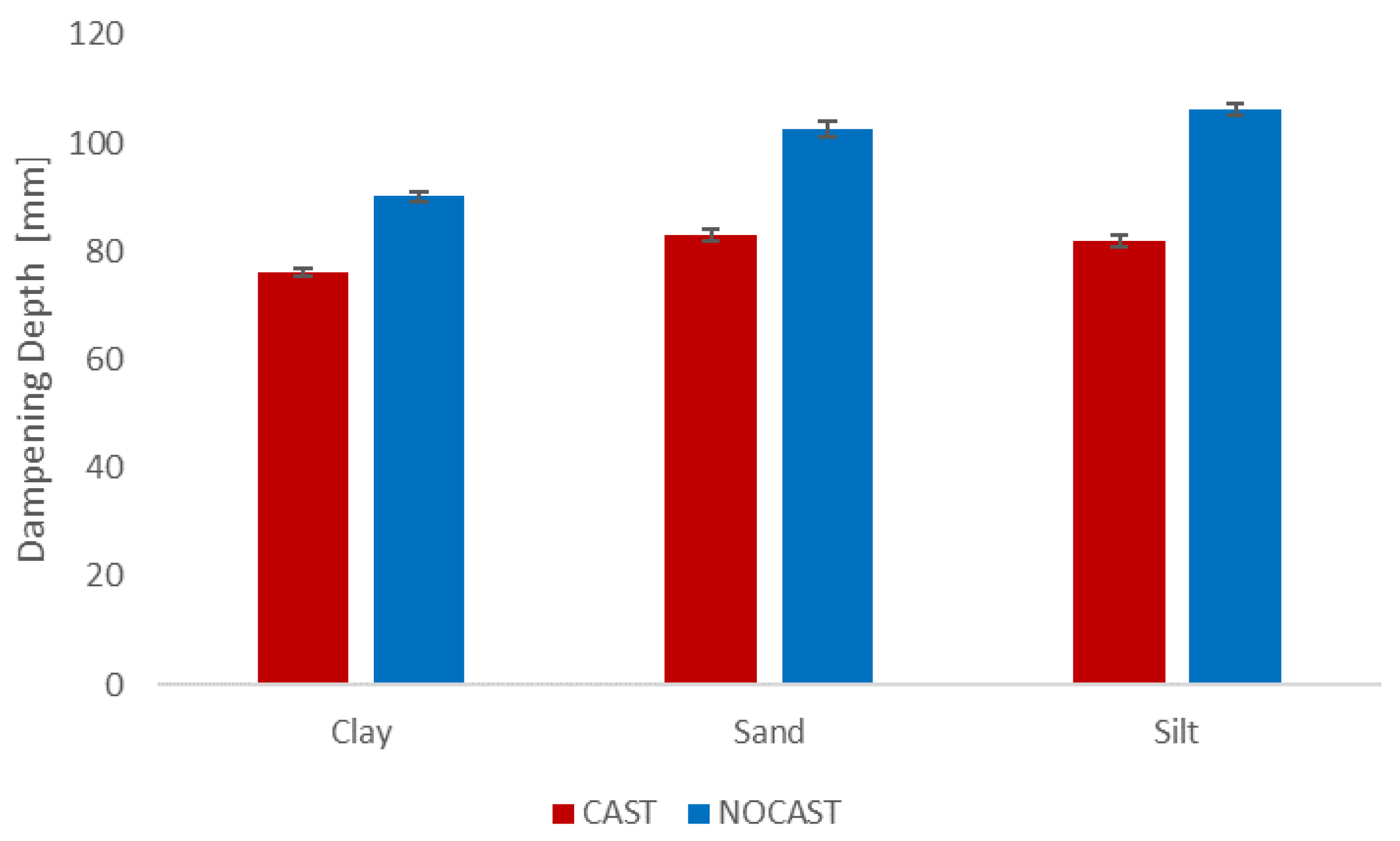
3.2. Thermal Properties
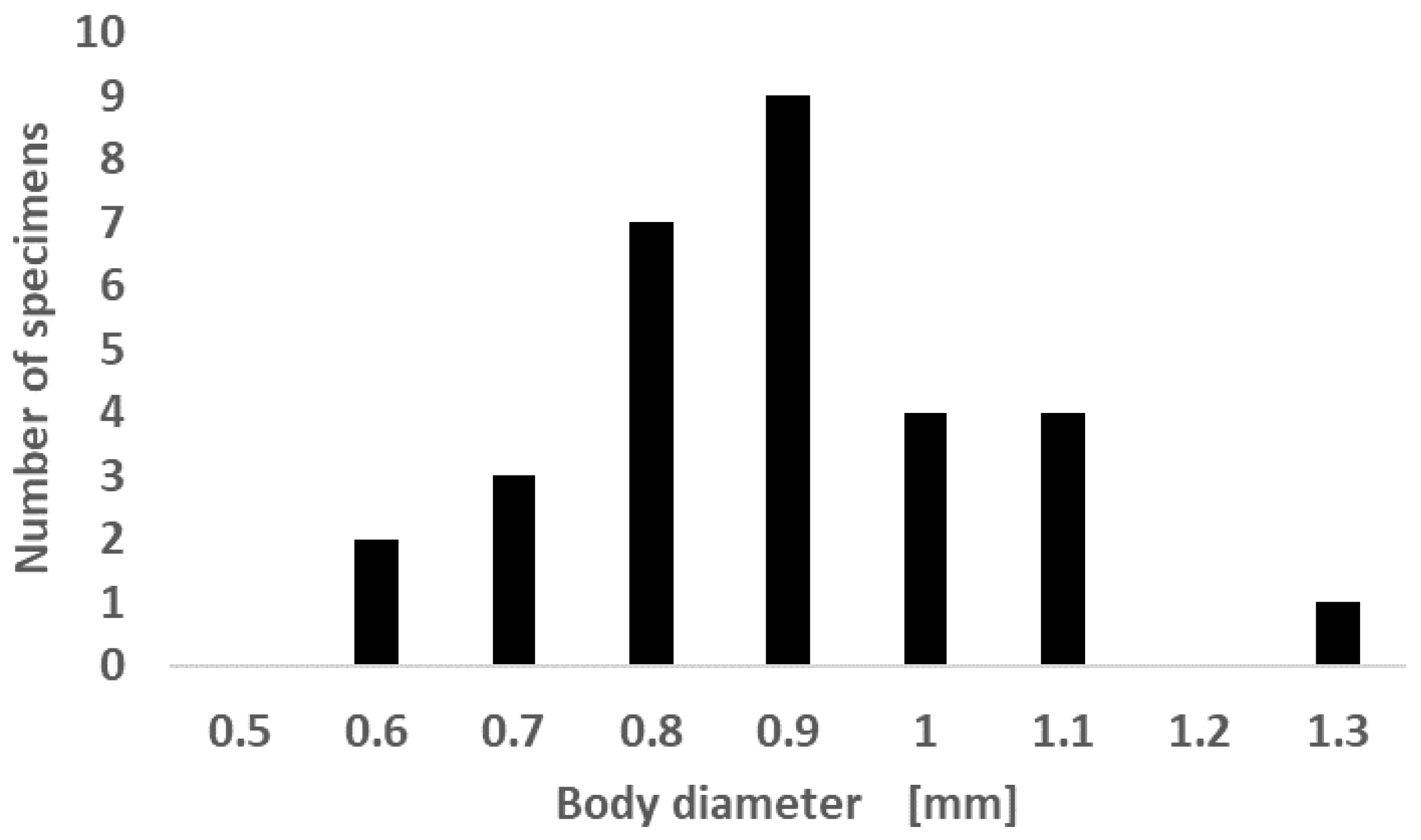
3.3. Pheretimoid Size
4. Discussion
4.1. Moisture Release and Thermal Properties
4.2. Ecological Relevance of Thermal Property Differences
5. Conclusions
- Significant differences between the thermal properties and macropore structure existed between the CAST and NOCAST soil.
- Production of CAST soil may improve pheretimoid survival when large daily temperature fluctuations about the mortality threshold occur, or when the soil is exposed to the extreme temperatures that may occur during forest fires.
- The casting layer may increase the invasiveness of pheretimoids in new environments.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A.
| ANOVA of Model | Effect Tests | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Earthworm | Tension | Soil | Earthworm X Tension | Earthworm X Soil | Tension X Soil | ||||||||||
| r2 | F-Ratio | p > F | F-Ratio | p > F | F-Ratio | p > F | F-Ratio | p > F | F-Ratio | p > F | F-Ratio | p > F | F-Ratio | p > F | |
| K | 0.83 | 36.56 | <0.0001 | 526.0 | <0.0001 | 23.68 | <0.0001 | 51.27 | <0.0001 | 1.48 | 0.209 | 15.53 | <0.0001 | 0.933 | <0.491 |
| C | 0.61 | 11.55 | <0.0001 | 57.65 | <0.0001 | 39.60 | <0.0001 | 3.66 | <0.0280 | 1.29 | 0.275 | 1.58 | <0.210 | 1.36 | <0.219 |
| ρ | 0.81 | 31.14 | <0.0001 | 550.4 | <0.0001 | 0.00 | 1.00 | 32.44 | <0.0001 | 0.00 | 1.00 | 19.38 | <0.0001 | 0.00 | 1.00 |
| θm | 0.82 | 34.17 | <0.0001 | 324.4 | <0.0001 | 65.03 | <0.0001 | 42.19 | <0.0001 | 8.59 | <0.0001 | 1.95 | 0.146 | 1.27 | 0.261 |
| Δm | 0.88 | 89.28 | <0.0001 | 24.34 | <0.0001 | 188.03 | <0.0001 | 24.55 | <0.0001 | 50.20 | <0.0001 | 4.63 | <0.0111 | 13.10 | <0.0001 |
| D | 0.77 | 25.03 | <0.0001 | 392.8 | <0.0001 | 0.798 | <0.528 | 51.95 | <0.0001 | 0.54 | 0.705 | 13.43 | <0.0001 | 0.408 | 0.9150 |
| Clay | CAST | NOCAST | ||||||||
| Tension [bar] | 0.0 | −0.01 | −0.03 | −0.063 | −0.1 | 0.0 | −0.01 | −0.03 | −0.063 | −0.1 |
| K [Wm−1k−1] | 0.593 | 0.501 | 0.438 | 0.402 | 0.395 | 0.925 | 0.890 | 0.822 | 0.716 | 0.678 |
| (0.042) | (0.030) | (0.015) | (0.025) | (0.018) | (0.055) | (0.085) | (0.094) | (0.045) | (0.076) | |
| C [MJm−3k−1] | 2.864 | 2.377 | 2.149 | 1.839 | 1.838 | 3.233 | 3.137 | 2.674 | 2.369 | 2.257 |
| (0.173) | (0.116) | (0.056) | (0.101) | (0.048) | (0.197) | (0.324) | (0.279) | (0.123) | (0.187) | |
| ρ [g/cm3] | 1.057 | 0.933 | 0.869 | 0.841 | 0.836 | 1.261 | 1.201 | 1.139 | 1.111 | 1.103 |
| (0.018) | (0.020) | (0.018) | (0.018) | (0.017) | (0.033) | (0.037) | (0.035) | (0.034) | (0.033) | |
| D [mm s−1] | 0.196 | 0.226 | 0.235 | 0.261 | 0.257 | 0.229 | 0.241 | 0.269 | 0.273 | 0.270 |
| (0.006) | (0.005) | (0.009) | (0.011) | (0.010) | (0.012) | (0.016) | (0.006) | (0.012) | (0.014) | |
| Sand | CAST | NOCAST | ||||||||
| Tension [bar] | 0.0 | −0.01 | −0.03 | −0.063 | −0.1 | 0.0 | −0.01 | −0.03 | −0.063 | −0.1 |
| K [Wm−1k−1] | 0.762 | 0.645 | 0.531 | 0.463 | 0.499 | 1.151 | 1.178 | 0.996 | 0.835 | 0.777 |
| (0.047) | (0.056) | (0.037) | (0.046) | (0.037) | (0.059) | (0.057) | (0.033) | (0.047) | (0.053) | |
| C [MJm−3k−1] | 3.065 | 2.540 | 2.081 | 1.849 | 1.994 | 3.272 | 3.098 | 2.597 | 2.040 | 2.013 |
| (0.114) | (0.155) | (0.158) | (0.116) | (0.054) | (0.065) | (0.087) | (0.105) | (0.140) | (0.084) | |
| ρ [g/cm3] | 1.199 | 1.100 | 0.991 | 0.950 | 0.940 | 1.423 | 1.388 | 1.305 | 1.223 | 1.204 |
| (0.027) | (0.031) | (0.031) | (0.030) | (0.029) | (0.038) | (0.038) | (0.040) | (0.036) | (0.036) | |
| D [mm s−1] | 0.208 | 0.231 | 0.261 | 0.262 | 0.266 | 0.247 | 0.275 | 0.296 | 0.339 | 0.321 |
| (0.009) | (0.012) | (0.015) | (0.013) | (0.014) | (0.008) | (0.012) | (0.010) | (0.019) | (0.016) | |
| Silt | CAST | NOCAST | ||||||||
| Tension [bar] | 0.0 | 0.01 | 0.03 | 0.063 | 0.1 | 0.0 | 0.01 | 0.03 | 0.063 | 0.1 |
| K [Wm−1k−1] | 0.726 | 0.621 | 0.565 | 0.484 | 0.490 | 1.235 | 1.248 | 1.160 | 1.150 | 1.082 |
| (0.035) | (0.042) | (0.051) | (0.048) | (0.042) | (0.059) | (0.061) | (0.048) | (0.093) | (0.080) | |
| C [MJm−3k−1] | 3.024 | 2.518 | 2.366 | 1.909 | 2.047 | 3.144 | 2.915 | 2.802 | 2.920 | 2.675 |
| (0.154) | (0.204) | (0.115) | (0.079) | (0.135) | (0.219) | (0.180) | (0.181) | (0.213) | (0.223) | |
| ρ [gcm−3] | 1.180 | 1.055 | 0.998 | 0.971 | 0.961 | 1.451 | 1.435 | 1.396 | 1.366 | 1.355 |
| (0.041) | (0.051) | (0.052) | (0.049) | (0.048) | (0.032) | (0.035) | (0.036) | (0.037) | (0.037) | |
| D [mm s−1] | 0.205 | 0.242 | 0.243 | 0.260 | 0.249 | 0.276 | 0.304 | 0.301 | 0.289 | 0.305 |
| (0.009) | (0.024) | (0.023) | (0.017) | (0.007) | (0.019) | (0.024) | (0.016) | (0.010) | (0.024) | |
References
- Jouquet, P.; Dauber, J.; Lagerlöf, J.; Lavelle, P.; Lepage, M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl. Soil Ecol. 2006, 32, 153–164. [Google Scholar] [CrossRef]
- Lawton, J.H. What do species do in ecosystems? Oikos 1994, 71, 367–374. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.; Brown, G.G.; De Deyn, G.B.; Van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, I.M.; Van Groenigen, K.J.; Fonte, S.J.; Six, J.; Brussaard, L.; Van Groenigen, J.W. Greenhouse-gas emissions from soils increased by earthworms. Nat. Clim. Chang. 2013, 3, 187. [Google Scholar] [CrossRef]
- Frelich, L.E.; Hale, C.M.; Reich, P.B.; Holdsworth, A.R.; Scheu, S.; Heneghan, L.; Bohlen, P.J. Earthworm invasion into previously earthworm-free temperate and boreal forests. In Biological Invasions Belowground: Earthworms as Invasive Species; Springer: Berlin/Heidelberg, Germany, 2006; pp. 35–45. [Google Scholar]
- Hendrix, P.F. Biological invasions belowground—Earthworms as invasive species. In Biological Invasions Belowground: Earthworms as Invasive Species; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–4. [Google Scholar]
- Bohlen, P.J.; Scheu, S.; Hale, C.M.; McLean, M.A.; Migge, S.; Groffman, P.M.; Parkinson, D. Non-Native invasive earthworms as agents of change in northern temperate forests. Front. Ecol. Environ. 2004, 2, 427–435. [Google Scholar] [CrossRef]
- Burtelow, A.E.; Bohlen, P.J.; Groffman, P.M. Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Appl. Soil Ecol. 1998, 9, 197–202. [Google Scholar] [CrossRef]
- Hale, C.M.; Frelich, L.E.; Reich, P.B. Changes in hardwood forest understory plant communities in response to European earthworm invasions. Ecology 2006, 87, 1637–1649. [Google Scholar] [CrossRef]
- Callaham, M.A.; Hendrix, P.F.; Phillips, R.J. Occurrence of an exotic earthworm (Amynthas agrestis) in undisturbed soils of the southern Appalachian Mountains, USA: The 7th international symposium on earthworm ecology· Cardiff· Wales· 2002. Pedobiologia 2003, 47, 466–470. [Google Scholar] [CrossRef]
- Moore, J.-D.; Görres, J.; Reynolds, J.W. Exotic Asian pheretimoid earthworms (Amynthas spp., Metaphire spp.): Potential for colonisation of south-eastern Canada and effects on forest ecosystems. Environ. Rev. 2018, 26, 113–120. [Google Scholar] [CrossRef]
- Görres, J.H.; Melnichuk, R.D. Asian invasive earthworms of the genus Amynthas Kinberg in Vermont. Northeast. Nat. 2012, 19, 313–322. [Google Scholar] [CrossRef]
- Chang, C.-H.; Snyder, B.A.; Szlavecz, K. Asian pheretimoid earthworms in North America north of Mexico: An illustrated key to the genera Amynthas, Metaphire, Pithemera, and Polypheretima (Clitellata: Megascolecidae). Zootaxa 2016, 4179, 495–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-H.; Johnston, M.R.; Görres, J.H.; Dávalos, A.; McHugh, D.; Szlavecz, K. Co-invasion of three Asian earthworms, Metaphire hilgendorfi, Amynthas agrestis and Amynthas tokioensis in the USA. Biol. Invasions 2018, 20, 843–848. [Google Scholar] [CrossRef]
- Hale, C.M.; Frelich, L.E.; Reich, P.B.; Pastor, J. Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota, USA. Ecosystems 2005, 8, 911–927. [Google Scholar] [CrossRef]
- Ponge, J.-F. Humus forms in terrestrial ecosystems: A framework to biodiversity. Soil Biol. Biochem. 2003, 35, 935–945. [Google Scholar] [CrossRef]
- Redmond, C.T.; Kesheimer, A.; Potter, D.A. Earthworm community composition, seasonal population structure, and casting activity on Kentucky golf courses. Appl. Soil Ecol. 2014, 75, 116–123. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H.; Reeder, R.C. Soil thermal conductivity effects of density, moisture, salt concentration, and organic matter. Soil Sci. Soc. Am. J. 2000, 64, 1285–1290. [Google Scholar] [CrossRef]
- Görres, J.H.; Bellitürk, K.; Melnichuk, R.D. Temperature and moisture variables affecting the earthworms of genus Amynthas Kinberg, 1867 (Oligachaeta: Megascolecidae) in a hardwood forest in the Champlain Valley, Vermont, USA. Appl. Soil Ecol. 2016, 104, 111–115. [Google Scholar] [CrossRef]
- Blackmon, J.H., IV. The Use of Fire in the Control of Invasive, Epigeic Earthworm Species in the Southeastern United States. Master’s Thesis, UGA, Athens, GA, USA, 2009. [Google Scholar]
- Nouri-Aiin, M.; Görres, J.H. Earthworm cocoons: The cryptic side of invasive earthworm populations. Appl. Soil Ecol. 2019, 141, 54–60. [Google Scholar] [CrossRef]
- Richardson, D.R.; Snyder, B.A.; Hendrix, P.F. Soil moisture and temperature: Tolerances and optima for a non-native earthworm species, Amynthas agrestis (Oligochaeta: Opisthopora: Megascolecidae). Southeast. Nat. 2009, 8, 325–334. [Google Scholar] [CrossRef]
- Johnston, M.R.; Herrick, B.M. Cocoon Heat Tolerance of Pheretimoid Earthworms Amynthas tokioensis and Amynthas agrestis. Am. Midl. Nat. 2019, 181, 299–309. [Google Scholar] [CrossRef]
- Darbyshire, J. Effect of water suctions on the growth in soil of the ciliate Colpoda steini, and the bacterium Azotobacter chroococcum. Eur. J. Soil Sci. 1976, 27, 369–376. [Google Scholar] [CrossRef]
- Griffiths, B.; Young, I.; Caul, S. Nematode and protozoan population dynamics on decomposing barley leaves incubated at soil matric potentials. Pedobiologia 1995, 39, 454–461. [Google Scholar]
- Görres, J.H.; Savin, M.C.; Neher, D.A.; Weicht, T.R.; Amador, J.A. Grazing in a porous environment: 1. The effect of soil pore structure on C and N mineralization. Plant Soil 1999, 212, 75–83. [Google Scholar] [CrossRef]
- Jury, W.A.; Horton, R. Soil Physics; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Abu-Hamdeh, N.H. Thermal properties of soils as affected by density and water content. Biosys. Eng. 2003, 86, 97–102. [Google Scholar] [CrossRef]
- Van Duin, R. The Influence of Soil Management on the Temperature Wave Near the Surface; Institute for Land and Water Management Research Technical Bulletin: Wageningen, The Netherlands, 1963. [Google Scholar]
- Ikeda, H.; Callaham, M.A., Jr.; O’Brien, J.J.; Hornsby, B.S.; Wenk, E.S. Can the invasive earthworm, Amynthas agrestis, be controlled with prescribed fire? Soil Biol. Biochem. 2015, 82, 21–27. [Google Scholar] [CrossRef]
- Blackmon, J.H.; Taylor, M.K.; Carrera-Martínez, R.; Snyder, B.A.; Callaham, M.A. Temperature Affects Hatching Success of Cocoons in the Invasive Asian Earthworm Amynthas agrestis from the Southern Appalachians. Southeast. Nat. 2019, 18, 270–280. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Görres, J.H.; Martin, C.; Nouri-Aiin, M.; Bellitürk, K. Physical Properties of Soils Altered by Invasive Pheretimoid Earthworms: Does Their Casting Layer Create Thermal Refuges? Soil Syst. 2019, 3, 52. https://doi.org/10.3390/soilsystems3030052
Görres JH, Martin C, Nouri-Aiin M, Bellitürk K. Physical Properties of Soils Altered by Invasive Pheretimoid Earthworms: Does Their Casting Layer Create Thermal Refuges? Soil Systems. 2019; 3(3):52. https://doi.org/10.3390/soilsystems3030052
Chicago/Turabian StyleGörres, Josef H., Christina Martin, Maryam Nouri-Aiin, and Korkmaz Bellitürk. 2019. "Physical Properties of Soils Altered by Invasive Pheretimoid Earthworms: Does Their Casting Layer Create Thermal Refuges?" Soil Systems 3, no. 3: 52. https://doi.org/10.3390/soilsystems3030052




