The Controls of Iron and Oxygen on Hydroxyl Radical (•OH) Production in Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Tundra Soil Cores Collection
2.2. Mesocosm Design
2.3. Soil Water Collection and Characterization
2.4. EDC and Iron Concentrations
2.5. CDOM and FDOM Analysis
2.6. •OH Concentrations
2.7. EDC, DOC and Iron Production
2.8. Dissolved O2 Consumption
2.9. •OH Production
3. Results
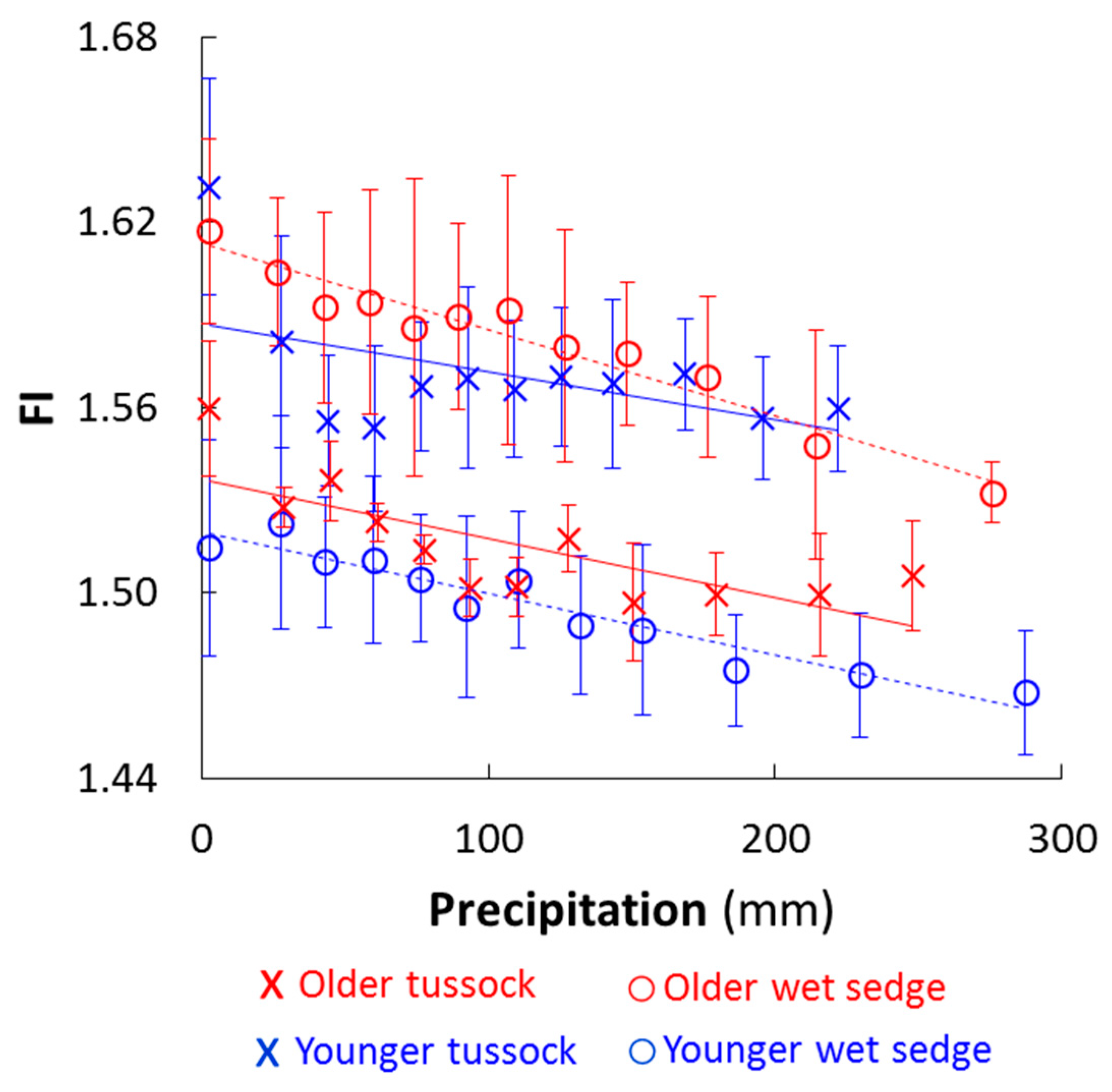
3.1. Soil and Soil Water Chemistry Differed by Landscape Age and Vegetation Type
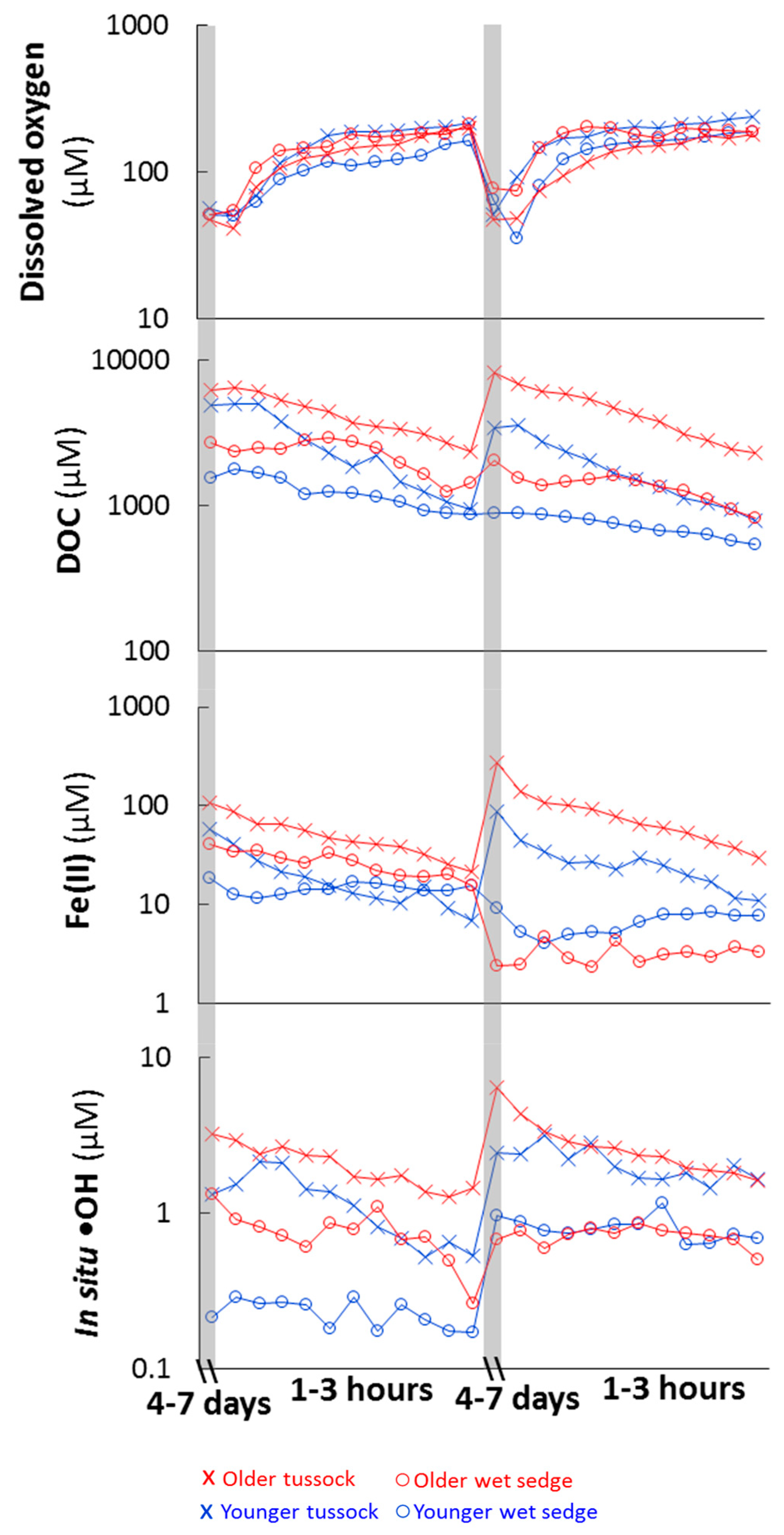
3.2. Change in Soil Water Chemistry During Precipitation Events
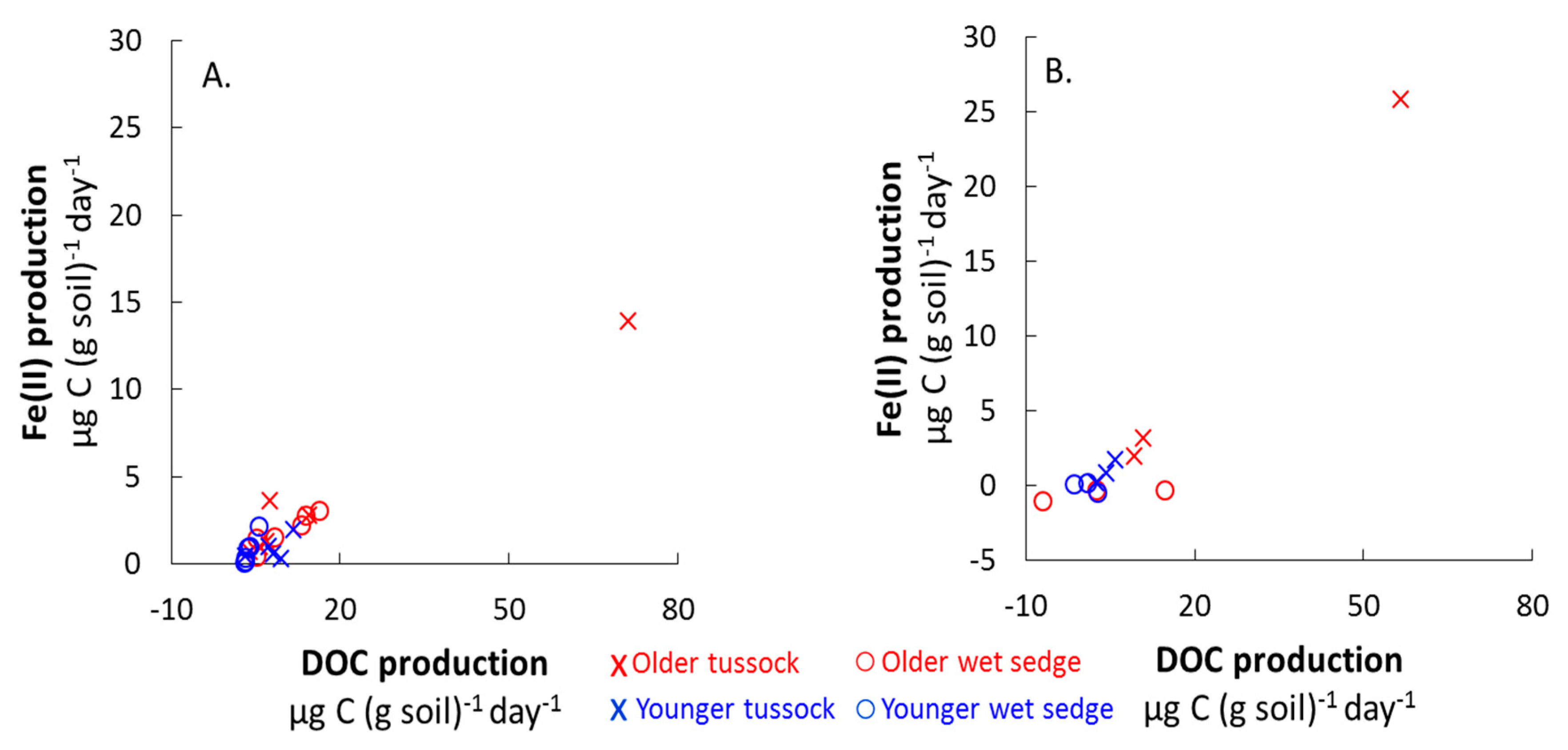
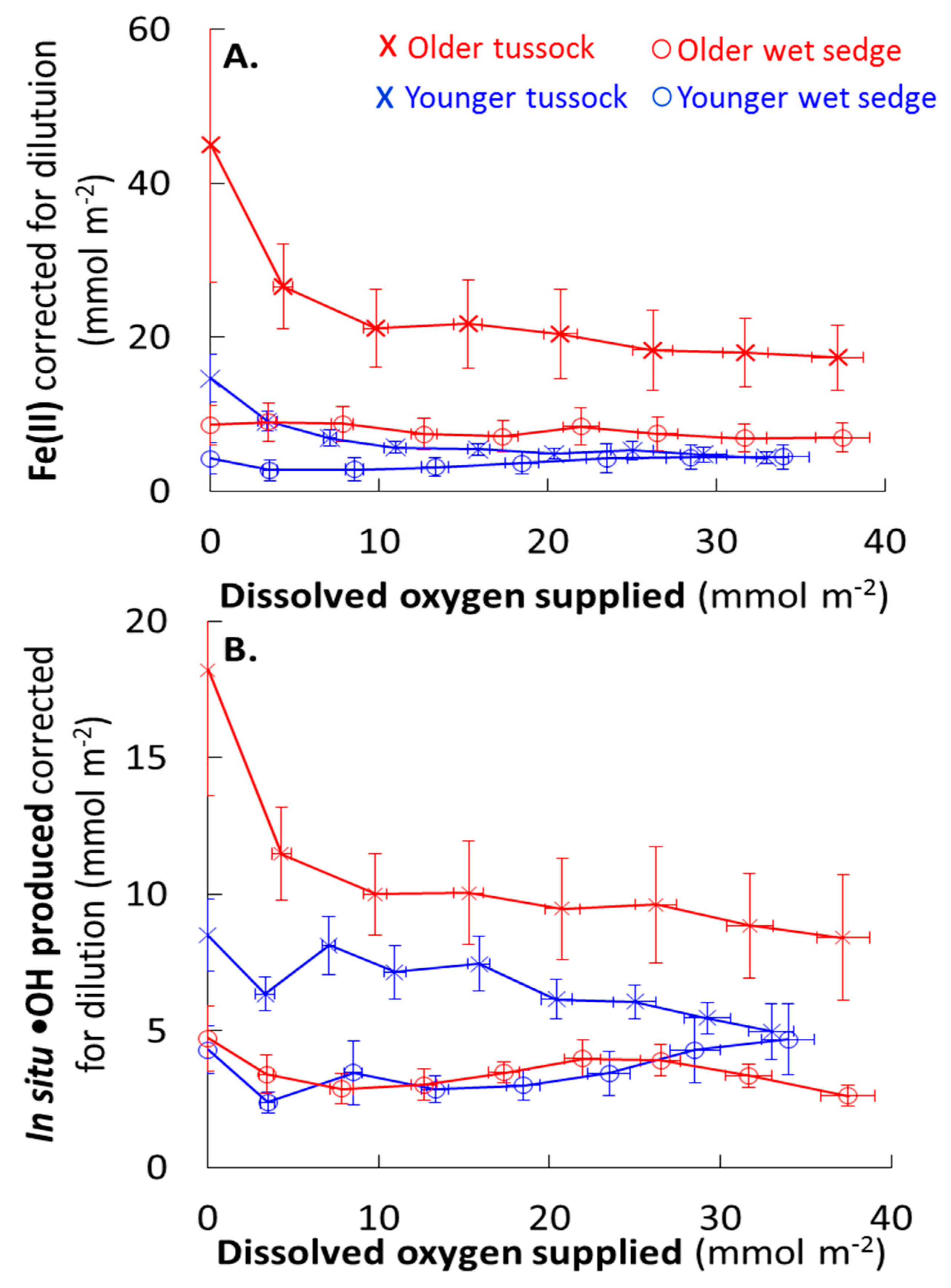
3.3. Consumption and Production from Waterlogged Soils
4. Discussion
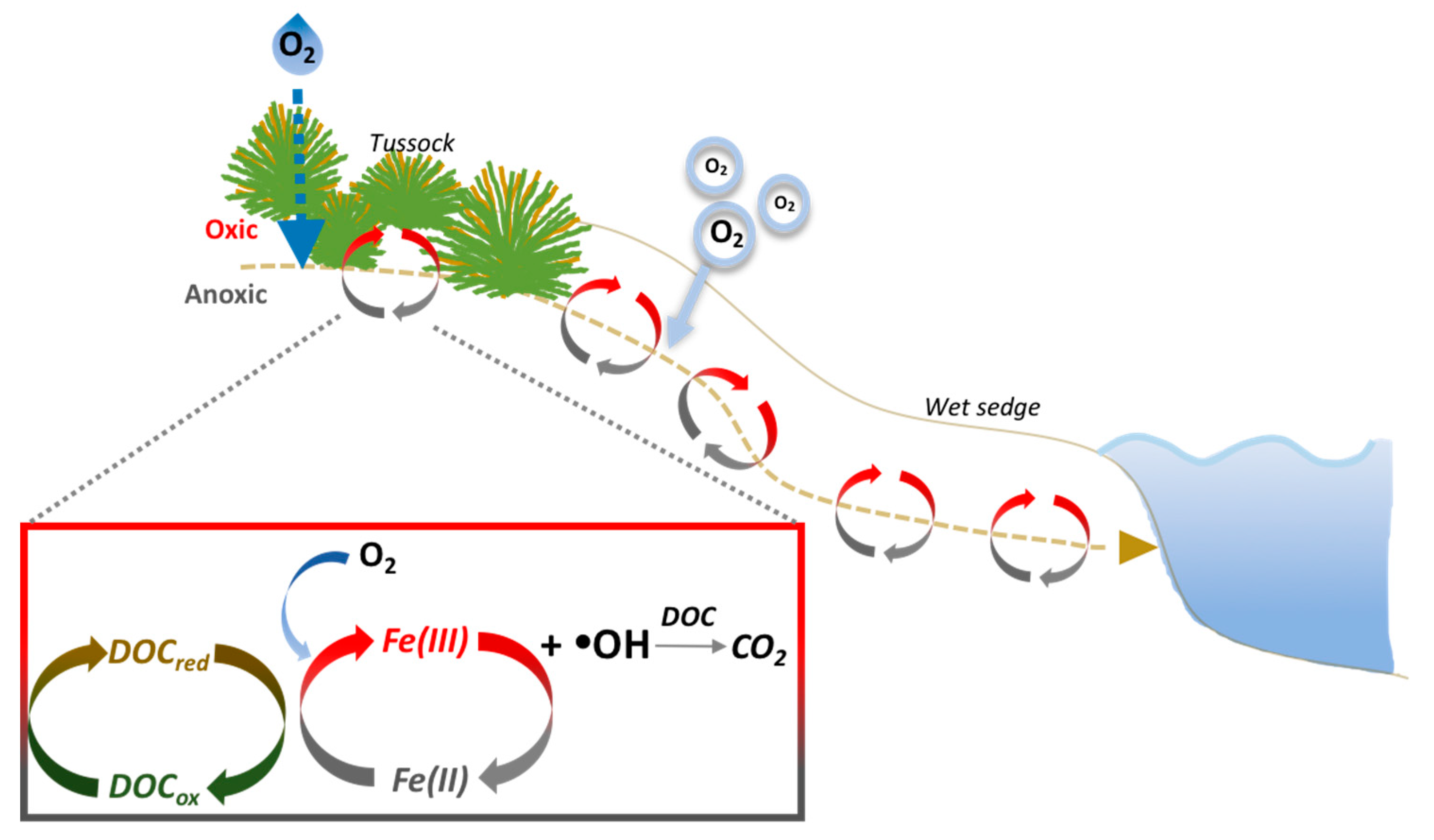
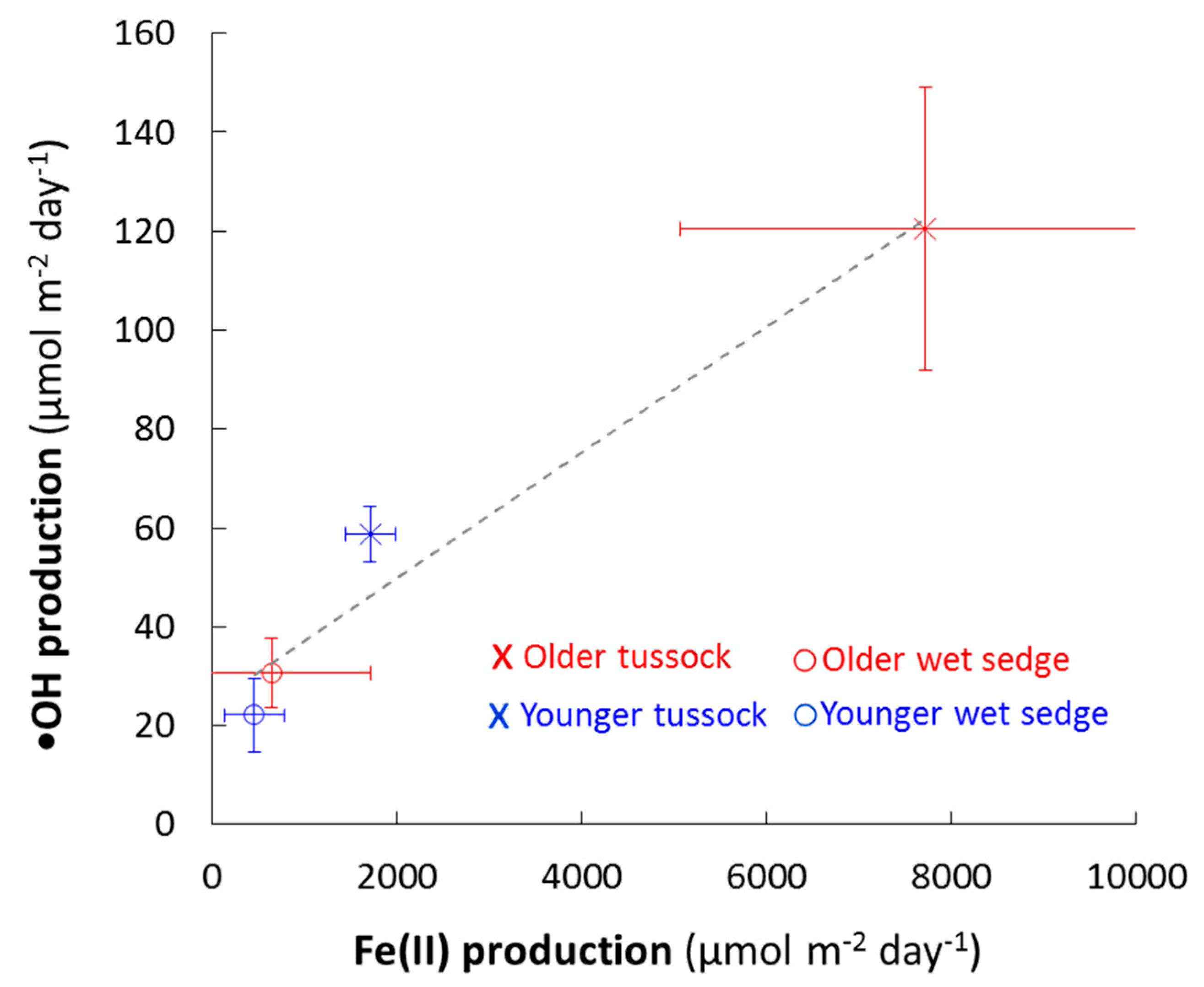
4.1. The Balance of Fe(II) Production and Consumption Controls •OH Production During Precipitation Events
4.2. O2 Supply Limits •OH Production During Waterlogged Conditions
4.3. •OH Mediated Oxidation of DOC to CO2 During Precipitation Versus Static Conditions
4.4. Landscape Controls on Fe(II) and •OH Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Calculation of •OH and CO2 Produced during a 4 mm Precipitation Event
Appendix B. Calculation of •OH and CO2 Produced during Static, Waterlogged Conditions
References
- Page, S.E.; Sander, M.; Arnold, W.A.; McNeill, K. Hydroxyl radical formation upon oxidation of reduced humic acids by oxygen in the dark. Environ. Sci. Technol. 2012, 46, 1590–1597. [Google Scholar] [CrossRef]
- Page, S.E.; Kling, G.W.; Sander, M.; Harrold, K.H.; Logan, J.R.; McNeill, K.; Cory, R.M. Dark formation of hydroxyl radical in arctic soil and surface waters. Environ. Sci. Technol. 2013, 47, 12860–12867. [Google Scholar] [CrossRef] [PubMed]
- Trusiak, A.; Treibergs, L.A.; Kling, G.W.; Cory, R.M. The role of iron and reactive oxygen species in the production of CO2 in arctic soil waters. Geochim. Cosmochim. Acta 2018, 224, 80–95. [Google Scholar] [CrossRef]
- Goldstone, J.V.; Pullin, M.J.; Bertilsson, S.; Voelker, B.M. Reactions of hydroxyl radical with humic substances: Bleaching, mineralization and production of bioavailable carbon substrates. Environ. Sci. Technol. 2002, 36, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.J.; Silver, W.L. Iron oxidation stimulates organic matter decomposition in humid tropical forest soils. Glob. Chang. Biol. 2013, 19, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Minella, M.; De Laurentiis, E.; Maurino, V.; Minero, C.; Vione, D. Dark production of hydroxyl radicals by aeration of anoxic lake water. Sci. Total Environ. 2015, 527–528, 322–327. [Google Scholar] [CrossRef]
- Tong, M.; Yuan, S.; Ma, S.; Jin, M.; Liu, D.; Cheng, D.; Wang, Y. Production of Abundant Hydroxyl Radicals from Oxygenation of Subsurface Sediments. Environ. Sci. Technol. 2016, 50, 214–221. [Google Scholar] [CrossRef]
- King, J.Y.; Reeburgh, W.S.; Regli, S.K. Methane emission and transport by arctic sedges in Alaska: Results of a vegetation removal experiment. J. Geophys. Res. Atmos. 1998, 103, 29083–29092. [Google Scholar] [CrossRef]
- Updegraff, K.; Bridgham, S.D.; Pastor, J.; Weishampel, P.; Harth, C. Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol. Appl. 2001, 11, 311–326. [Google Scholar]
- King, J.Y.; Reeburgh, W.S.; Thieler, K.K.; Kling, G.W.; Loya, W.M.; Johnson, L.C.; Nadelhoffer, K.J. Pulse-labeling studies of carbon cycling in Arctic tundra ecosystems: The contribution of photosynthates to methane emission. Glob. Biogeochem. Cycles 2002, 16, 1–8. [Google Scholar] [CrossRef]
- Zona, D.; Oechel, W.C.; Kochendorfer, J.; Paw, U.K.T.; Salyuk, A.N.; Olivas, P.C.; Lipson, D.A. Methane fluxes during the initiation of a large-scale water table manipulation experiment in the Alaskan Arctic tundra. Glob. Biogeochem. Cycles 2009, 23, 1–11. [Google Scholar] [CrossRef]
- Knorr, K.H.; Glaser, B.; Blodau, C. Fluxes and 13C isotopic composition of dissolved carbon and pathways of methanogenesis in a fen soil exposed to experimental drought. Biogeosciences 2008, 5, 1457–1473. [Google Scholar] [CrossRef]
- Knorr, K.H.; Oosterwoud, M.R.; Blodau, C. Experimental drought alters rates of soil respiration and methanogenesis but not carbon exchange in soil of a temperate fen. Soil Biol. Biochem. 2008, 40, 1781–1791. [Google Scholar] [CrossRef]
- Lipson, D.A.; Jha, M.; Raab, T.K.; Oechel, W.C. Reduction of iron (III) and humic substances plays a major role in anaerobic respiration in an Arctic peat soil. J. Geophys. Res. Biogeosci. 2010, 115, 1–13. [Google Scholar] [CrossRef]
- Stumm, W.; Sulzberger, B. The cycling of iron in natural environments: Considerations based on laboratory studies of heterogeneous redox processes. Geochim. Cosmochim. Acta 1992, 56, 3233–3257. [Google Scholar] [CrossRef]
- Luther, G.W.; Kostka, J.E.; Church, T.M.; Sulzberger, B.; Stumm, W. Seasonal iron cycling in the salt-marsh sedimentary environment: The importance of ligand complexes with Fe(II) and Fe(III) in the dissolution of Fe(III) minerals and pyrite, respectively. Mar. Chem. 1992, 40, 81–103. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef]
- Lipson, D.A.; Zona, D.; Raab, T.K.; Bozzolo, F.; Mauritz, M.; Oechel, W.C. Water-table height and microtopography control biogeochemical cycling in an Arctic coastal tundra ecosystem. Biogeosciences 2012, 9, 577–591. [Google Scholar] [CrossRef]
- Herndon, E.M.; Yang, Z.; Bargar, J.; Janot, N.; Regier, T.Z.; Graham, D.E.; Liang, L. Geochemical drivers of organic matter decomposition in arctic tundra soils. Biogeochemistry 2015, 126, 397–414. [Google Scholar] [CrossRef]
- Roden, E.E.; Wetzel, R.G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol. Oceanogr. 1996, 41, 1733–1748. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel Mode of Microbial Energy Metabolism: Organic Carbon Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [PubMed]
- Roden, E.E.; Kappler, A.; Bauer, I.; Jiang, J.; Paul, A.; Stoesser, R. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat. Geosci. 2010, 3, 417–421. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Organic-Matter Mineralization with Reduction of Ferric Iron in Anaerobic Sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [PubMed]
- Knorr, K.H. DOC-dynamics in a small headwater catchment as driven by redox fluctuations and hydrological flow paths—Are DOC exports mediated by iron reduction/oxidation cycles? Biogeosciences 2013, 10, 891–904. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Prairie, Y.T.; Tranvik, L.J. Browning of boreal freshwaters coupled to carbon-iron interactions along the aquatic continuum. PLoS ONE 2014, 9, e88104. [Google Scholar] [CrossRef] [PubMed]
- Judd, K.E.; Kling, G.W. Production and export of dissolved C in arctic tundra mesocosms: The roles of vegetation and water flow. Biogeochemistry 2002, 60, 213–234. [Google Scholar] [CrossRef]
- Ward, C.P.; Cory, R.M. Chemical composition of dissolved organic matter draining permafrost soils. Geochim. Cosmochim. Acta 2015, 167, 63–79. [Google Scholar] [CrossRef]
- Hamilton, T.D. Glacial Geology of the Toolik Lake and Upper Kuparuk River Regions; Biological Papers of the University of Alaska; University of Alaska: Fairbanks, Alaska, 2003; pp. 1–24. [Google Scholar]
- Kling, G.W.; Kipphut, G.W.; Miller, M.C. Arctic lakes and streams as gas conduits to the atmosphere: Implications for tundra carbon budgets. Science 1991, 251, 298–301. [Google Scholar] [CrossRef]
- Johnson, L.C.; Shaver, G.R.; Giblin, A.E.; Nadelhoffer, K.J.; Rastetter, E.R.; Laundre, J.A.; Murray, G.L. Effects of drainage and temperature on carbon balance of tussock tundra microcosms. Oecologia 1996, 108, 737–748. [Google Scholar] [CrossRef]
- Gebauer, R.L.E.; Tenhunen, J.D.; Reynolds, J.F. Soil aeration in relation to soil physical properties, nitrogen availability and root characteristics within an arctic watershed. Plant Soil 1996, 178, 37–48. [Google Scholar] [CrossRef]
- Hobbie, J.E.; Kling, G.W. (Eds.) A Changing Arctic: Ecological Consequences for Tundra, Streams and Lakes; Oxford University Press: Oxford, UK, 2014; p. 331. [Google Scholar]
- Robertson, G.P.; Bledsoe, C.S.; Coleman, D.C.; Sollins, P. (Eds.) Standard Soil Methods for Long-Term Ecological Research; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Kling, G.W.; Kipphut, G.W.; Miller, M.M.; O’Brien, W.J. Integration of lakes and streams in a landscape perspective: The importance of material processing on spatial patterns and temporal coherence. Freshwater Biol. 2000, 43, 477–497. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Cory, R.M.; Miller, M.P.; McKnight, D.M.; Guerard, J.J.; Miller, P.L. Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol. Oceanogr. Methods 2010, 8, 67–78. [Google Scholar]
- Miller, W.L. Recent Advances in the Photochemistry of Natural Dissolved Organic Matter. In Aquatic and Surface Photochemistry; CRC Press: Boca Raton, FL, USA, 2010; pp. 111–128. [Google Scholar]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Cory, R.M.; Mcknight, D.M.; Chin, Y.; Miller, P.; Jaros, C.L. Chemical characteristics of fulvic acids from Arctic surface waters: Microbial contributions and photochemical transformations. J. Geophys. Res. Biogeosci. 2007, 112, 1–14. [Google Scholar] [CrossRef]
- Page, S.E.; Arnold, W.A.; McNeill, K. Terephthalate as a probe for photochemically generated hydroxyl radical. J. Environ. Monit. JEM 2010, 12, 1658–1665. [Google Scholar] [CrossRef]
- Page, S.E.; Logan, J.R.; Cory, R.M.; McNeill, K. Evidence for dissolved organic matter as the primary source and sink of photochemically produced hydroxyl radical in arctic surface waters. Environ. Sci. Process. Impacts 2014, 16, 807–822. [Google Scholar] [CrossRef]
- Stumm, W.; Lee, F.G. Oxygenation of ferrous iron. Ind. Eng. Chem. 1961, 53, 143–146. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Graf, C.; Scwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Klüpfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Heitmann, T.; Goldhammer, T.; Beer, J.; Blodau, C. Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Glob. Chang. Biol. 2007, 13, 1771–1785. [Google Scholar] [CrossRef]
- Voelker, B.M.; Sulzberger, B. Effects of fulvic acid on Fe(II) oxidation by hydrogen peroxide. Environ. Sci. Technol. 1996, 30, 1106–1114. [Google Scholar] [CrossRef]
- Yarie, J.; Van Cleve, K.; Dyrness, C.T.; Oliver, L.; Levison, J.; Erickson, R. Soil-solution chemistry in relation to forest succession on the Tanana River floodplain, interior Alaska. Can. J. For. Res. 1993, 23, 928–940. [Google Scholar] [CrossRef]
- Petrone, K.C.; Jones, J.B.; Hinzman, L.D.; Boone, R.D. Seasonal export of carbon, nitrogen and major solutes from Alaskan catchments with discontinuous permafrost. J. Geophys. Res. Biogeosci. 2006, 111, 1–13. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Oechel, W.C. Landscape-Scale CO2, H2O Vapour and Energy Flux of Moist-Wet Coastal Tundra Ecosystems over Two Growing Seasons A. Br. Ecol. Soc. 1997, 85, 575–590. [Google Scholar]
- Liljedahl, A.K.; Hinzman, L.D.; Harazono, Y.; Zona, D.; Tweedie, C.E.; Hollister, R.D.; Engstrom, R. Nonlinear controls on evapotranspiration in arctic coastal wetlands. Biogeosciences 2011, 8, 3375–3389. [Google Scholar] [CrossRef]
- Liljedahl, A.K.; Boike, J.; Daanen, R.P.; Fedorov, A.N.; Frost, G.V.; Grosse, G.; Hinzmanm, L.D.; Iilma, Y.; Jorgenson, J.C.; Matveyeva, N.; et al. Pan-Arctic ice-wedge degradation in warming permafrost and its influence on tundra hydrology. Nat. Geosci. 2016, 9, 312. [Google Scholar] [CrossRef]
- Armstrong, W. Oxygen Diffusion from the Roots of Some British Bog Plants. Nature 1964, 204, 801–802. [Google Scholar] [CrossRef]
- Soukup, A.; Armstrong, W.; Schreiber, L.; Franke, R.; Votrubová, O. Apoplastic barriers to radial oxygen loss and solute penetration: A chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytol. 2007, 173, 264–278. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.-D. A global budget for fine root biomass, surface area and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.; Miller, P.C. Nordic Society Oikos Soil Oxygen Flux Measured Polarographically in an Alaskan Tussock Tundra A. Proc. Natl. Acad. Sci. USA 1981, 5, 139–144. [Google Scholar]
- Huemmrich, K.F.; Kinoshita, G.; Gamon, J.A.; Houston, S.; Kwon, H.; Oechel, W.C. Tundra carbon balance under varying temperature and moisture regimes. J. Geophys. Res. 2010, 115, G00I02. [Google Scholar] [CrossRef]
- Chivers, M.R.; Turetsky, M.R.; Waddington, J.M.; Harden, J.W.; McGuire, A.D. Effects of experimental water table and temperature manipulations on ecosystem CO2 fluxes in an Alaskan rich fen. Ecosystems 2009, 12, 1329–1342. [Google Scholar] [CrossRef]
- Gorski, C.A.; Aeschbacher, M.; Soltermann, D.; Voegelin, A.; Baeyens, B.; Fernandes, M.M.; Sander, M. Redox Properties of Structural Fe in Clay Minerals. 1. Electrochemical Quantification of Electron-Donating and -Accepting Capacities of Smectites. Environ. Sci. Technol. 2012, 9360–9368. [Google Scholar]
- Sander, M.; Hofstetter, T.B.; Gorski, C.A. Electrochemical analyses of redox-active iron minerals: A review of nonmediated and mediated approaches. Environ. Sci. Technol. 2015, 49, 5862–5878. [Google Scholar] [CrossRef]
- Lau, M.P.; Sander, M.; Gelbrecht, J.; Hupfer, M. Solid phases as important electron acceptors in freshwater organic sediments. Biogeochemistry 2015, 123, 49–61. [Google Scholar] [CrossRef]
- Keller, K.; Blum, J.D.; Kling, G.W. Geochemistry of Soils and Streams on Surfaces of Varying Ages in Arctic Alaska. Arct. Antarct. Alp. Res. 2007, 39, 84–98. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsieh, Y.H.; Cheng, K.Y.; Hsieh, L.L.; Cheng, T.C.; Yao, K.S. Effect of pH on Fenton process using estimation of hydroxyl radical with salicylic acid as trapping reagent. Water Sci. Technol. 2008, 58, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Ping, C.L.; Bockheim, J.G.; Kimble, J.M.; Michaelson, G.J.; Walker, D.A. Characteristics of cryogenic soils along a latitudinal transect in Arctic Alaska. J. Geophys. Res. 1998, 103, 28917–28928. [Google Scholar] [CrossRef]
- Stieglitz, M.; Shaman, J.; McNamara, J.; Engel, V.; Shanley, J.; Kling, G.W. An approach to understanding hydrologic connectivity on the hillslope and the implications for nutrient transport. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Voytek, E.B.; Rushlow, C.R.; Godsey, S.E.; Singha, K. Identifying hydrologic flowpaths on arctic hillslopes using electrical resistivity and self-potential. Geophysics 2016, 81, WA225–WA232. [Google Scholar] [CrossRef]
- Neilson, B.T.; Cardenas, M.B.; O’Connor, M.T.; Rasmussen, M.T.; King, T.V.; Kling, G.W. Groundwater flow and exchange across the land surface explain carbon export patterns in continuous permafrost watersheds. Geophys. Res. Lett. 2018, 45, 7596–7605. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Müller, R.A.; Norman, M.; Tranvik, L.J. Sensitivity of freshwaters to browning in response to future climate change. Clim. Chang. 2016, 134, 225–239. [Google Scholar] [CrossRef]
- Sarkkola, S.; Nieminen, M.; Koivusalo, H.; Laurén, A.; Kortelainen, P.; Mattsson, T.; Finér, L. Science of the Total Environment Iron concentrations are increasing in surface waters from forested headwater catchments in eastern Finland. Sci. Total Environ. 2013, 463–464, 683–689. [Google Scholar]
- Ekström, S.M.; Regnell, O.; Reader, H.E.; Nilsson, P.A.; Löfgren, S.; Kritzberg, E.S. Increasing concentrations of iron in surface waters as a consequence of reducing conditions in the catchment area. J. Geophys. Res. Biogeosci. 2016, 121, 479–493. [Google Scholar] [CrossRef]
- Koenigk, T.; Brodeau, L.; Graversen, R.G.; Karlsson, J.; Svensson, G.; Tjernstrom, M.; Wille, U. Arctic climate change in 21st century CMIP5 simulations with EC-Earth. Clin. Dyn. 2013, 40, 2719–2743. [Google Scholar] [CrossRef]
- Nilsson, J.; Sørensen, L.S.; Barletta, V.R.; Forsberg, R. Mass changes in Arctic ice caps and glaciers: Implications of regionalizing elevation changes. Cryosphere 2015, 9, 139–150. [Google Scholar] [CrossRef]
- Liljedahl, A.K.; Hinzman, L.D.; Kane, D.L.; Oechel, W.C.; Tweedie, C.E.; Zona, D. Tundra water budget and implications of precipitation underestimation. Water Resour. Res. 2017, 53, 6472–6486. [Google Scholar] [CrossRef]
- Barber, V.A.; Juday, G.P.; Finney, B.P. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Lett. Nat. 2000, 405, 668. [Google Scholar] [CrossRef]
- Al, D.E.T.; Inje, T.V.; Lekseev, G.A.; Aslowski, W.M. The Arctic Ocean Response to the North Atlantic Oscillation. Am. Meteorol. Soc. 2000, 95, 2671–2696. [Google Scholar]
- Olivas, P.C.; Oberbauer, S.F.; Tweedie, C.E.; Oechel, W.C.; Kuchy, A. Responses of CO2 flux components of Alaskan Coastal Plain tundra to shifts in water table. J. Geophys. Res. 2010, 115, 1–13. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; Mcfarland, J.; Blazewicz, S.J.; Jansson, J.K. Multi-omics of permafrost, active layer and thermokarst bog soilmicrobiomes. Nature 2015, 521, 208. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.J.; Caraco, N.F.; Kling, G.W.; Kratz, T.K. Carbon Dioxide Supersaturation in the Surface Waters of Lakes. Science 1994, 265, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355. [Google Scholar] [CrossRef] [PubMed]
- Kritzberg, E.S.; Ekstrom, S.M. Increasing iron concentrations in surface waters—a factor behind brownification? Biogeosciences 2012, 9, 1465–1478. [Google Scholar] [CrossRef]
| Variable | Older (Imnavait) | Younger (Toolik) | ||||
|---|---|---|---|---|---|---|
| Vegetation | Tussock | Wet sedge | Tussock | Wet sedge | ||
| Horizon | Organic | Mineral | Organic | Organic | Mineral | Organic |
| Depth (cm) | 19 ± 8 | 9 ± 4 | 27 ± 1 | 15 ± 7 | 18 ± 8 | 30 ± 1 |
| Bulk density (g dry soil cm−3) | 0.3 ± 0.04 A | 0.2 ± 0.01 B | 0.4 ± 0.08 A | 0.2 ± 0.01 B | ||
| Soil organic carbon (%) | 40 ± 3 A | 5 ± 0 A | 40 ± 1 B,C | 30 ± 6 A | 6 ± 1 A | 35 ± 1 B,D |
| Moisture (% water) | 82 ± 3 A | 30 ± 2 A | 83.1 ± 1.2 B | 70 ± 7 B | 38 ± 5 B | 82 ± 0 B |
| Porosity (% of total soil volume) | 80 ± 3 A | 30 ± 2 A | 90 ± 6 B | 70 ± 7 B | 40 ± 5 B | 80 ± 6 B |
| Variable | Older (Imnavait) | Younger (Toolik) | ||
|---|---|---|---|---|
| Vegetation | Tussock | Wet Sedge | Tussock | Wet Sedge |
| pH | 5.0 ± 0.1 A | 5.4 ± 0.1 B,C | 4.9 ± 0.1 A | 6.4 ± 0.1 B,D |
| Conductivity (µS cm−1) | 46 ± 3 | 34 ± 6 C | 44 ± 6 A | 151 ± 10 B,D |
| Dissolved oxygen (µM) | 47 ± 9 | 46 ± 4 | 49 ± 11 | 65 ± 12 |
| Electron donating capacity µmol (kg dry soil)−1 | 530 ± 210 C | 370 ± 90 | 120 ± 40 D | 190 ± 30 |
| DOC µg C (g dry soil)−1 | 120 ± 45 | 100 ± 16 C | 48 ± 7.1 | 49 ± 6.8 D |
| Fe(tot) µg (g dry soil)−1 | 22 ± 8.9 | 26 ± 6.2 C | 5.1 ± 1.2 | 11 ± 3.1 D |
| Fe(II) µg (g dry soil)−1 | 19 ± 7.5 C | 14 ± 3.0 C | 4.2 ± 1.2 D | 6.6 ± 2.6 D |
| Variable | Older (Imnavait) | Younger (Toolik) | ||
|---|---|---|---|---|
| Vegetation | Tussock | Wet Sedge | Tussock | Wet Sedge |
| Slope ratio | 0.75 ± 0.05 C | 0.71 ± 0.01 | 0.86 ± 0.00 D | 0.77 ± 0.12 |
| Fluorescence Index | 1.56 ± 0.02 | 1.62 ± 0.03 C | 1.63 ± 0.04 A | 1.52 ± 0.02 B,D |
| C/A | 0.52 ± 0.02 | 0.55 ± 0.02 | 0.54 ± 0.03 | 0.51 ± 0.02 |
| T/A | 0.23 ± 0.08 C | 0.45 ± 0.20 | 0.63 ± 0.05 D | 1.06 ± 0.73 |
| Variable | Older (Imnavait) | Younger (Toolik) | Older (Imnavait) | Younger (Toolik) | ||||
|---|---|---|---|---|---|---|---|---|
| Vegetation | Tussock | Wet sedge | Tussock | Wet sedge | Tussock | Wet sedge | Tussock | Wet sedge |
| Acclimation period | First | Second | ||||||
| Electron donating capacity production µmol (kg soil)−1 (day)−1 | 110 ± 57 | 60 ± 10 C,E | 24 ± 10 | 23 ± 4 D | 160 ± 120 | −1 ± 9 F | 20 ± 8 | 10 ± 7 |
| DOC production µg C (g soil)−1 (day)−1 | 18 ± 11 | 10 ± 1.9 C | 7.9 ± 1.4 A | 3.6 ± 0.4 B,D | 26 ± 16 | 3.3 ± 1.0 | 4.7 ± 0.9 A | 0.9 ± 1.4 B |
| Fe(tot) production g Fe (g soil)−1 (day)−1 | 4.5 ± 2.4 | 3.4 ± 0.8 C,E | 1.0 ± 0.3 | 1.3 ± 0.4 D | 11 ± 8.2 | −0.3 ± 0.4 C,F | 0.9 ± 0.3 | 0.6 ± 0.3 D |
| Fe(II) production µg Fe (g soil)−1 (day)−1 | 3.9 ± 2.1 C | 1.9 ± 0.4 C,E | 0.9 ± 0.1 D | 0.8 ± 0.3 D,E | 10 ± 7.8 | −0.5 ± 0.4 C,F | 0.9 ± 0.3 A | 0.1 ± 0.0 B,D,F |
| O2 consumption µg O2 (g soil)−1 (day)−1 | 3.0 ± 1.1 | 4.2 ± 0.3 C,E | 2.5 ± 0.5 | 2.8 ± 0.4 D | 2.1 ± 1.2 | 2 ± 0.9 F | 1.9 ± 0.8 | 2.6 ± 0.9 |
| Daily Production Rate | Precipitation Events | Waterlogged Soils |
|---|---|---|
| •OH (µmol m−2 day−1) | 200 ± 70 A | 60 ± 20 B |
| CO2 (µmol m−2 day−1) | 60 ± 20 A | 20 ± 6 B |
| Summer time production | ||
| •OH (mmol m−2) | 10 ± 5 | 4 ± 1 |
| CO2 (mmol m−2) | 4 ± 1 A | 1 ± 0.4 B |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trusiak, A.; Treibergs, L.A.; Kling, G.W.; Cory, R.M. The Controls of Iron and Oxygen on Hydroxyl Radical (•OH) Production in Soils. Soil Syst. 2019, 3, 1. https://doi.org/10.3390/soilsystems3010001
Trusiak A, Treibergs LA, Kling GW, Cory RM. The Controls of Iron and Oxygen on Hydroxyl Radical (•OH) Production in Soils. Soil Systems. 2019; 3(1):1. https://doi.org/10.3390/soilsystems3010001
Chicago/Turabian StyleTrusiak, Adrianna, Lija A. Treibergs, George W. Kling, and Rose M. Cory. 2019. "The Controls of Iron and Oxygen on Hydroxyl Radical (•OH) Production in Soils" Soil Systems 3, no. 1: 1. https://doi.org/10.3390/soilsystems3010001
APA StyleTrusiak, A., Treibergs, L. A., Kling, G. W., & Cory, R. M. (2019). The Controls of Iron and Oxygen on Hydroxyl Radical (•OH) Production in Soils. Soil Systems, 3(1), 1. https://doi.org/10.3390/soilsystems3010001





