Conversion of a Semiarid Nevada Soil to Irrigated Agriculture Preferentially Removes Labile Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Particle Size Fractionation
2.4. Density Fractionation
2.5. Soil C, N, δ13C and δ15N
2.6. Mid-Infrared Spectroscopy
2.7. Microbial Respiration
2.8. Statistical Analyses
3. Results
3.1. .Particle Size and Density Fraction Mass
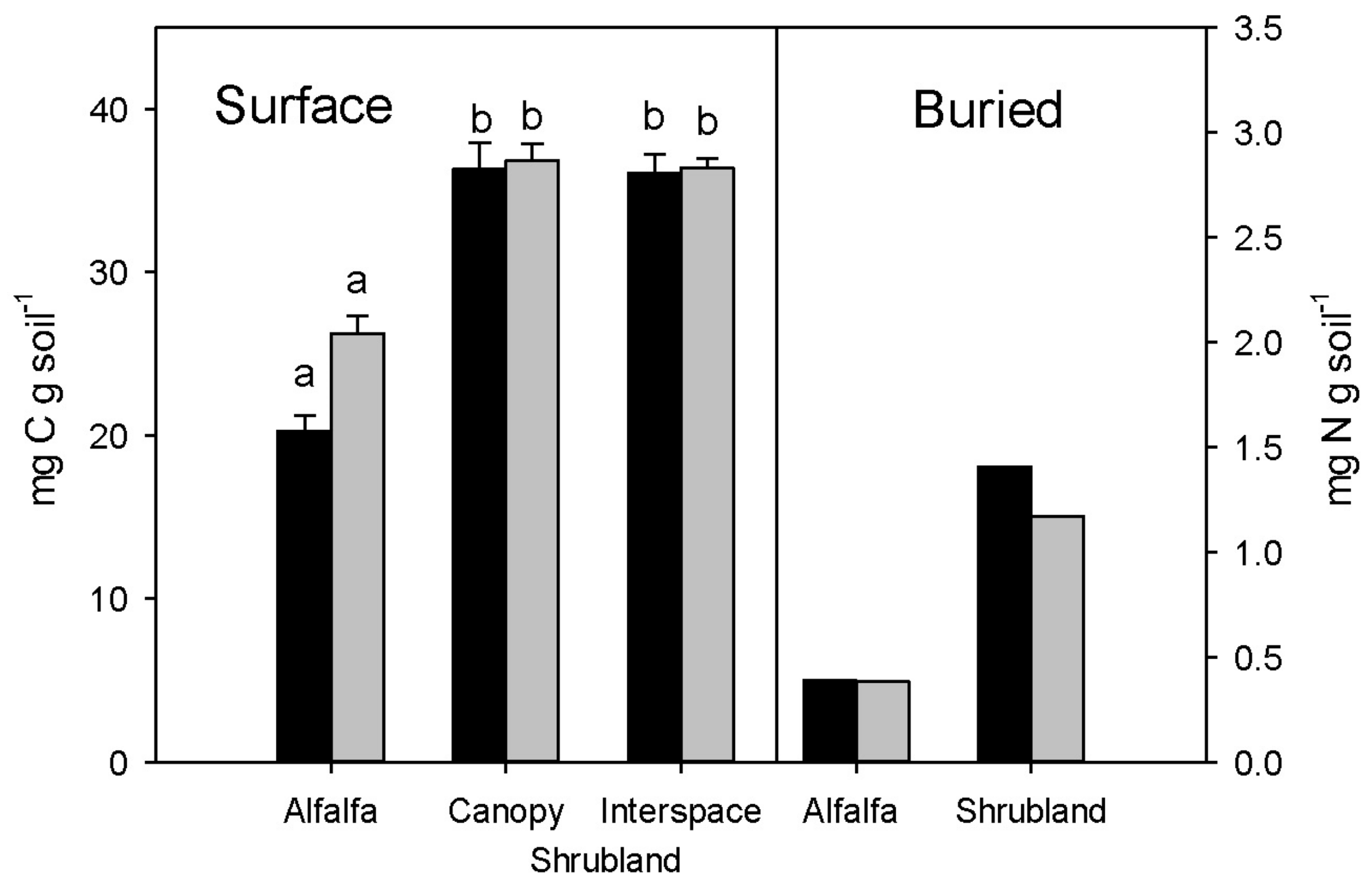
3.2. Carbon and Nitrogen
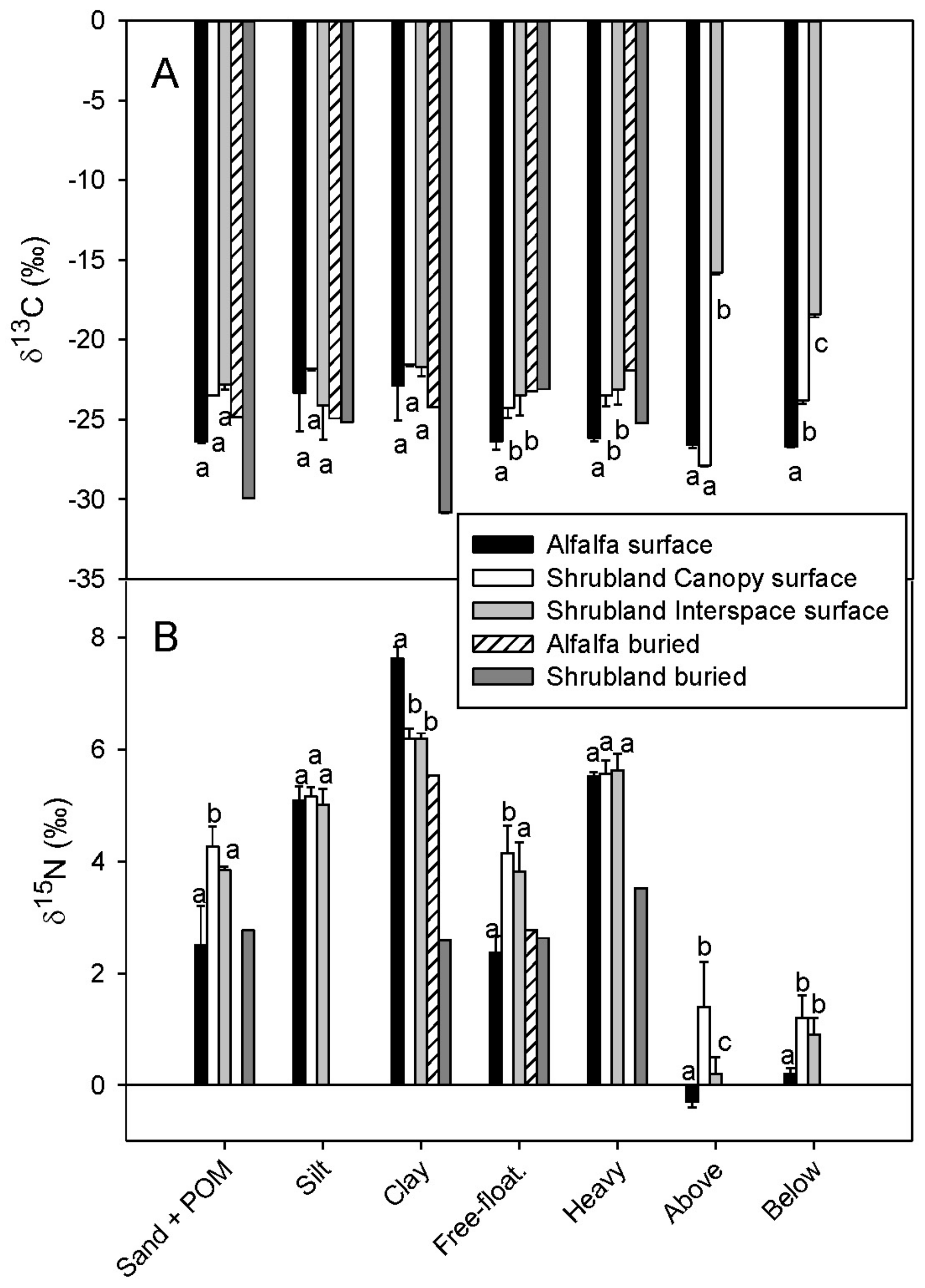
3.3. Stable Isotope Composition
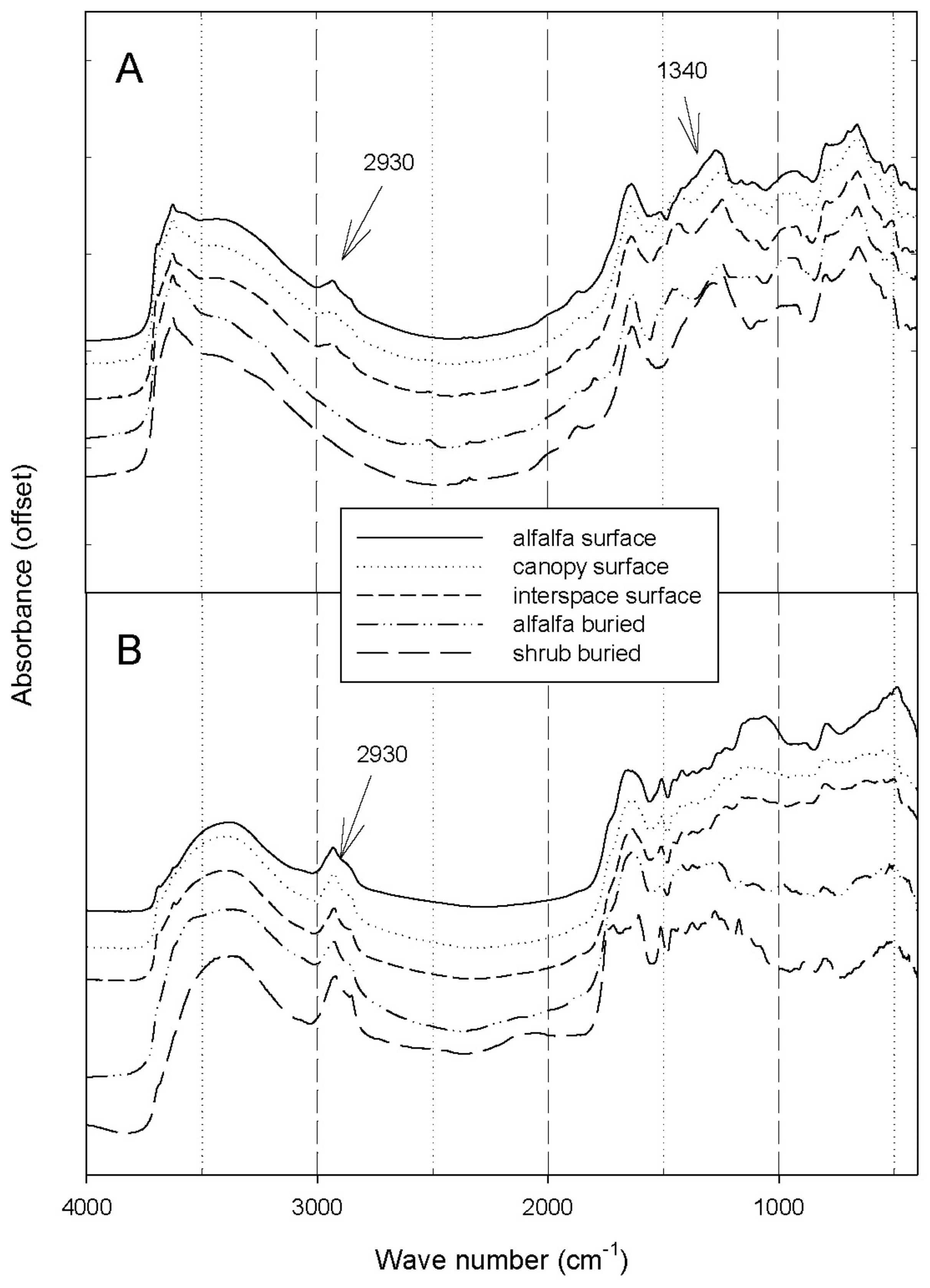
3.4. Mid-Infrared Spectroscopy
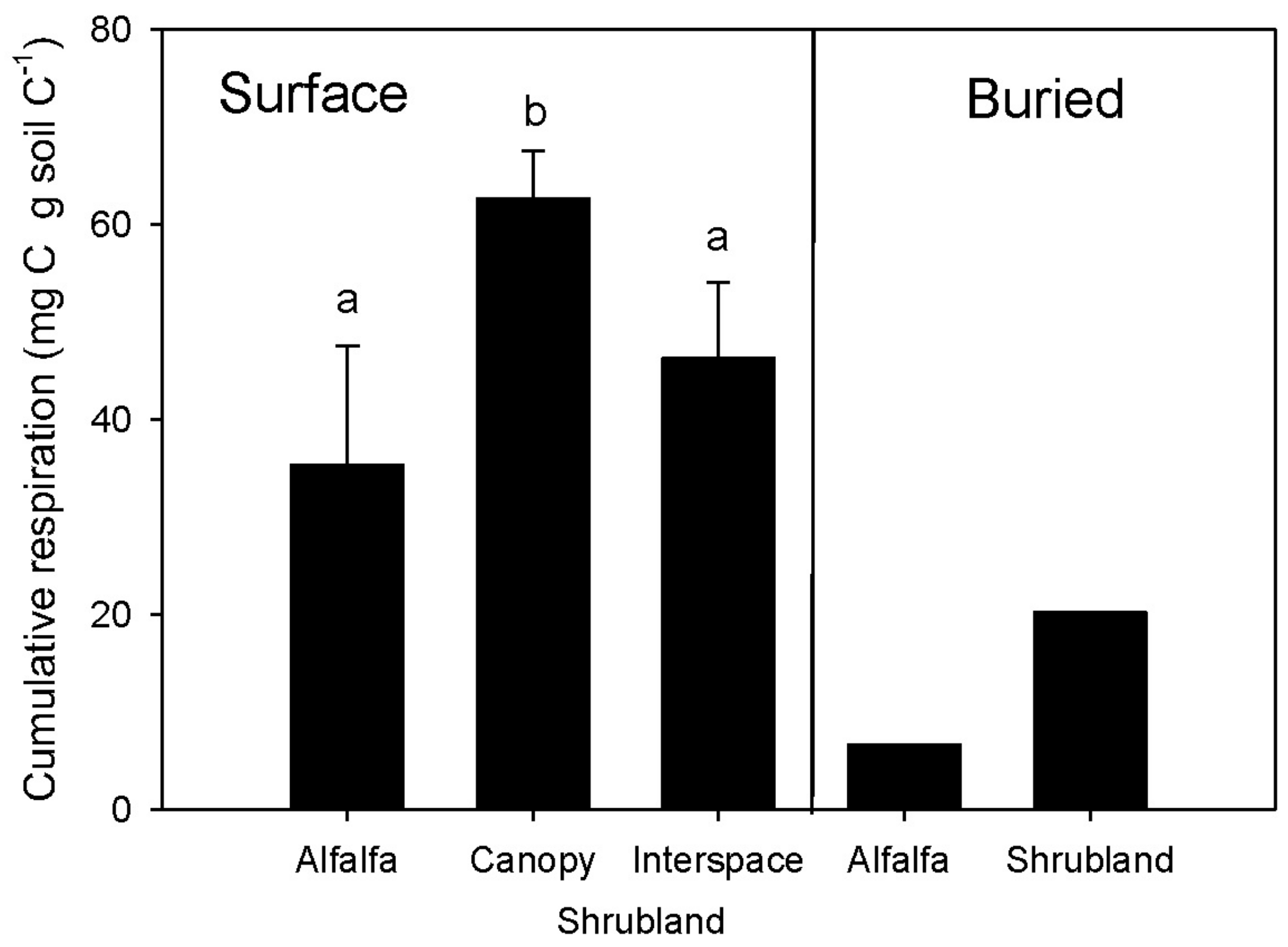
3.5. Microbial Respiration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emmerich, W.E. Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agric. For. Meteorol. 2003, 116, 91–102. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon dynamics in cropland and rangeland. Environ. Pollut. 2002, 116, 353–362. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, J. Impact of land use change on profile distributions of soil organic carbon fractions in the Yanqi Basin. Catena 2014, 115, 79–84. [Google Scholar] [CrossRef]
- Entry, J.A.; Sojka, R.E.; Shewmaker, G.E. Management of irrigated agriculture to increase organic carbon storage in soils. Soil Sci. Soc. Am. J. 2002, 66, 1957–1964. [Google Scholar] [CrossRef]
- Zach, A.; Tiessen, H.; Noellemeyer, E. Carbon turnover and carbon-13 natural abundance under land use change in semiarid savanna soils of La Pampa, Argentina. Soil Sci. Soc. Am. J. 2006, 70, 1541–1546. [Google Scholar] [CrossRef]
- Miller, R.E.; Huenneke, L.F. Size decline in Larrea tridentata (Creosotebush). Southwest Nat. 1996, 41, 248–250. [Google Scholar]
- Rundel, P.W.; Gibson, A.C. Ecological Communities and Processes in a Mojave Desert Ecosystem: Rock Valley, Nevada; Cambridge University Press: New York, NY, USA, 1996. [Google Scholar]
- Schlesinger, W.H.; Bernhardt, E.S. Biogeochemistry: An Analysis of Global Change, 3rd ed.; Academic Press: Oxford, UK, 2013. [Google Scholar]
- Lapenis, A.G.; Lawrence, G.B.; Bailey, S.W.; Aparin, B.F.; Shiklomanov, A.I.; Speranskaya, N.A.; Torn, M.S.; Calef, M. Climatically driven loss of calcium in steppe soil as a sink for atmospheric carbon. Glob. Biogeochem. Cycle 2008, 22. [Google Scholar] [CrossRef]
- Stevenson, B.A.; Verburg, P.S.J. Effluxed CO2-13C from sterilized and unsterilized treatments of a calcareous soil. Soil Biol. Biochem. 2006, 38, 1727–1733. [Google Scholar] [CrossRef]
- Xie, J.; Li, Y.; Zhai, C.; Li, C.; Lan, Z. CO2 absorption by alkaline soils and its implication to the global carbon cycle. Environ. Geol. 2009, 56, 953–961. [Google Scholar] [CrossRef]
- Li, X.R.; Wang, X.P.; Li, T.; Zhang, J.G. Microbiotic soil crust and its effect on vegetation and habitat on artificially stabilized desert dunes in Tengger Desert, North China. Biol. Fertil. Soils 2002, 35, 147–154. [Google Scholar]
- Li, X.R.; Zhang, P.; Su, Y.G.; Jia, R.L. Carbon fixation by biological soil crusts following revegetation of sand dunes in arid desert regions of China: A four-year field study. Catena 2012, 97, 119–126. [Google Scholar] [CrossRef]
- Su, Y.Z.; Wang, X.F.; Yang, R.; Lee, J. Effects of sandy desertified land rehabilitation on soil carbon sequestration and aggregation in an arid region in China. J. Environ. Manag. 2010, 91, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lin, W.; Zhang, Y. Characteristics of carbon flux in two biologically crusted soils in the Gurbantunggut Desert, Northwestern China. Catena 2012, 96, 41–48. [Google Scholar] [CrossRef]
- Prǎvǎlie, R. Drylands extent and environmental issues. A global approach. Earth-Sci. Rev. 2016, 161, 259–278. [Google Scholar] [CrossRef]
- Eswaran, H.; Reich, P.F.; Kimble, J.M.; Beinroth, F.H.; Padmanabhan, E.; Moncharoen, P. Global carbon stocks. In Global Climate Change and Pedogenic Carbonates; Lal, R., Kimble, J.M., Eswaran, H., Stewart, B.A., Eds.; CRC/Lewis: Boca Raton, FL, USA, 2000. [Google Scholar]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 4–21. [Google Scholar] [CrossRef]
- Jasoni, R.L.; Smith, S.D.; Arnone, J.A., III. Net ecosystem CO2 exchange in Mojave Desert shrublands during the eighth year of exposure to elevated CO2. Glob. Chang. Biol. 2005, 11, 749–756. [Google Scholar] [CrossRef]
- Wohlfahrt, G.; Fenstermaker, L.F.; Arnone, J.A., III. Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Glob. Chang. Biol. 2008, 14, 1475–1487. [Google Scholar] [CrossRef]
- Reid, W.V.; Mooney, H.A.; Cropper, A.; Capistrano, D.; Carpenter, S.R.; Chopra, K.; Dasgupta, P.; Dietz, T.; Duraiappah, A.K.; Hassan, R.; et al. Ecosystems and Human Well-Being: Synthesis, Millenium Ecosystem Assessment; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Wu, L.; Wood, Y.; Jiang, P.; Li, L.; Pan, G.; Lu, J.; Chang, A.C.; Enloe, H.A. Carbon sequestration and dynamics of two irrigated agricultural soils in California. Soil Sci. Soc. Am. J. 2008, 72, 808–814. [Google Scholar] [CrossRef]
- Albaladejo, J.; Ortiz, R.; Garcia-Franco, N.; Navarro, A.R.; Almagro, M.; Pintado, J.G.; Martínez-Mena, M. Land use and climate change impacts on soil organic carbon stocks in semi-arid Spain. J. Soils Sediments 2013, 13, 265–277. [Google Scholar] [CrossRef]
- Cochran, R.L.; Collins, H.P.; Kennedy, A.; Bezdicek, D.F. Soil carbon pools and fluxes after land conversion in a semiarid shrub-steppe ecosystem. Biol. Fertil. Soils 2007, 43, 479–489. [Google Scholar] [CrossRef]
- Tiessen, H.; Feller, C.; Sampaio, E.V.S.B.; Garin, P. Carbon sequestration and turnover in semiarid savannas and dry forest. Clim. Chang. 1998. [Google Scholar] [CrossRef]
- Urioste, A.M.; Hevia, G.G.; Hepper, E.N.; Anton, L.E.; Bono, A.A.; Buschiazzo, D.E. Cultivation effects on the distribution of organic carbon, total nitrogen and phosphorus in soils of the semiarid region of Argentinian Pampas. Geoderma 2006, 136, 621–630. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Gao, Q.; Peng, C. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 129, 413–421. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2015, 6, 166–172. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L., II; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global desertification: building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Galbally, I.; Meyer, C.P.; Wang, Y.-P.; Kirstine, W. Soil-atmosphere exchange of CH4, CO, N2O and NOx and the effects of land-use change in the semiarid Mallee system in Southeastern Australia. Glob. Chang. Biol. 2010, 16, 2407–2419. [Google Scholar]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Lemenih, M.; Itanna, F. Soil carbon stocks and turnovers in various vegetation types and arable lands along an elevation gradient in southern Ethiopia. Geoderma 2004, 123, 177–188. [Google Scholar] [CrossRef]
- Saha, D.; Kukal, S.S.; Sharma, S. Landuse impacts on SOC fractions and aggregate stability in typic ustochrepts of Northwest India. Plant Soil 2011, 339, 457–470. [Google Scholar] [CrossRef]
- Bonino, E.E. Changes in carbon pools associated with a land-use gradient in the Dry Chaco, Argentina. For. Ecol. Manag. 2006, 223, 183–189. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, X.; Zhang, X.; Cheng, J.; Gale, W.; Guo, C.; Long, T. Soil organic carbon losses due to land use change in a semiarid grassland. Plant Soil 2012, 355, 299–309. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Xiao, H.L.; Liu, Z.M.; Li, J. Soil degradation and restoration as affected by land use change in the semiarid Bashang area, northern China. Catena 2005, 59, 173–186. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, O.J.; Huang, J.; Li, L.; Liu, P.; Han, X. Soil carbon and nitrogen stores and storage potential as affected by land-use in an agro-pastoral ecotone of northern China. Biogeochemistry 2007, 82, 127–138. [Google Scholar] [CrossRef]
- Cates, A.M.; Ruark, M.D.; Hedtcke, J.L.; Posner, J.L. Long-term tillage, rotation and perennialization effects on particulate and aggregate soil organic matter. Soil Tillage Res. 2016, 155, 371–380. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: processes and potential. Glob. Chang. Biol. 2000, 6, 317–328. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Bronson, K.F. Impacts of land use change on nitrogen cycling archived in semiarid unsaturated zone nitrate profiles, Southern High Plains, Texas. Environ. Sci. Technol. 2008, 42, 7566–7572. [Google Scholar] [CrossRef] [PubMed]
- Smith, P. Land use change and soil organic carbon dynamics. Nutr. Cycl. Agroecosyst. 2008, 81, 169–178. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Belnap, J.; Marion, G. On carbon sequestration in desert ecosystems. Glob. Chang. Biol. 2009, 15, 1488–1490. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Peng, C. Land use induced changes of organic carbon storage in soils of China. Glob. Chang. Biol. 2003, 9, 305–315. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Gillabel, J.; Denef, K.; Brenner, J.; Merckx, R.; Paustian, K. Carbon sequestration and soil aggregation in center-pivot irrigated and dryland cultivated farming systems. Soil Sci. Soc. Am. J. 2007, 71, 1020–1028. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Wan, S. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Glob. Chang. Biol. 2009, 15, 184–195. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Courcelles, V.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Yan, M.; Zhou, G.; Zhang, X. Effects of irrigation on the soil CO2 efflux from different poplar clone plantations in arid northwest China. Plant Soil 2013, 375, 89–97. [Google Scholar] [CrossRef]
- Austin, A.T.; Yahdjian, L.; Stark, J.M.; Belnap, J.; Porporato, A.; Norton, U.; Ravetta, D.A.; Schaeffer, S.M. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 2004, 141, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Hastings, S.J.; Oechel, W.C.; Muhlia-Melo, A. Diurnal, seasonal and annual variation in the net ecosystem CO2 exchange of a desert shrub community (Sarcocaulescent) in Baja California, Mexico. Glob. Chang. Biol. 2005, 11, 927–939. [Google Scholar] [CrossRef]
- Huxman, T.E.; Snyder, K.A.; Tissue, D.; Leffler, A.J.; Ogle, K.; Pockman, W.T.; Sandquist, D.R.; Potts, D.L.; Schwinning, S. Precipitation pulses and carbon fluxes in semiarid and arid ecosystem. Oecologia 2004, 141, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Burke, I.C.; Reiners, W.A.; Schimel, D.S. Organic matter turnover in a sagebrush steppe landscape. Biogeochemistry 1989, 7, 11–31. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Evans, R.D. Pulse additions of soil carbon and nitrogen affect soil nitrogen dynamics in an arid Colorado Plateau shrubland. Oecologia 2005, 145, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Sponseller, R.A. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob. Chang. Biol. 2007, 13, 426–436. [Google Scholar] [CrossRef]
- DeMarco, J.; Filley, T.; Throop, H.L. Patterns of woody plant-derived soil carbon losses and persistence after brush management in a semi-arid grassland. Plant Soil 2016, 406, 277–293. [Google Scholar] [CrossRef]
- Hooker, T.D.; Stark, J.M. Carbon flow from plant detritus and soil organic matter to microbes-Linking carbon and nitrogen cycling in semiarid soils. Soil Sci. Soc. Am. J. 2012, 76, 903–914. [Google Scholar] [CrossRef]
- Throop, H.L.; Lajtha, K.; Kramer, M. Density fractionation and 13C reveal changes in soil carbon following woody encroachment in a desert ecosystem. Biogeochemistry 2013, 112, 409–422. [Google Scholar] [CrossRef]
- Plant Guide for Yellow-Flowered Alfalfa (Medicago sativa subsp. falcata (L.) Arcang. Available online: https://www.nrcs.usda.gov/Internet/FSE_PLANTMATERIALS/publications/ndpmcpg12773.pdf (accessed on 3 August 2018).
- Plant Guide for Rubber Rabbitbrush (Ericameria nauseosa). Available online: https://plants.usda.gov/factsheet/pdf/fs_erna10.pdf (accessed on 3 August 2018).
- Plant Guide for Yellow Rabbitbrush (Chrysothamnus viscidiflorus). Available online: https://plants.usda.gov/plantguide/pdf/pg_chvi8.pdf (accessed on 3 August 2018).
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-NRCS: Washington, DC, USA, 2014.
- Kettler, T.A.; Doran, J.W.; Gilbert, T.L. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef]
- Sollins, P.; Glassman, C.; Paul, E.A.; Swanston, C.; Lajtha, K.; Heil, J.W.; Elliott, E.T. Soil carbon and nitrogen: Pools and fractions. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Coleman, D.C., Bledsoe, C.S., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Harris, D.; Horwáth, W.R.; van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci. Soc. Am. J. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Werner, R.A.; Bruch, B.A.; Brand, W.A. ConFlo III—An interface for high precision δ13C and δ15N analysis with an extended dynamic range. Rapid Commun. Mass Spectrom. 1999, 13, 1237–1241. [Google Scholar] [CrossRef]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome, F.N.; Calderón, F.J. Soil chemical insights provided through vibrational spectroscopy. Adv. Agron. 2014, 126, 1–148. [Google Scholar]
- Haberhauer, G.; Feigl, B.; Gerzabek, M.H.; Cerri, C. FT-IR spectroscopy of organic matter in tropical soils: Changes induced through deforestation. J. Appl. Spectrosc. 2000, 54, 221–224. [Google Scholar] [CrossRef]
- Calderón, F.J.; Reeves, J., III; Collins, H.P.; Paul, E.A. Chemical differences in soil organic matter fractions determined by diffuse-reflectance mid-infrared spectroscopy. Soil Sci. Soc. Am. J. 2011, 75, 568–579. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Ding, G.; Novak, J.M.; Amarasiriwardena, D.; Hunt, P.G.; Xing, B. Soil organic matter characteristics as affected by tillage management. Soil Sci. Soc. Am. J. 2002, 66, 421–429. [Google Scholar] [CrossRef]
- Haberhauer, G.; Gerzabek, M.H. Drift and transmission FT-IR spectroscopy of forest soils: An approach to determine decomposition processes of forest litter. Vib. Spectrosc. 1999, 19, 413–417. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wang, T. Fourier transform Raman spectroscopic characterization of humic substances. Vib. Spectrosc. 1997, 14, 105–112. [Google Scholar] [CrossRef]
- Matamala, R.; Jastrow, J.; Hofmann, S.; Mishra, U.; Michaelson, G.; Calderon, F.; Ping, C.-L.; Zhaosheng, F. Influence of site and soil properties on the DRIFT spectra of northern cold-region soils. Geoderma 2017, 305, 80–91. [Google Scholar] [CrossRef]
- Matejkova, S.; Simon, T. Application of FTIR spectroscopy for evaluation of hydrophobic/hydrophilic organic components in arable soil. Plant Soil Environ. 2012, 58, 192–195. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Calderón, F.J.; Jackson, L.E. Rototillage, disking, and subsequent irrigation: effects on soil nitrogen dynamics, microbial biomass, and carbon dioxide efflux. J. Environ. Qual. 2002, 31, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- De Oliveira, S.P.; De Lacerda, N.B.; Blum, S.C.; Escobar, M.E.O.; De Oliveira, T.S. Organic carbon and nitrogen stocks in soils of northeastern Brazil converted to irrigated agriculture. Land Degrad. Dev. 2015, 26, 9–21. [Google Scholar] [CrossRef]
- Kocyigit, R.; Demirci, S. Long-term changes of aggregate-associated and labile soil organic carbon and nitrogen after conversion from forest to grassland and cropland in northern Turkey. Land Degrad. Dev. 2012, 23, 475–482. [Google Scholar] [CrossRef]
- Fallahzade, J.; Hajabbasi, M.A. The effects of irrigation and cultivation on the quality of desert soil in central Iran. Land Degrad. Dev. 2012, 23, 53–61. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycle 2003, 17, 1031. [Google Scholar] [CrossRef]
- Ariz, I.; Cruz, C.; Neves, T.; Irigoyen, J.J.; Garcia-Olaverri, C.; Nogués, S.; Aparicio-Tejo, P.M.; Aranjuelo, I. Leaf δ15N as a physiological indicator of the responsiveness of N2-fixing alfalfa plants to elevated [CO2], temperature and low water availability. Front. Plant Sci. 2015, 6, 574. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.; Ishizu, A.; Doucett, R.; Hart, S.C.; Schwartz, E.; Menyailo, O.V.; Hungate, B.A. 13C and 15N natural abundance of the soil microbial biomass. Soil Biol. Biochem. 2006, 38, 3257–3266. [Google Scholar] [CrossRef]
- Högberg, P.; Johannisson, C. 15N abundance of forests is correlated with losses of nitrogen. Plant Soil. 1993, 157, 147–150. [Google Scholar] [CrossRef]
- Kramer, M.G.; Sollins, P.; Sletten, R.S.; Swart, P.K. N isotope fractionation and measures of organic matter alteration during decomposition. Ecology 2003, 84, 2021–2025. [Google Scholar] [CrossRef]
- Werth, M.; Kuzyakov, Y. 13C fractionation at the root-microorganisms-soil interface: A review and outlook for partitioning studies. Soil Biol. Biochem. 2010, 42, 1372–1384. [Google Scholar] [CrossRef]
- Kozlovskii, F.I.; Ruhlmann, J.; Travnikova, L.S.; Kuzyakov, Y.V. Clay differentiation in initially homogeneous substrates upon long-term field experiments. Eurasian Soil Sci. 2001, 34, 130–138. [Google Scholar]


| Surface | Buried | ||||
|---|---|---|---|---|---|
| Alfalfa | Shrub Canopy | Shrub Interspace | Alfalfa | Shrub | |
| Sand + POM | 11.8 (0.2) a | 14.6 (0.3) b | 14.8 (0.3) b | 10.3 (0.9) | 17.5 (0.7) |
| Silt | 9.4 (0.1) a | 11.4 (0.2) a | 11.3 (0.1) a | 14.9 (2.6) | 15.5 (1.1) |
| Clay | 8.4 (0.2) a | 10.9 (0.7) b | 11.0 (0.4) b | 13.0 (0.1) | 14.8 (0.3) |
| Free floating | 14.0 (0.3) a | 16.9 (0.9) b | 16.2 (0.3) ab | 19.9 (0.9) | 26.7 (2.2) |
| Heavy | 9.8 (0.2) a | 11.1 (0.4) a | 11.1 (0.5) a | 12.9 (1.8) | 13.5 (0.2) |
| Aboveground | 8.7 (0.5) a | 65.5 (1.7) b | 69.4 (3.4) b | ||
| Belowground | 13.4 (0.3) a | 41.7 (1.1) b | 47.7 (1.9) c | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimble, B.R.; Calderon, F.J.; Poulson, S.R.; Verburg, P.S.J. Conversion of a Semiarid Nevada Soil to Irrigated Agriculture Preferentially Removes Labile Carbon. Soil Syst. 2018, 2, 38. https://doi.org/10.3390/soilsystems2030038
Trimble BR, Calderon FJ, Poulson SR, Verburg PSJ. Conversion of a Semiarid Nevada Soil to Irrigated Agriculture Preferentially Removes Labile Carbon. Soil Systems. 2018; 2(3):38. https://doi.org/10.3390/soilsystems2030038
Chicago/Turabian StyleTrimble, Brittany R., Francisco J. Calderon, Simon R. Poulson, and Paul S. J. Verburg. 2018. "Conversion of a Semiarid Nevada Soil to Irrigated Agriculture Preferentially Removes Labile Carbon" Soil Systems 2, no. 3: 38. https://doi.org/10.3390/soilsystems2030038
APA StyleTrimble, B. R., Calderon, F. J., Poulson, S. R., & Verburg, P. S. J. (2018). Conversion of a Semiarid Nevada Soil to Irrigated Agriculture Preferentially Removes Labile Carbon. Soil Systems, 2(3), 38. https://doi.org/10.3390/soilsystems2030038




