Abstract
Antimony (Sb) is an understudied pollutant with potentially toxic effects at particularly low concentrations. The fate of Sb in the environment is complicated because of its many chemical forms at varying oxidation states. Here, we validated an extraction method and an analytical technique to quantify inorganic and methylated Sb in bulk soil and soil solution. We identified and quantified trimethylantimony (TMSb) in shooting range soils for the first time, up to a concentration of 1.35 mg kg−1. Then, we evaluated the release of Sb species from soil to soil solution as well as the influence of manure addition upon flooding in an incubation experiment with fresh soils from shooting ranges. This incubation experiment showed an immediate and exhaustive Sb release into the soil solution (within 6 h), reaching over 3000 μg L−1 for one site, followed by a sharp decline and again a slow increase at the end of the incubation in Sb concentrations in the soil solution for two of the three sites. TMSb was also formed in the soil solution after 4 to 10 days. High dissolved organic carbon (DOC) concentrations and the dissolution of Fe- and Mn-(oxy-)hydroxides were the main drivers of Sb release, while the addition of organic matter (OM) drove TMSb formation.
1. Introduction
Strongly elevated antimony (Sb) concentrations, caused by recent human activities and the associated increased exposure of biota, have recently come into the focus of public interest. Sb is now considered a pollutant of primary concern by the Council of the European Communities and the US Environmental Protection Agency [1,2]. Global consumption of Sb was estimated to be about 188,000 tons in 2016, owing primarily to an increased use of flame retardants and heat stabilizers for plastics. Because of its compatibility with lead (Pb), Sb is also used as a hardening agent in batteries and ammunition [3]. Sb in shooting range soils can pose an environmental risk, since the weathering of bullets will gradually release Sb into the environment [4]. Furthermore, mining activities of Sb are a major source of soil contamination in Sb-producing countries [5]. Unfortunately, compared to other pollutants, such as arsenic (As) and mercury (Hg), Sb is much less studied. Nonetheless, it can be more mobile than other pollutants such as As and Pb [6,7]. Recent studies even show that Sb mobility in soils was underestimated in early studies, especially in contaminated areas [8,9,10,11,12]. This lack of knowledge is attributable to the fact that Sb is a redox-sensitive element with a complex biogeochemical cycle. Sb(III) and Sb(V) are the two forms that are mostly found in the environment. Sb(V) is the predominant and stable species in oxic systems, while Sb(III) is predominant under reducing conditions [13], although recent work shows that in contaminated sites, even under reducing conditions, Sb(V) can be dominant, because of thermodynamic constraints of the conversion of Sb(V) to Sb(III) [14]. In soils, Sb(III) is reported to be less mobile because of the immediate sorption onto iron (Fe), manganese (Mn), or aluminum (Al) (oxy-)hydroxides [15,16,17]. However, both Sb species bind to Fe- and Mn-(oxy-)hydroxides [15,18]. Since Fe- and Mn-(oxy-)hydroxides are widespread in soils of all climate zones [19], a low Sb mobility is expected under aerobic conditions [20]. This entails that under waterlogged (anoxic, reduced) conditions, Sb might be released by reductive dissolution of Fe- and Mn-(oxy-)hydroxides. However, anoxic conditions can also lead to the reduction of Sb(V) to Sb(III), in spite of the above-mentioned restrictions, and to precipitation and adsorption processes [21]. Hockmann and Schulin [22] showed a reduced sorption capacity of Sb onto Fe (oxy-)hydroxides when Sb(V) is added together with dissolved organic carbon (DOC), although the effects were less pronounced when Sb(V) was allowed to equilibrate with the Fe-(oxy-)hydroxides before the addition of DOC. Heier et al. [23] found a close correlation between Sb and DOC concentrations in soil solution of a contaminated peatland area in Norway. Furthermore, if the sorption of Sb is controlled by the metal (oxy-)hydroxide surfaces, other components competing for binding sites, such as DOC, can enhance Sb mobility [22]. The sorption to solid organic matter (OM) [24] and clay minerals [16] is hypothesized to play only a minor role in the retention of Sb in soils. Nonetheless, several recent studies have highlighted the fact that Sb can be mobilized in surface waters, even under oxic conditions [14,25,26,27].
Furthermore, Sb is subject to biomethylation under oxic and anoxic conditions [28,29,30]. This microbiological process transforms inorganic Sb to methylated Sb species. The products of Sb biomethylation are mono-, di-, and trimethylantimony (MMSb, DMSb, and TMSb, respectively). Despite the broad spectrum of microorganisms that are able to methylate Sb [29,30,31] and the inherent effects on Sb mobility [5,29], information about these processes in soils are still very scarce. Duester et al. [32] determined methylated Sb concentrations in different locations of the Ruhr basin in Germany and found that MMSb was the most abundant of all organic Sb species in agricultural and garden soils. Frohne et al. [33] revealed a linear correlation between decreasing MMSb concentrations and increasing redox potential (−300 to 600 mV). Another study about the occurrence of organic Sb investigated soils in farmlands and forests of an old mining area in China and found high concentrations of TMSb which contributed a large part to the total Sb concentrations in soils. [34]. Several other studies found methylated Sb mostly under anaerobic conditions [30,35,36]. The current hypotheses to explain the formation of TMSb in soils are the same as for the formation of methylated As compounds: either TMSb is formed as a by-product of the methane production or there is an active mechanism of methylation by microorganisms using S-adenosylmethionine of methylcobalamin as the methyl donor. Still, methylated Sb species are not well-studied, and this is related to the lack of user-friendly extraction and analysis methods. Furthermore, the lack of commercially available standards for MMSb and DMSb prevented us from validating our extraction and HPLC method for these two compounds.
In this study, we investigated the mobility and spatial distribution of Sb and other trace elements, such as As, Cu, Fe, and Mn, as well as the presence of TMSb in three Swiss shooting range soils that are prone to flooding and therefore to changing redox conditions. To do so, we tested and validated an extraction technique to measure TMSb concentrations in soils. This method was already used for plants and is compatible with the speciation analysis of Sb(III), Sb(V), and TMSb by HPLC–ICP–MS used in this study [37]. Furthermore, we conducted flooding experiments in order to understand the dynamics leading to the release of Sb from the soil solid phase into soil solution and the formation of TMSb in such soils. Such experiments are pertinent for several reasons. Firstly, Sb use and emission in the environment is a global issue, not restricted to shooting ranges soils [38]. Furthermore, it is known that Sb release is influenced by changing redox conditions in many soils such as wetlands and rice paddies [9,39] as well as by heavy precipitation [25,26,27]. Finally, climate change is thought to result in an increase in the frequency of extreme weather events including floods, in the future [40,41].
2. Materials and Methods
2.1. Reagents
Sb stock solutions were prepared from salts of potassium antimony (III) tartrate hydrate (C8H4K2O12Sb2x3H2O, 99.95%), potassium hexahydroxoantimonate(V) (KSb(OH)6, 99%), and trimethylantimony(V)dibromide ((CH3)3SbBr2) produced by Sigma–Aldrich (Buchs, Switzerland). These compounds were used to validate the extraction recoveries. For the total element concentration analysis, a multi-element standard from Roth (1000 mg L−1, Karlsruhe, Germany) was used. Soil extraction solutions were prepared with citric acid (99.5%), ascorbic acid (99.7%), and oxalic acid (99%) from Merck Millipore Inc. (Burlington, MA, USA). Ammonium tartrate (99.5%) from Sigma Aldrich (St Louis, MO, USA) and methanol (99.9%) form Merck Millipore Inc. (Burlington, MA, USA) were used for the HPLC eluent. Hydrochloric acid (HCl, 35%, supra), nitric acid (HNO3, 69%, supra), and hydrogen peroxide (30%, pure) were obtained from Roth (Karlsruhe, Germany). Water from an ultrapure water system (MilliQ, Merck Millipore Inc., Burlington, MA, USA) was used throughout the study.
2.2. Field Sites and Sampling
Several 25-, 50- and 300-m shooting ranges in Switzerland were evaluated in order to find three appropriate sites for this study. The final selection was based on the following criteria: soils should be physically undisturbed and allow coring to at least 35 cm depth. The area should be flat and water-influenced (nearby streams and high chance of flooding events). The three chosen sites were characterized by contrasting soil properties such as soil pH and OM concentration, offering three different scenarios for studying Sb behavior (Table 1). The first two shooting ranges were situated in the vicinity of Laupen (Site A) and in the municipality of Seedorf (Site B), both in the canton of Berne, and were bordered by a stream. The third Sb-polluted soil was near the city of Embrach (Site C) in the canton of Zurich and was situated across a groundwater-fed peatland, with the target area, however, situated on a mineral soil under pasture. According to the soil texture definition of the World Reference Base for Soil Resources (WRB) [42], the soils at Sites A and C were defined as loam, whereas the soil at Site B was classified as sandy loam.

Table 1.
Site properties (mean ± standard deviation, sd). Soil properties refer to the average of 0–5, 5–15, 15–25, 25–35 cm depth layers if not mentioned otherwise.
At each site (A, B, and C), three soil cores were sampled in the areas near the targets. The cores were 7.5 m apart at sites A and C, while they were only 1.5 m apart at the narrower Site B. Three further cores were taken at each site to measure Sb as well as TMSb concentrations away from the targets, in a gradient. These cores were taken in the middle of each field and were 3, 8, and 14 m away from the front core at Site A, and 3, 11, and 25 m at Sites B and C. Details can be found on the sampling plan (Figure S1). The cores were collected with a Pürckhauer corer (Ø 2 cm). The plant cover was removed, and the soil samples were divided into four depths (0–5 cm, 5–15 cm, 15–25 cm, 25–35 cm). The soil samples were dried for 10 h in an oven at 70 °C. The dried soils were sieved (<2 mm mesh size) to remove ammunition residues, stones, and larger organic particles. The dried and sieved samples were ground using a mortar and pestle. Additionally, 1–2 kg of soil was sampled for the incubation experiment from a 0–20 cm depth, sieved to 2 mm, and homogeneously mixed. The samples were kept fresh (no drying) at 4 °C for a maximum of 5 days prior to the start of the incubation experiment.
2.3. Microwave-Assisted Digestion for Total Concentrations
Microwave digestion (Ethos, contFLOW 1600, MLS, Leutkirch, Germany) was used to determine total element concentrations in the solid phase. After digestion, the pressurized vessels were allowed to cool before they were opened to prevent possible loss of volatile Sb. The digests were diluted with ultrapure water and stored at 4 °C until analysis. Different acid compositions were tested, since Sb is challenging to digest [43,44]. The acid composition finally used for digestion was a mixture of 7.5 mL HNO3 and 2.5 mL HCl. The details of the microwave digestion procedure can be found in Table S1. Certified reference materials (CRM, National Research Council Canada, PACS-3 with 17.4 mg/kg Sb) were digested with each batch and showed consistent recoveries of 70.3% ± 4.7% (n = 13). The blanks were also digested with each batch to check for possible contamination. The method limit of detection of Sb for this digestion procedure was calculated at 0.24 mg kg−1 (average blanks plus 3x standard deviation).
2.4. Soil Extractions for Methylated Sb Species
Different extraction methods for methylated Sb species were tested. The procedures are described in part 3.1, if different from the procedure of Potin-Gautier et al. [45]. Samples consisting of 100 mg of soil were precisely weighed and filled into a 20 mL borosilicate glass vial (Wheaton, Millville, NJ, USA), then 10 mL of 200 mM oxalic acid (OA) mixed with 100 mM ascorbic acid (AA) was added. Sample and liquid were shaken by hand to mix them uniformly. The glass vials were then sonicated for 30 min (Sonorex Super RK 106, Bandelin, Berlin, Germany). After the ultrasonic bath, the extraction solution was separated from the soil using a centrifuge (Z323, Wehingen, Germany) at 3500 rotations per minute for 5 min (relative centrifugal force: 6000× g at 20 °C). The extraction solution was finally diluted in 150 mM ammonium tartrate (AT) and stored at 4 °C prior to analysis. We spiked a selected soil with low native Sb concentration (0.7 ± 0.01 mg kg−1, n = 3) with different Sb species in order to validate the extraction method. The extracts of the spiked soil were then analyzed to assess the recoveries and possible species conversion. The method limit of detection of methylated Sb for the chosen extraction was 50 µg kg−1.
2.5. Incubation Experiments
Eighteen Erlenmeyer flasks (250 mL) filled with fresh soil from the shooting range sites (<2 mm sieved and homogenized), were placed in a growth chamber (KBW 720, Binder, Tuttlingen, Germany) at an average Swiss summer temperature of 18 °C and a humidity level of 70%. Nine Erlenmeyer flasks were filled with 78.4 g field-fresh soil from the three different shooting ranges (3x A, B, and C) and supplemented with 1.6 g (2%) OM in the form of cow dung (Hauert HBG Dünger AG, Grossaffoltern, Switzerland, dried and finely chopped). The remaining nine flasks were filled with field-fresh and homogenized shooting range soil without any further supplementation (80 g). All flasks were equipped with a Rhizonsampler (Rhizonsphere Research Product, Wageningen, Netherlands: Rhizon flex, 5 cm porous material, pore size 0.2 μm, 2.5 mm outer diameter, complemented with 20 cm polyvinyl chloride/polyethylene (PVC/PE) tubing and injection needle) in order to sample soil solutions. The Erlenmeyer flasks were closed with a plastic lid to avoid dust contamination and water loss. At the start of the experiment, all 18 Erlenmeyer flasks were flooded with 120 mL of ultrapure water. For 15 days, the soil solution was sampled 10 times (after 6 h, 1, 2, 3, 4, 6, 8, 10, 12, and 15 days). The soil solution was sampled by applying a vacuum to the Rhizon sampler using a 5 mL vacutainer (Plastipak, Becton Dickinson, Franklin Lakes, NJ, USA). The sampled volume was distributed into three subsamples (1: total Sb and other trace elements, diluted in 1% HNO3; 2: Sb species, diluted in 150 mM AT; 3: DOC analysis, diluted in ultrapure water). The remaining soil solution in the vacutainer was used to determine redox potentials and pH values. The soil solution samples were stored in a fridge at 4 °C prior to analysis. The soil used for the incubation experiment was analyzed for total element concentration and Sb speciation before and after the experiment. The soils remained fully water-saturated for the duration of the experiment.
2.6. Soil Characterization
The organic matter concentration in the soils was measured by loss on ignition (550 °C for two hours in a muffle furnace). The pH was measured in a solution containing 10 g of soils and 25 mL of 0.01 M CaCl2 after equilibration for two hours. The texture was determined after treatment with H2O2 to remove the organic matter. The moisture content was obtained by weighing the soil before and after drying to constant mass. The bulk density was measured using 100 cm3 stainless steel rings.
2.7. Analysis
The total concentration of trace elements (Sb, Pb, Cu, As, Fe, and Mn) in the digests of the solid and the soil solution samples was determined by Inductively-Coupled Plasma Mass Spectrometry (ICP–MS 7700x ICP-MS, Agilent Technologies, Waldbronn, Germany). The instrument’s settings are presented in Table S2. Indium (m/z 115) was used as an internal standard and injected continuously through a T-piece. Sb was measured at m/z 121. Further analytical details concerning rinsing between samples to avoid carryover of Sb can be found in Mestrot et al. [37]. The concentrations of Sb species (Sb(III), Sb(V) and TMSb) were measured through coupling a High-Pressure Liquid Chromatograph (HPLC 1200 Series, Agilent Technologies, Santa Clara, CA, USA) to the ICP–MS. The details of the method, initially developed by Ge and Wei [46], are presented in Mestrot et al. [37] and in Table S2. Briefly, the mobile phase was 150 mM AT with 4% methanol at pH 5 and was used at a flow rate of 1 mL/min. Because of the high salt content of the mobile phase, the HPLC flow was split (1:1) with a microsplitter (P-451 Idex, H&S, Middleborough, MA, USA). In this way, 50% of the flow went to the waste, while the rest was mixed online with a solution of 1% HNO3. The calibration standards were prepared daily by diluting the Sb stock solution of each species in 150 mM AT. The C, N, and S concentrations were measured on milled soils using an elemental analyzer (vario El cube, Elementar Analysensysteme, Langenselbold, Germany). DOC concentrations in soil solution were measured using a vario TOC cube (Elementar Analysensysteme, Langenselbod, Germany). Redox potential and pH were measured using a SenTix ORP electrode and a pH meter (pH 330i), both from WTW (Dinslaken, Germany). The texture was measured with a MasterSizer 2000 (Malvern Panalytical Ltd., Malvern, UK) after dispersion of the sample with pyrophosphate (NaP2O7).
3. Results and Discussion
3.1. TMSb Extraction Method Validation
First, we assessed the extraction of Sb species from the soils by different methods from the literature [45,46,47], using a sediment CRM (PACS-3: 14.7 ± 2.2 mg kg−1 Sb, Figure 1a). The total Sb recovery from the CRM with 200 mM AT as an extractant was 21.4 ± 1.8% (n = 3), while, when using a mixture of 200 mM OA and 100 mM AA, it was 45.2 ± 4.6% (n = 4). The OA+AA method was chosen because of the better extraction efficiency of total Sb. The experimental conditions were further modified to improve the extraction efficiency. Using 200 mg instead of 100 mg of CRM in 10 mL or 5 mL led to lower recoveries: 32.8 ± 4.0% (n = 3) and 31.1 ± 2.7% (n = 3), respectively. An extraction time of 2 h was also tested but did not significantly improve the recoveries (49.0 ± 2.5%, n = 3). The 30-min extraction was therefore chosen to keep the sample processing time short. The extraction efficiency of single species was also tested with soil spiking experiments (Figure 1b). The OA+AA extraction provided high recoveries for Sb(III)-spiked soil (83.2 ± 4.3%, n = 3), Sb(V)-spiked soil (76.8 ± 4.7%, n = 3), TMSb-spiked soil (78.9 ± 11.0%, n = 3), and for a mixture of all three (85.6 ± 5.3%, n = 3).
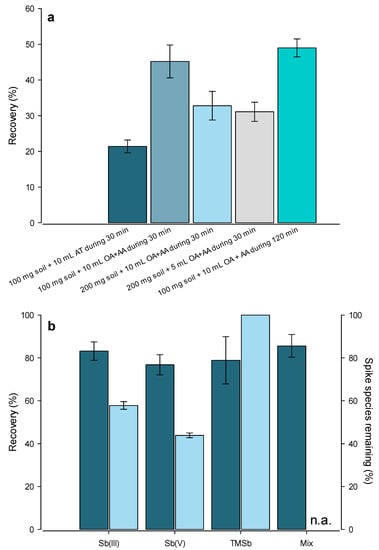
Figure 1.
(a) Recovery of the certified total Sb concentrations in the Certified Reference Material (CRM) PACS-3 (mean ± sd, n ≥ 3); (b) Extraction recovery after spiking Sb(III), Sb(V), TMSb, and a mix of these three Sb species in a low-Sb soil (left column, total Sb by ICP–MS). The percentage of the spike species remaining after extraction (right column) was measured by HPLC–ICP–MS (mean ± sd, n = 3). AT: ammonium tartrate, OA: oxalic acid, AA: ascorbic acid, TMSb; trimethylantimony.
The higher recovery from the soil spiking experiment compared to the total Sb recovery from the CRM is attributable to the amount of Sb that is contained in fractions that are not available in the PACS-3 CRM. Indeed, most of the Sb recovered from the soil used for the spiking experiment was added. The speciation analysis revealed conversions of Sb(III) to Sb(V) and of Sb(V) to Sb(III) during the extraction (Figure 1b). However, the TMSb standard did not transform into inorganic species. Therefore, the method is well-suited for TMSb analysis in soils. The blank values (n = 14) for the extraction method were below the detection limit of the HPLC–ICP–MS (0.2 µg L−1) for all species.
3.2. Total Sb Concentrations in the Shooting Range Soils
The total concentrations of Sb and Pb in the soil at all three sites were high (up to 2000 mg kg−1 for Sb and 60,000 mg kg−1 for Pb), and most of the samples situated near the surface exceeded the Swiss remediation values (50 mg kg−1 Sb and 1000 mg kg−1 Pb). For Sb, these concentrations are well above soil background values of 0.3–8.6 mg kg−1 [48]. However, they are in line with previously found concentrations of Sb in shooting range and mining sites that range up to 17,500 mg kg−1 [5,8,22,34,49,50]. It must be noted, however, that extremely small bullet residues (<100 μm) can be present in the soil [4]. This is possibly the reason for high relative standard deviations (RSD) of the total Sb and total Pb concentrations in replicate digests (as high as 15.8% and 19.1%, respectively), although we did not directly assess the presence of such small bullet residues. Conversely, concentrations of naturally occurring Fe and Mn are characterized by low Relative Standard Deviation (RSD) values (2.1% and 2.7%, respectively). As a result, it is difficult to assess the mobility of Sb and Pb down the soil profile for Site A because the observed concentration changes fell into the error margin of their measurement (Figure 2a). The analysis also revealed that the percentage of Sb from the bullets (1–5% Sb, 95–99% Pb) is conserved in the soil profiles of all three sites with Sb percentages of 1 to 5.2% calculated as 100 × Sb/(Sb + Pb). This result is similar to previously found ratios in shooting range soils [12,50]. Compared to the surface concentrations, the decreasing Sb and Pb concentrations with increasing depth in the soils at Sites B and C indicate that both elements are strongly bound in the OM-rich topsoil and little leached down the soil profile. This decrease in concentration with depth was also found in previous studies [27,51,52,53,54,55]. Nonetheless, Sb and Pb concentrations were elevated, even at a depth of 30 cm (20 to 130 mg kg−1 Sb and 800 to 5600 mg kg−1 Pb). The concentrations at 30 cm were higher than the remediation values for Sb at Site C and for Pb at Sites B and C.
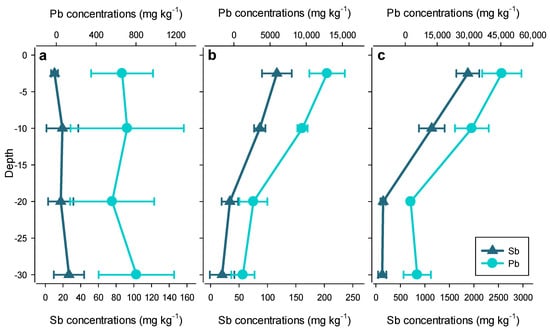
Figure 2.
Depth profile of Sb and Pb concentrations (mean ± sd, n = 3) at (a) Site A; (b) Site B; (c) Site C.
3.3. TMSb in Shooting Range Soils
TMSb was detected in 15 of the 48 extracted samples as a distinct peak in the HPLC–ICP–MS chromatograms at all three sites (Figure 3).
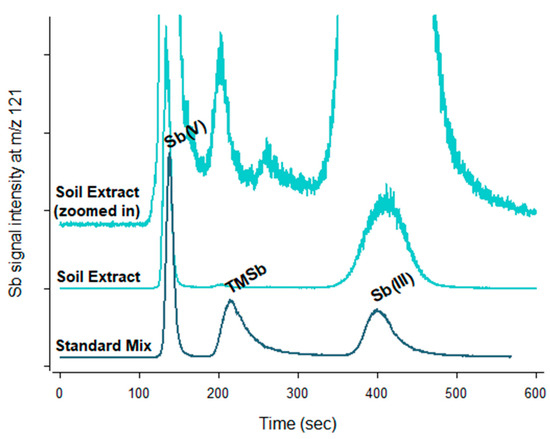
Figure 3.
Chromatograms of a soil extract compared to that of the standard Sb mixture.
However, the low concentration of TMSb in most of the samples meant that this peak could not be quantified. The reason for the small peak was a high dilution necessary to prevent the buildup of inorganic Sb in the HPLC–ICP–MS system. In order to quantify the TMSb concentration present in the samples showing the highest peak, a subset of five soil samples were measured again but at a lower dilution. Since these were only five, the lower dilution did not lead to a buildup of Sb in the instrument. This allowed for a proper quantification of TMSb in these samples, where it represented up to 0.6% of the total Sb (Table 2).

Table 2.
TMSb concentrations in selected soil samples from Sites A, B, and C. The instrumental limit of detection (LOD) was 0.3 µg L−1.
Our results showed that TMSb was present in shooting range soils at concentrations of up to 1.35 mg kg−1. Because of the lack of information regarding the mobility and toxicity of this compound, further research is necessary to understand the implications of these findings.
3.4. Sb Mobility under Flooded Conditions
The change in the total Sb concentration in the soil solutions of the incubated soils was similar in all three fields and in both treatments (with and without manure amendments). It followed two distinct phases in the three studied soils, with a third phase present only at Sites A and B. The Sb concentrations were elevated at the beginning of the experiment, with values around 250 µg L−1 at Sites A and B and 3000 µg L−1 at Site C (Figure 4a,b). Then, for a duration of 3 to 8 days, the concentration of Sb stayed high or slightly increased (Phase I). After this period, a sharp decrease was observed when Sb concentrations diminished to 30–50 µg L−1 at Sites A and B, and to 800–1000 µg L−1 at Site C (Phase II). Thereafter, Sb concentrations slightly increased again at Sites A and B (Figure 5a,b) for the remainder of the experiment (Phase III). During the incubation period, the pH showed a slight increase (from 7.4 to 7.8 for Site A and Site A with 2% OM, from 6 to 6.9 for Site B and Site B with 2% OM, and from 7.2 to 7.6 for Site C and Site C with 2% OM, respectively).
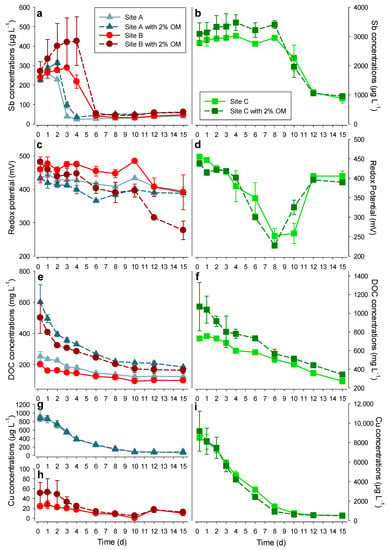
Figure 4.
Course of Sb concentrations (a,b), redox potential (c,d), dissolved organic carbon (DOC) concentrations (e,f) and Cu concentrations (g–i) in soil solutions during the flooding experiment. The left panels (a,c,e,g,h) represent Sites A and B. The right panels (b,d,f,i) represent Site C (mean ± sd, n = 3 incubators). OM: organic matter.
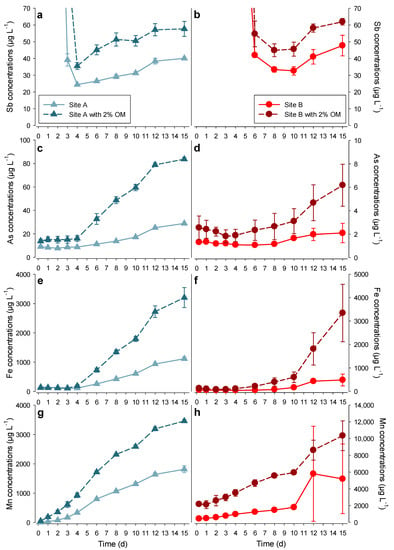
Figure 5.
Course of Sb (a,b), As (c,d), Fe (e,f), and Mn (g,h) concentrations in soil solutions during the flooding experiment: (a) Sb concentrations in the soil solution from Site A (zoomed in from Figure 4a); (b) Sb concentrations in the soil solution from Site B (zoomed in from Figure 4a). The left panels (a,c,e,g) represent Site A. The right panels (b,d,f,h) represent Site B (mean ± sd, n = 3 incubators).
Phase I: The Sb contained in these contaminated soils is released into solution, presumably mainly by simple dissolution. The increasing concentrations with time can be attributed to kinetic restrictions of the dissolution reactions or diffusion from locations away from the solid soil–solution interface. Importantly, the amount of Sb released into soil solution, which can be considered to be mostly available to plants, microorganisms, and animals, was high and sudden (from 228 to 3089 µg L−1 within the first 6 h, Figure 4a,b). The high mobility of Sb in shooting range soils has already been established in the field [11,12,50] and in column experiments [56]. Clausen and Korte [50] reported up to 330 µg/L of Sb in the soil solution from the target area of a shooting range. The column experiments of Okkenhaug et al. [56] showed a high initial Sb release, similar to our experiment, with Sb concentrations in the hundreds of µg L−1 in soil solution. Furthermore, a field study on the same soils as used for the column experiment by Okkenhaug et al. [56] also showed soil solution concentrations of Sb in the hundreds of µg L−1 [12]. Hockmann et al. [57] found a similar pattern in a lysimeter experiment with shooting range soils, although at much lower concentrations. However, a direct comparison of the results of Hockmann et al. [57] with ours is difficult, because their study was run for a much longer time than ours (i.e., months versus days). Others measured an increase of Sb concentrations in streams draining shooting ranges after heavy precipitation events, reaching concentrations of several tens of µg L−1 [25,26,27]. Another incidence of high Sb release to soil solution upon flooding was reported in the study of Okkenhaug et al. [9]. The researchers flooded a Sb-impacted rice paddy and measured Sb concentration in the soil solution after 2, 4, 6, and 8 weeks. They found concentrations ranging from 500 to 2500 µg L−1, which is similar to our results, but Okkenhaug et al. [9] even reported increasing Sb concentrations over time. High Sb mobility in soils can, however, not be generalized, since there are also studies showing a limited mobilization of Sb upon flooding [18,58]. In our case, we believe that using fresh soil, instead of dried or frozen soil, provided a more accurate picture of the environmental processes involved, since the microbial communities were less disturbed, as shown in previous studies investigating the effect of soil drying on microbial communities [59,60].
The high DOC concentration in the soil solution also suggests a possible competition of organic molecules with Sb for binding sites on the metal (oxy-)hydroxides (Figure 4e,f), which would explain why Sb is not immediately readsorbed to the soil. Indeed, both Sb and DOC are known to form inner sphere complexes with metal (oxy-)hydroxides [21,49,61,62,63,64]. Furthermore, our results show a close positive correlation between the concentration of Sb and DOC in the two experimental treatments (both p < 0.0001, Figure S2a,b). This is similar to previous findings from Hockmann et al. [57] during a leaching experiment of a shooting range soil (non-waterlogged calcareous soil). Additionally, the Sb concentrations in a stream draining a contaminated shooting range in Norway also correlated closely positively with the DOC concentrations [23]. However, a microcosm study of waterlogged shooting range soil lacked the correlation of Sb and DOC in soil solution. Instead, a fairly stable DOC concentration was measured in combination with a high initial concentration increase of Sb [64]. In the present study, the DOC is defined as the 0.2 μm-filtered organic carbon fraction. We did not differentiate between macromolecules or colloidal particles, and this might be the reason for the differing research findings, although the usual definition of dissolved organic carbon includes a filtration < 0.45 µm [65].
Phase II: The second phase, characterized by a sharp decrease in Sb concentrations in the soil solutions, has already been described in column experiments [56]. This behavior can possibly be explained by two mechanisms. [A] Reduction of Sb(V) to less soluble Sb(III) and subsequent immobilization by adsorption. Indeed, most of the Sb in the soil solutions was found as Sb(V) at the beginning of the incubation experiment, while Sb(III) concentrations remained low and relatively stable in the soil solutions during the whole experiment, most likely because of the adsorption to metal (oxy-)hydroxides (Figure S3). However, without solid-state speciation analysis by determining the X-ray absorption near edge structure (XANES), this hypothesis cannot be confirmed. Our assumption is nonetheless supported by the finding that Sb(III) was the major Sb species in the soil measured by XANES in the solid phase after flooding incubations [57,58]. [B] Another hypothesis for the sudden decrease in soluble Sb is the formation of colloids, defined as solid particles with a diameter between 1 nm and 1 µm [66]. Unfortunately, our setup did not allow for accurate colloid measurements, since we only sampled soil solutions with a rhizon sampler (diameter < 0.2 µm). Therefore, we could not assess whether colloids of a diameter > 0.2 µm were formed. Although detailed studies of Sb colloids are scarce, Klitzke et al. [67] found over 12% of total dissolved Sb in colloidal form in batch studies with shooting range soils. Furthermore, the release pattern of Sb matched that of Cu (Figure 4g–i) which is known for forming colloids under similar redox conditions [68].
Phase III: For Sites A and B, the increase of Sb concentrations corresponded to a simultaneous increase of Fe and Mn concentrations in the soil solutions (Figure 5). Here, we hypothesize that the reductive dissolution of Fe- and Mn-(oxy-)hydroxides is the responsible mechanisms for the release of Sb into the soil solutions. Indeed, (oxy-)hydroxides provide binding sites for many trace elements, and, under low redox potential (e.g., during flooding), they are dissolved, and the associated metals are released [18]. Here, Site A provided enough data points in Phase III to perform a regression analysis of Sb on Mn and Fe concentrations (Figure S3c–f). The Sb concentrations in the soil solution of Site A after four days of incubation correlated with both Fe and Mn concentrations, especially when no OM was added (r = 0.996, p < 0.0001 and r = 0.962, p < 0.005, respectively). Our hypothesis was further supported by similarities between the release patterns of As, an element whose mobility is mainly linked to Fe- and Mn- (oxy-)hydroxides and Sb (Figure 5c,d). Interestingly, although the redox potential was lower at the end than at the beginning of the experiment (Figure 4c,d), it did not continuously decrease and did not reach the Eh values needed for reductive dissolution of Fe- and Mn- (oxy-)hydroxides, corresponding to 150 and 400 mV, respectively (Figure 5e–h). We suggest that this is the result of soil heterogeneity, leading to Fe and Mn being released in minute anaerobic pockets, while the redox potential was measured in a bigger volume of soil solution, also reflecting less anaerobic microsites (2–3 mL).
3.5. TMSb Formation under Flooded Conditions
Dissolved TMSb was detected in soil solution after four days of waterlogging in Site A soils, while in Site B and Site C soils, it was only detected after 10 and 12 days, respectively. Furthermore, the increase of TMSb formation in OM-amended incubators was significant for Sites A (p < 0.005) and B (p < 0.05). Up to 3.5 µg L−1 of TMSb was found in the OM-amended soil from Site A after six days of incubation, which corresponded to 7.8 ± 1.1% (n = 3) of the total amount of Sb found in the soil solution. This constitutes the first report of TMSb formation in the soil solution of a Sb-polluted soil. We assume a higher TMSb production rate because of the lower redox potentials towards the end of the experiment (Figure 4). This is in accordance with previous experiments showing an increased formation of MMSb under lower redox potential [33]. Also, the higher production rate of TMSb in OM-amended soils (Figure 6) highlights the influence of C source and nutrient availability and the role of microorganisms. These two results therefore confirmed the hypothesis that methylated Sb compounds are formed by microorganisms under low redox potential and in the presence of OM. Furthermore, TMSb concentrations were below the detection limit in the soil extracts performed with our new method before and after the incubation. This, however, does not mean that all the TMSb formed was in the soil solution, but rather that if any TMSb was bound to the soil, its amount was too low to be detected.
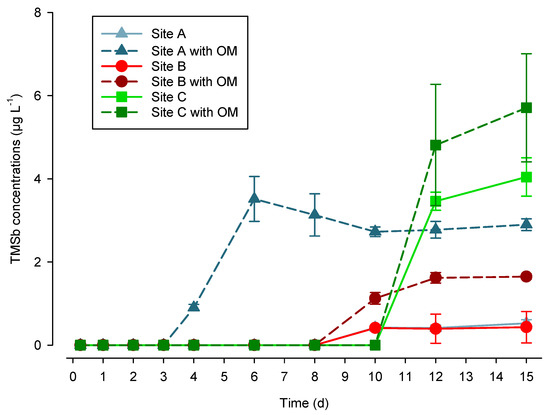
Figure 6.
TMSb concentration during the flooding experiment (mean ± sd, n = 3 incubators).
4. Conclusions
To better understand the chemical transformations and mobility of Sb in soils, it is important to investigate the occurrence and properties of the different Sb species, including the methylated ones. To this end, our new method allowed for a differentiation between organic (TMSb) and inorganic (Sb(V)/Sb(III)) Sb species in soils. Using this method, we were able to demonstrate for the first time the presence of TMSb in all the examined shooting range soils.
In our incubation experiment, the Sb release was fast and immediately reached a high Sb concentration. The release kinetics can be described by three distinct phases. The formation of TMSb was detected for the first time during an incubation experiment of polluted soils and was more pronounced in OM-amended soils. The incubation experiment provided valuable information about key processes driving Sb release into soil solution under waterlogged conditions and highlighted the need for further studies. Such studies are highly relevant, since Sb is a global pollutant found in many environments that are prone to changing redox conditions, such as wetlands, floodplains, shooting ranges, landfills, and rice paddies. The processes controlling Sb release into soil solution in response to flooding must be assessed thoroughly in the future, especially since climate change is likely to bring more extreme weather events and will therefore increase the number of floods which might ultimately lead to elevated concentrations of these elements in soil solution, ground, and surface waters.
Supplementary Materials
The following are available online at http://www.mdpi.com/2571-8789/2/2/34/s1, Table S1: Microwave programming for the digestion procedure, Table S2: ICP–MS and HPLC–ICP–MS instrumental settings for Sb analysis, Figure S1. Sampling plan. A 2 cm diameter core was taken at each sampling point. The core was divided into four depth layers: 0–5 cm, 5–15 cm, 15–25 cm and 25–35 cm, Figure S2. Relationships between the concentrations of Sb in soil solutions and those of (a) DOC in the treatment without addition of OM (cow dung); (b) DOC in the treatment with addition of 2% OM; (c) Fe (without OM); (d) Fe (with OM); (e) Mn (without OM); (f) Mn (with OM). (a) and (b): data from the whole experiment duration were used. (c), (d), (e), and (f): only data from after four days of incubation were used, Figure S3. Course of the soil solution concentrations during the flooding experiment (mean ± sd, n = 3 incubators): (a) Sb(III) concentrations in the soil solutions from Sites A and B; (b) Sb(III) concentrations in the soil solution from Site C; (c) Sb(V) concentrations in the soil solutions from Sites A and B; (d) Sb(V) concentrations in the soil solution from Site C.
Author Contributions
Conceptualization, M.G. and A.M.; Methodology, M.G. and A.M.; Validation, M.G., A.M., and W.W.; Formal Analysis, M.G. and A.M.; Investigation, M.G., A.M., and W.W.; Resources, A.M. and W.W.; Writing-Original Draft Preparation, M.G.; Writing-Review & Editing, A.M. and W.W.; Visualization, M.G. and A.M.; Supervision, A.M. and W.W.; Project Administration, A.M.; Funding Acquisition, A.M.
Acknowledgments
This study was supported by funding from the Swiss Federal Office for Environment (FOEN), the Swiss Federal Office for Defense Procurement (armasuisse), the Intra-European Fellowship from the People Program (Marie Curie Actions) of the European Union’s Seventh Framework Program FP7/2007–2013/under REA grant agreement No. 326736, and the Swiss National Science Foundation (SNSF, nr. PP00P2_163661). The authors also extend gratitude to Daniela Fischer and Michael Wendler for their analytical and technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Communities. Richtlinie 98/83/EG des Rates vom 3. November 1998 Über die Qualität von Wasser für den Menschlichen Gebrauch. 1998. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:330:0032:0054:de:PDF (accessed on 22 February 2016).
- United States Environmental Protection Agency. Ambient Water Quality Criteria Document for Antimony; Prepared by the Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office Cincinnati, OH for the Office of Air Quality Planning and Standards; EPA 440/5-80-020; EPA: Washington, DC, USA, 1980.
- USGS. Mineral Commodity Summaries 2012. Available online: http://minerals.usgs.gov/minerals/pubs/mcs/2012/mcs2012.pdf (accessed on 22 February 2016).
- Ackermann, S.; Gieré, R.; Newville, M.; Majzlan, J. Antimony Sinks in the Weathering Crust of Bullets from Swiss Shooting Ranges. Sci. Total Environ. 2009, 407, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, M. Adsorption of Methylantimony and Methylarsenic on Soils, Sediments, and Mine Tailings from Antimony Mine Area. Microchem. J. 2015, 123, 158–163. [Google Scholar] [CrossRef]
- Cidu, R.; Biddau, R.; Dore, E.; Vacca, A.; Marini, L. Antimony in the Soil–Water–Plant System at the Su Suergiu Abandoned Mine (Sardinia, Italy): Strategies to Mitigate Contamination. Sci. Total Environ. 2014, 497–498, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, K.; MacKinnon, G.; Farmer, J.G.; Graham, M.C. Mobility of Antimony, Arsenic and Lead at a Former Mine, Glendinning, Scotland. Sci. Total Environ. 2015, 529, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, G.; Zhu, Y.G.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, Speciation and Availability of Antimony (Sb) in Soils and Terrestrial Plants from an Active Sb Mining Area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, G.; Zhu, Y.; He, J.; Li, X.; Luo, L.; Mulder, J. Antimony (Sb) and Arsenic (As) in Sb Mining Impacted Paddy Soil from Xikuangshan, China: Differences in Mechanisms Controlling Soil Sequestration and Uptake in Rice. Environ. Sci. Technol. 2012, 46, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Hammel, W.; Debus, R.; Steubing, L. Mobility of Antimony in Soil and its Availability to Plants. Chemosphere 2000, 41, 1791–1798. [Google Scholar] [CrossRef]
- Mariussen, E.; Johnsen, I.V.; Stromseng, A.E. Application of sorbents in different soil types from small arms shooting ranges for immobilization of lead (Pb), copper (Cu), zinc (Zn), and antimony (Sb). J. Soils Sediments 2018, 18, 1558–1568. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Gebhardt, K.G.; Amstaetter, K.; Bue, H.L.; Herzel, H.; Mariussen, E.; Almas, A.R.; Cornelissen, G.; Breedveld, G.D.; Rasmussen, G.; et al. Antimony (Sb) and lead (Pb) in contaminated shooting range soils: Sb and Pb mobility and immobilization by iron based sorbents, a field study. J. Hazard. Mater. 2016, 307, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the Environment: A Review Focused on Natural Waters. II. Relevant Solution Chemistry. Earth Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Z.; Wang, H.; Song, F.; Wu, F.; Giesy, J.P. Environmental Geochemical and Spatial/Temporal Behavior of Total and Speciation of Antimony in Typical Contaminated Aquatic Environment from Xikuangshan, China. Microchem. J. 2018, 137, 181–189. [Google Scholar] [CrossRef]
- Leuz, A.-K.; Mönch, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to Goethite: Influence on Sb(III) Oxidation and Mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
- Blay, K. Sorption Wässeriger Antimon-Spezies an Bodenbildende Festphasen und Remobilisierung Durch Natürliche Komplexbildner. Ph.D. Thesis, Technische Universität München, Munich, Germany, 1999. [Google Scholar]
- Rakshit, S.; Sarkar, D.; Punamiya, P.; Datta, R. Antimony Sorption at Gibbsite–Water Interface. Chemosphere 2011, 84, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Tighe, M.; Lockwood, P.; Wilson, S. Adsorption of Antimony(V) by Floodplain Soils, Amorphous Iron(III) Hydroxide and Humic Acid. J. Environ. Monit. 2005, 7, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Blume, H.-P.; Brümmer, G.W. Scheffer/Schachtschabel: Lehrbuch der Bodenkunde, 16th ed.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2009; ISBN 978-3-8274-1444-1. [Google Scholar]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Thige, M. The Chemistry and Behaviour of Antimony in the Soil Environment with Comparisons to Arsenic: A Critical Review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Mitsunobu, S.; Harada, T.; Takahashi, Y. Comparison of Antimony Behavior with that of Arsenic under Various Soil Redox Conditions. Environ. Sci. Technol. 2006, 40, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Hockmann, K.; Schulin, R. Leaching of Antimony from Contaminated Soils. In Competitive Sorption and Transport of Heavy Metals in Soils and Geological Media, 1st ed.; Magdi Selim, H., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 119–145. ISBN 9781138073395. [Google Scholar]
- Heier, L.S.; Meland, S.; Ljønes, M.; Salbu, B.; Strømseng, A.E. Short-term Temporal Variations in Speciation of Pb, Cu, Zn and Sb in a Shooting Range Runoff Stream. Sci. Total Environ. 2010, 408, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Conesa, H.M.; Wieser, M.; Gasser, M. Effects of three Amendments on Extractability and Fractionation of Pb, Cu, Ni and Sb in two Shooting Range Soils. J. Hazard. Mater. 2010, 181, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Stromseng, A.E.; Ljones, M.; Bakka, L.; Mariussen, E. Episodic discharge of lead, copper and antimony from a Norwegian small arm shooting range. J. Environ. Monit. 2009, 11, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Mariussen, E.; Ljones, M.; Stromseng, A.E. Use of sorbents for purification of lead, copper and antimony in runoff water from small arms shooting ranges. J. Hazard. Mater. 2012, 243, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Mariussen, E.; Johnsen, I.V.; Stromseng, A.E. Distribution and mobility of lead (Pb), copper (Cu), zinc (Zn), and antimony (Sb) from ammunition residues on shooting ranges for small arms located on mires. Environ. Sci. Pollut. Res. 2017, 24, 10182–10196. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.; Chasteen, T.G. Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth. Microbiol. Mol. Biol. Rev. 2002, 66, 250–271. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.S. Review: Biological Methylation of Less-studied Elements. Appl. Organomet. Chem. 2002, 16, 677–691. [Google Scholar] [CrossRef]
- Jenkins, R.O.; Craig, P.J.; Miller, D.P.; Stoop, L.C.A.M.; Ostah, N.; Morris, T.A. Antimony Biomethylation by Mixed Cultures of Micro-organisms under Anaerobic Conditions. Appl. Organomet. Chem. 1998, 12, 449–455. [Google Scholar] [CrossRef]
- Fatoki, O. Biomethylation in the Natural Environment: A Review. S. Afr. J. Sci. 1997, 93, 366–370. [Google Scholar]
- Duester, L.; Diaz-Bone, R.A.; Kösters, J.; Hirner, A.V. Methylated Arsenic, Antimony and Tin Species in Soils. J. Environ. Monit. 2005, 7, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Frohne, T.; Rinklebe, J.; Diaz-Bone, R.A.; Du Laing, G. Controlled Variation of Redox Conditions in a Floodplain Soil: Impact on Metal Mobilization and Biomethylation of Arsenic and Antimony. Geoderma 2011, 160, 414–424. [Google Scholar] [CrossRef]
- Wei, C.; Ge, Z.; Chu, W.; Feng, R. Speciation of Antimony and Arsenic in the Soils and Plants in an Old Antimony Mine. Environ. Exp. Bot. 2015, 109, 31–39. [Google Scholar] [CrossRef]
- Gurleyuk, H.; Van Fleet Stalder, V.; Chasteen, T.G. Confirmation of the Biomethylation of Antimony Compounds. Appl. Organomet. Chem. 1997, 11, 471–483. [Google Scholar] [CrossRef]
- Michalke, K.; Wickenheiser, E.B.; Mehring, M.; Hirner, A.V.; Hensel, R. Production of Volatile Derivatives of Metal(loid)s by Microflora Involved in Anaerobic Digestion of Sewage Sludge. Appl. Environ. Microbiol. 2000, 66, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Mestrot, A.; Ji, Y.; Tandy, S.; Wilcke, W. A Novel Method to Determine Trimethylantimony Concentrations in Plant Tissue. Environ. Chem. 2016, 13, 919–926. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, J.; Zhu, C.; Zhao, D.; Gao, J.; Hao, J.; He, M.; Liu, K.; Wang, K.; Hua, S. A Comprehensive Global Inventory of Atmospheric Antimony Emissions from Anthropogenic Activities, 1995–2010. Environ. Sci. Technol. 2014, 48, 10235–10241. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, Y.M.; Altansuvd, J. Speciation and Bioavailability of Selenium and Antimony in Non-flooded and Wetland Soils: A Review. Chemosphere 2014, 111, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Henne, P.D.; Bigalke, M.; Büntgen, U.; Colombaroli, D.; Conedera, M.; Feller, U.; Frank, D.; Fuhrer, J.; Grosjean, M.; Heiri, O.; et al. An Empirical Perspective for Understanding Climate Change Impacts in Switzerland. Reg. Environ. Chang. 2018, 18, 205–221. [Google Scholar] [CrossRef]
- Fischer, E.M.; Knutti, R. Anthropogenic Contribution to Global Occurrence of Heavy Precipitation and High Temperature Extremes. Nat. Clim. Chang. 2015, 5, 560–564. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015, World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 21 February 2016).
- Link, D.D.; Walter, P.J.; Kingston, H.M. Development and Validation of the new IPA Microwave-assisted Beach Method 3051A. Environ. Sci. Technol. 1998, 32, 3628–3632. [Google Scholar] [CrossRef]
- Link, D.D.; Walter, P.J.; Kingston, H.M. Wastewater Standards and Extraction Chemistry in Validation of Microwave-assisted EPA Method 3015A. Environ. Sci. Technol. 1999, 33, 2469–2473. [Google Scholar] [CrossRef]
- Potin-Gautier, M.; Pannier, F.; Quiroz, W.; Pinochet, H.; de Gregori, I. Antimony Speciation Analysis in Sediment Reference Materials Using High-performance Liquid Chromatography Coupled to Hydride Generation Atomic Fluorescence Spectrometry. Anal. Chim. Acta 2005, 553, 214–222. [Google Scholar] [CrossRef]
- Ge, Z.; Wei, C. Simultaneous Analysis of Sb III, Sb V and TMSb by High Performance Liquid Chromatography–Inductively Coupled Plasma–Mass Spectrometry Detection: Application to Antimony Speciation in Soil Samples. J. Chromatogr. Sci. 2012, 51, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.R.; Pergantis, S.A. Identification of Sb(V) Complexes in Biological and Food Matrixes and their Stibine Formation Efficiency during Hydride Generation with ICPMS Detection. Anal. Chem. 2007, 79, 5304–5311. [Google Scholar] [CrossRef] [PubMed]
- Tschan, M.; Robinson, B.H.; Schulin, R. Antimony in the Soil-plant System: A Review. Environ. Chem. 2009, 6, 106–115. [Google Scholar] [CrossRef]
- Johnson, C.A.; Moench, H.; Wersin, P.; Kugler, P.; Wenger, C. Solubility of Antimony and other Elements in Samples Taken from Shooting Ranges. J. Environ. Qual. 2005, 34, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.; Korte, N. The Distribution of Metals in Soils and Pore Water at Three U.S. Military Training Facilities. Soil Sediment Contam. 2009, 18, 546–563. [Google Scholar] [CrossRef]
- Van Vleek, B.; Amarasiriwardena, D.; Xing, B. Investigation of Distribution of Soil Antimony Using Sequential Extraction and Antimony Complexed to Soil-derived Humic Acids Molar Mass Fractions Extracted from Various Depths in a Shooting Range Soil. Microchem. J. 2011, 97, 68–73. [Google Scholar] [CrossRef]
- Jorgensen, S.S.; Willems, M. The Fate of Lead in Soils: The Transformation of Lead Pellets in Shooting-Range Soils. AMBIO 1987, 16, 11–15. [Google Scholar]
- Knechtenhofer, L.; Xifra, I.; Scheinost, A.; Fluhler, H.; Kretzschmar, R. Fate of heavy metals in a strongly acidic shooting-range soil: small-scale metal distribution and its relation to preferential water flow. J. Plant Nutr. Soil Sci. 2003, 166, 84–92. [Google Scholar] [CrossRef]
- Sanderson, P.; Naidu, R.; Bolan, N.; Bowman, M.; Mclure, S. Effect of soil type on distribution and bioaccessibility of metal contaminants in shooting range soils. Sci. Total Environ. 2012, 438, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, L.; Chen, M.; Hardison, D.; Harris, W. Lead transformation and distribution in the soils of shooting ranges in Florida, USA. Sci. Total Environ. 2003, 307, 179–189. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Amstatter, K.; Bue, H.L.; Cornelissen, G.; Breedveld, G.D.; Henriksen, T.; Mulder, J. Antimony (Sb) Contaminated Shooting Range Soil: Sb Mobility and Immobilization by Soil Amendments. Environ. Sci. Technol. 2013, 47, 6431–6439. [Google Scholar] [CrossRef] [PubMed]
- Hockmann, K.; Tandy, S.; Lenz, M.; Reiser, R.; Conesa, H.M.; Keller, M.; Studer, B.; Schulin, R. Antimony Retention and Release from Drained and Waterlogged Shooting Range Soil under Field Conditions. Chemosphere 2015, 134, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Tandy, S.; Hockmann, K.; Schulin, R. Changes in Sb Speciation with Waterlogging of Shooting Range Soils and Impacts on Plant Uptake. Environ. Pollut. 2013, 172, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tzeneva, V.A.; Salles, J.F.; Naumova, N.; De Vos, W.A.; Kuikman, P.J.; Dolfing, J.; Smidt, H. Effect of Soil Sample Preservation, Compared to the Effect of Other Environmental Variables, on Bacterial and Eukaryotic Diversity. Res. Microbiol. 2009, 160, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Vangestel, M.; Merckx, R.; Vlassak, K. Microbial Biomass Responses to Soil Drying and Rewetting—The Fate of Fast-Growing and Slow-Growing Microorganisms in Soils from Different Climates. Soil Biol. Biochem. 1993, 25, 109–123. [Google Scholar] [CrossRef]
- Tipping, E. The Adsorption of Aquatic Humic Substances by Iron Oxides. Geochim. Cosmochim. Acta 1981, 45, 191–199. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and Desorption of Natural Organic Matter on Iron Oxide: Mechanisms and Models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Scheinost, A.C.; Rossberg, A.; Vatelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.K.; Funke, H.; Johnson, A. Quantitative Antimony Speciation in Shooting-range Soils by EXAFS Spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- Hockmann, K.; Lenz, M.; Tandy, S.; Nachtegaal, M.; Janousch, M.; Schulin, R. Release of Antimony from Contaminated Soil Induced by Redox Changes. J. Hazard. Mater. 2014, 275, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the Dynamics of Dissolved Organic Matter in Soils: A Review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; Mclaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Klitzke, S.; Lang, F.; Kirby, J.; Lombi, E.; Hamon, R. Lead, Antimony and Arsenic in Dissolved and Colloidal Fractions from an Amended Shooting-range Soil as Characterised by Multi-stage Tangential Ultrafiltration and Centrifugation. Environ. Chem. 2012, 9, 462–473. [Google Scholar] [CrossRef]
- Abgottspon, F.; Bigalke, M.; Wilcke, W. Fast Colloidal and Dissolved Release of Trace Elements in a Carbonatic Soil after Experimental Flooding. Geoderma 2015, 259–260, 156–163. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).