Arsenic Speciation of Contaminated Soils/Solid Wastes and Relative Oral Bioavailability in Swine and Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Solid Waste Preparation
2.2. In Vivo Bioavailability
2.3. Arsenic Speciation Methods
2.4. Statistical Analyses
3. Results
Arsenic Speciation
4. Discussion
4.1. Arsenic Speciation and Bioavailability
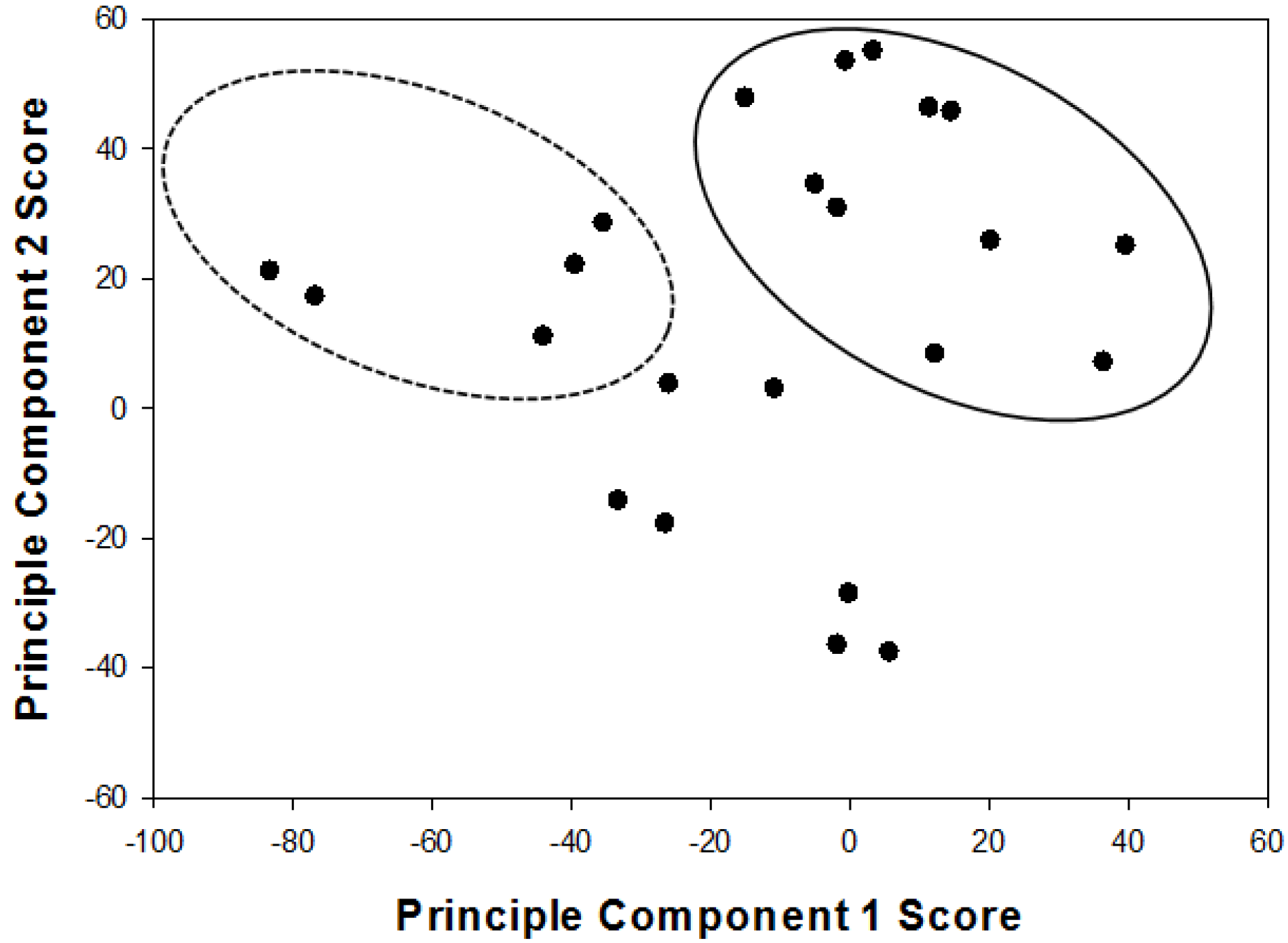
4.2. Species Groupings via Principle Component Analysis
4.3. Predicting Bioavailability Using Arsenic Speciation
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services. Toxicological Profile for Arsenic. In Environment and Occupational Medicine; US Department of Health and Human Services: Washington, DC, USA, 2007; pp. 1006–1017. [Google Scholar]
- ATSDR, Division of Toxicology & Human Health Sciences. Summary Data for 2015 Priority List of Hazardous Substances; ATSDR: Atlanta, GA, USA, 2015; pp. 1–2.
- Ruby, M.V.; Schoof, R.; Brattin, W.; Mosby, D.E.; Casteel, S.W.; Berti, W.; Carpenter, M.; Edwards, D.; Cragin, D.; Chappell, W. Advances in Evaluating the Oral Bioavailability of Inorganics in Soil for Use in Human Health Risk Assessment. Environ. Sci. Technol. 1999, 33, 3697–3705. [Google Scholar] [CrossRef]
- Basta, N.T.; Juhasz, A. Using In Vivo Bioavailability and/or In Vitro Gastrointestinal Bioaccessibility Testing to Adjust Human Exposure to Arsenic from Soil Ingestion. Rev. Mineral. Geochem. 2014, 79, 451–472. [Google Scholar] [CrossRef]
- Casteel, S.W.; Weis, C.P.; Henningsen, G.M.; Brattin, W.J. Estimation of relative bioavailability of lead in soil and soil-like materials using young swine. Environ. Health Perspect. 2006, 114, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Weis, C.P.; Lavelle, J.M.; Wels, C.P.; Lavelle, J.M. Characteristics to Consider when Choosing an Animal Model for the Study of Lead Bioavailability Characteristics to Consider when Choosing an Animal Model for the Study of Lead Bioavailability. Chem. Speciat. Bioavailab. 1991, 3, 113–119. [Google Scholar] [CrossRef][Green Version]
- Bradham, K.D.; Scheckel, K.G.; Nelson, C.M.; Seales, P.E.; Lee, G.E.; Hughes, M.F.; Miller, B.W.; Yeow, A.; Gilmore, T.; Harper, S.; et al. Relative Bioavailability and Bioaccessibility and Speciation of Arsenic in Contaminated Soils. Environ. Health Perspect. 2011, 119, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Bradham, K.D.; Diamond, G.L.; Scheckel, K.G.; Hughes, M.F.; Casteel, S.W.; Miller, B.W.; Klotzbach, J.M.; Thayer, W.C.; Thomas, D.J. Mouse Assay for Determination of Arsenic Bioavailability in Contaminated Soils. J. Toxicol. Environ. Health Part A 2013, 76, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Brattin, W.; Drexler, J.; Lowney, Y.; Griffin, S.; Diamond, G.; Woodbury, L. An In Vitro Method for Estimation of Arsenic Relative Bioavailability in Soil. J. Toxicol. Environ. Health Part A 2013, 76, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.L.; Kim, C.S. Arsenic Speciation in Solids Using X-ray Absorption Spectroscopy. Rev. Mineral. Geochem. 2014, 79, 257–369. [Google Scholar] [CrossRef]
- Foster, A.L.; Alpers, C.; Burlak, T.; Blum, A.; Petersen, E.; Basta, N.; Whitacre, S.; Casteel, S.; Kim, C.S.; Brown, A. Arsenic Chemistry, Mineralogy, Speciation, and Bioavailability/Bioaccessibilty in Soils and Mine Waste from the Empire Mine, CA, USA. In Proceedings of the Goldschmidt 2014, Sacramento, CA, USA, 8–13 June 2014; p. 726. [Google Scholar]
- Meunier, L.; Walker, S.R.; Wragg, J.; Parsons, M.B.; Koch, I.; Jamieson, H.E.; Reimer, K.J. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of nova scotia. Environ. Sci. Technol. 2010, 44, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- McClure, F.D. A statistical model to evaluate analyte homogeneity for a material. J. AOAC Int. 2001, 84, 947–954. [Google Scholar] [PubMed]
- Brattin, W.; Casteel, S.W. Measurement of Arsenic Relative Bioavailability in Swine. J. Toxicol. Environ. Health Part A 2013, 7638, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Klementiev, K.V. XAFSmass. Available online: www.cells.es/Beamlines/CLAESS/software/xafsmass.html (accessed on 25 April 2018).
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.M. SIXPack a Graphical User Interface for XAS Analysis Using IFEFFIT. Phys. Scr. 2005, T115, 1011–1014. [Google Scholar] [CrossRef]
- Foster, A.L.; Brown, G.E., Jr.; Tingle, T.N.; Parks, G.A. Quantitative arsenic speciation in mine tailings using X-ray absorption spectroscopy. Am. Mineral. 1998, 83, 553–568. [Google Scholar] [CrossRef]
- Manceau, A. The mechanism of anion adsorption on iron oxides: Evidence for the bonding of arsenate tetrahedra on free Fe(O, OH)6 edges. Geochim. Cosmochim. Acta 1995, 59, 3647–3653. [Google Scholar] [CrossRef]
- Foster, A.L. Spectroscopic Investigations of Arsenic Species in Solid Phases. In Arsenic in Ground Water; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 27–65. [Google Scholar]
- Fieller, E.C. Some Problems in Interval Estimation. J. R. Stat. Soc. Ser. B 1954, 16, 175–185. [Google Scholar]
- Minitab 17 Statistical Software. Minitab, Inc.: State College, PA, USA, 2010. Available online: www.minitab.com (accessed on 4 April 2018).
- Manning, B. Arsenic speciation in As(III)- and As(V)-treated soil using XANES spectroscopy. Microchim. Acta 2005, 151, 181–188. [Google Scholar] [CrossRef]
- Deschamps, E.; Ciminelli, V.S.T.; Weidler, P.G.; Ramos, A.Y. Arsenic sorption onto soils enriched in Mn and Fe minerals. Clay. Clay Miner. 2003, 51, 197–204. [Google Scholar] [CrossRef]
- Reynolds, J.G.; Naylor, D.V.; Fendorf, S.E. Arsenic Sorption in Phosphate-Amended Soils during Flooding and Subsequent Aeration. Soil Sci. Soc. Am. J. 1999, 63, 1149–1156. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Shan, X.-Q.; Jiang, W.; Zhu, Y.-G.; Liu, T.; McLaren, R.G. Arsenate Sorption on Two Chinese Red Soils Evaluated with Macroscopic Measurements and Extended X-Ray Absorption Fine-Structure Spectroscopy. Environ. Toxicol. Chem. 2006, 25, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Arčon, I.; Van Eiteren, J.T.; Glass, H.J.; Kodre, A.; Šlejkovec, Z. EXAFS and XANES study of arsenic in contaminated soil. X-Ray Spectrom. 2005, 34, 435–438. [Google Scholar] [CrossRef]
- Beaulieu, B.T.; Savage, K.S. Arsenate adsorption structures on aluminum oxide and phyllosilicate mineral surfaces in smelter-impacted soils. Environ. Sci. Technol. 2005, 39, 3571–3579. [Google Scholar] [CrossRef]
- Ritchie, V.J.; Ilgen, A.G.; Mueller, S.H.; Trainor, T.P.; Goldfarb, R.J. Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district, Denali National Park and Preserve, Alaska. Chem. Geol. 2013, 335, 172–188. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Compilation and Review of Data on Relative Bioavailability of Arsenic in Soil; OSWER 9200.1-113; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Meunier, L.; Koch, I.; Reimer, K.J. Effects of dissolution kinetics on bioaccessible arsenic from tailings and soils. Chemosphere 2011, 84, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L. Physico-Chemical Parameters Influencing the Bioaccessibility of Arsenic from Tailings and Soils; Royal Military College of Canada: Kingston, ON, Canada, 2011. [Google Scholar]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
| As Species | Molecular Formula | As Oxidation and Covalence Type |
|---|---|---|
| Arsenopyrite | FeAsS | As(-I) |
| Arsenite coppt with pyrite (syn) | FeS2-As | |
| Loellingite | FeAs2 | |
| Orpiment | As2S3 | As(III)-S |
| Realgar | As4S4 | |
| Arsenolite | As2O3 | As(III)-O |
| As (III) ads 1 Ferrihydrite (syn 2) 3 | FeOOH•0.4(H2O)-As(III) | |
| As(III) ads Al2O3 (syn) 3 | Al2O3-As(III) | |
| As(III) ads Montmorillonite (syn) 3 | (Na,Ca)0.33(Al,Mg)2 (Si4O10)(OH)2·nH2O-As(III) | |
| Arseniosiderite | Ca2Fe3(AsO4)3O2•3H2O | As(V)-O |
| Pharmacosiderite | KFe4(AsO4)3(OH)4•6H2O | |
| Scorodite 4 | FeAsO4•2H2O | |
| Parascorodite 4 | FeAsO4•2H2O | |
| Kankite 4 | FeAsO4•3.5H2O | |
| Amorphous ferric arsenate (syn) | FeAsO4•4-7H2O | |
| Arsenate coppt with jarosite (syn) | Na,KFe3(SO4)2(OH)6-As(V) | |
| Arsenate coppt with calcite (syn) | CaCO3-As(V) | |
| Lead Arsenate | PbHAsO4 | |
| As (V) ads Goethite (syn) 5 | α-FeO(OH)-As(V) | |
| As (V) ads Ferrihydrite (syn) 5 | FeOOH•0.4(H2O)-As(V) | |
| As (V) ads Birnessite (syn) 5 | MnO2-As(V) | |
| As(V) ads Gibbsite (syn) 5 | Al(OH)3-As(V) |
| Sample | As Source | Total As (mg kg−1) | Mouse RBA | Swine RBA | Arsenopyrite | Arseniosiderite | Ferric Arsenate (Scorodite, Kankite) | Am. Ferric Arsenate | As(V) Coppt Jarosite | As(V) Coppt Calcite | As(III) Adsorbed | As(V) Adsorbed | R-Factor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 33 | Au mining | 302 | 8.55 | 23.7 | 7 | 93 | 0.060 | ||||||
| 37 | Au mining | 370 | 9.83 | 11.7 | 54 | 30 | 16 | 0.084 | |||||
| 35 | Au mining | 633 | 16.1 | 19.2 | 46 | 54 | 0.046 | ||||||
| 34 | Au mining | 2541 | 6.37 | 15.3 | 38 | 42 | 20 | 0.029 | |||||
| 36 | Au mining | 10,482 | 4 | 70 | 30 | 0.158 | |||||||
| 38 | Au mining | 12,041 | 23 | 19 | 24 | 56 | 0.026 | ||||||
| 30 | Glass Works | 3996 | 26 | 36 | 41 | 23 | 0.167 | ||||||
| 29 | Glass Works | 4553 | 48 | 63 | 37 | 0.057 | |||||||
| 11 | Mining | 249 | 44.8 | 60 | 75 | 25 | 0.026 | ||||||
| 6 | Mining | 839 | 41.7 | 42 | 12 | 46 | 0.032 | ||||||
| 12 | Mining | 1236 | 39.7 | 63 | 13 | 24 | 0.137 | ||||||
| 10 | Mining | 3913 | 12.9 | 19 | 64 | 15 | 21 | 0.012 | |||||
| 13 | Mining | 12,483 | 7.87 | 63 | 37 | 0 | 0.147 | ||||||
| 3 | Pesticide | 222 | 43.5 | 100 | 0.110 | ||||||||
| 18 | Pesticide | 283 | 30 | 31 | 100 | 0.114 | |||||||
| 7 | Pesticide | 332 | 34 | 54.3 | 54 | 32 | 14 | 0.020 | |||||
| 19 | Pesticide | 353 | 46.1 | 41 | 100 | 0.105 | |||||||
| 21 | Pesticide | 375 | 39.4 | 53 | 100 | 0.329 | |||||||
| 20 | Pesticide | 391 | 21.5 | 49 | 100 | 0.259 | |||||||
| 1 | Pesticide | 464 | 20.2 | 90 | 10 | 0.418 | |||||||
| 2 | Pesticide | 641 | 29.1 | 39.5 | 47 | 53 | 0.037 | ||||||
| 8 | Smelter | 162 | 29.9 | 54.9 | 47 | 41 | 12 | 0.063 | |||||
| 16 | Spiked | 226 | 81.2 | 30 | 14 | 56 | 0.093 | ||||||
| 14 | Spiked | 238 | 79.7 | 52 | 48 | 0.072 | |||||||
| 15 | Spiked | 259 | 69.7 | 66 | 34 | 0.149 | |||||||
| 17 | SRM | 1540 | 41.4 | 41.8 | 23 | 26 | 51 | 0 | 0.040 | ||||
| 9 | Tailings | 521 | 14 | 32 | 39 | 29 | 0.062 |
| Species Group | Mineral Phase | ||
|---|---|---|---|
| 1 | Sulfides | Arsenopyrite | Least Bioaccessible |
| Realgar | |||
| Pyrite | |||
| 2 | Iron Arsenates | Scorodite | |
| Kankite | |||
| Pharmacosiderite | |||
| Amorphous | |||
| 3 | Arsenic bearing Iron(oxy) Hydroxides | Goethite | |
| Lepidocrocite | |||
| Akaganeite | |||
| Amorphous | |||
| 4 | Roaster Iron Oxides | Hematite | |
| Maghemite | |||
| 5 | Sulfates | Tooeleite | |
| Jarosite | |||
| Schwertmannite | |||
| 6 | Clay minerals—Generally Iron Bearing | Undifferentiated | |
| 7 | Calcium-Iron Arsenates | Yukonite | Most Bioaccessible |
| amorphous | |||
| Adapted from Meunier et al. [12] | |||
| Mouse RBA (%) | Swine RBA (%) | |||||
|---|---|---|---|---|---|---|
| ID | Mean | CI a | Predicted RBA | Mean | CI a | Predicted RBA |
| 1 | 20.2 | 18.1, 22.4 | 21.8 *,# | |||
| 2 | 29.1 | 26.0, 32.3 | 18.8 | 39.5 + | 35.8, 43.1 + | 41.7 *,# |
| 3 | 43.5 | 37.9, 49.2 | 36.2 | |||
| 6 | 41.7 | 34.5, 48.8 | 43.2 *,# | |||
| 7 | 34.0 | 29.8, 38.3 | 49.1 # | 52.3 + | 54.3, 58.4 + | 42.8 |
| 8 | 29.9 | 26.6, 33.3 | 20.2 | 54.9 + | 50.4, 59.4 + | 52.6 * |
| 9 | 14 | 13, 15 | 30.0 # | |||
| 10 | 12.5 | 2.57, 22.4 | 17.4*,# | 19 | 17, 20 | 27.4 # |
| 11 | 44.8 | 41.6, 48.2 | 21.1 | 60 | 56, 65 | 40.1 |
| 12 | 39.7 | 38.7, 40.7 | 27.0 | |||
| 13 | 7.87 | 4.33, 11.4 | 21.1 # | |||
| 14 | 79.7 | 73.8, 85.9 | 51.2 | |||
| 15 | 69.7 | 65.9, 73.6 | 55.3 | |||
| 16 | 81.2 | 70.9, 91.7 | 54.0 | |||
| 17 | 41.4 | 39.1, 43.6 | 27.6 | 41.8 | 39, 45 | 26.4 |
| 18 | 30.0 | 27.4, 32.7 | 36.2 # | 31 | 25, 38 | 38.3 # |
| 19 | 46.1 | 41.8, 50.5 | 36.2 | 41 | 38, 44 | 38.3 * |
| 20 | 21.5 | 17.6, 25.3 | 36.2 # | 49 | 42, 57 | 38.3 |
| 21 | 39.4 | 36.1, 42.8 | 36.2 * | 53 | 49, 57 | 38.3 |
| 29 | 48 | 45, 51 | 20.3 | |||
| 30 | 26 | 24, 28 | 30.9 # | |||
| 33 | 8.55 | 6.51, 10.6 | 35.0 # | 23.7 | 10.9, 36.5 | 40.6 # |
| 34 | 6.37 | 5.33, 7.43 | 38.7 # | 15.3 | 11.7, 18.8 | 28.4 # |
| 35 | 16.1 | 15.2, 17.0 | 26.9 # | 19.2 | 16.9, 21.4 | 39.4 # |
| 36 | 4 | 3.3, 4.6 | 4.00 *,# | |||
| 37 | 9.83 | 8.82, 10.9 | 45.8 # | 11.7 | 8.3, 15.2 | 23.5 # |
| 38 | 23 | 17.6, 28.5 | 23.1 *,# | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, B.N.; Betts, A.R.; Miller, B.W.; Scheckel, K.G.; Anderson, R.H.; Bradham, K.D.; Casteel, S.W.; Thomas, D.J.; Basta, N.T. Arsenic Speciation of Contaminated Soils/Solid Wastes and Relative Oral Bioavailability in Swine and Mice. Soil Syst. 2018, 2, 27. https://doi.org/10.3390/soilsystems2020027
Stevens BN, Betts AR, Miller BW, Scheckel KG, Anderson RH, Bradham KD, Casteel SW, Thomas DJ, Basta NT. Arsenic Speciation of Contaminated Soils/Solid Wastes and Relative Oral Bioavailability in Swine and Mice. Soil Systems. 2018; 2(2):27. https://doi.org/10.3390/soilsystems2020027
Chicago/Turabian StyleStevens, Brooke N., Aaron R. Betts, Bradley W. Miller, Kirk G. Scheckel, Richard H. Anderson, Karen D. Bradham, Stan W. Casteel, David J. Thomas, and Nicholas T. Basta. 2018. "Arsenic Speciation of Contaminated Soils/Solid Wastes and Relative Oral Bioavailability in Swine and Mice" Soil Systems 2, no. 2: 27. https://doi.org/10.3390/soilsystems2020027
APA StyleStevens, B. N., Betts, A. R., Miller, B. W., Scheckel, K. G., Anderson, R. H., Bradham, K. D., Casteel, S. W., Thomas, D. J., & Basta, N. T. (2018). Arsenic Speciation of Contaminated Soils/Solid Wastes and Relative Oral Bioavailability in Swine and Mice. Soil Systems, 2(2), 27. https://doi.org/10.3390/soilsystems2020027






